The Effects of Model Insoluble Copper Compounds in a Sedimentary Environment on Denitrifying Anaerobic Methane Oxidation (DAMO) Enrichment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum and the Enrichment of DAMO Microorganisms
2.2. The Tests for Copper Influence and the Associated Copper Compound Synthesis
2.3. Slurry Sampling, Staining, DNA Extraction, Library Preparation and Metagenome Sequencing, and Bioinformatics
2.4. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.5. Instrumental Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Toxicity Tests and Copper Speciation
3.1.1. Enrichment and Variations in DAMO Activity
3.1.2. Variation of Copper Speciation

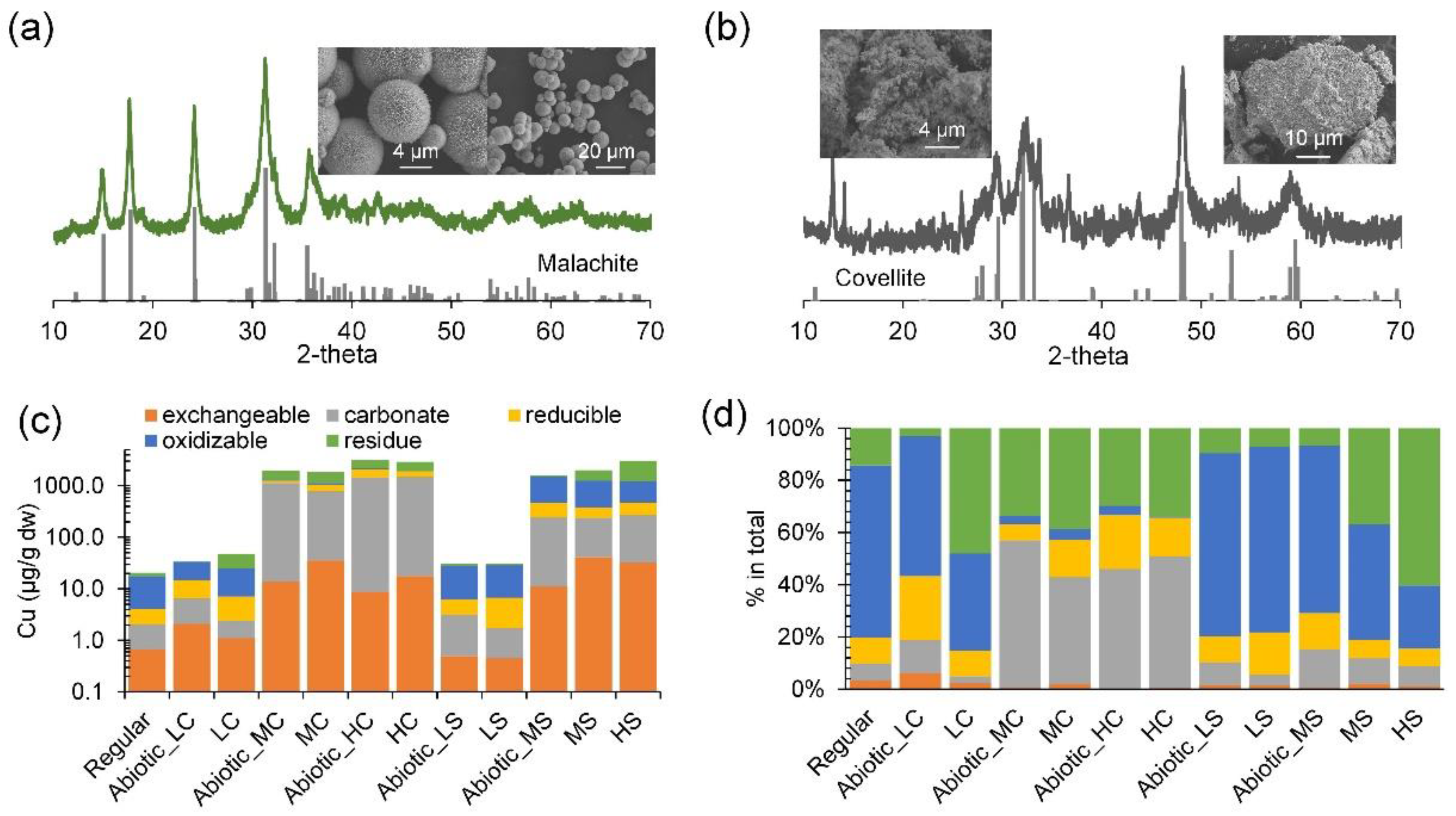
3.2. Shift in Microbial Composition
3.2.1. Overall Microbial Composition Changes
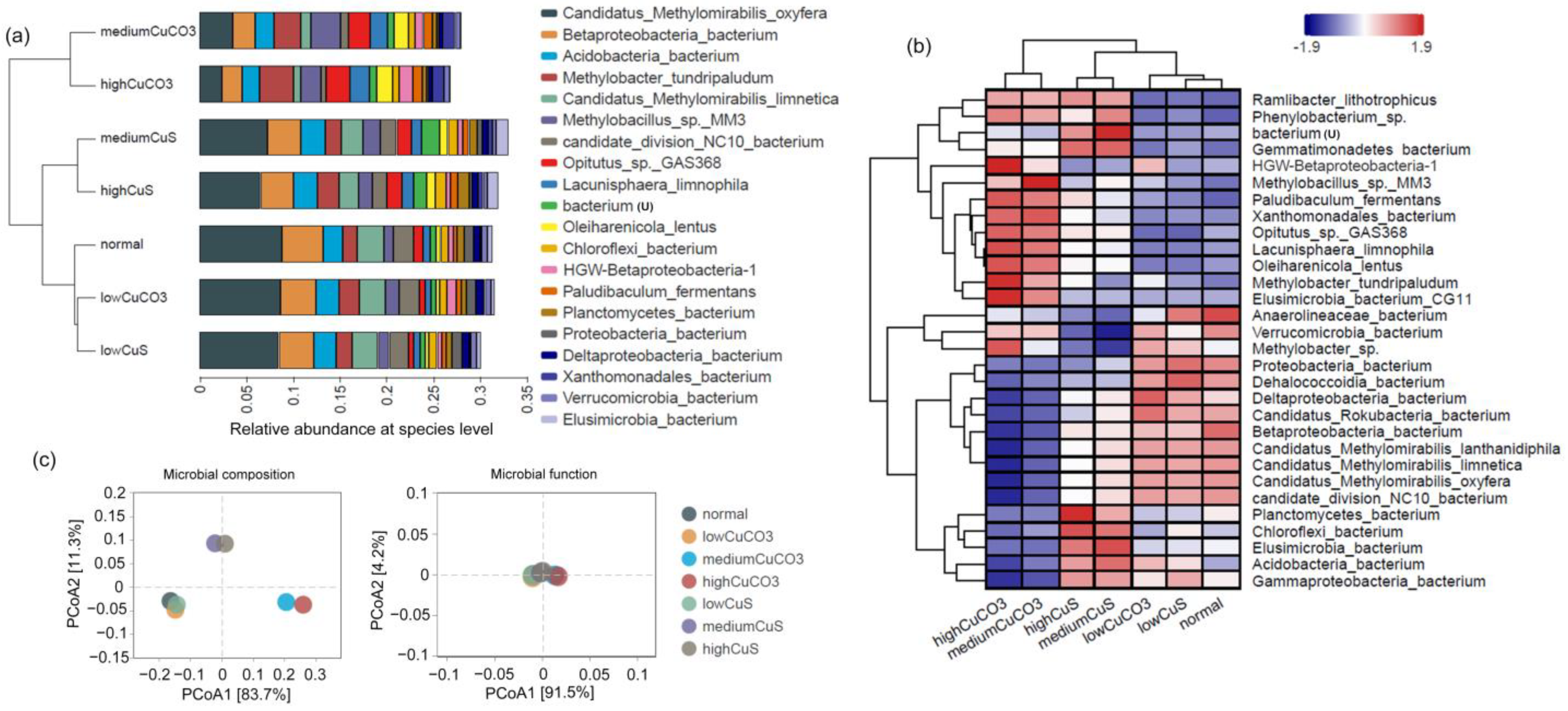
3.2.2. Variation in Representative DAMO Microorganisms
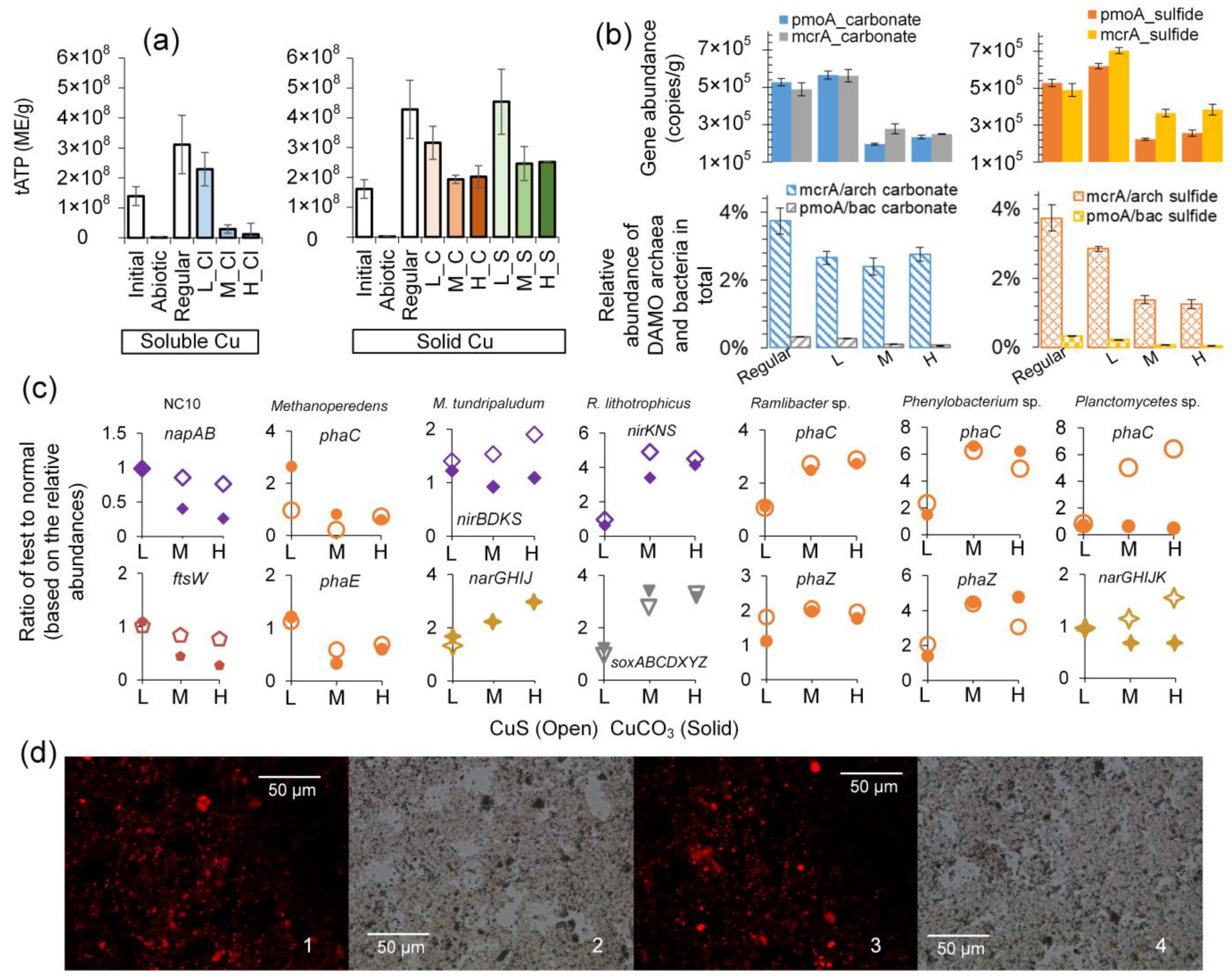
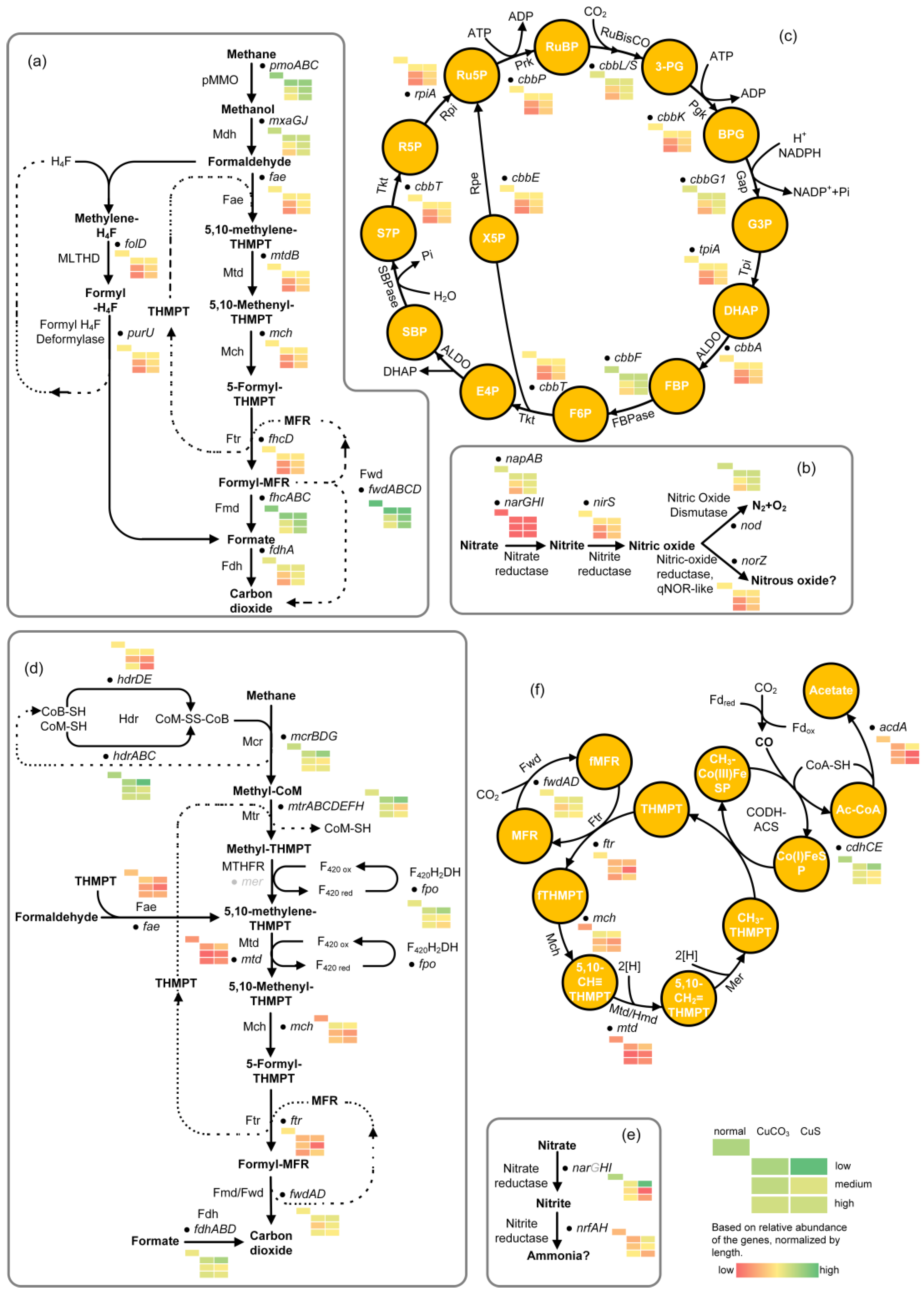
3.3. Effects of Copper on Major DAMO Pathways
3.4. Difference in Energy Content Could Be a Determining Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.-L.; Shen, L.-D.; Lian, X.; Zhu, Q.; Liu, S.; Huang, Q.; He, Z.-F.; Geng, S.; Cheng, D.-Q.; Lou, L.-P.; et al. Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Natl. Acad. Sci. USA 2014, 111, 4495. [Google Scholar] [CrossRef] [PubMed]
- Segarra, K.E.A.; Schubotz, F.; Samarkin, V.; Yoshinaga, M.Y.; Hinrichs, K.U.; Joye, S.B. High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat. Commun. 2015, 6, 7477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hou, L.; Chen, F.; Zhou, J.; Liu, M.; Yin, G.; Gao, J.; Han, P. Denitrifying anaerobic methane oxidation in intertidal marsh soils: Occurrence and environmental significance. Geoderma 2020, 357, 113943. [Google Scholar] [CrossRef]
- Yang, W.-T.; Wang, W.-Q.; Shen, L.-D.; Bai, Y.-N.; Liu, X.; Tian, M.-H.; Wang, C.; Feng, Y.-F.; Liu, Y.; Yang, Y.-L.; et al. Potential role of nitrite-dependent anaerobic methane oxidation in methane consumption and nitrogen removal in Chinese paddy fields. Sci. Total Environ. 2022, 838, 156534. [Google Scholar] [CrossRef]
- Padilla, C.C.; Bristow, L.A.; Sarode, N.; Garcia-Robledo, E.; Gómez Ramírez, E.; Benson, C.R.; Bourbonnais, A.; Altabet, M.A.; Girguis, P.R.; Thamdrup, B.; et al. NC10 bacteria in marine oxygen minimum zones. ISME J. 2016, 10, 2067–2071. [Google Scholar] [CrossRef]
