Understanding the Pathogenesis, Biocontrol Mechanisms, and Factors Influencing Biocontrol Effectiveness for Soil-Borne Diseases in Panax Plants
Abstract
:1. Introduction
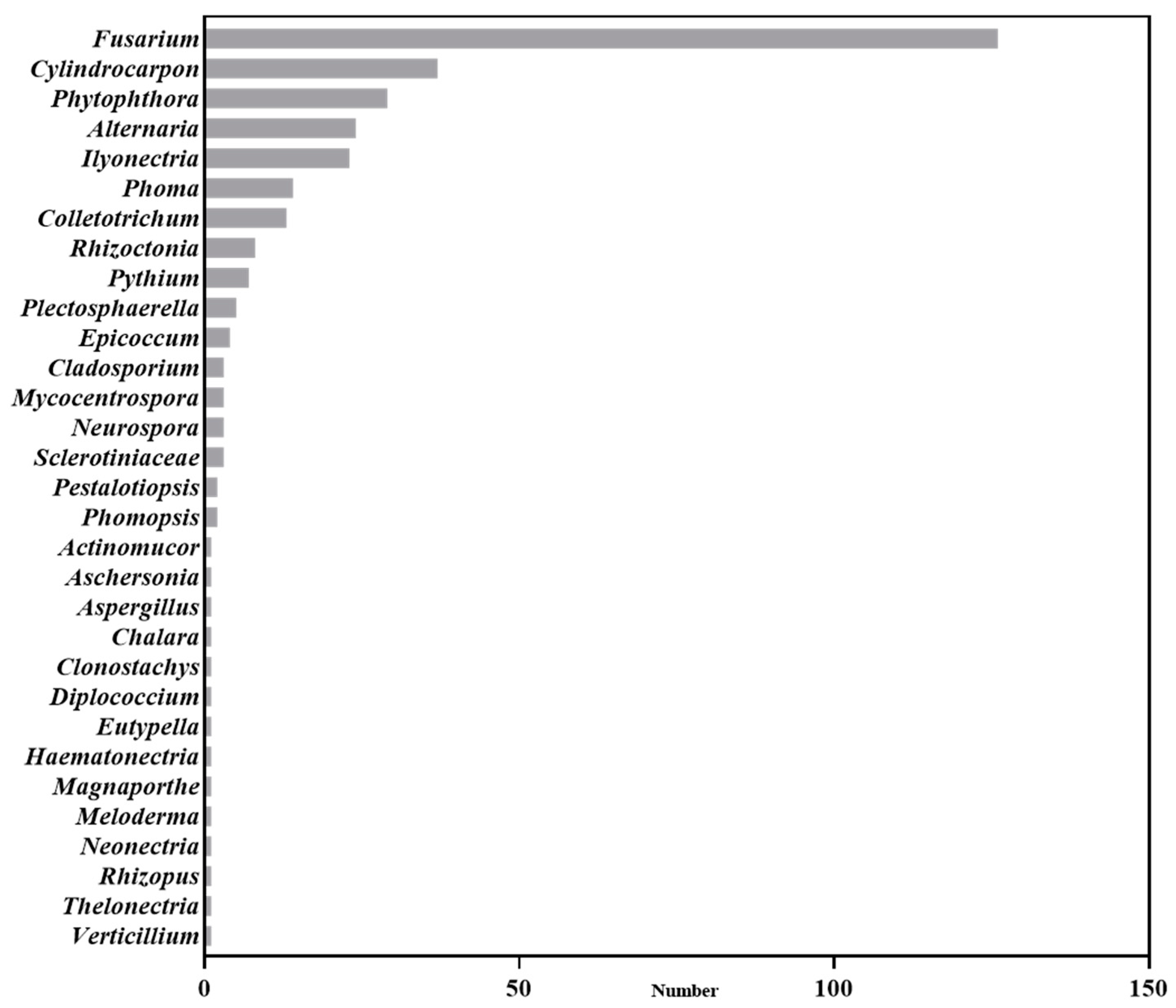
2. Pathogen Diversity of Soil-Borne Disease in Panax Plants
3. Pathogenesis of Soil-Borne Diseases in Panax Plants
3.1. Autotoxin Secretion of Panax Plants
3.2. Toxic Effects of Pathogen
3.3. Environmental Factors
4. Microbial Diversity for Biocontrol of Soil-Borne Diseases in Panax Plants
5. Biocontrol Mechanisms of Soil-Borne Disease Suppression
5.1. Synthesis of Antagonistic Substances
5.2. Competition for Ecological Niches
5.3. Induction of Host Resistance
5.4. Reshaping the Soil Microbiome
6. Factors Influencing Biocontrol Efficacy in Panax Plants
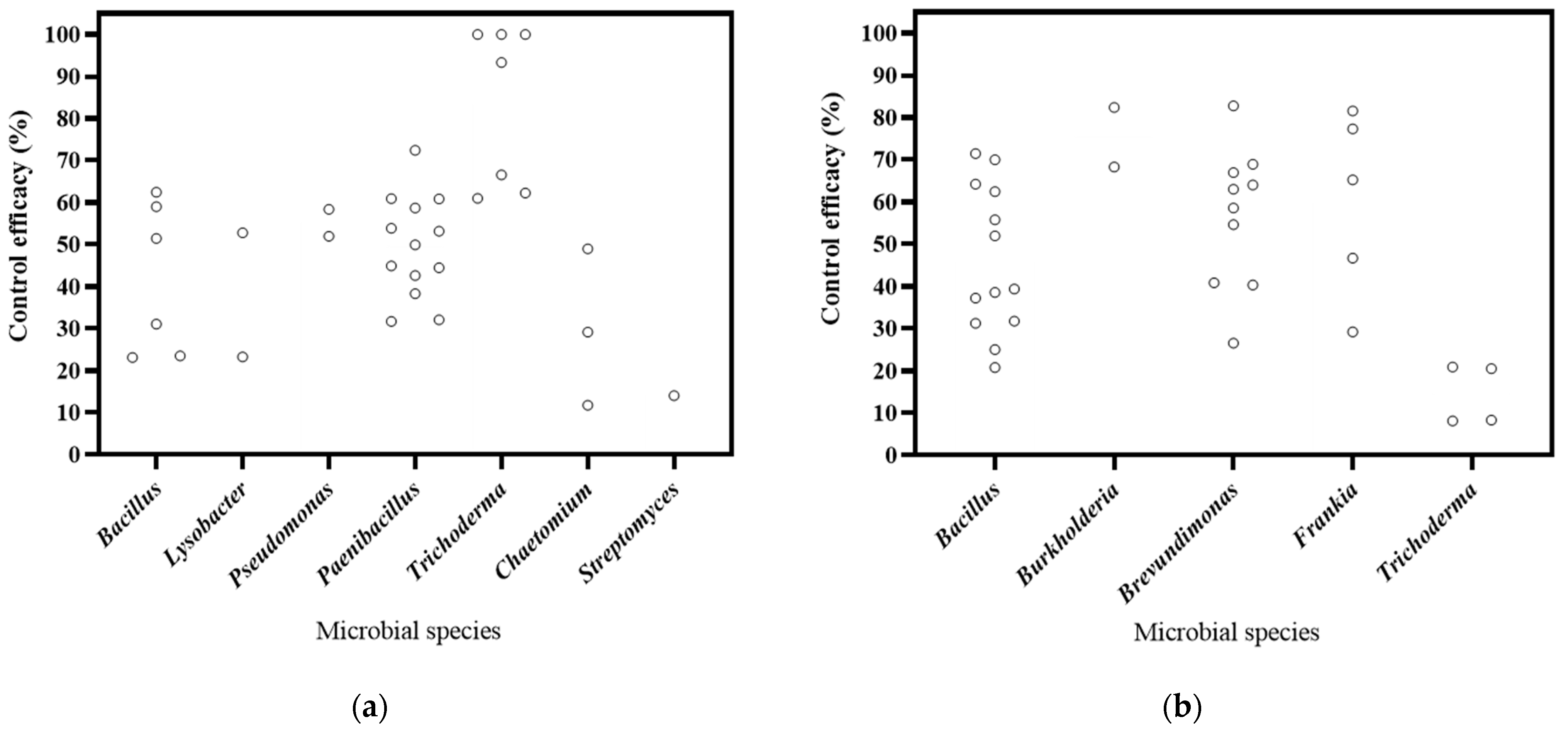
6.1. Microbial Species Effects on Biocontrol Effectiveness
6.2. Effects of Inoculation Method on Biocontrol Effectiveness
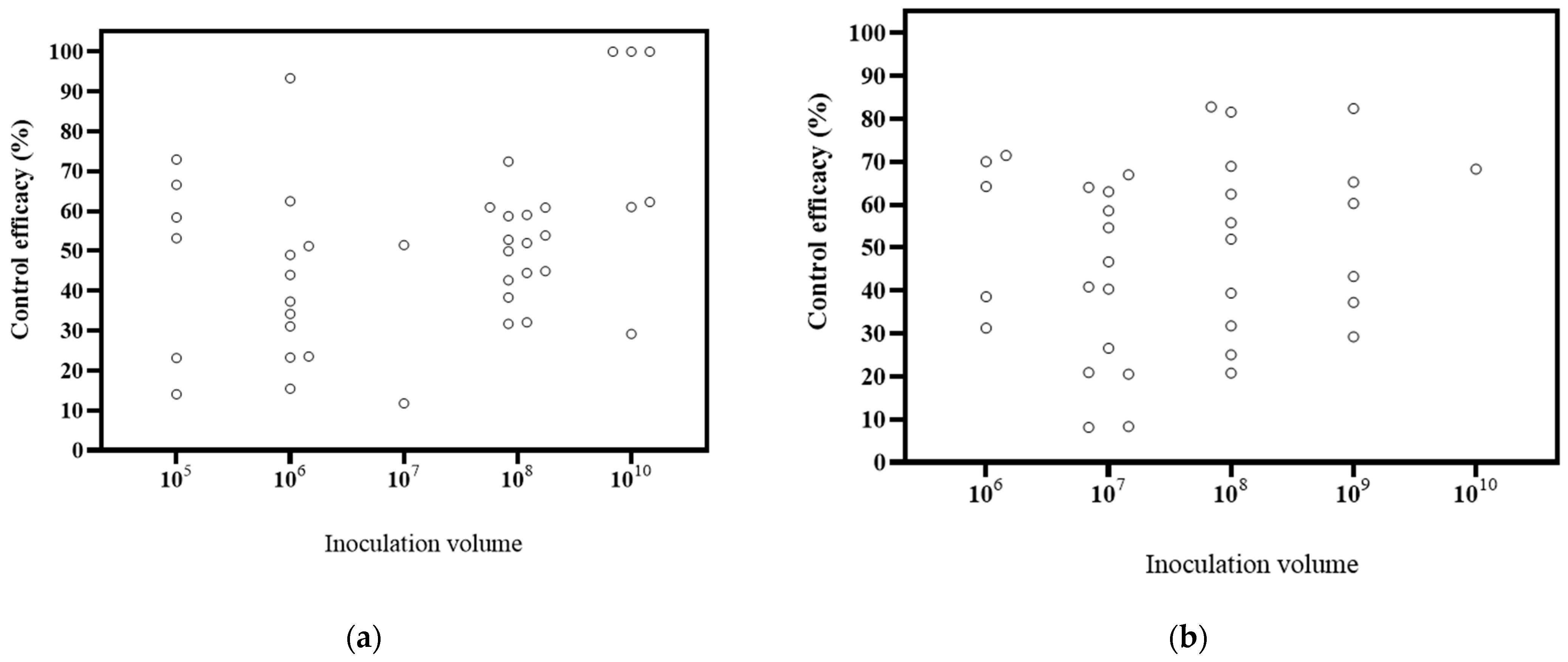
6.3. Effects of Inoculation Volume on Biocontrol Effectiveness
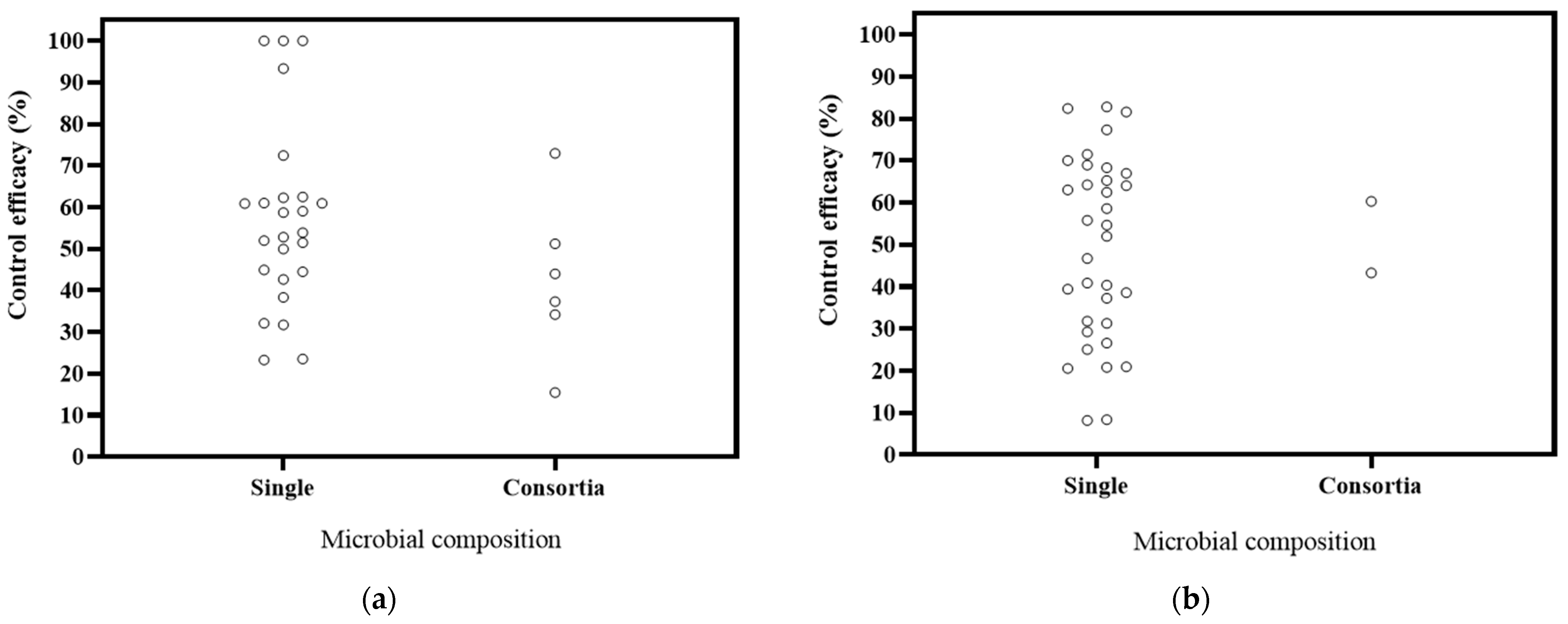
6.4. Effects of Microbial Composition of Biocontrol Effectiveness
7. Conclusions
8. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Plunkett, G.M.; Lowry, P.P.; Frodin, D.G.; Wen, J. Phylogeny and geography of Schefflera: Pervasive polyphyly in the largest genus of Araliaceae. Ann. Mo. Bot. Gard. 2005, 92, 202–224. [Google Scholar]
- Shim, D.; Bak, Y.; Choi, H.G.; Lee, S.; Park, S.C. Effects of Panax species and their bioactive components on allergic airway diseases. J. Ginseng Res. 2024, 48, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.K.; Shao, S.; Wang, D.D.; Zhao, D.Q.; Wang, M.X. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jeong, W.S. Red ginseng (Panax ginseng Meyer) oil: A comprehensive review of extraction technologies, chemical composition, health benefits, molecular mechanisms, and safety. J. Ginseng Res. 2022, 46, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, X.Q.; Qin, X.L.; Zhu, J.H.; Xu, J.D.; Zhou, S.S.; Kong, M.; Shen, H.; Huo, J.G.; Li, S.L.; et al. Metals/bisulfite system involved generation of 24-sulfonic-25-ene ginsenoside Rg1, a potential quality control marker for sulfur-fumigated ginseng. Food Chem. 2024, 448, 139112. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.L.; Wang, J.H.; Wang, Y.P. Effects of different straw biochar combined with microbial inoculants on soil environment in pot experiment. Sci. Rep. 2021, 11, 14685. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Bello, S.K. Biological control of soil-borne pathogens in arid lands: A review. J. Plant Dis. Prot. 2024, 131, 293–313. [Google Scholar] [CrossRef]
- Jin, Y.L.; Jiang, S.L.; Jiang, X.L. Occurrence of root rot of Panax notoginseng caused by Fusarium oxysporum in China. Int. J. Agric. Biol. 2018, 20, 2175–2180. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhang, X.M.; Jiao, X.L.; Hao, J.J.; Li, S.D.; Gao, W.W. Genetic diversity and population structure of Cylindrocarpon-like fungi infecting Ginseng roots in northeast China. J. Fungi 2022, 8, 814. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.M.; Han, M.; Han, Z.M.; Yang, L.; Cheng, L.; Yang, X.; Lv, Z.L. Biological control ginseng grey mold and plant colonization by antagonistic bacteria isolated from rhizospheric soil of Panax ginseng Meyer. Biol. Control 2019, 138, 104048. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.M.; Zhang, L.X.; Han, M. An investigation of Panax ginseng Meyer growth promotion and the biocontrol potential of antagonistic bacteria against ginseng black spot. J. Ginseng Res. 2018, 42, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Picco, A.M.; Angelini, P.; Ciccarone, C.; Franceschini, A.; Ragazzi, A.; Rodolfi, M.; Varese, G.C.; Zotti, M. Biodiversity of emerging pathogenic and invasive fungi in plants, animals and humans in Italy. Plant Biosyst. 2011, 145, 988–996. [Google Scholar] [CrossRef]
