Abstract
Panax plants are known for their significant medicinal and economic value. Being perennial, they are prone to soil-borne diseases during cultivation. However, there has been limited research on the pathogenesis of soil-borne diseases and the diversity of pathogens. While biological control has gained attention for its efficacy and environmental benefits, the factors affecting its efficiency still need thorough evaluation. This review summarizes the influence of biotic factors, such as pathogens and hosts, and environmental factors on the occurrence of soil-borne diseases and pathogen diversity. Additionally, we synthesized bacterial, actinobacterial, and fungal diversity for the biocontrol of soil-borne diseases and their functional mechanisms. Moreover, the review delves into the factors influencing the efficacy of biocontrol, including microbial species, the inoculation method and inoculation volume, and inoculant composition. This article serves as a valuable resource for enhancing the efficiency of biological control and optimizing strategies for managing soil-borne diseases in Panax cultivation in the future.
1. Introduction
The Araliaceae family encompasses a wide array of plants, consisting of over 1500 species [1], with 114 of these species having medicinal properties that hold significant importance for human health. Among these Araliaceae plants, Panax species, such as Panax ginseng, P. quinquefolium, P. notoginseng, P. japonicum, and P. trifolium, possess valuable medicinal attributes, including tumor inhibition, blood lipid reduction, antithrombotic effects, promotion of blood circulation, and removal of blood stasis [2,3]. Moreover, Panax plants have been widely used as raw materials for healthcare products, cosmetics and dietary supplements [4]. For example, Panax ginseng alone is projected to reach a global trade volume of around $17.7 billion by 2030 [5]. Consequently, the market demand for these plants continues to grow, necessitating large-scale cultivation. However, their perennial nature renders them susceptible to frequent occurrence of soil-borne diseases, which significantly impacts the yield and quality of the plants [6].
Soil-borne diseases are plant diseases that spread through soil [7]. Soil-borne pathogens could be bacteria, fungi, or viruses and can cause different plant diseases. It is widely recognized that fungi are the primary pathogens responsible for soil-borne diseases in Panax [8,9], causing root or foliar diseases [10,11]. Understanding the diversity of pathogens is crucial for effective prevention and control of soil borne diseases [12]. Regrettably, there is currently a lack of systematic knowledge regarding pathogen diversity of Panax plants. Additionally, when a disease occurs, in addition to pathogens, there is a lack of knowledge regarding the susceptible host and favorable environments that allow the pathogens to thrive [7]. However, a comprehensive summary of these factors specific to Panax plants’ soil-borne diseases is currently lacking.
Traditionally, chemical control has been widely used in the management of soil-borne diseases. However, the extensive use of pesticides not only causes cost increases, drug residues, environmental pollution, and pathogen resistance, but also affects soil microecological balance [13]. Biological control, on the other hand, is an important alternative to chemical control, as it provides an effective and environmentally friendly strategy for managing soil-borne diseases [14]. Studies have reported that biocontrol microorganisms play a crucial role by either antagonizing pathogens or regulating plant disease resistance [15]. Understanding and analyzing the biocontrol mechanisms against pathogens is of great significance in developing more efficient biocontrol strategies. However, the exploration of soil-borne disease biocontrol in Panax plants is still limited and requires further investigation to enhance our understanding.
In this study, we firstly present a comprehensive summary of soil-borne disease pathogenesis and the diversity of pathogens in Panax plants. Then, we hypothesize that biological control is effective in managing soil-borne diseases and that biological control factors play a significant role in disease control. We focus on antagonistic microbial diversity, biocontrol mechanisms employed by antagonistic microorganisms, and systematically assess various factors in relation to soil-borne diseases’ biocontrol efficiency, aiming to provide a technical reference that can enhance the effectiveness of biocontrol measures and optimize strategies for managing soil-borne diseases in the future.
2. Pathogen Diversity of Soil-Borne Disease in Panax Plants
All data are based on published data regarding Panax plants from 1995 to 2024 from Web of Science, PubMed, and Google Scholar. To clarify the pathogen diversity, we used three sets of keywords, “Panax” and “root-rot” (or “blight” or “black-spot”), to search the relevant literature on soil-borne diseases. A total of 268 references about pathogen diversity were obtained.
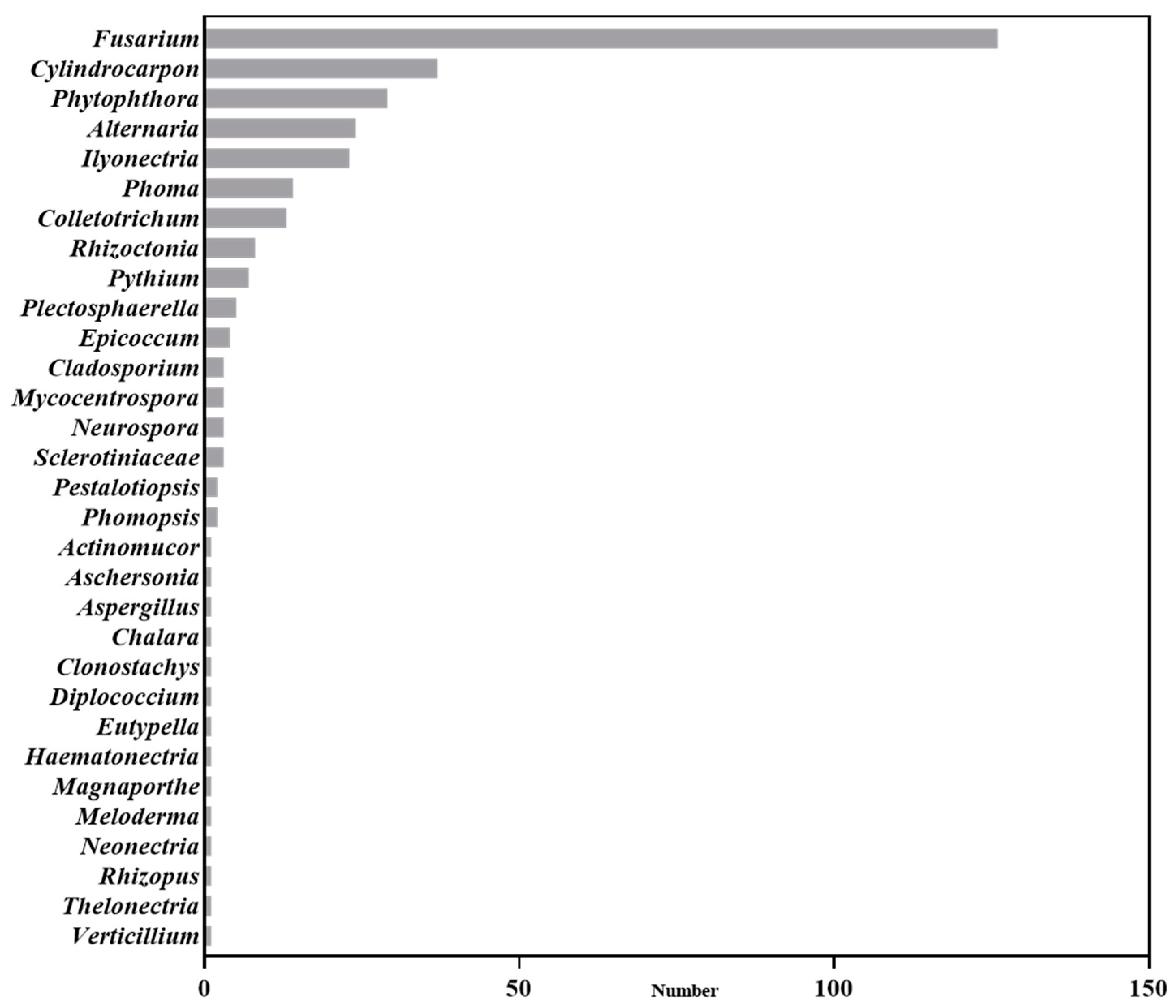
Based on an analysis of current publications in the field of Panax plants, our findings reveal that Panax plant pathogens encompass thirty-one fungal genera, mainly including Fusarium, Cylindrocarpon, Ilyonectria, Alternaria, and Phytophthora (Figure 1). A substantial portion of the research has focused on Fusarium (37.5%), Cylindrocarpon (11.01%), and Phytophthora (8.63%). The above pathogens did not only exist for Panax plants. For example, Fusarium can infect various plants, causing root rot, wilt, or necrosis [16]. But they all infect Panax plants [17,18], and some Panax-specific pathogens were also found. For example, the P. ginseng-specific type of F. oxysporum was studied in 2022 [19]. Cylindrocarpon and Ilyonectria are known as both root rot and rust rot pathogens [20]. Among the Cylindrocarpon species, C. destructans has been reported especially in regard to Panax plants [21]. Although there are no Panax-specific Phytophthora, Phytophthora cactorum has caused both root and foliar diseases [22] in Panax plants.
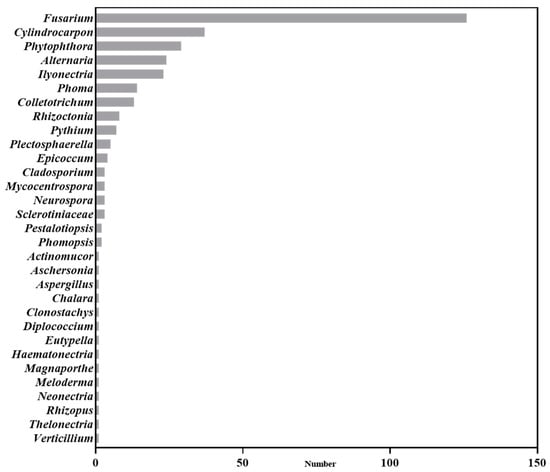
Figure 1.
Soil-borne disease pathogens reported in Panax plants.
3. Pathogenesis of Soil-Borne Diseases in Panax Plants
After wintering in resting soil, soil-borne pathogens could spread to the plants in the next season. Disease occurrence involves the following aspects.