- Steinle, L.; Maltby, J.; Treude, T.; Kock, A.; Bange, H.W.; Engbersen, N.; Zopfi, J.; Lehmann, M.F.; Niemann, H. Effects of low oxygen concentrations on aerobic methane oxidation in seasonally hypoxic coastal waters. Biogeosciences 2017, 14, 1631–1645. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Tong, T.; Xie, S.; Liu, Y. Influences of eutrophication on methanogenesis pathways and methanogenic microbial community structures in freshwater lakes. Environ. Pollut. 2020, 260, 114106. [Google Scholar] [CrossRef]
- Jilbert, T.; Cowie, G.; Lintumäki, L.; Jokinen, S.; Asmala, E.; Sun, X.; Mörth, C.-M.; Norkko, A.; Humborg, C. Anthropogenic Inputs of Terrestrial Organic Matter Influence Carbon Loading and Methanogenesis in Coastal Baltic Sea Sediments. Front. Earth Sci. 2021, 9, 716416. [Google Scholar] [CrossRef]
- Raghoebarsing, A.A.; Pol, A.; Van de Pas-Schoonen, K.T.; Smolders, A.J.; Ettwig, K.F.; Rijpstra, W.I.C.; Schouten, S.; Damsté, J.S.S.; Op den Camp, H.J.; Jetten, M.S.M.; et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 2006, 440, 918–921. [Google Scholar] [CrossRef]
- Ettwig, K.F.; Butler, M.K.; Le Paslier, D.; Pelletier, E.; Mangenot, S.; Kuypers, M.M.M.; Schreiber, F.; Dutilh, B.E.; Zedelius, J.; De Beer, D.; et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010, 464, 543–548. [Google Scholar] [CrossRef]
- Haroon, M.F.; Hu, S.; Shi, Y.; Imelfort, M.; Keller, J.; Hugenholtz, P.; Yuan, Z.; Tyson, G.W. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 2013, 500, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Lee, H.-S.; Jin, P. Significance of anaerobic oxidation of methane (AOM) in mitigating methane emission from major natural and anthropogenic sources: A review of AOM rates in recent publications. Environ. Sci. Adv. 2022, 1, 401–425. [Google Scholar] [CrossRef]
- Yang, W.-T.; Shen, L.-D.; Bai, Y.-N. Role and regulation of anaerobic methane oxidation catalyzed by NC10 bacteria and ANME-2d archaea in various ecosystems. Environ. Res. 2023, 219, 115174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, X.; Jia, Z.; Zhu, L.; Zheng, P.; Kartal, B.; Hu, B. Nitrogen input promotes denitrifying methanotrophs’ abundance and contribution to methane emission reduction in coastal wetland and paddy soil. Environ. Pollut. 2022, 302, 119090. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zheng, Y.; Hou, L.; Niu, Y.; Gao, D.; An, Z.; Zhou, J.; Yin, G.; Dong, H.; Han, P.; et al. Microbial abundance and activity of nitrite/nitrate-dependent anaerobic methane oxidizers in estuarine and intertidal wetlands: Heterogeneity and driving factors. Water Res. 2021, 190, 116737. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, L.; Bai, Y.; Zhao, X.; Wang, S.; Liu, J.; Liu, X.; Tian, M.; Yang, W.; Jin, J.; et al. Response of potential activity, abundance and community composition of nitrite-dependent anaerobic methanotrophs to long-term fertilization in paddy soils. Environ. Microbiol. 2022, 24, 5005–5018. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; Wang, Y.; Li, T.; Huang, J. NO3− is an important driver of nitrite-dependent anaerobic methane oxidation bacteria and CH4 fluxes in the reservoir riparian zone. Environ. Sci. Pollut. Res. 2022, 29, 16138–16151. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.-G.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Cui, F.; Zhu, M.-Y.; Shen, L.-Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Macklin, M.G.; Thomas, C.J.; Mudbhatkal, A.; Brewer, P.A.; Hudson-Edwards, K.A.; Lewin, J.; Scussolini, P.; Eilander, D.; Lechner, A.; Owen, J.; et al. Impacts of metal mining on river systems: A global assessment. Science 2023, 381, 1345–1350. [Google Scholar] [CrossRef]
- Rader, K.J.; Carbonaro, R.F.; van Hullebusch, E.D.; Baken, S.; Delbeke, K. The Fate of Copper Added to Surface Water: Field, Laboratory, and Modeling Studies. Environ. Toxicol. Chem. 2019, 38, 1386–1399. [Google Scholar] [CrossRef]
- Shao, M.-F.; Zhang, T.; Fang, H.H.-P. Sulfur-driven autotrophic denitrification: Diversity, biochemistry, and engineering applications. Appl. Microbiol. Biotechnol. 2010, 88, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.R.; Bennett, P.C. Mineral stimulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Dooley, D.M.; Chan, J.M. Copper Enzymes in Denitrification. In Encyclopedia of Inorganic Chemistry; Scott, R.A., Ed.; JohnWiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kulczycki, E.; Fowle, D.A.; Kenward, P.A.; Leslie, K.; Graham, D.W.; Roberts, J.A. Stimulation of Methanotroph Activity by Cu-Substituted Borosilicate Glass. Geomicrobiol. J. 2011, 28, 1–10. [Google Scholar] [CrossRef]
- Kampman, C.; Temmink, H.; Hendrickx, T.L.G.; Zeeman, G.; Buisman, C.J.N. Enrichment of denitrifying methanotrophic bacteria from municipal wastewater sludge in a membrane bioreactor at 20 °C. J. Hazard. Mater. 2014, 274, 428–435. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Geng, S.; Pan, Y.; Cai, C.; Wang, J.; Wang, L.; Liu, S.; Zheng, P.; Xu, X.; Hu, B. Improvement of the trace metal composition of medium for nitrite-dependent anaerobic methane oxidation bacteria: Iron (II) and copper (II) make a difference. Water Res. 2015, 85, 235–243. [Google Scholar] [CrossRef]