- Song, M.; Yun, H.Y.; Kim, Y.H. Antagonistic Bacillus species as a biological control of ginseng root rot caused by Fusarium cf. incarnatum. J. Ginseng Res. 2014, 38, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.W.; Suo, M.; Qiu, Z.J.; Wu, H.; Zhao, M.; Yang, H.Y. Regulating root fungal community using Mortierella alpina for Fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 2022, 13, 850917. [Google Scholar] [CrossRef]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L. Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res. Microbiol. 2016, 167, 222–233. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.R.; Liu, H.; Wang, Z.H.; Yun, Y.Z. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Guo, R.J.; Liu, X.Z.; Li, S.D.; Miao, Z.Q. In vitro Inhibition of Fungal Root-Rot Pathogens of Panax notoginseng by Rhizobacteria. Plant Pathol. J. 2009, 25, 70–76. [Google Scholar] [CrossRef]
- Reeleder, R.D. The ginseng root pathogens Cylindrocarpon destructans and Phytophthora cactorum are not pathogenic to the medicinal herbs Hydrastis canadensis and Actaea racemosa. Can. J. Plant Pathol. 2003, 25, 218–221. [Google Scholar] [CrossRef]
- Wang, J.; Feng, S.; Lu, B.H.; Yang, L.N.; Wang, X.; Zhang, Y.J.; Gao, J. Fusarium oxysporum f. sp. ginseng, a new forma specialis causing Fusarium root rot of Panax ginseng. Phytopathol. Mediterr. 2022, 61, 417–429. [Google Scholar] [CrossRef]
- Farh, M.E.; Kim, Y.J.; Kim, Y.J.; Yang, D.C. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: Causative agent of ginseng root-rot disease and rusty symptoms. J. Ginseng Res. 2018, 42, 9–15. [Google Scholar] [CrossRef]
- Nunes, I.B.; Goodwin, P.H. Interaction of ginseng with Ilyonectria root rot pathogens. Plants 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Qu, S.; Dong, K.; Wang, S.H.; Xu, C.; Wang, Y.P.; Hua, S. Integrated Metabolome and Transcriptome Analysis Unveils the Underlying Molecular Response of Panax ginseng Plants to the Phytophthora cactorum Infection. Agriculture 2023, 13, 509. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-Glucosidase activity of Lentilactobacillus buchneri URN103L and its potential to convert ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, K.G. Allelochemicals: Biological Control of Plant Pathogens and Diseases; Springer Nature: Berlin, Germany, 2006; Volume 2, pp. 157–175. [Google Scholar] [CrossRef]
- Zhao, X.S.; Gao, J.; Song, C.C.; Fang, Q.; Wang, N.; Zhao, T.J.; Liu, D.B.; Zhou, Y.F. Fungal sensitivity to and enzymatic deglycosylation of ginsenosides. Phytochemistry 2012, 78, 65–71. [Google Scholar] [CrossRef]
- Qiu, C.D.; Wang, W.Q.; Liu, Z.Y. Genome Resource of American Ginseng Black Spot Pathogen Alternaria panax. Plant Dis. 2022, 106, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Fadli, A.; Benyahia, H.; Hussain, S.; Khan, R.I.; Rao, M.J.; Ahmed, T.; Ancona, V.; Khalid, M.F. Phytophthora-citrus interactions and management strategies: A review. Turk. J. Agric. For. 2022, 46, 730–742. [Google Scholar] [CrossRef]
- Falkow, S. Molecular Koch’s postulates applied to bacterial pathogenicity—A personal recollection 15 years later. Nat. Rev. Microbiol. 2004, 2, 67–72. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, A.H.J.; Jennings, M.P. Discovery of virulence factors of pathogenic bacteria. Curr. Opin. Chem. Biol. 2008, 12, 93–101. [Google Scholar] [CrossRef]