3.1. Autotoxin Secretion of Panax Plants
Autotoxicity in plant species is a phenomenon where intraspecific allelopathy occurs. It involves the release of compounds such as phenolic compounds, terpenoids, and nitrogenous organic compounds [23]. Panax plants, however, exhibit a distinct characteristic of continuously accumulating saponins, which are secondary metabolites known for their key active components extensively used in food, healthcare products, and pharmaceuticals [24]. Saponins play a crucial role in inducing disease occurrence, potentially aiding in the growth of pathogens [25]. Pathogens reported, such as Pythium cactorum, P. irregulare, and Cylindrocarpon destructans, display a strong chemotaxis towards saponins [26]. They are capable of rapidly degrading or utilizing saponins as growth-promoting factors to facilitate their invasion and reproduction. It is reported that ginsenosides belonging to propanaxanol group can promote C. destructanans growth by nearly 130% [27].
3.2. Toxic Effects of Pathogen
When the condition is appropriate, the hyphae or conidia of pathogens in soil begin to infect from the roots. Some of them directly attack the roots, causing disease from the roots, which could lead to Panax plants’ withering and death, such as Fusarium and Cylindrocarpon [20]. Alternatively, some pathogens can colonize into the root and invade the whole plant, leading to disease occurrence in the aboveground sections of Panax plants, such as wilting or spot disease, including pathogens Alternaria and Phytophthora [28,29]. While invading, pathogens could secrete virulence factor (VF), which is a pathogen-produced factor that causes diseases [30,31]. In the case of Panax plants, studies on F. oxysporum and Ilyonectria morpanacis have reported the functions of VF. In F. oxysporum infection, the pathogen first secretes fusaric acid, which accelerates colonization. It then invades the plant’s exosomes by producing cell wall degradation enzymes, thereby compromising the plant’s immune system [32,33]. For I. morpanacis infection, it also rapidly secretes substantial quantities of hydrolases such as cellulase and pectinase, which aid in the rapid invasion of the epidermis and the subsequent spread through the cortex and internal tissues [20].
3.3. Environmental Factors
Apart from plant and pathogen factors, abiotic factors also play a significant role in root rot. Environmental changes can create a favorable environment for pathogen growth and increase the susceptibility of plants to pathogenic infections [20]. Temperature and humidity particularly play a crucial role in root rot occurrence, as the disease tends to thrive in seasons characterized by high temperatures and humidity [34]. A study conducted on P. notoginseng has confirmed temperature and humidity as the main factors influencing root rot [8]. Additionally, soil pH has a profound impact on the severity of root rot. The disease is more likely to occur in acidic soils with a pH below 5. This is supported by direct evidence from findings involving C. destructans. The development of root lesions was significantly reduced at pH 7.0 in comparison to a pH of 5.0 [35].
4. Microbial Diversity for Biocontrol of Soil-Borne Diseases in Panax Plants
Biological control is an environmentally friendly approach to plant disease management that utilizes live microorganisms to regulate soil microecosystems, effectively safeguarding plants against pathogenic microorganisms and guiding the progression of the microbial community towards balanced succession [10]. Bacteria, actinomyces, and fungi have all been used for biological control of plant soil-borne diseases.
In terms of bacteria, Bacillus spp., Pseudomonas spp., and Burkholderia spp. are the most commonly employed for disease control in Panax plants, given their potent antagonistic effects. For instance, Bacillus amylophilus AK-0 has been found to significantly inhibit the growth of C. destructans [36]. Similarly, B. velezensis has demonstrated inhibitory effects against F. oxysporum [23]. P. aeruginosa strain D4 effectively combats Ilyonectria sp., Cladosporium sp., Aschersonia sp., and Fusarium sp. [37]. Moreover, Burkholderia sp. has shown remarkable inhibition of Fusarium and C. destructans [38,39]. In addition, Brevundimonas and Paenibacillus have exhibited strong antagonistic effects on Alternaria spp. and F. oxysporum [11,40,41].
Actinobacteria, as the primary sources of antibiotics [42], have been discovered to possess the ability to control soil-borne diseases, including gray mold [43] and rust rot [44] in Panax plants. In terms of root rot control, Streptomyces has displayed exceptional efficacy [45]. For instance, bioactive substances extracted from S. cellulosae YIM PH20352 exhibited effectiveness against the pathogen Alternaria in P. ginseng [46]. Additionally, Huang et al. identified that Streptomyces G7 produced polyketide lydicamycinsand other active metabolites, inhibiting pathogenic organisms such as F. graminearum, Ustilaginoidea virens and Magnaporthe oryzae pathogenic to plants [47].
Among the fungi, Trichoderma, Chaetomium, and Penicillium have been found to effectively control soil-borne diseases in Panax plants [48,49]. Trichoderma has been employed to manage those caused by Phytophthora. cactorum in P. notoginseng [50]. Chaetomium, on the other hand, demonstrates inhibitory effects against root rot pathogens such as F. flocciferum, Phoma herbarum and Plectosphaerella cucumerina, which are isolated from P. notoginseng [51]. Moreover, Penicillium has proven effective in controlling root rot caused by F. oxysporum in P. ginseng [52]. Recently, Mortierella has been identified as having the ability to enhance the resistance of P. ginseng against root rot [14].
5. Biocontrol Mechanisms of Soil-Borne Disease Suppression
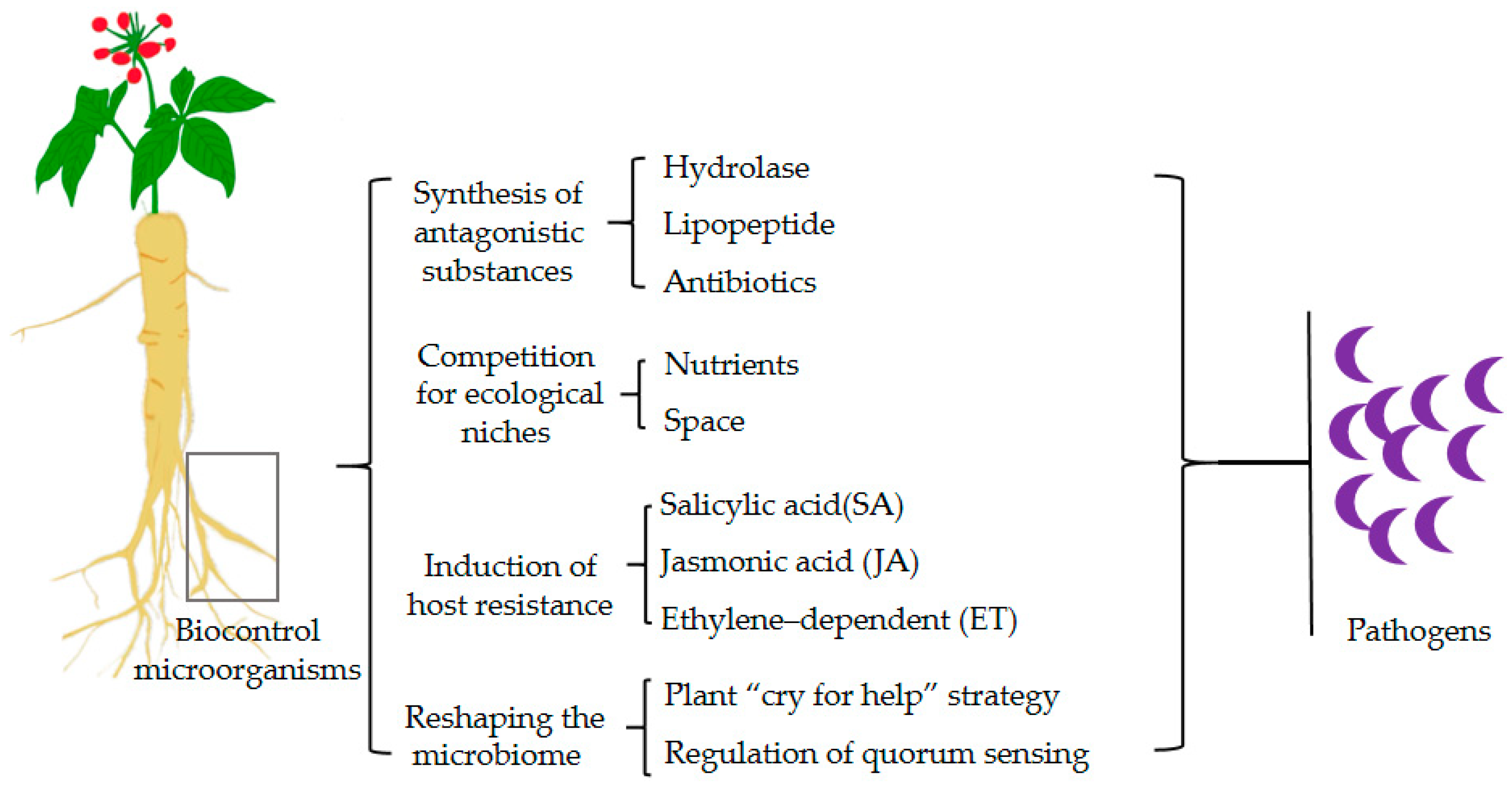
In the context of soil-borne diseases, biocontrol microorganisms can operate through various mechanisms, including the synthesis of antagonistic substances, competition for ecological niches, and the induction of host resistance (Figure 2). The typical microorganisms and their mechanisms are listed in Table 1.
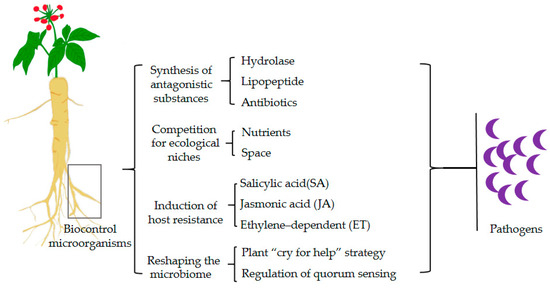
Figure 2.
Biocontrol mechanisms of soil-borne disease suppression.