- Hatamoto, M.; Nemoto, S.; Yamaguchi, T. Effects of Copper and PQQ on the Denitrification Activities of Microorganisms Facilitating Nitrite- and Nitrate-Dependent DAMO Reaction. Int. J. Environ. Res. 2018, 12, 749–753. [Google Scholar] [CrossRef]
- Fu, L.; Li, S.-W.; Ding, Z.-W.; Ding, J.; Lu, Y.-Z.; Zeng, R.J. Iron reduction in the DAMO/Shewanella oneidensis MR-1 coculture system and the fate of Fe(II). Water Res. 2016, 88, 808–815. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Fu, L.; Ding, J.; Ding, Z.-W.; Li, N.; Zeng, R.J. Cr(VI) reduction coupled with anaerobic oxidation of methane in a laboratory reactor. Water Res. 2016, 102, 445–452. [Google Scholar] [CrossRef]
- Liang, L.; Sun, C.; Jin, Z.; Wang, M.; Yu, Q.; Zhao, Z.; Zhang, Y. Magnetite-mediated electrically connected community for shortening startup of methane-dependent denitrification in a membrane biofilm reactor. Chem. Eng. J. 2022, 428, 132004. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, G.; Li, Z.; Xu, F.; Yang, N.; Sun, X.; Xu, R.; Sun, W. Effects of antimony on anaerobic methane oxidization and microbial community in an antimony-contaminated paddy soil: A microcosm study. Sci. Total Environ. 2021, 784, 147239. [Google Scholar] [CrossRef]
- Cervi, E.C.; Clark, S.; Boye, K.E.; Gustafsson, J.P.; Baken, S.; Burton, G.A. Copper transformation, speciation, and detoxification in anoxic and suboxic freshwater sediments. Chemosphere 2021, 282, 131063. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, J.; Li, X.; Wu, T.; Hu, Z.; Wang, S. Ecological risk by heavy metal contents in sediments within the Wei River Basin, China. Environ. Earth Sci. 2019, 78, 101. [Google Scholar] [CrossRef]
- Cai, C.; Leu, A.O.; Xie, G.; Guo, J.; Feng, Y.; Zhao, J.; Tyson, G.W.; Yuan, Z.; Hu, S. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 12, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Ettwig, K.F.; Alen, T.v.; Pas-Schoonen, K.T.v.d.; Jetten, M.S.M.; Strous, M. Enrichment and Molecular Detection of Denitrifying Methanotrophic Bacteria of the NC10 Phylum. Appl. Environ. Microbiol. 2009, 75, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, F.; Schroth, M.H.; Bürgmann, H.; Zeyer, J. Physical Extraction of Microorganisms from Water-Saturated, Packed Sediment. Water Environ. Res. 2014, 86, 407–416. [Google Scholar] [CrossRef]