- Li, J.B.; Ai, M.T.; Hou, J.E.; Zhu, P.Q.; Cui, X.M.; Yang, Q. Plant-pathogen interaction with root rot of Panax notoginseng as a model: Insight into pathogen pathogenesis, plant defence response and biological control. Mol. Plant Pathol. 2024, 25, e13427. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Da-Ran, K.; Youn-Sig, K. Isolation and characterization of beneficial microbe against ginseng root rot pathogens. Korean J. Pestic. Sci. 2020, 24, 296–303. [Google Scholar] [CrossRef]
- Rahman, M.; Punja, Z.K. Factors influencing development of root rot on ginseng caused by Cylindrocarpon destructans. Phytopathology 2005, 95, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Balaraju, K.; Jeon, Y.H. Biological characteristics of Bacillus amyloliquefaciens AK-0 and suppression of ginseng root rot caused by Cylindrocarpon destructans. J. Appl. Microbiol. 2017, 122, 166–179. [Google Scholar] [CrossRef]
- Durairaj, K.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Senthilkumar, P.; Choi, K.M.; Lee, J.H.; Oh, B.T. An investigation of biocontrol activity Pseudomonas and Bacillus strains against Panax ginseng root rot fungal phytopathogens. Biol. Control 2018, 125, 138–146. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.J.; Tu, J.L.; Li, Y.; Guan, H.L. Comprehensive genomic analysis of Burkholderia arboris PN-1 reveals its biocontrol potential against Fusarium solani induced root rot in Panax notoginseng. Curr. Genet. 2024, 70, 4. [Google Scholar] [CrossRef]
- Kim, H.; Mohanta, T.K.; Park, Y.H.; Park, S.C.; Shanmugam, G.; Park, J.S.; Jeon, J.; Bae, H. Complete genome sequence of the mountain-cultivated ginseng endophyte Burkholderia stabilis and its antimicrobial compounds against ginseng root rot disease. Biol. Control 2020, 140, 104126. [Google Scholar] [CrossRef]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Lee, S.W.; Jo, I.H. Diversity of bacterial endophytes in Panax ginseng and their protective effects against pathogens. 3 Biotech 2018, 8, 397. [Google Scholar] [CrossRef]
- Son, S.H.; Khan, Z.; Kim, S.G.; Kim, Y.H. Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and fusarium wilt fungus. J. Appl. Microbiol. 2009, 107, 524–532. [Google Scholar] [CrossRef]
- Wei, J.H.; He, L.; Niu, G.Q. Regulation of antibiotic biosynthesis in actinomycetes: Perspectives and challenges. Synth. Syst. Biotechnol. 2018, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, I.K.; Yun, B.S. Antagonistic effect of Streptomyces sp. BS062 against Botrytis diseases. Mycobiology 2015, 43, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhou, G.; Lei, F.; Zhang, L. Optimization of fermentation medium for antagonistic actinomycetes F05 of American ginseng rust rot pathogen. China J. Chin. Mater. Medica 2009, 34, 2296–2298. [Google Scholar]
- Torres-Rodriguez, J.A.; Reyes-Perez, J.J.; Quinones-Aguilar, E.E.; Hernandez-Montiel, L.G. Actinomycete potential as biocontrol agent of phytopathogenic fungi: Mechanisms, source, and applications. Plants 2022, 11, 3201. [Google Scholar] [CrossRef]
- Xu, X.D.; Zhao, Y.; Bao, K.; Miao, C.P.; Tang, S.K.; Wu, S.H.; Li, Y.Q. Isolation, structure elucidation and antifungal activity of angucycline antibiotics from Streptomycete cellulosae. Appl. Biochem. Microbiol. 2023, 59, 456–461. [Google Scholar] [CrossRef]