Table 1.
Microbial biocontrol mechanisms.
5.1. Synthesis of Antagonistic Substances
Biocontrol microorganisms possess the ability to directly inhibit pathogens through the secretion of antagonistic substances, including both non-volatile and volatile compounds. Non-volatile substances, such as hydrolases, lipopeptides, and antibiotics play a significant role in this inhibition. Hydrolases could degrade the cell walls of a number of phytopathogenic fungi [63]. For instance, Paenibacillus polymyxa SY42 produces cellulase and protease to effectively degrade the cell wall of F. oxysporum, thus protecting plants against infection [53]. Similarly, Actinomyces BX5, BX17, and BX26 hinder the growth of F. graminis by targeting the cell wall integrity using hydrolases [60]. Lipopeptides can function by the cell membrane and form aggregates with phospholipids, thereby changing the permeability of the cell membrane of target pathogens [64]. Taking B. amyloliquefaciens FS6 as an example, it can inhibit F. solani in P. ginseng [65]. Antibiotics function by comprehensive mechanisms. Recently, a new antibiotic echinosporin from Amycolatopsis showed antifungal activity against root-rot pathogens such as F. oxysporum and Alternaria panax of the P. notoginseng [66].
Volatile organic compounds (VOCs) possess unique characteristics such as low molecular weight, low boiling points, and significant volatility, enabling them to disperse into the atmosphere and soil, thereby directly inhibiting pathogens [67,68]. The main mechanisms underlying the antifungal effects of VOCs are the disruption of cell walls and membrane structures, leading to intracellular lysate leakage and oxidative stress induction [69]. Research has shown that VOCs produced by B. subtilis, S. setonii, and Nocardiopsis sp. exhibit inhibitory effects on Curvularia lunata in maize, Ceratocystis fimbriata in sweet potato, and Ganoderma sp. in palm, respectively [70,71,72]. In Panax plants, two studies have reported the inhibitory effects of VOCs, with the VOC of T. koningiopsis T-403 inhibiting C. destructans by 84% [59] and the VOC from B. velezensis W17 inhibiting F. oxysporum by 31.89% [23].
5.2. Competition for Ecological Niches
Niche competition refers to the competitive interactions that take place between individuals as they vie for limited resources, such as food, space, and light. Competition for nutrients and space is a prominent manifestation of niche competition [73]. Biocontrol microorganisms have the capacity to suppress pathogens by engaging in competition for these crucial nutrients and space. For instance, Streptomyces could compete with Fusarium for resources and nutrients [61]. Chaetomium globosum LB-2 significantly inhibits the growth of F. oxysporum and Exserohilum turcicum, potentially through competition for resources [74]. The successful colonization of biocontrol microorganisms on the root surface is a crucial step in repelling soil-borne pathogens from occupying ecological spaces and invasion sites [75]. For example, Bacillus can colonize tissues, occupy space, and deplete nutrients before pathogen infection, thus gaining competitive advantages [76,77,78]. Biofilm formation on roots by Bacillus agents contributes to spatial competition with plant pathogens and ultimately suppresses disease development [79,80].
Iron is an essential micronutrient that is present in a high percentage of soils; however, it has low solubility in soil with a pH > 6 and is not suitable for uptake by microorganisms [81]. Therefore, iron bioavailability usually becomes a limiting factor that causes nutrient competition among living microbes [82]. Some strains of Bacillus spp. and Pseudomonas spp. can compete with pathogens for iron by producing siderophores, which effectively chelate the available iron (Fe) in the plant, thus depriving the pathogen of this vital nutrient [54]. Due to iron deficiency, pathogenic fungal spore germination is inhibited and hyphal growth restrained, effectively lowering the chance that the plants become infected, and reducing disease incidence and severity [83].
5.3. Induction of Host Resistance
Systemic resistance is triggered by necrotizing pathogenic microorganisms as well as non-pathogenic rhizobacteria, providing protection against a wide range of pathogens [84]. There are two types of systemic immunity studied in the context of local plant-microbe interactions: systemic acquired resistance (SAR) and induced systemic resistance (ISR), which depend on the site of induction and the lifestyle of the inducing microorganism. SAR is induced by pathogens that interact with plant leaves, while ISR is elicited by beneficial microorganisms that interact with plant roots [85]. For instance, the interaction of some Bacillus strains with plant roots elicits ISR and enhances the resistance of the entire plant against pathogens [55]. ISR mediated by biocontrol microorganisms can trigger the up-regulation of defense genes in P. ginseng, such as PgPR5, PgPR10, PgCAT, ultimately inducing systemic resistance [86,87]. Among actinomyces, Streptomyces AcH 505 is another example that can induce plant resistance to Erysiphe necator by activating salicylic acid (SA) and jasmonic acid (JA)/ethylene–dependent (ET) signaling pathways [62]. Among fungi, Trichodema citrinoviride can enhance P. ginseng resistance against B. cinerea by up-regulating the expression of defense-related genes, including PR2, PR4, PR5, and PR10 [58].
5.4. Reshaping the Soil Microbiome
Reshaping the structure and function of the soil microbiome can lead to the suppression of plant diseases, which could be performed by the plant “cry for help” strategy and the regulation of quorum sensing (QS).
When pathogens attack, plants will develop “cry for help” strategies to attract beneficial microorganisms to the rhizosphere or roots, thus aiding in disease resistance [88]. The “cry for help” response represents a potential mechanism through which rhizosphere microbiota actively inhibit disease progression [89]. It has been reported that P. notoginseng recruits potentially beneficial microorganisms with disease-inhibiting functions, such as Sphingobium, Pseudoxanthomonas, Pseudomonas, Stenotrophomonas, and Flavobacterium into the rhizosphere. Increasing relevant biocontrol microorganisms could serve to combat the invasion of F. oxysporum, F. solani, and Ilyonectria pathogens [57]. In P. notoginseng, it has been documented that probiotic consortia consisting of eight microbial species can alter the soil microbiota and inhibit root rot disease [56].
QS might be an effective mechanism by which various microorganisms can regulate their gene expression and accordingly synchronize their biological behavior according to their population density. Among Gram-negative bacteria such as Pseudomonas, the most widely reported QS signal is acylhomoserinolactone (AHL) [90]. It has been proved that, for P. ginseng, the use of AHL can reshape the soil microflora and offers potential for promoting growth and enhancing resistance [91].
Understanding biocontrol mechanisms, combined with the pathogenesis of soil-borne diseases, will aid to provide guidance for better applying biocontrol microorganisms, improving biocontrol effectiveness and formulating better biocontrol strategies [32].
6. Factors Influencing Biocontrol Efficacy in Panax Plants
Biological control has been proved to be a successful approach in the prevention of soil-borne diseases. However, there is currently a lack of systematic investigations on the factors influencing the effectiveness of biocontrol in Panax plants. To address this knowledge gap, based on publications as described as Section 2, we further scrutinized related studies. Inclusion was based on four screening criteria: (1) the study’s focus on the efficacy of biological control against soil-borne diseases in Panax plants (excluding reviews and meta-analyses), (2) the presence of experimental and control groups, (3) the use of disease incidence (DI) or the disease severity index (DSI) to assess the effects of biological control, and (4) considering results from different trials within the same article as independent observations. Data from tables and article descriptions were directly extracted, whereas data from figures was extracted using GetData Graph Digitizer 2.20 (https://getdata-graph-digitizer.software.informer.com/2.2/, accessed on 6 November 2024). Subsequently, the decrease rate was calculated based on the means of DI or DSI, according to the formula: control efficacy (%) = (DI or DSI in treatment with pathogen—DI or DSI in treatment with biocontrol microorganisms)/(DI or DSI in treatment with pathogen) × 100 [92].
6.1. Microbial Species Effects on Biocontrol Effectiveness
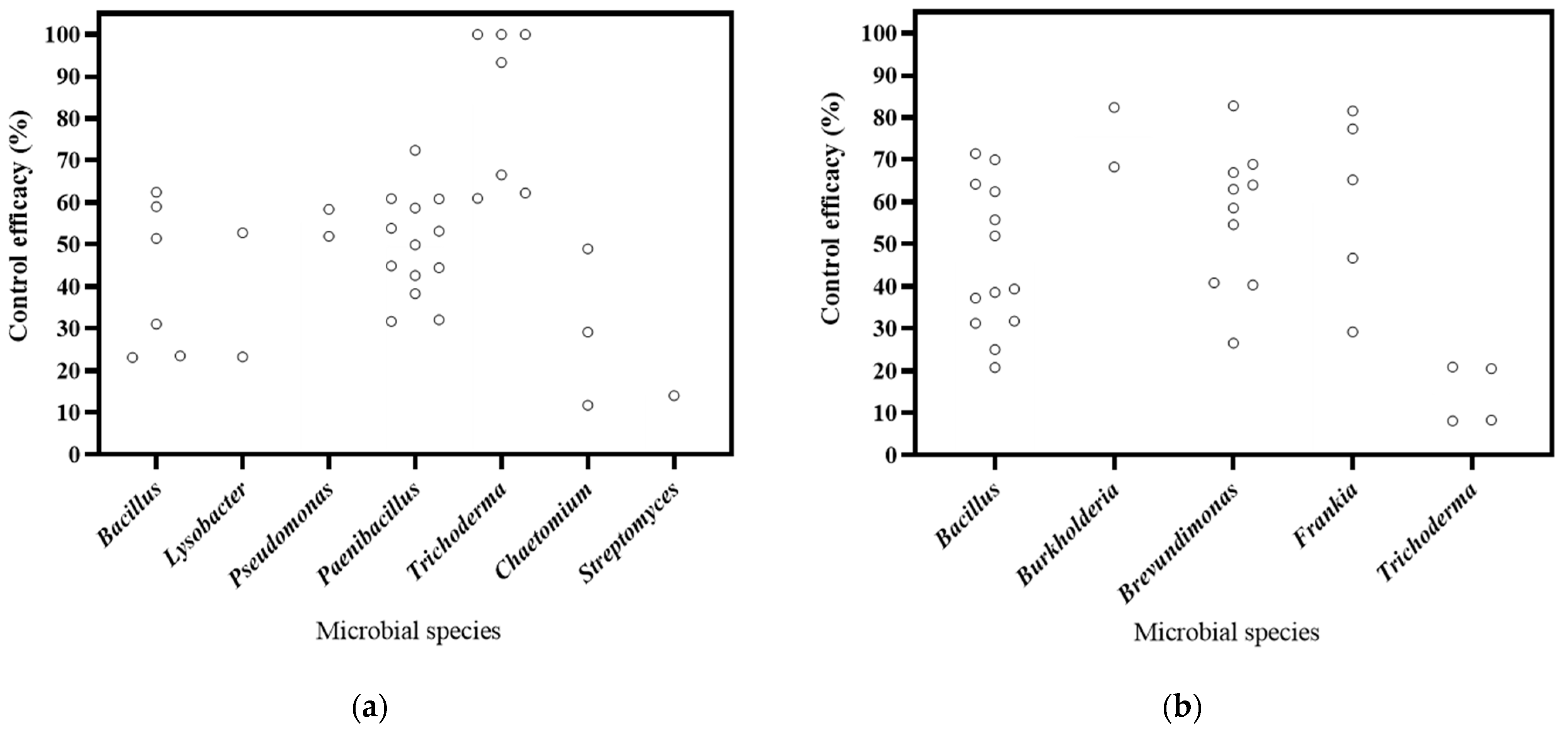
The literature included in this study focuses on biocontrol bacteria, fungi, and actinomyces, in 15, 7, and 3 publications, respectively (Figure 3). Based on the DI, the decrease rates of bacteria, fungi, and actinobacteria ranged from 23.29% to 72.46%, from 61.01% to 100.00%, and 14.09%, respectively. The most substantial decrease was observed in Trichoderma. As for DSI, the decrease rates of for bacteria, fungi, and actinobacteria ranged from 25.05% to 82.77%, from 43.29% to 60.32%, and from 29.25% to 81.59%, respectively. And Burkholderia exhibited the highest efficacy in disease control. Therefore, the findings of this study suggest that these microorganisms have significant potential for controlling soil-borne diseases in Panax plants.