- Riis, V.; Lorbeer, H.; Babel, W. Extraction of microorganisms from soil: Evaluation of the efficiency by counting methods and activity measurements. Soil Biol. Biochem. 1998, 30, 1573–1581. [Google Scholar] [CrossRef]
- Luesken, F.A.; Zhu, B.; van Alen, T.A.; Butler, M.K.; Diaz, M.R.; Song, B.; Op den Camp, H.J.M.; Jetten, M.S.M.; Ettwig, K.F. pmoA primers for detection of anaerobic methanotrophs. Appl. Environ. Microbiol. 2011, 77, 3877–3880. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Jetten, M.S.M.; Ettwig, K.F.; Lüke, C. McrA primers for the detection and quantification of the anaerobic archaeal methanotroph Candidatus Methanoperedens nitroreducens. Appl. Microbiol. Biotechnol. 2017, 101, 1631–1641. [Google Scholar] [CrossRef]
- Shen, L.-D.; Ouyang, L.; Zhu, Y.; Trimmer, M. Active pathways of anaerobic methane oxidation across contrasting riverbeds. ISME J. 2019, 13, 752–766. [Google Scholar] [CrossRef]
- Gao, Y.; Lee, J.; Neufeld, J.D.; Park, J.; Rittmann, B.E.; Lee, H.-S. Anaerobic oxidation of methane coupled with extracellular electron transfer to electrodes. Sci. Rep. 2017, 7, 5099. [Google Scholar] [CrossRef]
- Gao, Y.; Trueman, B.F.; Li, B.; Earle, M.R.; Gagnon, G.A. Release and migration of Pb from Pb(ii) and Pb(iv) compounds in the presence of microbiological activity. Environ. Sci. Water Res. Technol. 2022, 8, 2905–2916. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Gardham, S.; Chariton, A.A.; Hose, G.C. Direct and indirect effects of copper-contaminated sediments on the functions of model freshwater ecosystems. Ecotoxicology 2015, 24, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Huguenot, D.; Bois, P.; Cornu, J.Y.; Jezequel, K.; Lollier, M.; Lebeau, T. Remediation of sediment and water contaminated by copper in small-scaled constructed wetlands: Effect of bioaugmentation and phytoextraction. Environ. Sci. Pollut. Res. 2015, 22, 721–732. [Google Scholar] [CrossRef]
- Guo, Q.; Li, N.; Bing, Y.; Chen, S.; Zhang, Z.; Chang, S.; Chen, Y.; Xie, S. Denitrifier communities impacted by heavy metal contamination in freshwater sediment. Environ. Pollut. 2018, 242, 426–432. [Google Scholar] [CrossRef]
- Sakadevan, K.; Zheng, H.; Bavor, H.J. Impact of heavy metals on denitrification in surface wetland sediments receiving wastewater. Water Sci. Technol. 1999, 40, 349–355. [Google Scholar] [CrossRef]