- Huang, J.Q.; Li, X.J.; Zhan, X.L.; Pan, S.Y.; Pan, C.; Li, J.X.; Fan, S.T.; Zhang, L.E.; Du, K.H.; Du, Z.Y.; et al. A Streptomyces species from the ginseng rhizosphere exhibits biocontrol potential. Plant Physiol. 2024, 194, 2709–2723. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanisms of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Prakasam, V.; Jonathan, E.I.; Paramathma, M.; Samiyappan, R. Combining Pseudomonas, Bacillus and Trichoderma strains with organic amendments and micronutrient to enhance suppression of collar and root rot disease in physic nut. Appl. Soil Ecol. 2011, 49, 215–223. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Wang, J.H.; Liu, L.; Zhao, Y.Q.; Jiang, Z.T.; Wang, Y.X.; Li, Z.K.; Fu, L.; Cui, Z.L. Biocontrol potential of Burkholderia sp. BV6 against the rice blast fungus Magnaporthe oryzae. J. Appl. Microbiol. 2022, 133, 883–897. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.Q.; Yang, Y.B.; Duang, R.T.; Chen, G.Y.; Li, X.Z.; Li, Q.L.; Qin, S.H.; Li, S.Q.; Zhao, L.X.; et al. Anti-phytopathogen, multi-target acetylcholinesterase inhibitory and antioxidant activities of metabolites from endophytic Chaetomium globosum. Nat. Prod. Res. 2016, 30, 2616–2619. [Google Scholar] [CrossRef]
- Wang, L.W.; Zhang, Y.B.; Wang, Y.; Suo, M.; Wu, H.; Zhao, M.; Yang, H.Y. Inoculation with Penicillium citrinum aids ginseng in resisting Fusarium oxysporum by regulating the root and rhizosphere microbial communities. Rhizosphere 2022, 22, 100535. [Google Scholar] [CrossRef]
- Xie, S.Y.; Si, H.; Xue, Y.Y.; Zhou, R.; Wang, S.Q.; Duan, Y.Z.; Niu, J.F.; Wang, Z.Z. Efficacy of rhizobacteria Paenibacillus polymyxa SY42 for the biological control of Atractylodes chinensis root rot. Microb. Pathog. 2024, 187, 106517. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A review on mechanisms and prospects of endophytic bacteria in biocontrol of plant pathogenic fungi and their plant growth-promoting activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wei, L.F.; Yang, J.; Ahmed, W.; Wang, Y.T.; Fu, L.N.; Ji, G.H. Probiotic Consortia: Reshaping the Rhizospheric Microbiome and Its Role in Suppressing Root-Rot Disease of Panax notoginseng. Front. Microbiol. 2020, 11, 701. [Google Scholar] [CrossRef]
- Wang, B.Y.; Xia, Q.; Lin, Y.L.; Wei, F.G.; Yang, S.Z.; Dai, C.C.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; Zhao, J. Root rot induces a core assemblage of bacterial microbiome to prevent disease infection in Sanqi ginseng. Appl. Soil Ecol. 2024, 198, 105371. [Google Scholar] [CrossRef]
- Park, Y.H.; Mishra, R.C.; Yoon, S.; Kim, H.; Park, C.; Seo, S.T.; Bae, H. Endophytic Trichoderma citrinoviride isolated from mountain-cultivated ginseng (Panax ginseng) has great potential as a biocontrol agent against ginseng pathogens. J. Ginseng Res. 2019, 43, 408–420. [Google Scholar] [CrossRef]
- Hussein, K.A.; Lee, Y.D.; Joo, J.H. Effect of rosemary essential oil and Trichoderma koningiopsis T-403 VOCs on pathogenic fungi responsible for ginseng root rot disease. J. Microbiol. Biotechnol. 2020, 30, 1018–1026. [Google Scholar] [CrossRef]
- Cabrera, R.; García-López, H.; Aguirre-von-Wobeser, E.; Orozco-Avitia, J.A.; Gutiérrez-Saldaña, A.H. Amycolatopsis BX17: An actinobacterial strain isolated from soil of a traditional milpa agroecosystem with potential biocontrol against Fusarium graminearum. Biol. Control 2020, 147, 104285. [Google Scholar] [CrossRef]
- Essarioui, A.; LeBlanc, N.; Kistler, H.C.; Kinkel, L.L. Plant community richness mediates inhibitory interactions and resource competition between Streptomyces and Fusarium populations in the rhizosphere. Microb. Ecol. 2017, 74, 157–167. [Google Scholar] [CrossRef]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Grosse, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant-Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Aktuganov, G.E.; Galimzyanova, N.F.; Melent’ev, A.I.; Kuz’mina, L.Y. Extracellular hydrolases of strain Bacillus sp 739 and their involvement in the lysis of micromycete cell walls. Microbiology 2007, 76, 413–420. [Google Scholar] [CrossRef]