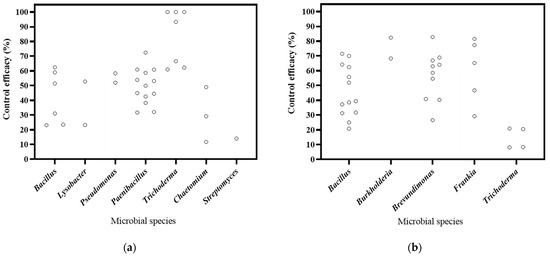
Figure 3.
Effects of microbial species on biocontrol effectiveness. (a) control efficacy in disease incidence (DI) [57,58,93,94,95,96,97,98,99,100,101,102]; (b) control efficacy in disease severity index (DSI) [11,13,103,104,105,106,107,108,109,110].
6.2. Effects of Inoculation Method on Biocontrol Effectiveness
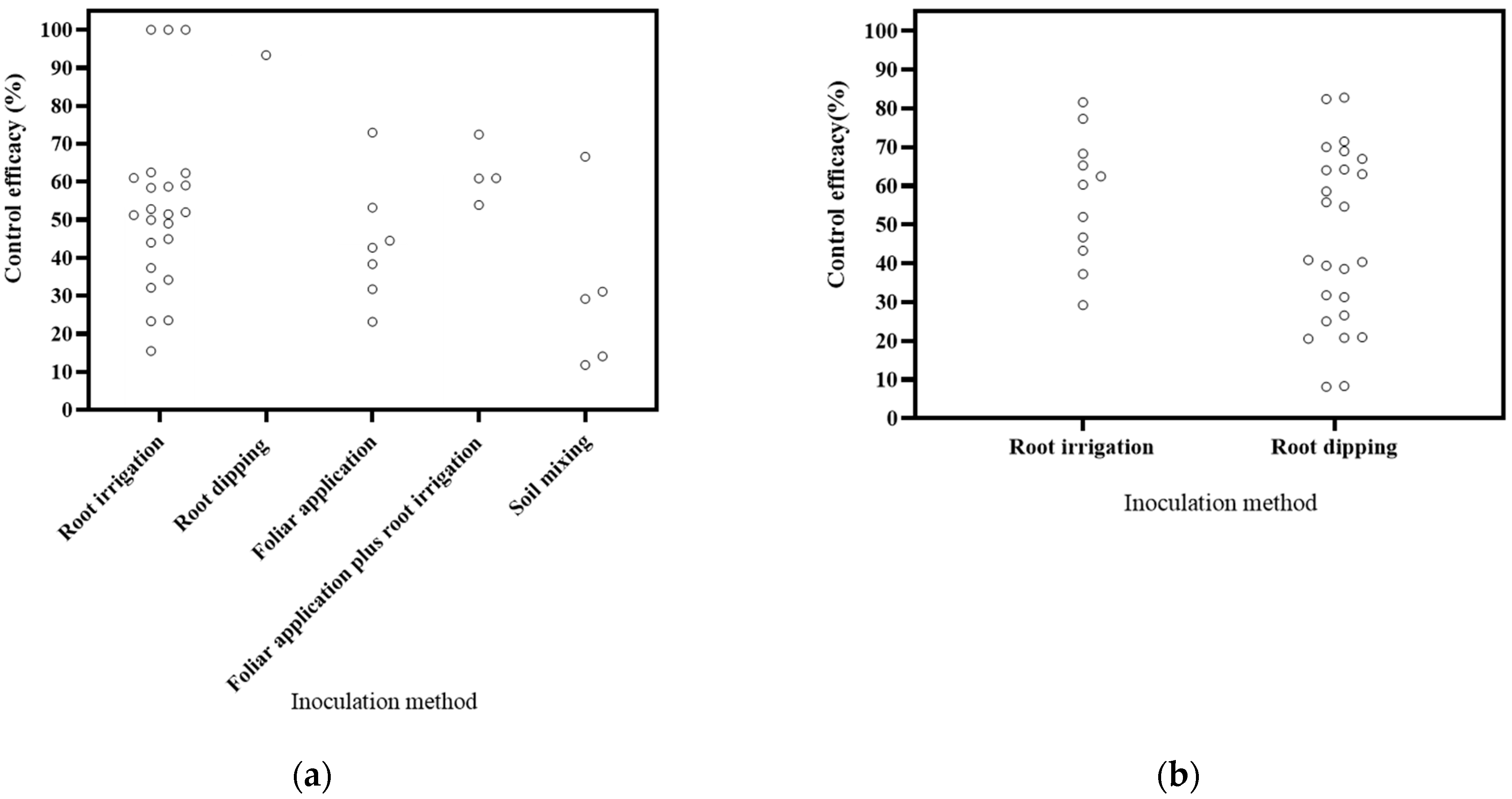
Various inoculation methods were employed, including root irrigation, foliar application, root dipping, soil mixing, and a combination of foliar application and root irrigation (Figure 4). The literature covered 16 publications on root irrigation application, 2 on foliar application, 6 on root dipping application, and 1 on foliar application plus root irrigation application. Based on the DI, root irrigation application exhibited a reduction range of 15.45–100%, foliar application showed a range of 23.15–72.95%, root dipping application resulted in a reduction of 93.34%, foliar application plus root irrigation application yielded a range of 53.89–72.46%, and soil mixing resulted in a reduction a range of 11.81–66.57%. Notably, root irrigation application demonstrated the highest effectiveness in disease control. Based on the DSI, root irrigation application led to a reduction range of 29.25–81.59%, while root dipping application showed a range of 8.19–82.77%. Root dipping application exhibited the most effective control. These results align with the commonly held assumption in soil-borne disease control that methods directly targeting the roots, such as root dipping and root irrigation, tend to be more efficient [111].
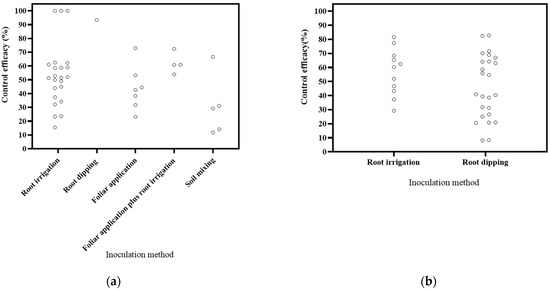
Figure 4.
Effects of inoculation method on biocontrol effectiveness. (a) control efficacy in disease incidence (DI) [56,57,58,93,94,95,96,97,98,99,100,101,102,112]; (b) control efficacy in disease severity index (DSI) [11,13,50,103,104,105,106,107,108,109,110].
