Endophytic Bacterial Community, Core Taxa, and Functional Variations Within the Fruiting Bodies of Laccaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Method and Storage
2.2. Species Identification of Laccaria Samples
2.3. Laccaria Fruiting Body Separation
2.4. Fruiting Body Bacteriome and Quantitative Real-Time PCR Analysis
2.5. Statistical Analysis
3. Results
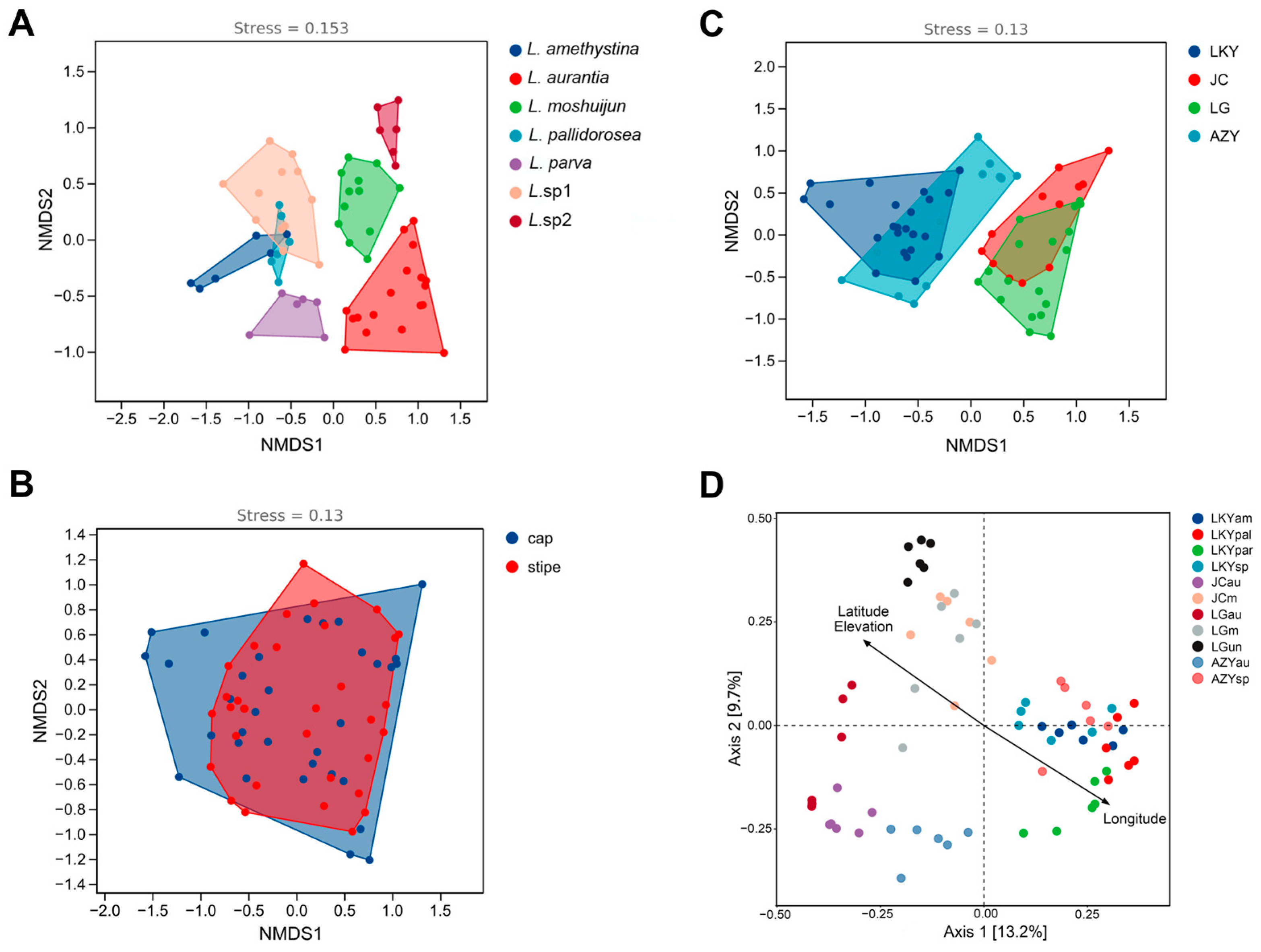
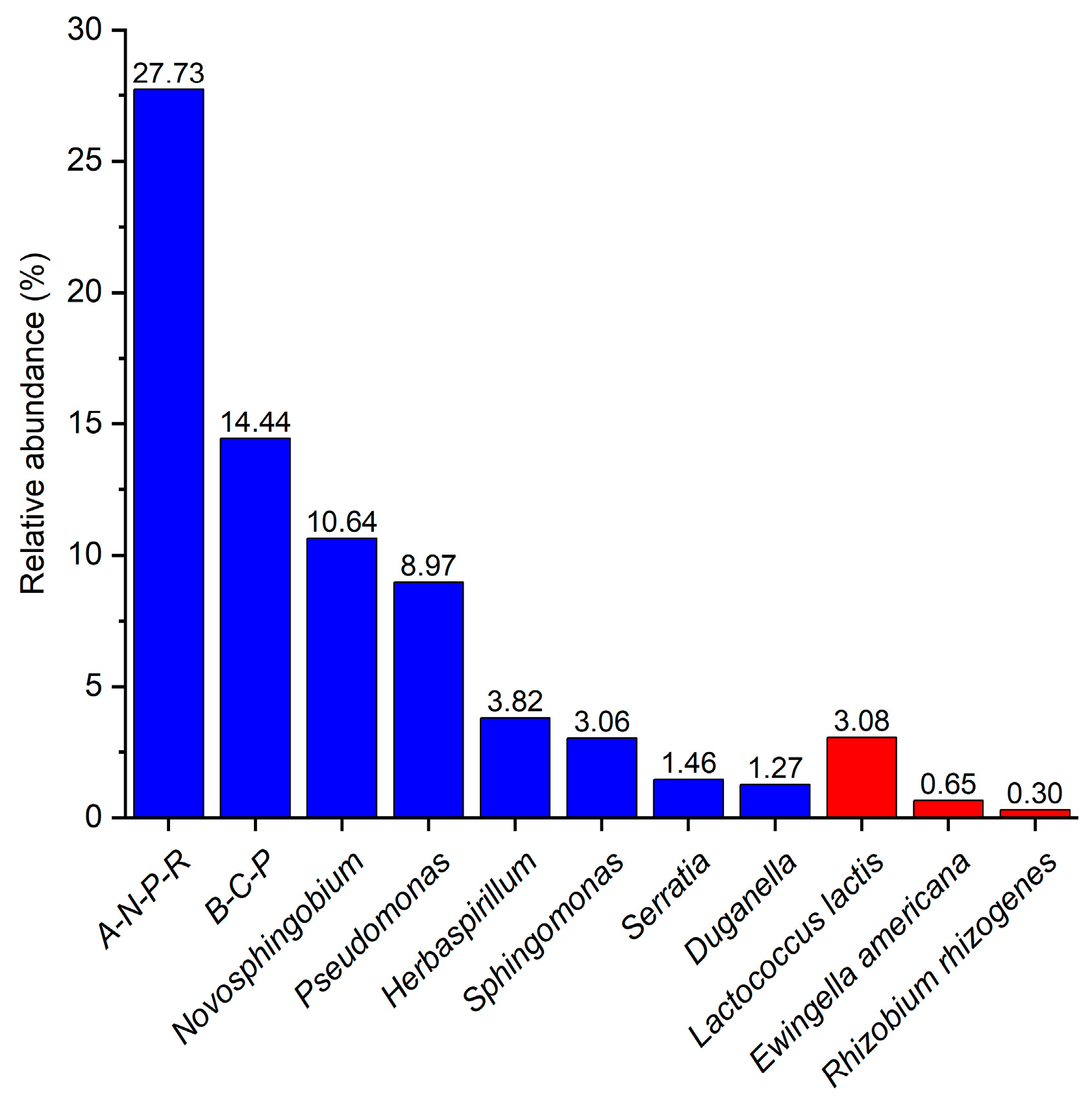
3.1. Analysis of Community Composition and Diversity of Abundant and Rare Bacteria in the Fruiting Body
3.2. Identification of the Main Drivers of Bacterial Diversity and Structure Within Laccaria Fruiting Bodies
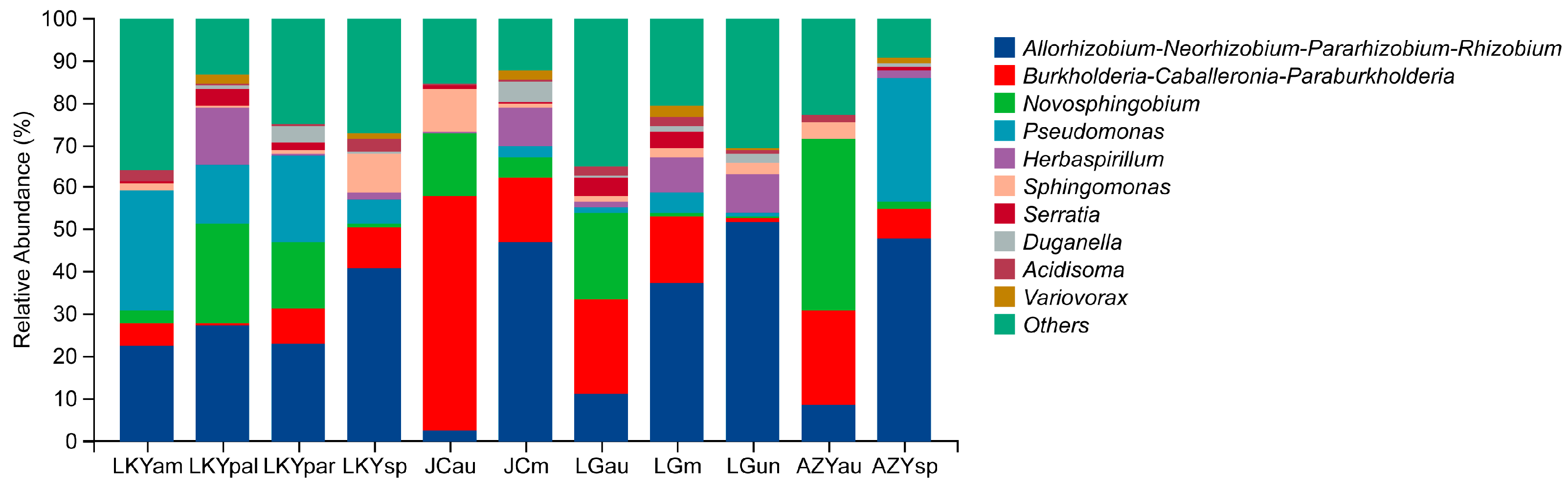
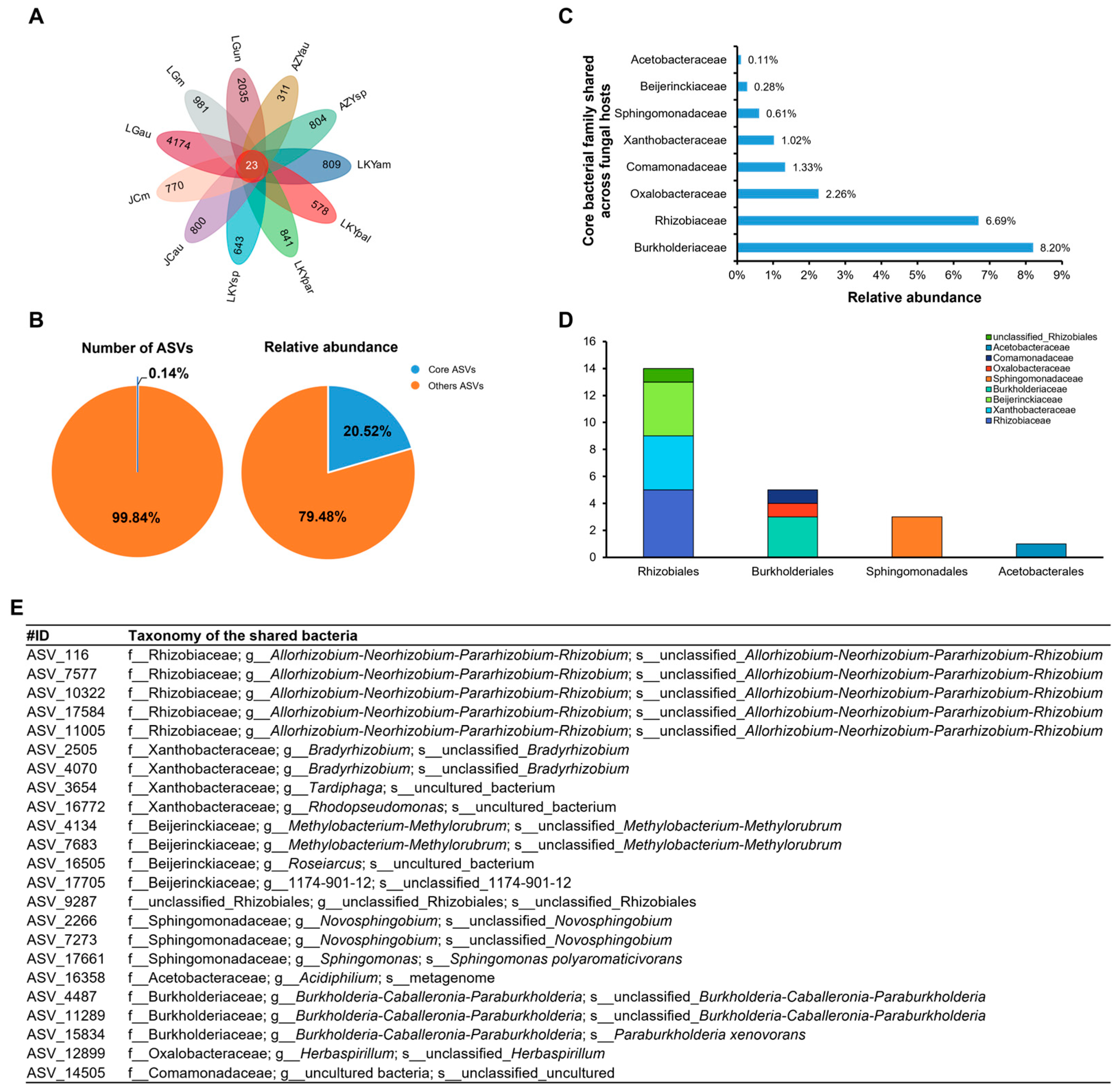
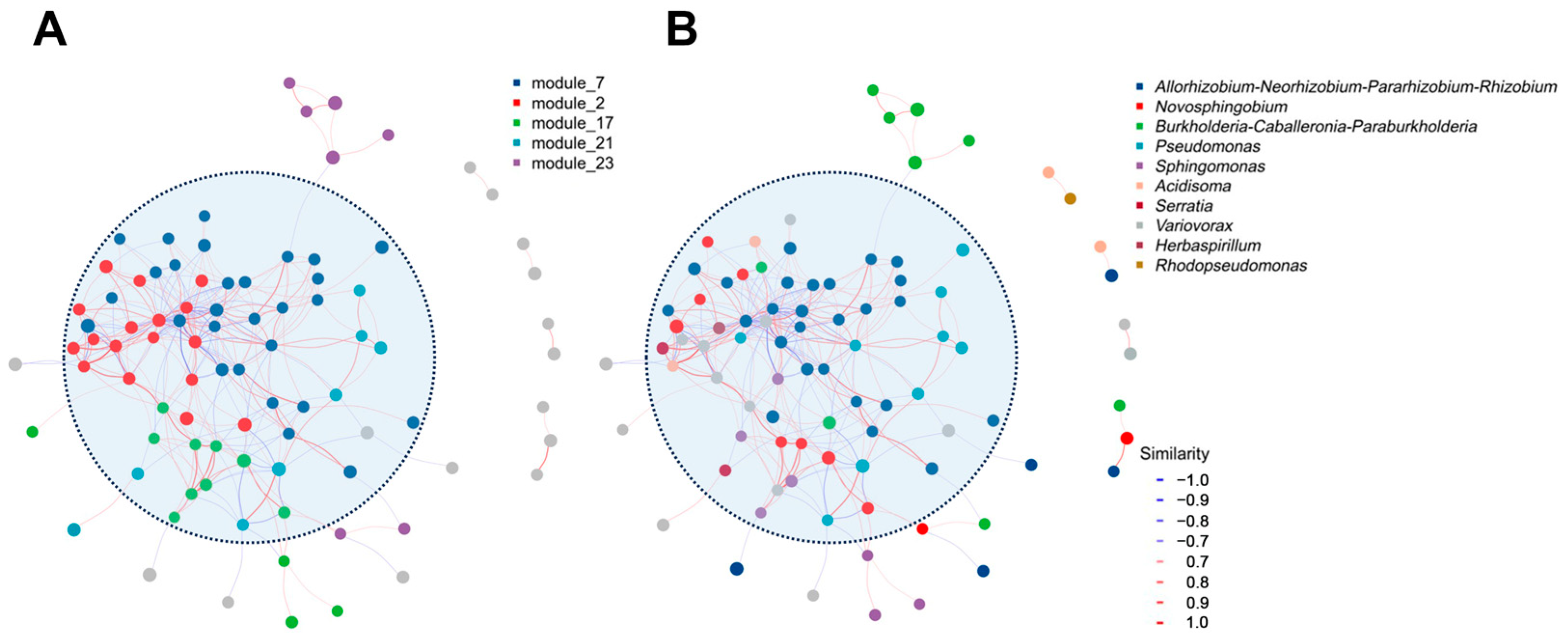
3.3. Core and Keystone Microbes in the Laccaria Fruiting Bodies
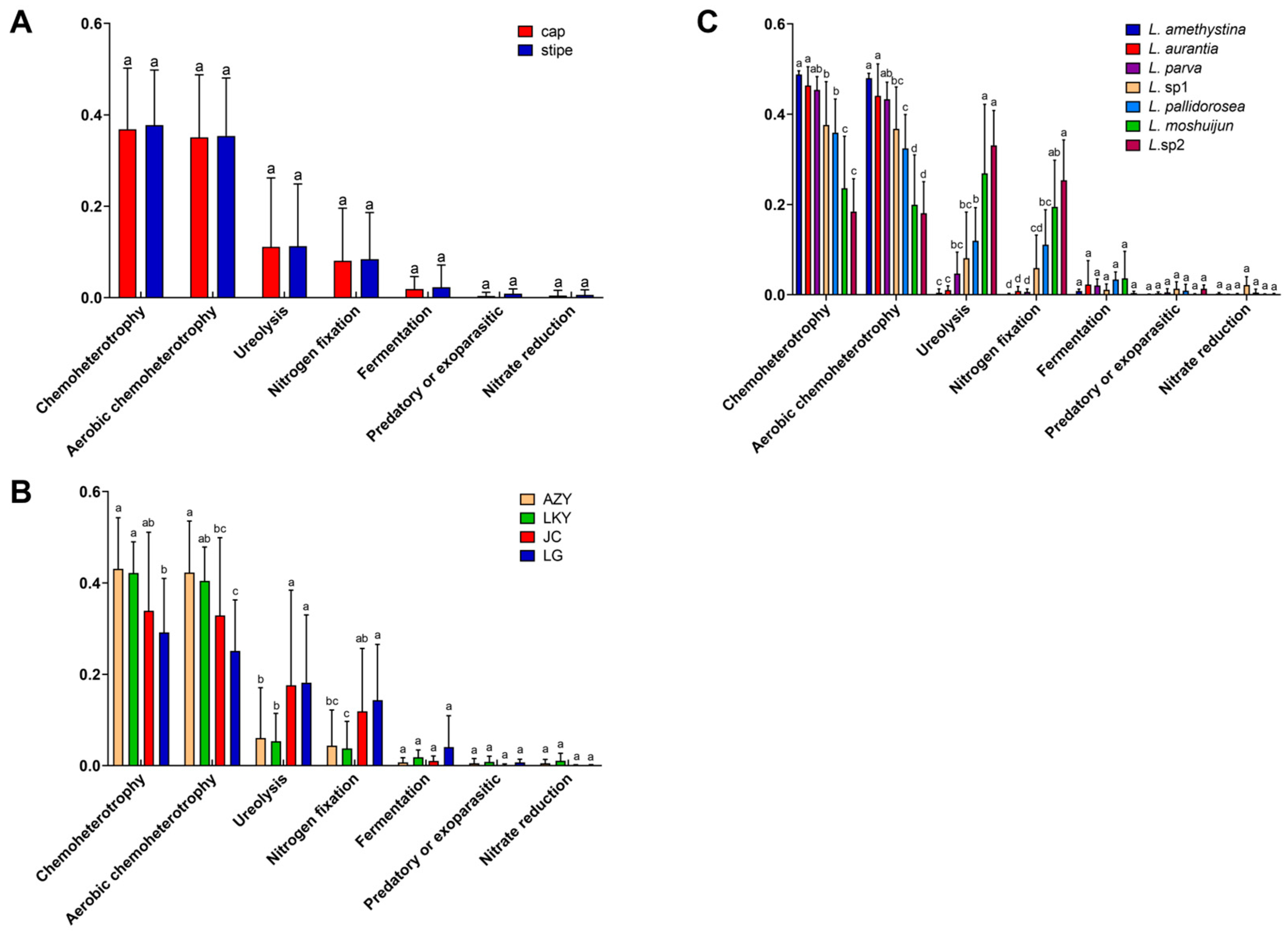
3.4. Bacteriome Function
4. Discussion
4.1. Laccaria Endophytic Bacterial Abundance and Composition
4.2. The Key Bacterial Genera Within Laccaria Fruiting Bodies
4.3. Prediction of Ecological Function Within Laccaria Fruiting Body
4.4. Implication of Laccaria Endophytic Bacteria on Plant and Human Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partida-Martínez, L.P. The fungal holobiont: Evidence from early diverging fungi. Environ. Microbiol. 2017, 19, 2919–2923. [Google Scholar] [CrossRef] [PubMed]
- Gohar, D.; Põldmaa, K.; Tedersoo, L.; Aslani, F.; Furneaux, B.; Henkel, T.W.; Saar, I.; Smith, M.E.; Bahram, M. Global diversity and distribution of mushroom-inhabiting bacteria. Environ. Microbiol. Rep. 2022, 14, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.; Chater, C.C.C.; Perez-Moreno, J.; Yu, F. Microbiome community structure and functional gene partitioning in different micro-niches within a sporocarp-forming fungus. Front. Microbiol. 2021, 12, 629352. [Google Scholar] [CrossRef]
- Haq, I.U.; Hillmann, B.; Moran, M.; Willard, S.; Knights, D.; Fixen, K.R.; Schilling, J.S. Bacterial communities associated with wood rot fungi that use distinct decomposition mechanisms. ISME Commun. 2022, 2, 26. [Google Scholar] [CrossRef]
- Xing, R.; Zhang, H.-C.; Gao, Q.-B.; Zhang, F.; Chi, X.; Chen, S.-L. Bacterial communities associated with mushrooms in the Qinghai-Tibet Plateau are shaped by soil parameters. Int. Microbiol. 2022, 26, 231–242. [Google Scholar] [CrossRef]
- Liu, D.; Chater, C.C.C.; Yu, F.; Perez-Moreno, J. Tuber pseudohimalayense ascomata-compartments strongly select their associated bacterial microbiome from nearby pine forest soils independently of their maturation stage. Pedobiologia 2021, 87–88, 150743. [Google Scholar] [CrossRef]
- Liu, D.; Pérez-Moreno, J.; He, X.; Garibay-Orijel, R.; Yu, F.; McMahon, K. Truffle microbiome is driven by fruit body compartmentalization rather than soils conditioned by different host trees. mSphere 2021, 6, e0003921. [Google Scholar] [CrossRef]
- Braat, N.; Koster, M.C.; Wösten, H.A.B. Beneficial interactions between bacteria and edible mushrooms. Fungal Biol. Rev. 2022, 39, 60–72. [Google Scholar] [CrossRef]