- Jacinthe, P.-A.; Tedesco, L.P. Impact of Elevated Copper on the Rate and Gaseous Products of Denitrification in Freshwater Sediments. J. Environ. Qual. 2009, 38, 1183–1192. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Machado, A.; Matos, P.; Bordalo, A.A. Impact of copper on the diversity, abundance and transcription of nitrite and nitrous oxide reductase genes in an urban European estuary. FEMS Microbiol. Ecol. 2011, 77, 274–284. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Buchner, D.; Wang, H.; Xu, H.; Haderlein, S.B.; Zhu, Y. Increased copper levels inhibit denitrification in urban soils. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 109, 421–427. [Google Scholar] [CrossRef]
- Black, A.; Hsu, P.-C.L.; Hamonts, K.E.; Clough, T.J.; Condron, L.M. Influence of copper on expression of nirS, norB and nosZ and the transcription and activity of NIR, NOR and N2OR in the denitrifying soil bacteria Pseudomonas stutzeri. Microb. Biotechnol. 2016, 9, 381–388. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, Z.; Wang, Y.; Ru, D.; Li, J.; Fan, J. Effect of trace elements on the development of co-cultured nitrite-dependent anaerobic methane oxidation and methanogenic bacteria consortium. Bioresour. Technol. 2018, 268, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lai, C.-Y.; Wu, M.; Lu, X.; Hu, S.; Yuan, Z.; Guo, J. Copper stimulation on methane-supported perchlorate reduction in a membrane biofilm reactor. J. Hazard. Mater. 2022, 425, 127917. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Herrera, V.; León, G.; Banihani, Q.; Field, J.A.; Sierra-Alvarez, R. Toxicity of copper(II) ions to microorganisms in biological wastewater treatment systems. Sci. Total Environ. 2011, 412–413, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; McIlroy, S.J.; Tyson, G.W.; Guo, J. Phylogenetic and metabolic diversity of microbial communities performing anaerobic ammonium and methane oxidations under different nitrogen loadings. ISME Commun. 2023, 3, 39. [Google Scholar] [CrossRef]
- Macey, M.C.; Pratscher, J.; Crombie, A.; Murrell, J.C. Draft Genome Sequences of Obligate Methylotrophs Methylovorus sp. Strain MM2 and Methylobacillus sp. Strain MM3, Isolated from Grassland Soil. Microbiol. Resour. Announc. 2018, 7, 10–1128. [Google Scholar] [CrossRef]
- Kulichevskaya, I.S.; Suzina, N.E.; Rijpstra, W.I.C.; Damsté, J.S.S.; Dedysh, S.N. Paludibaculum fermentans gen. nov., sp. nov., a facultative anaerobe capable of dissimilatory iron reduction from subdivision 3 of the Acidobacteria. Int. J. Syst. Evol. Microbiol. 2014, 64, 2857–2864. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Karst, S.M.; Nierychlo, M.; Dueholm, M.S.; Albertsen, M.; Kirkegaard, R.H.; Seviour, R.J.; Nielsen, P.H. Genomic and in situ investigations of the novel uncultured Chloroflexi associated with 0092 morphotype filamentous bulking in activated sludge. ISME J. 2016, 10, 2223–2234. [Google Scholar] [CrossRef]