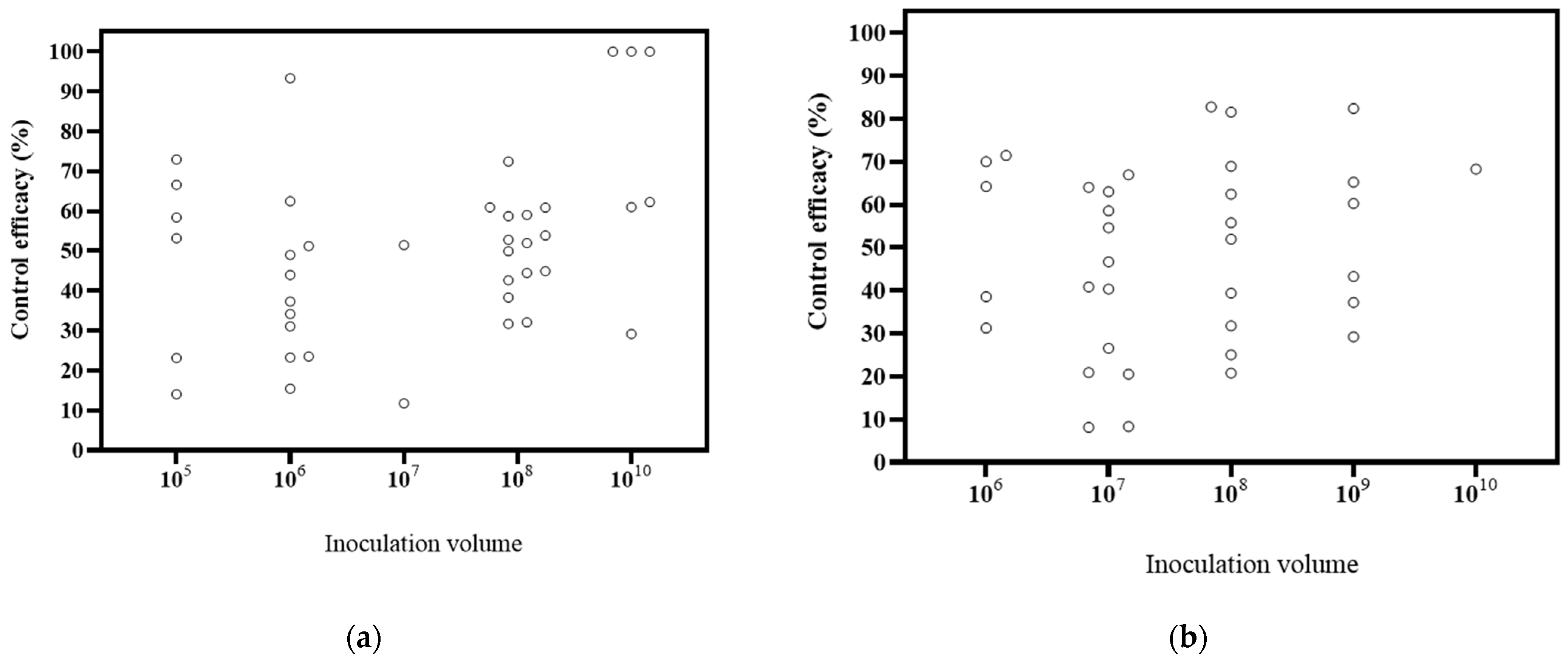
6.3. Effects of Inoculation Volume on Biocontrol Effectiveness
The papers included in the study covered different inoculation volumes, including 105 CFU/mL, 106 CFU/mL, 107 CFU/mL, 108 CFU/mL, 109 CFU/mL, and 1010 CFU/mL, with respective counts of 3, 8, 5, 9, 4, and 3 publications (Figure 5). Based on the DI, the decrease ranges were 14.09–66.57% at 105 CFU/mL, 15.45–93.34% at 106 CFU/mL, 11.80–51.52% at 107 CFU/mL, 31.74–72.46% at 108 CFU/mL, and 29.21–100% at 1010 CFU/mL. The highest biological effectiveness was observed at 1010 CFU/mL. Based on the DSI, the decrease ranges of were 31.30–71.52% at 106 CFU/mL, 8.19–67.00% at 107 CFU/mL, 20.80–82.77% at 108 CFU/mL, 29.25–82.42% at 109 CFU/mL, and 68.29% at 1010 CFU/mL. The greatest biological control effectiveness was achieved at 108 CFU/mL. These findings, combined with the DI and DSI, suggest that higher inoculation volumes tend to result in better effects. It is widely accepted that higher inoculation volumes lead to improved control effects [113]. For instance, a study suggested that inoculation volumes of 106 and 107 CFU/mL did not achieve the desired inhibitory activity and recommended a higher concentration for an appropriate effect [78]. The results of this study support this conclusion.
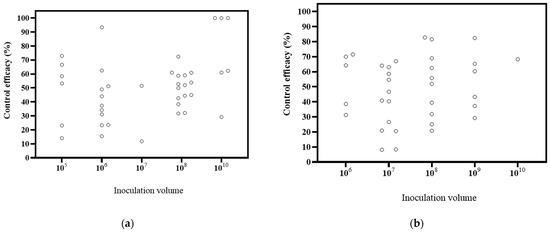
Figure 5.
Effects of microbial volume on biocontrol effectiveness. (a) control efficacy in disease incidence (DI) [56,57,58,93,94,95,96,97,98,99,100,101,102,112]; (b) control efficacy in disease severity index (DSI) [11,13,50,103,104,105,106,107,108,109,110].
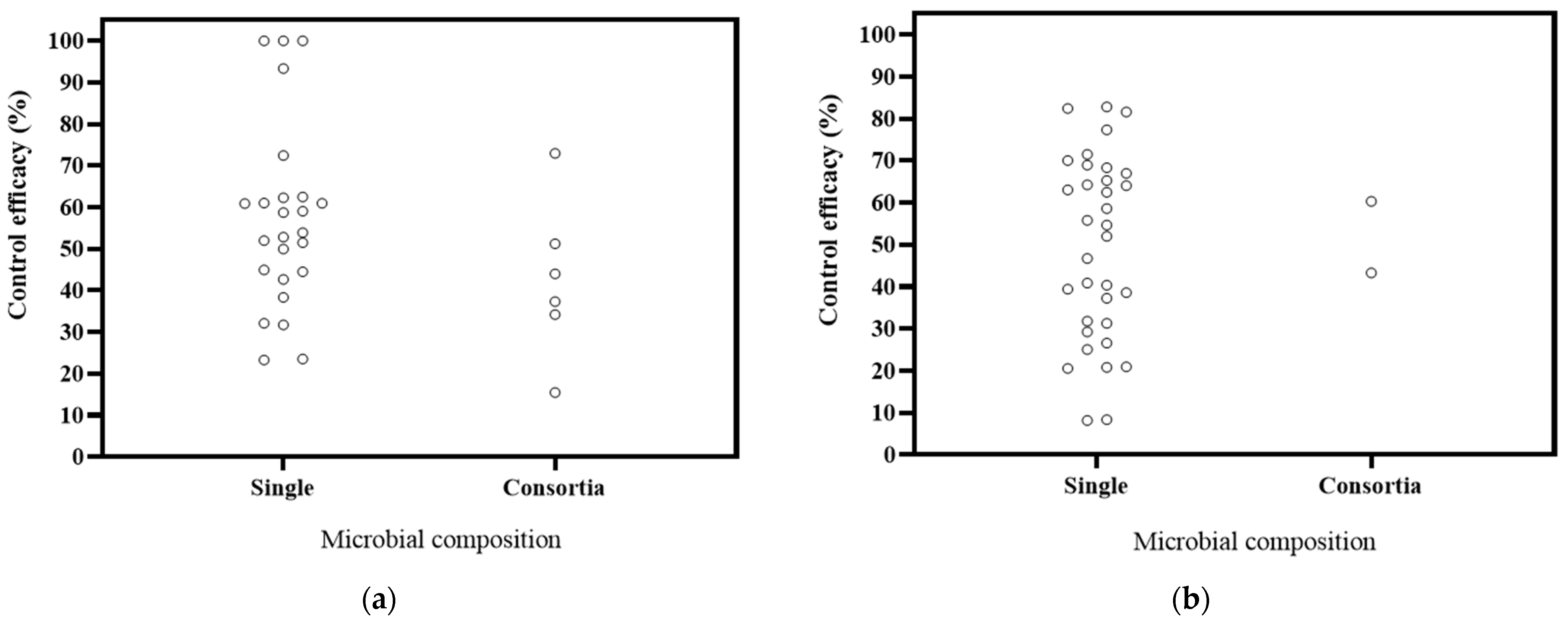
6.4. Effects of Microbial Composition of Biocontrol Effectiveness
The literature on microbial composition comprised 24 studies focusing on single microorganisms and 3 studies on microbial consortia (Figure 6). The decrease rates in DI were 23.29–100% for single microorganisms and 15.45–75.95% for consortia. In DSI, the decrease ranges were 8.19–82.77% for single microorganisms and 43.29–60.32% for consortia. Combining the DI with the DSI, it is evident that single microorganisms exhibit better control for Panax plants. These findings support previous studies that have reported similar or even lower effectiveness in disease control when comparing applied microbial populations to single microorganisms [114,115,116]. Most of the available data on Panax plants are based on diverse, potted experiments. Therefore, in the future, a systematic comparison should be conducted to assess the biocontrol effectiveness of single microorganisms and microbial consortia within the same cultivation system. Additionally, the effectiveness of microbial control measures should be better tested in field conditions [117].
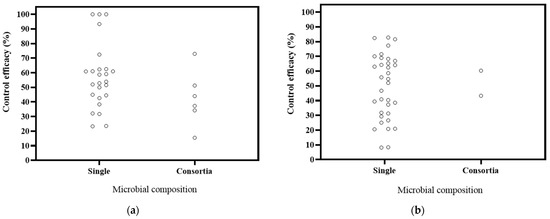
Figure 6.
Effects of microbial composition on biocontrol effectiveness. (a) control efficacy in disease incidence (DI) [56,57,58,93,94,95,96,97,98,99,100,101,102,112]; (b) control efficacy in disease severity index (DSI) [11,13,50,103,104,105,106,107,108,109,110].
7. Conclusions
Soil-borne disease poses a significant threat to the quality and quantity of Panax plants. In this review, we initially explored the pathogenesis of soil-borne disease and the diversity of pathogens. Furthermore, we conducted a comprehensive review focusing on the diversity of biocontrol microbes, specifically bacteria, actinobacteria, and fungi. We examined their respective function mechanisms, emphasizing the synthesis of antagonistic substances, niche competition, and the induction of host resistance. In addition, we investigated the effects of microbial species, inoculation methods, inoculation volume, and microbial composition on the control efficiency of soil-borne diseases. We found that combining Trichoderma or Burkholderia with dipping or irrigation at higher inoculation volumes could lead to better results (Table 2). At present, research data related to Panax plants are limited, and more research data are needed in the future to verify the applicability of our conclusions to Panax plants.

Table 2.
Suggested biocontrol strategies in this study.