- Kang, Y.M.; Cho, K.M. Identification of auxin from Pseudomonas sp. P7014 for the rapid growth of Pleurotus eryngii mycelium. Korean J. Microbiol. 2014, 50, 15–21. [Google Scholar] [CrossRef]
- Colauto, N.B.; Fermor, T.R.; Eira, A.F.; Linde, G.A. Pseudomonas putida stimulates primordia on Agaricus bitorquis. Curr. Microbiol. 2016, 72, 482–488. [Google Scholar] [CrossRef]
- Baars, J.J.P.; Scholtmeijer, K.; Sonnenberg, A.S.M.; van Peer, A.V. Critical factors involved in primordia building in Agaricus bisporus: A review. Molecules 2020, 25, 2984. [Google Scholar] [CrossRef] [PubMed]
- Šantrić, L.; Potočnik, I.; Radivojević, L.; Umiljendić, J.G.; Rekanović, E.; Duduk, B.; Milijašević-Marčić, S. Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus bisporus. J. Environ. Sci. Health B 2018, 53, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Büchner, R.; Vörös, M.; Allaga, H.; Varga, A.; Bartal, A.; Szekeres, A.; Varga, S.; Bajzát, J.; Bakos-Barczi, N.; Misz, A.; et al. Selection and characterization of a bacillus strain for potential application in industrial production of white button mushroom (Agaricus bisporus). Agronomy 2022, 12, 467. [Google Scholar] [CrossRef]
- McGee, C.F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl. Microbiol. Biot. 2017, 102, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2019, 22, 858–872. [Google Scholar] [CrossRef]
- Martin, F.M.; van der Heijden, M.G.A. The mycorrhizal symbiosis: Research frontiers in genomics, ecology, and agricultural application. New Phytol. 2024, 242, 1486–1506. [Google Scholar] [CrossRef]
- Lewis, J.D. Mycorrhizal fungi, evolution and diversification of. In Encyclopedia of Evolutionary Biology; Kliman, R.M., Ed.; Academic Press: Oxford, UK, 2016; pp. 94–99. [Google Scholar]
- Siddiqui, Z.A.; Pichtel, J. Mycorrhizae: An overview. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z.A., Akhtar, M.S., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–35. [Google Scholar]
- Garbaye, J. Tansley review no. 76. Helper bacteria: A new dimension to the mycorrhizal symbiosis. New Phytol. 1994, 128, 197–210. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Labbé, J.L.; Weston, D.J.; Dunkirk, N.; Pelletier, D.A.; Tuskan, G.A. Newly identified helper bacteria stimulate ectomycorrhizal formation in Populus. Front. Plant Sci. 2014, 5, 579. [Google Scholar] [CrossRef]
- Gong, S.; Feng, B.; Jian, S.-P.; Wang Geng, S.; Ge, Z.-W.; Yang Zhu, L. Elevation matters more than season in shaping the heterogeneity of soil and root associated ectomycorrhizal fungal community. Microbiol. Spectr. 2022, 10, e0195021. [Google Scholar] [CrossRef] [PubMed]