- Dimkic, I.; Janakiev, T.; Petrovic, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Wang, R.; Liang, X.; Long, Z.; Wang, X.; Yang, L.; Lu, B.; Gao, J. An LCI-like protein APC protects ginseng root from Fusarium solani infection. J. Appl. Microbiol. 2021, 130, 165–178. [Google Scholar] [CrossRef]
- Xu, X.D.; Han, L.; Zhao, L.X.; Chen, X.; Miao, C.P.; Hu, L.F.; Huang, X.S.; Chen, Y.W.; Li, Y.Q. Echinosporin antibiotics isolated from Amycolatopsis strain and their antifungal activity against root-rot pathogens of the Panax notoginseng. Folia Microbiol. 2019, 64, 171–175. [Google Scholar] [CrossRef]
- Razo-Belmán, R.; Angeles-López, Y.I.; Garcia-Ortega, L.F.; León-Ramírez, C.G.; Ortiz-Castellanos, L.; Yu, H.L.; Martínez-Soto, D. Fungal volatile organic compounds: Mechanisms involved in their sensing and dynamic communication with plants. Front. Plant Sci. 2023, 14, 1257098. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2742. [Google Scholar] [CrossRef]
- Zhao, X.X.; Zhou, J.Y.; Tian, R.F.; Liu, Y.L. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Xie, S.S.; Liu, J.; Gu, S.Y.; Chen, X.J.; Jiang, H.Y.; Ding, T. Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol. 2020, 70, 2. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.Q.; Xu, M.J.; Zhang, C.M.; Gao, J.; Li, C.G.; Xing, K.; Qin, S. Antifungal volatile organic compounds from Streptomyces setonii WY228 control black spot disease of sweet potato. Appl. Environ. Microbiol. 2022, 88, e02317–e02321. [Google Scholar] [CrossRef]
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Lee, Y.H. High-throughput identification of genes influencing the competitive ability to obtain nutrients and performance of biocontrol in Pseudomonas putida JBC17. Sci. Rep. 2022, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, Z. Identification of the biocontrol strain LB-2 and determination of its antifungal effects on plant pathogenic fungi. J. Plant Pathol. 2018, 100, 25–32. [Google Scholar] [CrossRef]
- Soni, R.; Keharia, H.J.P. Phytostimulation and biocontrol potential of Gram-positive endospore-forming Bacilli. Planta 2021, 254, 49. [Google Scholar] [CrossRef]
- Perea-Molina, P.A.; Pedraza-Herrera, L.A.; Beauregard, P.B.; Uribe-Vélez, D. A biocontrol Bacillus velezensis strain decreases pathogen Burkholderia glumae population and occupies a similar niche in rice plants. Biol. Control 2022, 176, 105067. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Wang, S.Y.; Herrera-Balandrano, D.D.; Wang, Y.X.; Shi, X.C.; Chen, X.; Jin, Y.; Liu, F.Q.; Laborda, P. Biocontrol ability of the Bacillus amyloliquefaciens group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the management of fungal postharvest diseases: A review. J. Agric. Food Chem. 2022, 70, 6591–6616. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Molina-Santiago, C.; Pearson, J.R.; Navarro, Y.; Berlanga-Clavero, M.V.; Caraballo-Rodriguez, A.M.; Petras, D.; García-Martín, M.L.; Lamon, G.; Haberstein, B.; Cazorla, F.M.; et al. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat. Commun. 2019, 10, 1919. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F.J. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Zhu, X.X.; Chen, W.J.; Bhatt, K.; Zhou, Z.; Huang, Y.H.; Zhang, L.H.; Chen, S.H.; Wang, J.X. Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Front. Plant Sci. 2023, 13, 1063393. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.A.; Pieterse, C.M. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Jin, H.C.; Lee, S.; Nam, M.H.; Chung, J.H.; Kwon, S.I.; Ryu, C.M.; Park, O.K. GDSL lipase-like 1 regulates systemic resistance associated with ethylene signaling in Arabidopsis. Plant J. 2009, 58, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Lee, B.D.; Dutta, S.; Ryu, H.; Yoo, S.J.; Suh, D.S.; Park, K. Induction of systemic resistance in Panax ginseng against Phytophthora cactorum by native Bacillus amyloliquefaciens HK34. J. Ginseng Res. 2015, 39, 213–220. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Wang, J.; Gao, W.; Sun, X.; Xiong, Q.; Shu, X.; Miao, Y.; Shen, Q.; Xun, W.; et al. Nonpathogenic Pseudomonas syringae derivatives and its metabolites trigger the plant “cry for help” response to assemble disease suppressing and growth promoting rhizomicrobiome. Nat. Commun. 2024, 15, 1907. [Google Scholar] [CrossRef]
- Kalia, V.C.; Gong, C.J.; Patel, S.K.S.; Lee, J.K. Regulation of Plant Mineral Nutrition by Signal Molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef]
- Ibal, J.C.; Park, M.K.; Park, G.S.; Jung, B.K.; Park, T.H.; Kim, M.S.; Kang, G.U.; Park, Y.J.; Shin, J.H. Use of Acyl-Homoserine Lactones Leads to Improved Growth of Ginseng Seedlings and Shifts in Soil Microbiome Structure. Agronomy 2021, 11, 2177. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.W.; Zuo, B.; Liang, X.Y.; Zhang, D.N.; Liu, R.X.; Yang, L.N.; Lu, B.H.; Wang, X.; Gao, J. Novel Biocontrol Strain Bacillus amyloliquefaciens FS6 for Excellent Control of Gray Mold and Seedling Diseases of Ginseng. Plant Dis. 2021, 105, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Zhao, X.L.; Wu, K.; Liang, C.Y.; Liu, J.; Yang, H.; Wang, C.M.; Yang, B.; Yin, F.; Zhang, W.D. Isolation and characterization of Bacillus velezensis strain B19 for biocontrol of Panax notoginseng root rot. Biol. Control 2023, 185, 105311. [Google Scholar] [CrossRef]
- Dong, Y.J.; Tang, B.B.; He, M.M.; Wang, L.L.; Wu, K.; Yang, S.X.; Liu, J.F.; Yang, H.; Wang, C.M.; Yin, F.; et al. High concentrations of antagonistic bacterial strains from diseased sanqi ginseng rhizosphere suppressed Fusarium root rot. Eur. J. Plant Pathol. 2022, 163, 143–153. [Google Scholar] [CrossRef]
- Liu, T.T.; Zhang, J.Y.; Wang, T.; Li, Z.Y.; Liang, H.J.; Jiang, C.Y.; Tang, H.; Gao, J.; Jiang, Y.; Chen, C.Q. The novel Pseudomonas thivervalensis strain JI6 promotes growth and controls rusty root rot disease in Panax ginseng. Biol. Control 2024, 193, 105514. [Google Scholar] [CrossRef]
- Dong, L.L.; Xu, J.; Zhang, L.J.; Cheng, R.Y.; Wei, G.F.; Su, H.; Yang, J.; Qian, J.; Xu, R.; Chen, S.L. Rhizospheric microbial communities are driven by Panax ginseng at different growth stages and biocontrol bacteria alleviates replanting mortality. Acta Pharm. Sin. B 2018, 8, 272–282. [Google Scholar] [CrossRef]
- Chen, J.L.; Sun, S.Z.; Miao, C.P.; Wu, K.; Chen, Y.W.; Xu, L.H.; Guan, H.L.; Zhao, L.X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2016, 40, 315–324. [Google Scholar] [CrossRef]
- Cho, G.; Kim, D.R.; Kwak, Y.S. Transition from Ginseng Root Rot Disease-Conducive Soil to -Suppressive Soil Mediated by Pseudomonadaceae. Microbiol. Spectr. 2023, 11, e01150-23. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Lu, Y.L.; Miao, C.P.; Guan, H.L.; Wang, R.; Wang, H.J.; Tian, L.Y.; Wei, F.G.; Xu, W.M. Mitigating root rot in Panax notoginseng: The synergistic effects of biochar and Chaetomium globosum YIM PH30719. Ind. Crops Prod. 2024, 222, 119805. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Q.; Gao, Y.G.; Zang, P.; Zheng, T. Effects of a co-bacterial agent on the growth, disease control, and quality of ginseng based on rhizosphere microbial diversity. BMC Plant Biol. 2024, 24, 647. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, S.; Piao, X.; Yan, M.; Cui, L.; Wang, Y.J.A.J.o.B. Control of ginseng leaf black spot disease by endophytic fungi. Afr. J. Biotechnol. 2021, 20, 308–312. [Google Scholar]