8. Future Prospects
Although biological control has enormous potential benefits, its limitations cannot be ignored. The safety of the biological control microorganism is extremely important, due to the microorganism playing a crucial role in shaping disease outcomes in agriculture and having the potential for siderophore production by PGPR against a wide range of phytopathogens, making them an attractive and sustainable alternative to chemical fungicides and bactericides [118]. Moreover, when evaluating the effectiveness of biological control, the choice of quantitative indicators can significantly influence the results. In our opinion, DSI is a more recommendable evaluation criterion. Having sufficient data, it is crucial to perform a comprehensive analysis of the factors involved in biocontrol effectiveness in Panax plants. Examining the interaction among these factors and assessing the contribution of each can offer valuable insights for the application of biological control agents. Additionally, at present, there is a lack of specific research on nematodes in the prevention and control of disease in Panax plants. However, it has been reported that predatory nematodes can be vital in managing plant parasitic nematodes [119]. Therefore, future research should pay greater attention to this topic and further enrich investigations in this field.
Author Contributions
Z.W., Writing—original draft, Visualization, Data curation. S.W.: Visualization, Writing—original draft. H.Y.: Conceptualization, Writing—review and editing, Funding acquisition, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Joint Funds of Natural Science Foundation of Heilongjiang Province, grant number LH2022C013, and the Key Research and Development Program of Heilongjiang Province, grant number JD2023SJ14.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plunkett, G.M.; Lowry, P.P.; Frodin, D.G.; Wen, J. Phylogeny and geography of Schefflera: Pervasive polyphyly in the largest genus of Araliaceae. Ann. Mo. Bot. Gard. 2005, 92, 202–224. [Google Scholar]
- Shim, D.; Bak, Y.; Choi, H.G.; Lee, S.; Park, S.C. Effects of Panax species and their bioactive components on allergic airway diseases. J. Ginseng Res. 2024, 48, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.K.; Shao, S.; Wang, D.D.; Zhao, D.Q.; Wang, M.X. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jeong, W.S. Red ginseng (Panax ginseng Meyer) oil: A comprehensive review of extraction technologies, chemical composition, health benefits, molecular mechanisms, and safety. J. Ginseng Res. 2022, 46, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, X.Q.; Qin, X.L.; Zhu, J.H.; Xu, J.D.; Zhou, S.S.; Kong, M.; Shen, H.; Huo, J.G.; Li, S.L.; et al. Metals/bisulfite system involved generation of 24-sulfonic-25-ene ginsenoside Rg1, a potential quality control marker for sulfur-fumigated ginseng. Food Chem. 2024, 448, 139112. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.L.; Wang, J.H.; Wang, Y.P. Effects of different straw biochar combined with microbial inoculants on soil environment in pot experiment. Sci. Rep. 2021, 11, 14685. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Bello, S.K. Biological control of soil-borne pathogens in arid lands: A review. J. Plant Dis. Prot. 2024, 131, 293–313. [Google Scholar] [CrossRef]
- Jin, Y.L.; Jiang, S.L.; Jiang, X.L. Occurrence of root rot of Panax notoginseng caused by Fusarium oxysporum in China. Int. J. Agric. Biol. 2018, 20, 2175–2180. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhang, X.M.; Jiao, X.L.; Hao, J.J.; Li, S.D.; Gao, W.W. Genetic diversity and population structure of Cylindrocarpon-like fungi infecting Ginseng roots in northeast China. J. Fungi 2022, 8, 814. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.M.; Han, M.; Han, Z.M.; Yang, L.; Cheng, L.; Yang, X.; Lv, Z.L. Biological control ginseng grey mold and plant colonization by antagonistic bacteria isolated from rhizospheric soil of Panax ginseng Meyer. Biol. Control 2019, 138, 104048. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.M.; Zhang, L.X.; Han, M. An investigation of Panax ginseng Meyer growth promotion and the biocontrol potential of antagonistic bacteria against ginseng black spot. J. Ginseng Res. 2018, 42, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Picco, A.M.; Angelini, P.; Ciccarone, C.; Franceschini, A.; Ragazzi, A.; Rodolfi, M.; Varese, G.C.; Zotti, M. Biodiversity of emerging pathogenic and invasive fungi in plants, animals and humans in Italy. Plant Biosyst. 2011, 145, 988–996. [Google Scholar] [CrossRef]
- Song, M.; Yun, H.Y.; Kim, Y.H. Antagonistic Bacillus species as a biological control of ginseng root rot caused by Fusarium cf. incarnatum. J. Ginseng Res. 2014, 38, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.W.; Suo, M.; Qiu, Z.J.; Wu, H.; Zhao, M.; Yang, H.Y. Regulating root fungal community using Mortierella alpina for Fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 2022, 13, 850917. [Google Scholar] [CrossRef]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L. Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res. Microbiol. 2016, 167, 222–233. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.R.; Liu, H.; Wang, Z.H.; Yun, Y.Z. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Guo, R.J.; Liu, X.Z.; Li, S.D.; Miao, Z.Q. In vitro Inhibition of Fungal Root-Rot Pathogens of Panax notoginseng by Rhizobacteria. Plant Pathol. J. 2009, 25, 70–76. [Google Scholar] [CrossRef]
- Reeleder, R.D. The ginseng root pathogens Cylindrocarpon destructans and Phytophthora cactorum are not pathogenic to the medicinal herbs Hydrastis canadensis and Actaea racemosa. Can. J. Plant Pathol. 2003, 25, 218–221. [Google Scholar] [CrossRef]
- Wang, J.; Feng, S.; Lu, B.H.; Yang, L.N.; Wang, X.; Zhang, Y.J.; Gao, J. Fusarium oxysporum f. sp. ginseng, a new forma specialis causing Fusarium root rot of Panax ginseng. Phytopathol. Mediterr. 2022, 61, 417–429. [Google Scholar] [CrossRef]
- Farh, M.E.; Kim, Y.J.; Kim, Y.J.; Yang, D.C. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: Causative agent of ginseng root-rot disease and rusty symptoms. J. Ginseng Res. 2018, 42, 9–15. [Google Scholar] [CrossRef]
- Nunes, I.B.; Goodwin, P.H. Interaction of ginseng with Ilyonectria root rot pathogens. Plants 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Qu, S.; Dong, K.; Wang, S.H.; Xu, C.; Wang, Y.P.; Hua, S. Integrated Metabolome and Transcriptome Analysis Unveils the Underlying Molecular Response of Panax ginseng Plants to the Phytophthora cactorum Infection. Agriculture 2023, 13, 509. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-Glucosidase activity of Lentilactobacillus buchneri URN103L and its potential to convert ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, K.G. Allelochemicals: Biological Control of Plant Pathogens and Diseases; Springer Nature: Berlin, Germany, 2006; Volume 2, pp. 157–175. [Google Scholar] [CrossRef]
- Zhao, X.S.; Gao, J.; Song, C.C.; Fang, Q.; Wang, N.; Zhao, T.J.; Liu, D.B.; Zhou, Y.F. Fungal sensitivity to and enzymatic deglycosylation of ginsenosides. Phytochemistry 2012, 78, 65–71. [Google Scholar] [CrossRef]
- Qiu, C.D.; Wang, W.Q.; Liu, Z.Y. Genome Resource of American Ginseng Black Spot Pathogen Alternaria panax. Plant Dis. 2022, 106, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Fadli, A.; Benyahia, H.; Hussain, S.; Khan, R.I.; Rao, M.J.; Ahmed, T.; Ancona, V.; Khalid, M.F. Phytophthora-citrus interactions and management strategies: A review. Turk. J. Agric. For. 2022, 46, 730–742. [Google Scholar] [CrossRef]
- Falkow, S. Molecular Koch’s postulates applied to bacterial pathogenicity—A personal recollection 15 years later. Nat. Rev. Microbiol. 2004, 2, 67–72. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, A.H.J.; Jennings, M.P. Discovery of virulence factors of pathogenic bacteria. Curr. Opin. Chem. Biol. 2008, 12, 93–101. [Google Scholar] [CrossRef]
- Li, J.B.; Ai, M.T.; Hou, J.E.; Zhu, P.Q.; Cui, X.M.; Yang, Q. Plant-pathogen interaction with root rot of Panax notoginseng as a model: Insight into pathogen pathogenesis, plant defence response and biological control. Mol. Plant Pathol. 2024, 25, e13427. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Da-Ran, K.; Youn-Sig, K. Isolation and characterization of beneficial microbe against ginseng root rot pathogens. Korean J. Pestic. Sci. 2020, 24, 296–303. [Google Scholar] [CrossRef]
- Rahman, M.; Punja, Z.K. Factors influencing development of root rot on ginseng caused by Cylindrocarpon destructans. Phytopathology 2005, 95, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Balaraju, K.; Jeon, Y.H. Biological characteristics of Bacillus amyloliquefaciens AK-0 and suppression of ginseng root rot caused by Cylindrocarpon destructans. J. Appl. Microbiol. 2017, 122, 166–179. [Google Scholar] [CrossRef]