- Zak, D.R.; Pellitier, P.T.; Argiroff, W.; Castillo, B.; James, T.Y.; Nave, L.E.; Averill, C.; Beidler, K.V.; Bhatnagar, J.; Blesh, J.; et al. Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytol. 2019, 223, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Dini-Andreote, F.; van Elsas, J.D. Transcriptional responses of the bacterium Burkholderia terrae bs001 to the fungal host Lyophyllum sp. strain karsten under soil-mimicking conditions. Microb. Ecol. 2017, 73, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.; Brule, C.; Palin, B.; Champmartin, D.; Rubini, P.; Garbaye, J.; Sarniguet, A.; Frey-Klett, P. Role of fungal trehalose and bacterial thiamine in the improved survival and growth of the ectomycorrhizal fungus Laccaria bicolor S238N and the helper bacterium Pseudomonas fluorescens BBc6R8. Environ. Microbiol. Rep. 2010, 2, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Boersma, F.G.H.; Otten, R.; Warmink, J.A.; Nazir, R.; van Elsas, J.D. Selection of Variovorax paradoxus-like bacteria in the mycosphere and the role of fungal-released compounds. Soil. Biol. Biochem. 2010, 42, 2137–2145. [Google Scholar] [CrossRef]
- Bai, H.Y.; Zhang, A.Y.; Mei, Y.; Xu, M.; Lu, X.L.; Dai, C.C.; Jia, Y. Effects of ectomycorrhizal fungus bolete identity on the community assemblages of endofungal bacteria. Environ. Microbiol. Rep. 2021, 13, 852–861. [Google Scholar] [CrossRef]
- Liu, D.; Perez-Moreno, J.; Zhang, P.; Wang, R.; Chater, C.C.C.; Yu, F. Distinct compartmentalization of microbial community and potential metabolic function in the fruiting body of Tricholoma matsutake. J. Fungi 2021, 7, 586. [Google Scholar] [CrossRef]
- Pent, M.; Bahram, M.; Põldmaa, K. Fruitbody chemistry underlies the structure of endofungal bacterial communities across fungal guilds and phylogenetic groups. ISME J. 2020, 14, 2131–2141. [Google Scholar] [CrossRef]
- Rigamonte, T.A.; Pylro, V.S.; Duarte, G.F. The role of mycorrhization helper bacteria in the establishment and action of ectomycorrhizae associations. Braz. J. Microbiol. 2010, 41, 832–840. [Google Scholar] [CrossRef]
- Pent, M.; Poldmaa, K.; Bahram, M. Bacterial communities in boreal forest mushrooms are shaped both by soil parameters and host identity. Front. Microbiol. 2017, 8, 836. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, X.-L.; Mu, L.-Q.; Deng, W.-Q. Morphology and molecular phylogeny reveal five new species of Laccaria (hydnangiaceae, agaricales) from Southern China. J. Fungi 2023, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhang, M.; Cao, G.; Zhu, J.; Zhang, A.; Bai, H.; Dai, C.; Jia, Y. Endofungal bacteria and ectomycorrhizal fungi synergistically promote the absorption of organic phosphorus in Pinus massoniana. Plant Cell Environ. 2024, 47, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, H.; Park, M.S.; Park, K.H.; Park, J.H.; Cho, Y.; Kim, C.; Lim, Y.W. Two new species of Laccaria (Agaricales, Basidiomycota) from Korea. Mycobiology 2020, 48, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-Y.; Cai, Q.; Li, J.; Yang, Z.L. Two new Laccaria species from China based on molecular and morphological evidence. Mycol. Prog. 2021, 20, 567–576. [Google Scholar] [CrossRef]
- Li, J.; Che, N.J.; Cui, Y.Y. Three new species of Laccaria (Agaricales, Basidiomycota) from Southwest China (Yunnan) based on morphological and multi-gene sequence data. Front. Microbiol. 2024, 15, 1411488. [Google Scholar] [CrossRef]