- Liu, D.F.; Sun, H.J.; Ma, H.W. Deciphering Microbiome Related to Rusty Roots of Panax ginseng and Evaluation of Antagonists Against Pathogenic Ilyonectria. Front. Microbiol. 2019, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.R.; Liu, T.T.; Li, X.; Gao, J.; Jiang, Y.; Chen, C.Q. Bacillus amyloliquefaciens FG14 as a potential biocontrol strain against rusty root rot of Panax ginseng, and its impact on the rhizosphere microbial community. Biol. Control 2023, 182, 105221. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.G.; Kim, Y.H. Biocontrol Efficacies of Bacillus Species Against Cylindrocarpon destructans Causing Ginseng Root Rot. Plant Pathol. J. 2011, 27, 333–341. [Google Scholar] [CrossRef]
- Li, X.Q.; Wang, J.R.; Shen, H.; Xing, C.X.; Kong, L.X.; Song, Y.; Hou, W.P.; Gao, J.; Jiang, Y.; Chen, C.Q. Biocontrol and growth promotion potential of Bacillus velezensis NT35 on Panax ginseng based on the multifunctional effect. Front. Microbiol. 2024, 15, 1447488. [Google Scholar] [CrossRef]
- Chu, Y.; Li, Y.; Wang, J.H.; Wang, C.Y. Study on the growth-promoting potenial and disease resistance of the antagonistic bacterium Frankia francese F1 on ginseng root rot and rust rot Appl. Ecol. Environ. Res. 2023, 21, 2055–2074. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Liu, H.L.; Zhang, B.P.; Geng, M.X.; Cai, X.X.; Wang, J.H.; Wang, Y.P. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Appl. Soil Ecol. 2022, 172, 104369. [Google Scholar] [CrossRef]
- Kim, H.; Rim, S.O.; Bae, H. Antimicrobial potential of metabolites extracted from ginseng bacterial endophyte Burkholderia stabilis against ginseng pathogens. Biol. Control 2019, 128, 24–30. [Google Scholar] [CrossRef]
- Hong, S.C. The Efficacy of Trichoderma, Benomyl, and Propiconazole: Treatments for the Control of Cylindrocarpon Destructans Diseases in North American Ginseng (Panax quinquefolius L.); National Library of Canada: Ottawa, ON, Canada, 2001. [Google Scholar]
- Gastol, M.; Domagala-Swiatkiewicz, I.; Bijak, M. The effect of mycorrhizal inoculation and phosphorus application on the growth and mineral nutrient status of apple seedlings. J. Plant Nutr. 2016, 39, 288–299. [Google Scholar] [CrossRef]
- Gao, Y.G.; Liu, Q.; Zang, P.; Li, X.; Ji, Q.; He, Z.M.; Zhao, Y.; Yang, H.; Zhao, X.L.; Zhang, L.X. An endophytic bacterium isolated from Panax ginseng CA Meyer enhances growth, reduces morbidity, and stimulates ginsenoside biosynthesis. Phytochem. Lett. 2015, 11, 132–138. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, Y.X.; Zhang, Z.P.; Li, J.Q.; Zhu, S.S.; Yang, M.; Luo, L.X. Application of plant survival-promoting and pathogen-suppressing Trichoderma species for crop biofertilization and biocontrol of root rot in Panax notoginseng. J. Plant Pathol. 2022, 104, 1361–1369. [Google Scholar] [CrossRef]
- Serrao, C.P.; Ortega, J.C.G.; Rodrigues, P.C.; de Souza, C.R.B. Bacillus species as tools for biocontrol of plant diseases: A meta-analysis of twenty-two years of research, 2000–2021. World J. Microbiol. Biotechnol. 2024, 40, 110. [Google Scholar] [CrossRef] [PubMed]