- Durairaj, K.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Senthilkumar, P.; Choi, K.M.; Lee, J.H.; Oh, B.T. An investigation of biocontrol activity Pseudomonas and Bacillus strains against Panax ginseng root rot fungal phytopathogens. Biol. Control 2018, 125, 138–146. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.J.; Tu, J.L.; Li, Y.; Guan, H.L. Comprehensive genomic analysis of Burkholderia arboris PN-1 reveals its biocontrol potential against Fusarium solani induced root rot in Panax notoginseng. Curr. Genet. 2024, 70, 4. [Google Scholar] [CrossRef]
- Kim, H.; Mohanta, T.K.; Park, Y.H.; Park, S.C.; Shanmugam, G.; Park, J.S.; Jeon, J.; Bae, H. Complete genome sequence of the mountain-cultivated ginseng endophyte Burkholderia stabilis and its antimicrobial compounds against ginseng root rot disease. Biol. Control 2020, 140, 104126. [Google Scholar] [CrossRef]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Lee, S.W.; Jo, I.H. Diversity of bacterial endophytes in Panax ginseng and their protective effects against pathogens. 3 Biotech 2018, 8, 397. [Google Scholar] [CrossRef]
- Son, S.H.; Khan, Z.; Kim, S.G.; Kim, Y.H. Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and fusarium wilt fungus. J. Appl. Microbiol. 2009, 107, 524–532. [Google Scholar] [CrossRef]
- Wei, J.H.; He, L.; Niu, G.Q. Regulation of antibiotic biosynthesis in actinomycetes: Perspectives and challenges. Synth. Syst. Biotechnol. 2018, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, I.K.; Yun, B.S. Antagonistic effect of Streptomyces sp. BS062 against Botrytis diseases. Mycobiology 2015, 43, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhou, G.; Lei, F.; Zhang, L. Optimization of fermentation medium for antagonistic actinomycetes F05 of American ginseng rust rot pathogen. China J. Chin. Mater. Medica 2009, 34, 2296–2298. [Google Scholar]
- Torres-Rodriguez, J.A.; Reyes-Perez, J.J.; Quinones-Aguilar, E.E.; Hernandez-Montiel, L.G. Actinomycete potential as biocontrol agent of phytopathogenic fungi: Mechanisms, source, and applications. Plants 2022, 11, 3201. [Google Scholar] [CrossRef]
- Xu, X.D.; Zhao, Y.; Bao, K.; Miao, C.P.; Tang, S.K.; Wu, S.H.; Li, Y.Q. Isolation, structure elucidation and antifungal activity of angucycline antibiotics from Streptomycete cellulosae. Appl. Biochem. Microbiol. 2023, 59, 456–461. [Google Scholar] [CrossRef]
- Huang, J.Q.; Li, X.J.; Zhan, X.L.; Pan, S.Y.; Pan, C.; Li, J.X.; Fan, S.T.; Zhang, L.E.; Du, K.H.; Du, Z.Y.; et al. A Streptomyces species from the ginseng rhizosphere exhibits biocontrol potential. Plant Physiol. 2024, 194, 2709–2723. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanisms of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Prakasam, V.; Jonathan, E.I.; Paramathma, M.; Samiyappan, R. Combining Pseudomonas, Bacillus and Trichoderma strains with organic amendments and micronutrient to enhance suppression of collar and root rot disease in physic nut. Appl. Soil Ecol. 2011, 49, 215–223. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Wang, J.H.; Liu, L.; Zhao, Y.Q.; Jiang, Z.T.; Wang, Y.X.; Li, Z.K.; Fu, L.; Cui, Z.L. Biocontrol potential of Burkholderia sp. BV6 against the rice blast fungus Magnaporthe oryzae. J. Appl. Microbiol. 2022, 133, 883–897. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.Q.; Yang, Y.B.; Duang, R.T.; Chen, G.Y.; Li, X.Z.; Li, Q.L.; Qin, S.H.; Li, S.Q.; Zhao, L.X.; et al. Anti-phytopathogen, multi-target acetylcholinesterase inhibitory and antioxidant activities of metabolites from endophytic Chaetomium globosum. Nat. Prod. Res. 2016, 30, 2616–2619. [Google Scholar] [CrossRef]
- Wang, L.W.; Zhang, Y.B.; Wang, Y.; Suo, M.; Wu, H.; Zhao, M.; Yang, H.Y. Inoculation with Penicillium citrinum aids ginseng in resisting Fusarium oxysporum by regulating the root and rhizosphere microbial communities. Rhizosphere 2022, 22, 100535. [Google Scholar] [CrossRef]
- Xie, S.Y.; Si, H.; Xue, Y.Y.; Zhou, R.; Wang, S.Q.; Duan, Y.Z.; Niu, J.F.; Wang, Z.Z. Efficacy of rhizobacteria Paenibacillus polymyxa SY42 for the biological control of Atractylodes chinensis root rot. Microb. Pathog. 2024, 187, 106517. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A review on mechanisms and prospects of endophytic bacteria in biocontrol of plant pathogenic fungi and their plant growth-promoting activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wei, L.F.; Yang, J.; Ahmed, W.; Wang, Y.T.; Fu, L.N.; Ji, G.H. Probiotic Consortia: Reshaping the Rhizospheric Microbiome and Its Role in Suppressing Root-Rot Disease of Panax notoginseng. Front. Microbiol. 2020, 11, 701. [Google Scholar] [CrossRef]
- Wang, B.Y.; Xia, Q.; Lin, Y.L.; Wei, F.G.; Yang, S.Z.; Dai, C.C.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; Zhao, J. Root rot induces a core assemblage of bacterial microbiome to prevent disease infection in Sanqi ginseng. Appl. Soil Ecol. 2024, 198, 105371. [Google Scholar] [CrossRef]
- Park, Y.H.; Mishra, R.C.; Yoon, S.; Kim, H.; Park, C.; Seo, S.T.; Bae, H. Endophytic Trichoderma citrinoviride isolated from mountain-cultivated ginseng (Panax ginseng) has great potential as a biocontrol agent against ginseng pathogens. J. Ginseng Res. 2019, 43, 408–420. [Google Scholar] [CrossRef]
- Hussein, K.A.; Lee, Y.D.; Joo, J.H. Effect of rosemary essential oil and Trichoderma koningiopsis T-403 VOCs on pathogenic fungi responsible for ginseng root rot disease. J. Microbiol. Biotechnol. 2020, 30, 1018–1026. [Google Scholar] [CrossRef]
- Cabrera, R.; García-López, H.; Aguirre-von-Wobeser, E.; Orozco-Avitia, J.A.; Gutiérrez-Saldaña, A.H. Amycolatopsis BX17: An actinobacterial strain isolated from soil of a traditional milpa agroecosystem with potential biocontrol against Fusarium graminearum. Biol. Control 2020, 147, 104285. [Google Scholar] [CrossRef]
- Essarioui, A.; LeBlanc, N.; Kistler, H.C.; Kinkel, L.L. Plant community richness mediates inhibitory interactions and resource competition between Streptomyces and Fusarium populations in the rhizosphere. Microb. Ecol. 2017, 74, 157–167. [Google Scholar] [CrossRef]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Grosse, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant-Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Aktuganov, G.E.; Galimzyanova, N.F.; Melent’ev, A.I.; Kuz’mina, L.Y. Extracellular hydrolases of strain Bacillus sp 739 and their involvement in the lysis of micromycete cell walls. Microbiology 2007, 76, 413–420. [Google Scholar] [CrossRef]
- Dimkic, I.; Janakiev, T.; Petrovic, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Wang, R.; Liang, X.; Long, Z.; Wang, X.; Yang, L.; Lu, B.; Gao, J. An LCI-like protein APC protects ginseng root from Fusarium solani infection. J. Appl. Microbiol. 2021, 130, 165–178. [Google Scholar] [CrossRef]
- Xu, X.D.; Han, L.; Zhao, L.X.; Chen, X.; Miao, C.P.; Hu, L.F.; Huang, X.S.; Chen, Y.W.; Li, Y.Q. Echinosporin antibiotics isolated from Amycolatopsis strain and their antifungal activity against root-rot pathogens of the Panax notoginseng. Folia Microbiol. 2019, 64, 171–175. [Google Scholar] [CrossRef]
- Razo-Belmán, R.; Angeles-López, Y.I.; Garcia-Ortega, L.F.; León-Ramírez, C.G.; Ortiz-Castellanos, L.; Yu, H.L.; Martínez-Soto, D. Fungal volatile organic compounds: Mechanisms involved in their sensing and dynamic communication with plants. Front. Plant Sci. 2023, 14, 1257098. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2742. [Google Scholar] [CrossRef]
- Zhao, X.X.; Zhou, J.Y.; Tian, R.F.; Liu, Y.L. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Xie, S.S.; Liu, J.; Gu, S.Y.; Chen, X.J.; Jiang, H.Y.; Ding, T. Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol. 2020, 70, 2. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.Q.; Xu, M.J.; Zhang, C.M.; Gao, J.; Li, C.G.; Xing, K.; Qin, S. Antifungal volatile organic compounds from Streptomyces setonii WY228 control black spot disease of sweet potato. Appl. Environ. Microbiol. 2022, 88, e02317–e02321. [Google Scholar] [CrossRef]
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Lee, Y.H. High-throughput identification of genes influencing the competitive ability to obtain nutrients and performance of biocontrol in Pseudomonas putida JBC17. Sci. Rep. 2022, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, Z. Identification of the biocontrol strain LB-2 and determination of its antifungal effects on plant pathogenic fungi. J. Plant Pathol. 2018, 100, 25–32. [Google Scholar] [CrossRef]
- Soni, R.; Keharia, H.J.P. Phytostimulation and biocontrol potential of Gram-positive endospore-forming Bacilli. Planta 2021, 254, 49. [Google Scholar] [CrossRef]