- Wilson, A.W.; Hosaka, K.; Perry, B.A.; Mueller, G.M. Laccaria (Agaricomycetes, Basidiomycota) from Tibet (Xizang autonomous region, China). Mycoscience 2013, 54, 406–419. [Google Scholar] [CrossRef]
- Popa, F.; Rexer, K.-H.; Donges, K.; Yang, Z.L.; Kost, G. Three new Laccaria species from Southwest China (Yunnan). Mycol. Prog. 2014, 13, 1105–1117. [Google Scholar] [CrossRef]
- Luo, X.; Ye, L.; Chen, J.; Karunarathna, S.C.; Xu, J.; Hyde, K.D.; Mortimer, P.E. Laccaria rubroalba sp. nov. (Hydnangiaceae, Agaricales) from Southwestern China. Phytotaxa 2016, 284, 41. [Google Scholar] [CrossRef]
- Vincenot, L.; Popa, F.; Laso, F.; Donges, K.; Rexer, K.H.; Kost, G.; Yang, Z.L.; Nara, K.; Selosse, M.A. Out of Asia: Biogeography of fungal populations reveals asian origin of diversification of the Laccaria amethystina complex, and two new species of violet Laccaria. Fungal Biol. 2017, 121, 939–955. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, M.S.; Lee, H.; Oh, S.-Y.; Wilson, A.W.; Mueller, G.M.; Lim, Y.W. A systematic revision of the ectomycorrhizal genus Laccaria from Korea. Mycologia 2018, 110, 948–961. [Google Scholar] [CrossRef]
- Li, F. Two new species of Laccaria from South China, with a note on Hodophilus glaberipes. Mycol. Prog. 2020, 19, 525–539. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.; Guerin-Laguette, A.; Arzú, R.F.; Yu, F.-Q. Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences; Pérez-Moreno, J., Guerin-Laguette, A., Arzú, R.F., Yu, F.-Q., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wilson, A.W.; Hosaka, K.; Mueller, G.M. Evolution of ectomycorrhizas as a driver of diversification and biogeographic patterns in the model mycorrhizal mushroom genus Laccaria. New Phytol. 2017, 213, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.M.; Aerts, A.; Ahrén, D.; Brun, A.; Danchin, E.G.J.; Duchaussoy, F.; Gibon, J.; Kohler, A.; Lindquist, E.; Pereda, V.; et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 2008, 452, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, L.; Ye, L.; Zhou, J.; Zhang, B.; Peng, W.; Zhang, X.; Li, X. Chinese black truffle (Tuber indicum) alters the ectomycorrhizosphere and endoectomycosphere microbiome and metabolic profiles of the host tree Quercus aliena. Front. Microbiol. 2018, 9, 2202. [Google Scholar] [CrossRef]
- Baragatti, M.; Grollemund, P.M.; Montpied, P.; Dupouey, J.L.; Gravier, J.; Murat, C.; Le Tacon, F. Influence of annual climatic variations, climate changes, and sociological factors on the production of the Périgord black truffle (Tuber melanosporum Vittad.) from 1903-1904 to 1988-1989 in the Vaucluse (France). Mycorrhiza 2019, 29, 113–125. [Google Scholar] [CrossRef]
- Büntgen, U.; Oliach, D.; Martínez-Peña, F.; Latorre, J.; Egli, S.; Krusic, P.J. Black truffle winter production depends on Mediterranean summer precipitation. Environ. Res. Lett. 2019, 14, 074004. [Google Scholar] [CrossRef]
- Garcia-Barreda, S.; Camarero, J.J.; Vicente-Serrano, S.M.; Serrano-Notivoli, R. Variability and trends of black truffle production in Spain (1970–2017): Linkages to climate, host growth, and human factors. Agr. Forest Meteorol. 2020, 287, 107951. [Google Scholar] [CrossRef]