- Minchev, Z.; Kostenko, O.; Soler, R.; Pozo, M.J. Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Front. Plant Sci. 2021, 12, 756368. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.A.M.; Hashem, M.; Ali, E.H. Integrated control of cotton root rot disease by mixing fungal biocontrol agents and resistance inducers. Crop Prot. 2009, 28, 295–301. [Google Scholar] [CrossRef]
- Elliott, M.; Shamoun, S.F.; Sumampong, G.; James, D.; Masri, S.; Varga, A. Evaluation of several commercial biocontrol products on European and North American populations of Phytophthora ramorum. Biocontrol Sci. Technol. 2009, 19, 1007–1021. [Google Scholar] [CrossRef]
- Cernava, T. How microbiome studies could further improve biological control. Biol. Control 2021, 160, 104669. [Google Scholar] [CrossRef]
- Deb, C.R.; Tatung, M. Siderophore producing bacteria as biocontrol agent against phytopathogens for a better environment: A review. S. Afr. J. Bot. 2024, 165, 153–162. [Google Scholar] [CrossRef]
- Kanwar, R.S.; Patil, J.A.; Yadav, S. Prospects of using predatory nematodes in biological control for plant parasitic nematodes—A review. Biol. Control 2021, 160, 104668. [Google Scholar] [CrossRef]


| Types | Strain | Inhibition Mechanisms | References |
|---|---|---|---|
| Bacteria | Bacillus | Synthesis of antagonistic substances; competition for ecological niches; induction of host resistance, reshaping the soil microbiome | [10,53,54,55,56] |
| Pseudomonas | Competition for ecological niches, reshaping the soil microbiome | [54,56,57] | |
| Burkholderia | Synthesis of antagonistic substances | [50] | |
| Fungi | Trichoderma | Synthesis of antagonistic substances; induction of host resistance | [58,59] |
| Chaetomium | Competition for ecological niches | [51] | |
| Penicillium | Synthesis of antagonistic sub-stances | [52] | |
| Actinomyces | Streptomyces | Synthesis of antagonistic substances; competition for ecological niches; induction of host resistance | [60,61,62] |
| Factor | Treatment | Biological Effect | Choice |
|---|---|---|---|
| Microbial species | Bacillus | DI/DSI | Trichoderma/ Burkholderia |
| Pseudomonadaceae | |||
| Chaetomium globosum | |||
| Burkholderia | |||
| Brevundimonas | |||
| Lysobacter | |||
| Pseudomonas | |||
| Frankia | |||
| Trichoderma | |||
| Streptomyces | |||
| Inoculation method | Rootirrigation | DI/DSI | Root irrigation/root dipping |
| Soil mixing | |||
| Root dipping | |||
| Foliar application | |||
| Foliar application plus root irrigation | |||
| Inoculation volume | 105 | DI/DSI | 1010/108 |
| 106 | |||
| 107 | |||
| 108 | |||
| 109 | |||
| 1010 | |||
| Microbial composition | Single | DI/DSI | Single |
| Consortia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, S.; Yang, H. Understanding the Pathogenesis, Biocontrol Mechanisms, and Factors Influencing Biocontrol Effectiveness for Soil-Borne Diseases in Panax Plants. Microorganisms 2024, 12, 2278. https://doi.org/10.3390/microorganisms12112278
Wang Z, Wang S, Yang H. Understanding the Pathogenesis, Biocontrol Mechanisms, and Factors Influencing Biocontrol Effectiveness for Soil-Borne Diseases in Panax Plants. Microorganisms. 2024; 12(11):2278. https://doi.org/10.3390/microorganisms12112278
Chicago/Turabian StyleWang, Zhaobei, Shuoye Wang, and Hongyan Yang. 2024. "Understanding the Pathogenesis, Biocontrol Mechanisms, and Factors Influencing Biocontrol Effectiveness for Soil-Borne Diseases in Panax Plants" Microorganisms 12, no. 11: 2278. https://doi.org/10.3390/microorganisms12112278
APA StyleWang, Z., Wang, S., & Yang, H. (2024). Understanding the Pathogenesis, Biocontrol Mechanisms, and Factors Influencing Biocontrol Effectiveness for Soil-Borne Diseases in Panax Plants. Microorganisms, 12(11), 2278. https://doi.org/10.3390/microorganisms12112278