- Perea-Molina, P.A.; Pedraza-Herrera, L.A.; Beauregard, P.B.; Uribe-Vélez, D. A biocontrol Bacillus velezensis strain decreases pathogen Burkholderia glumae population and occupies a similar niche in rice plants. Biol. Control 2022, 176, 105067. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Wang, S.Y.; Herrera-Balandrano, D.D.; Wang, Y.X.; Shi, X.C.; Chen, X.; Jin, Y.; Liu, F.Q.; Laborda, P. Biocontrol ability of the Bacillus amyloliquefaciens group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the management of fungal postharvest diseases: A review. J. Agric. Food Chem. 2022, 70, 6591–6616. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Molina-Santiago, C.; Pearson, J.R.; Navarro, Y.; Berlanga-Clavero, M.V.; Caraballo-Rodriguez, A.M.; Petras, D.; García-Martín, M.L.; Lamon, G.; Haberstein, B.; Cazorla, F.M.; et al. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat. Commun. 2019, 10, 1919. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F.J. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Zhu, X.X.; Chen, W.J.; Bhatt, K.; Zhou, Z.; Huang, Y.H.; Zhang, L.H.; Chen, S.H.; Wang, J.X. Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Front. Plant Sci. 2023, 13, 1063393. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.A.; Pieterse, C.M. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Jin, H.C.; Lee, S.; Nam, M.H.; Chung, J.H.; Kwon, S.I.; Ryu, C.M.; Park, O.K. GDSL lipase-like 1 regulates systemic resistance associated with ethylene signaling in Arabidopsis. Plant J. 2009, 58, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Lee, B.D.; Dutta, S.; Ryu, H.; Yoo, S.J.; Suh, D.S.; Park, K. Induction of systemic resistance in Panax ginseng against Phytophthora cactorum by native Bacillus amyloliquefaciens HK34. J. Ginseng Res. 2015, 39, 213–220. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Wang, J.; Gao, W.; Sun, X.; Xiong, Q.; Shu, X.; Miao, Y.; Shen, Q.; Xun, W.; et al. Nonpathogenic Pseudomonas syringae derivatives and its metabolites trigger the plant “cry for help” response to assemble disease suppressing and growth promoting rhizomicrobiome. Nat. Commun. 2024, 15, 1907. [Google Scholar] [CrossRef]
- Kalia, V.C.; Gong, C.J.; Patel, S.K.S.; Lee, J.K. Regulation of Plant Mineral Nutrition by Signal Molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef]
- Ibal, J.C.; Park, M.K.; Park, G.S.; Jung, B.K.; Park, T.H.; Kim, M.S.; Kang, G.U.; Park, Y.J.; Shin, J.H. Use of Acyl-Homoserine Lactones Leads to Improved Growth of Ginseng Seedlings and Shifts in Soil Microbiome Structure. Agronomy 2021, 11, 2177. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.W.; Zuo, B.; Liang, X.Y.; Zhang, D.N.; Liu, R.X.; Yang, L.N.; Lu, B.H.; Wang, X.; Gao, J. Novel Biocontrol Strain Bacillus amyloliquefaciens FS6 for Excellent Control of Gray Mold and Seedling Diseases of Ginseng. Plant Dis. 2021, 105, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Zhao, X.L.; Wu, K.; Liang, C.Y.; Liu, J.; Yang, H.; Wang, C.M.; Yang, B.; Yin, F.; Zhang, W.D. Isolation and characterization of Bacillus velezensis strain B19 for biocontrol of Panax notoginseng root rot. Biol. Control 2023, 185, 105311. [Google Scholar] [CrossRef]
- Dong, Y.J.; Tang, B.B.; He, M.M.; Wang, L.L.; Wu, K.; Yang, S.X.; Liu, J.F.; Yang, H.; Wang, C.M.; Yin, F.; et al. High concentrations of antagonistic bacterial strains from diseased sanqi ginseng rhizosphere suppressed Fusarium root rot. Eur. J. Plant Pathol. 2022, 163, 143–153. [Google Scholar] [CrossRef]
- Liu, T.T.; Zhang, J.Y.; Wang, T.; Li, Z.Y.; Liang, H.J.; Jiang, C.Y.; Tang, H.; Gao, J.; Jiang, Y.; Chen, C.Q. The novel Pseudomonas thivervalensis strain JI6 promotes growth and controls rusty root rot disease in Panax ginseng. Biol. Control 2024, 193, 105514. [Google Scholar] [CrossRef]
- Dong, L.L.; Xu, J.; Zhang, L.J.; Cheng, R.Y.; Wei, G.F.; Su, H.; Yang, J.; Qian, J.; Xu, R.; Chen, S.L. Rhizospheric microbial communities are driven by Panax ginseng at different growth stages and biocontrol bacteria alleviates replanting mortality. Acta Pharm. Sin. B 2018, 8, 272–282. [Google Scholar] [CrossRef]
- Chen, J.L.; Sun, S.Z.; Miao, C.P.; Wu, K.; Chen, Y.W.; Xu, L.H.; Guan, H.L.; Zhao, L.X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2016, 40, 315–324. [Google Scholar] [CrossRef]
- Cho, G.; Kim, D.R.; Kwak, Y.S. Transition from Ginseng Root Rot Disease-Conducive Soil to -Suppressive Soil Mediated by Pseudomonadaceae. Microbiol. Spectr. 2023, 11, e01150-23. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Lu, Y.L.; Miao, C.P.; Guan, H.L.; Wang, R.; Wang, H.J.; Tian, L.Y.; Wei, F.G.; Xu, W.M. Mitigating root rot in Panax notoginseng: The synergistic effects of biochar and Chaetomium globosum YIM PH30719. Ind. Crops Prod. 2024, 222, 119805. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Q.; Gao, Y.G.; Zang, P.; Zheng, T. Effects of a co-bacterial agent on the growth, disease control, and quality of ginseng based on rhizosphere microbial diversity. BMC Plant Biol. 2024, 24, 647. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, S.; Piao, X.; Yan, M.; Cui, L.; Wang, Y.J.A.J.o.B. Control of ginseng leaf black spot disease by endophytic fungi. Afr. J. Biotechnol. 2021, 20, 308–312. [Google Scholar]
- Liu, D.F.; Sun, H.J.; Ma, H.W. Deciphering Microbiome Related to Rusty Roots of Panax ginseng and Evaluation of Antagonists Against Pathogenic Ilyonectria. Front. Microbiol. 2019, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.R.; Liu, T.T.; Li, X.; Gao, J.; Jiang, Y.; Chen, C.Q. Bacillus amyloliquefaciens FG14 as a potential biocontrol strain against rusty root rot of Panax ginseng, and its impact on the rhizosphere microbial community. Biol. Control 2023, 182, 105221. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.G.; Kim, Y.H. Biocontrol Efficacies of Bacillus Species Against Cylindrocarpon destructans Causing Ginseng Root Rot. Plant Pathol. J. 2011, 27, 333–341. [Google Scholar] [CrossRef]
- Li, X.Q.; Wang, J.R.; Shen, H.; Xing, C.X.; Kong, L.X.; Song, Y.; Hou, W.P.; Gao, J.; Jiang, Y.; Chen, C.Q. Biocontrol and growth promotion potential of Bacillus velezensis NT35 on Panax ginseng based on the multifunctional effect. Front. Microbiol. 2024, 15, 1447488. [Google Scholar] [CrossRef]
- Chu, Y.; Li, Y.; Wang, J.H.; Wang, C.Y. Study on the growth-promoting potenial and disease resistance of the antagonistic bacterium Frankia francese F1 on ginseng root rot and rust rot Appl. Ecol. Environ. Res. 2023, 21, 2055–2074. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Liu, H.L.; Zhang, B.P.; Geng, M.X.; Cai, X.X.; Wang, J.H.; Wang, Y.P. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Appl. Soil Ecol. 2022, 172, 104369. [Google Scholar] [CrossRef]
- Kim, H.; Rim, S.O.; Bae, H. Antimicrobial potential of metabolites extracted from ginseng bacterial endophyte Burkholderia stabilis against ginseng pathogens. Biol. Control 2019, 128, 24–30. [Google Scholar] [CrossRef]
- Hong, S.C. The Efficacy of Trichoderma, Benomyl, and Propiconazole: Treatments for the Control of Cylindrocarpon Destructans Diseases in North American Ginseng (Panax quinquefolius L.); National Library of Canada: Ottawa, ON, Canada, 2001. [Google Scholar]
- Gastol, M.; Domagala-Swiatkiewicz, I.; Bijak, M. The effect of mycorrhizal inoculation and phosphorus application on the growth and mineral nutrient status of apple seedlings. J. Plant Nutr. 2016, 39, 288–299. [Google Scholar] [CrossRef]
- Gao, Y.G.; Liu, Q.; Zang, P.; Li, X.; Ji, Q.; He, Z.M.; Zhao, Y.; Yang, H.; Zhao, X.L.; Zhang, L.X. An endophytic bacterium isolated from Panax ginseng CA Meyer enhances growth, reduces morbidity, and stimulates ginsenoside biosynthesis. Phytochem. Lett. 2015, 11, 132–138. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, Y.X.; Zhang, Z.P.; Li, J.Q.; Zhu, S.S.; Yang, M.; Luo, L.X. Application of plant survival-promoting and pathogen-suppressing Trichoderma species for crop biofertilization and biocontrol of root rot in Panax notoginseng. J. Plant Pathol. 2022, 104, 1361–1369. [Google Scholar] [CrossRef]
- Serrao, C.P.; Ortega, J.C.G.; Rodrigues, P.C.; de Souza, C.R.B. Bacillus species as tools for biocontrol of plant diseases: A meta-analysis of twenty-two years of research, 2000–2021. World J. Microbiol. Biotechnol. 2024, 40, 110. [Google Scholar] [CrossRef] [PubMed]
- Minchev, Z.; Kostenko, O.; Soler, R.; Pozo, M.J. Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Front. Plant Sci. 2021, 12, 756368. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.A.M.; Hashem, M.; Ali, E.H. Integrated control of cotton root rot disease by mixing fungal biocontrol agents and resistance inducers. Crop Prot. 2009, 28, 295–301. [Google Scholar] [CrossRef]
- Elliott, M.; Shamoun, S.F.; Sumampong, G.; James, D.; Masri, S.; Varga, A. Evaluation of several commercial biocontrol products on European and North American populations of Phytophthora ramorum. Biocontrol Sci. Technol. 2009, 19, 1007–1021. [Google Scholar] [CrossRef]
- Cernava, T. How microbiome studies could further improve biological control. Biol. Control 2021, 160, 104669. [Google Scholar] [CrossRef]
- Deb, C.R.; Tatung, M. Siderophore producing bacteria as biocontrol agent against phytopathogens for a better environment: A review. S. Afr. J. Bot. 2024, 165, 153–162. [Google Scholar] [CrossRef]
- Kanwar, R.S.; Patil, J.A.; Yadav, S. Prospects of using predatory nematodes in biological control for plant parasitic nematodes—A review. Biol. Control 2021, 160, 104668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).