- Liu, D.; Herrera, M.; Yu, F.; Pérez-Moreno, J. Provenances originate morphological and microbiome variation of Tuber pseudobrumale in Southwestern China despite strong genetic consistency. Mycol. Prog. 2020, 19, 1545–1558. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Horton, M.W.; Bodenhausen, N.; Beilsmith, K.; Meng, D.; Muegge, B.D.; Subramanian, S.; Vetter, M.M.; Vilhjálmsson, B.J.; Nordborg, M.; Gordon, J.I.; et al. Genome-wide association study of Arabidopsis thaliana leaf microbial community. Nat. Commun. 2014, 5, 5320. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Zhu, Y.-J.; Wang, B.; Liu, D.; Bai, H.; Jin, L.; Wang, B.-T.; Ruan, H.-H.; Mao, L.; Jin, F.-J.; et al. Effects of nitrogen addition on rhizospheric soil microbial communities of poplar plantations at different ages. Forest Ecol. Manag. 2021, 494, 119328. [Google Scholar] [CrossRef]
- Ricotta, C.; Pavoine, S. A new parametric measure of functional dissimilarity: Bridging the gap between the Bray-Curtis dissimilarity and the Euclidean distance. Ecol. Model. 2022, 466, 109880. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Li, P.; Tedersoo, L.; Crowther, T.W.; Dumbrell, A.J.; Dini-Andreote, F.; Bahram, M.; Kuang, L.; Li, T.; Wu, M.; Jiang, Y.; et al. Fossil-fuel-dependent scenarios could lead to a significant decline of global plant-beneficial bacteria abundance in soils by 2100. Nat Food 2023, 4, 996–1006. [Google Scholar] [CrossRef]
- Carlino, N.; Blanco-Miguez, A.; Puncochar, M.; Mengoni, C.; Pinto, F.; Tatti, A.; Manghi, P.; Armanini, F.; Avagliano, M.; Barcenilla, C.; et al. Unexplored microbial diversity from 2500 food metagenomes and links with the human microbiome. Cell 2024, 187, 5775–5795.e15. [Google Scholar] [CrossRef]
- Cerigini, E.; Palma, F.; Barbieri, E.; Buffalini, M.; Stocchi, V. The Tuber borchii fruiting body-specific protein TBF-1, a novel lectin which interacts with associated Rhizobium species. FEMS Microbiol. Lett. 2008, 284, 197–203. [Google Scholar] [CrossRef]
- Qian, J.M.; Bai, Y. Stuck on you: Bacterial-auxin-mediated bacterial colonization of plant roots. Cell Host Microbe 2021, 29, 1471–1473. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520.e1504. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Pehkonen, K.; Sinkko, H.; Tamminen, M.V.; Timonen, S. Archaea are prominent members of the prokaryotic communities colonizing common forest mushrooms. Can. J. Microbiol. 2018, 64, 716–726. [Google Scholar] [CrossRef]
- Gohar, D.; Pent, M.; Põldmaa, K.; Bahram, M. Bacterial community dynamics across developmental stages of fungal fruiting bodies. FEMS Microbiol. Ecol. 2020, 96, fiaa175. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Zhang, Z.-Y.; Dong, C.-B.; Han, Y.-F.; Deshmukh, S.K.; Liang, Z.-Q. Bacterial community analysis and potential functions of core taxa in different parts of the fungus Cantharellus cibarius. Pol. J. Microbiol. 2021, 70, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Calixto, R.O.d.R.; Yang, P.; dos Santos, G.M.P.; Barreto-Bergter, E.; van Elsas, J.D.; de Boer, W. Chemotaxis and adherence to fungal surfaces are key components of the behavioral response of Burkholderia terrae BS001 to two selected soil fungi. FEMS Microbiol. Ecol. 2016, 92, fiw164. [Google Scholar] [CrossRef] [PubMed]