- Méheust, R.; Castelle, C.J.; Matheus Carnevali, P.B.; Farag, I.F.; He, C.; Chen, L.-X.; Amano, Y.; Hug, L.A.; Banfield, J.F. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. ISME J. 2020, 14, 2907–2922. [Google Scholar] [CrossRef]
- Dumont, M.G.; Pommerenke, B.; Casper, P. Using stable isotope probing to obtain a targeted metatranscriptome of aerobic methanotrophs in lake sediment. Environ. Microbiol. Rep. 2013, 5, 757–764. [Google Scholar] [CrossRef]
- Svenning, M.M.; Hestnes, A.G.; Wartiainen, I.; Stein, L.Y.; Klotz, M.G.; Kalyuzhnaya, M.G.; Spang, A.; Bringel, F.; Vuilleumier, S.; Lajus, A.; et al. Genome Sequence of the Arctic Methanotroph Methylobacter tundripaludum SV96. J. Bacteriol. 2011, 193, 6418–6419. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Line, L.; Schrameyer, V.; Jakobsen, T.H.; Rybtke, M.L.; Toyofuku, M.; Nomura, N.; Kolpen, M.; Tolker-Nielsen, T.; Kühl, M.; et al. Nitric-oxide-driven oxygen release in anoxic Pseudomonas aeruginosa. iScience 2021, 24, 103404. [Google Scholar] [CrossRef] [PubMed]
- Nayfach, S.; Pollard, K.S. Toward Accurate and Quantitative Comparative Metagenomics. Cell 2016, 166, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; McFarland, A.G.; Young, V.B.; Hayden, M.K.; Hartmann, E.M. Toward Accurate and Robust Environmental Surveillance Using Metagenomics. Front. Genet. 2021, 12, 600111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dumont, M.G.; Bodelier, P.L.E.; Adams, J.M.; He, D.; Chu, H. DNA stable-isotope probing highlights the effects of temperature on functionally active methanotrophs in natural wetlands. Soil Biol. Biochem. 2020, 149, 107954. [Google Scholar] [CrossRef]
- Cai, C.; Shi, Y.; Guo, J.; Tyson, G.W.; Hu, S.; Yuan, Z. Acetate Production from Anaerobic Oxidation of Methane via Intracellular Storage Compounds. Environ. Sci. Technol. 2019, 53, 7371–7379. [Google Scholar] [CrossRef]
- Müller-Santos, M.; Koskimäki, J.J.; Alves, L.P.S.; de Souza, E.M.; Jendrossek, D.; Pirttilä, A.M. The protective role of PHB and its degradation products against stress situations in bacteria. FEMS Microbiol. Rev. 2020, 45, fuaa058. [Google Scholar] [CrossRef]