- Kothe, E.; Ghodsalavi, B.; Svenningsen, N.B.; Hao, X.; Olsson, S.; Nicolaisen, M.H.; Al-Soud, W.A.; Sørensen, S.J.; Nybroe, O. A novel baiting microcosm approach used to identify the bacterial community associated with Penicillium bilaii hyphae in soil. PLoS ONE 2017, 12, e0187116. [Google Scholar] [CrossRef]
- Shin, D.; Lee, Y.; Park, J.; Moon, H.S.; Hyun, S.P. Soil microbial community responses to acid exposure and neutralization treatment. J. Environ. Manag. 2017, 204, 383–393. [Google Scholar] [CrossRef]
- Stopnisek, N.; Bodenhausen, N.; Frey, B.; Fierer, N.; Eberl, L.; Weisskopf, L. Genus-wide acid tolerance accounts for the biogeographical distribution of soil Burkholderia populations. Environ. Microbiol. 2013, 16, 1503–1512. [Google Scholar] [CrossRef]
- Nazir, R.; Warmink, J.A.; Boersma, H.; van Elsas, J.D. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol. Ecol. 2010, 71, 169–185. [Google Scholar] [CrossRef]
- Nazir, R.; Boersma, F.G.H.; Warmink, J.A.; van Elsas, J.D. Lyophyllum sp. strain Karsten alleviates pH pressure in acid soil and enhances the survival of Variovorax paradoxus HB44 and other bacteria in the mycosphere. Soil. Biol. Biochem. 2010, 42, 2146–2152. [Google Scholar] [CrossRef]
- Yuan, J.; Lai, Q.; Zheng, T.; Shao, Z. Novosphingobium indicum sp. nov., a polycyclic aromatic hydrocarbon-degrading bacterium isolated from a deep-sea environment. Int. J. Syst. Evol. Microbiol. 2009, 59, 2084–2088. [Google Scholar] [CrossRef]
- Sohn, J.H.; Kwon, K.K.; Kang, J.-H.; Jung, H.-B.; Kim, S.-J. Novosphingobium pentaromativorans sp. nov., a high-molecular-mass polycyclic aromatic hydrocarbon-degrading bacterium isolated from estuarine sediment. Int. J. Syst. Evol. Microbiol. 2004, 54, 1483–1487. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Plett, J.M.; Engle, N.L.; Deveau, A.; Cushman, K.C.; Martin, M.Z.; Doktycz, M.J.; Tuskan, G.A.; Brun, A.; Kohler, A.; et al. Populus trichocarpa and populus deltoides exhibit different metabolomic responses to colonization by the symbiotic fungus Laccaria bicolor. Mol. Plant-Microbe Interact. 2014, 27, 546–556. [Google Scholar] [CrossRef]
- Warmink, J.A.; Nazir, R.; Van Elsas, J.D. Universal and species-specific bacterial ‘fungiphiles’ in the mycospheres of different basidiomycetous fungi. Environ. Microbiol. 2009, 11, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Zagriadskaia, Y.A.; Lysak, L.V.; Sidorova, I.I.; Aleksandrova, A.V.; Voronina, E.Y. Bacterial complexes of the fruiting bodies and hyphosphere of certain basidiomycetes. Biol. Bull. Russ. Acad. Sci. 2013, 40, 358–364. [Google Scholar] [CrossRef]
- Xing, R.; Yan, H.y.; Gao, Q.b.; Zhang, F.q.; Wang, J.l.; Chen, S.l. Microbial communities inhabiting the fairy ring of Floccularia luteovirens and isolation of potential mycorrhiza helper bacteria. J. Basic. Microb. 2018, 58, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Finley, B.K.; Mau, R.L.; Hayer, M.; Stone, B.W.; Morrissey, E.M.; Koch, B.J.; Rasmussen, C.; Dijkstra, P.; Schwartz, E.; Hungate, B.A. Soil minerals affect taxon-specific bacterial growth. ISME J. 2022, 16, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, E.I.; Cervantes, F.J. The role of humic substances in mitigating greenhouse gases emissions: Current knowledge and research gaps. Sci. Total Environ. 2021, 750, 141677. [Google Scholar] [CrossRef]
- Jin, Y.; Yuan, Y.; Liu, Z.; Gai, S.; Cheng, K.; Yang, F. Effect of humic substances on nitrogen cycling in soil-plant ecosystems: Advances, issues, and future perspectives. J. Environ. Manag. 2024, 351, 119738. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Erlacher, A.; Cernava, T.; Cardinale, M.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Rhizobiales as functional and endosymbiontic members in the lichen symbiosis of Lobaria pulmonaria L. Front. Microbiol. 2015, 6, 53. [Google Scholar] [CrossRef]
- Wang, D.; Dong, W.; Murray, J.; Wang, E. Innovation and appropriation in mycorrhizal and rhizobial symbioses. Plant Cell 2022, 34, 1573–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Peng, J.; Chen, C.; Xiong, C.; Li, S.; Ge, A.; Wang, E.; Liesack, W. Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci. 2023, 28, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil. 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmstrom, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dodd, I.C.; Belimov, A.A.; Jiang, F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016, 43, 161–172. [Google Scholar] [CrossRef]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef]
- Elliott, G.N.; Chou, J.H.; Chen, W.M.; Bloemberg, G.V.; Bontemps, C.; Martínez-Romero, E.; Velázquez, E.; Young, J.P.W.; Sprent, J.I.; James, E.K. Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ. Microbiol. 2009, 11, 762–778. [Google Scholar] [CrossRef]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Sahu, P.K.; Pandey, A.; White, J.F.; Verma, S.K. Endophytic Burkholderia: Multifunctional roles in plant growth promotion and stress tolerance. Microbiol. Res. 2022, 265, 127201. [Google Scholar] [CrossRef]
- Stopnisek, N.; Zühlke, D.; Carlier, A.; Barberán, A.; Fierer, N.; Becher, D.; Riedel, K.; Eberl, L.; Weisskopf, L. Molecular mechanisms underlying the close association between soil Burkholderia and fungi. ISME J. 2016, 10, 253–264. [Google Scholar] [CrossRef]
- Morya, R.; Salvachúa, D.; Thakur, I.S. Burkholderia: An untapped but promising bacterial genus for the conversion of aromatic compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, L.; Wang, J.; Zhang, Y.; Xiao, C. Effects of warming on the bacterial community and its function in a temperate steppe. Sci. Total Environ. 2021, 792, 148409. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.X.; Ren, H.; Zhang, J. Composition and functional diversity of microbial community across a mangrove-inhabited mudflat as revealed by 16S rDNA gene sequences. Sci. Total Environ. 2018, 633, 518–528. [Google Scholar] [CrossRef]