- Macey, M.C.; Pratscher, J.; Crombie, A.T.; Murrell, J.C. Impact of plants on the diversity and activity of methylotrophs in soil. Microbiome 2020, 8, 31. [Google Scholar] [CrossRef]
- Gao, Y.; Trueman, B.F.; Gagnon, G.A. Early phase effects of silicate and orthophosphate on lead (Pb) corrosion scale development and Pb release. J. Environ. Manag. 2022, 321, 115947. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken Under the Auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Hu, S.; Hu, J.; Sun, Y.; Zhu, Q.; Wu, L.; Liu, B.; Xiao, K.; Liang, S.; Yang, J.; Hou, H. Simultaneous heavy metal removal and sludge deep dewatering with Fe(II) assisted electrooxidation technology. J. Hazard. Mater. 2021, 405, 124072. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Sahuquillo, A.; Lopez Sanchez, J.F. A Review of the Different Methods Applied in Environmental Geochemistry for Single and Sequential Extraction of Trace Elements in Soils and Related Materials. Water Air Soil Pollut. 2008, 189, 291–333. [Google Scholar] [CrossRef]
- Gao, Y.; Ryu, H.; Rittmann, B.E.; Hussain, A.; Lee, H.-S. Quantification of the methane concentration using anaerobic oxidation of methane coupled to extracellular electron transfer. Bioresour. Technol. 2017, 241, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Wang, Y.; Yao, P.; Ryu, H.; Dong, Z.; Tan, C.; Deng, S.; Liao, H.; Gao, Y. The Effects of Model Insoluble Copper Compounds in a Sedimentary Environment on Denitrifying Anaerobic Methane Oxidation (DAMO) Enrichment. Microorganisms 2024, 12, 2259. https://doi.org/10.3390/microorganisms12112259
Xia L, Wang Y, Yao P, Ryu H, Dong Z, Tan C, Deng S, Liao H, Gao Y. The Effects of Model Insoluble Copper Compounds in a Sedimentary Environment on Denitrifying Anaerobic Methane Oxidation (DAMO) Enrichment. Microorganisms. 2024; 12(11):2259. https://doi.org/10.3390/microorganisms12112259
Chicago/Turabian StyleXia, Longfei, Yong Wang, Peiru Yao, Hodon Ryu, Zhengzhong Dong, Chen Tan, Shihai Deng, Hongjian Liao, and Yaohuan Gao. 2024. "The Effects of Model Insoluble Copper Compounds in a Sedimentary Environment on Denitrifying Anaerobic Methane Oxidation (DAMO) Enrichment" Microorganisms 12, no. 11: 2259. https://doi.org/10.3390/microorganisms12112259
APA StyleXia, L., Wang, Y., Yao, P., Ryu, H., Dong, Z., Tan, C., Deng, S., Liao, H., & Gao, Y. (2024). The Effects of Model Insoluble Copper Compounds in a Sedimentary Environment on Denitrifying Anaerobic Methane Oxidation (DAMO) Enrichment. Microorganisms, 12(11), 2259. https://doi.org/10.3390/microorganisms12112259