- Muñoz-Gómez, S.A. Energetics and evolution of anaerobic microbial eukaryotes. Nat. Microbiol. 2023, 8, 197–203. [Google Scholar] [CrossRef]
- Hogberg, M.N.; Hogberg, P.; Wallander, H.; Nilsson, L.O. Carbon-nitrogen relations of ectomycorrhizal mycelium across a natural nitrogen supply gradient in boreal forest. New Phytol. 2021, 232, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Högberg, P.; Näsholm, T.; Franklin, O.; Högberg, M.N. Tamm Review: On the nature of the nitrogen limitation to plant growth in Fennoscandian boreal forests. Forest Ecol. Manag. 2017, 403, 161–185. [Google Scholar] [CrossRef]
- Jorgensen, K.; Clemmensen, K.E.; Wallander, H.; Lindahl, B.D. Ectomycorrhizal fungi are more sensitive to high soil nitrogen levels in forests exposed to nitrogen deposition. New Phytol. 2024, 242, 1725–1738. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Compant, S.; Nowak, J.; Coenye, T.; Clement, C.; Ait Barka, E. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol. Rev. 2008, 32, 607–626. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 2003, 5, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Esmaeel, Q.; Jacquard, C.; Sanchez, L.; Clement, C.; Ait Barka, E. The mode of action of plant associated Burkholderia against grey mould disease in grapevine revealed through traits and genomic analyses. Sci. Rep. 2020, 10, 19393. [Google Scholar] [CrossRef] [PubMed]
- Khojandi, N.; Haselkorn, T.S.; Eschbach, M.N.; Naser, R.A.; DiSalvo, S. Intracellular Burkholderia Symbionts induce extracellular secondary infections; driving diverse host outcomes that vary by genotype and environment. ISME J. 2019, 13, 2068–2081. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Wang, B.-J.; Liu, Y.-H.; Liu, S.-J. Novosphingobium taihuense sp. nov., a novel aromatic-compound-degrading bacterium isolated from Taihu Lake, China. Int. J. Syst. Evol. Microbiol. 2005, 55, 1229–1232. [Google Scholar] [CrossRef]
- D’Argenio, V.; Notomista, E.; Petrillo, M.; Cantiello, P.; Cafaro, V.; Izzo, V.; Naso, B.; Cozzuto, L.; Durante, L.; Troncone, L.; et al. Complete sequencing of Novosphingobium sp. PP1Y reveals a biotechnologically meaningful metabolic pattern. BMC Genom. 2014, 15, 384. [Google Scholar] [CrossRef]
- Liu, Y.; Pei, T.; Du, J.; Huang, H.; Deng, M.-R.; Zhu, H. Comparative genomic analysis of the genus Novosphingobium and the description of two novel species Novosphingobium aerophilum sp. nov. and Novosphingobium jiangmenense sp. nov. Syst. Appl. Microbiol. 2021, 44, 126202. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; Gomez-Cadenas, A.; Perez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Zhang, M.L.; Zhu, J.L.; Yan, M.; Xu, F.J.; Bai, H.Y.; Sun, K.; Zhang, W.; Dai, C.C.; Jia, Y. Endofungal bacterial microbiota promotes the absorption of chelated inorganic phosphorus by host pine through the ectomycorrhizal system. Microbiol. Spectr. 2023, 11, e00162-23. [Google Scholar] [CrossRef]
- Mao, R.; Wu, D.; Wang, Y. Surface display on lactic acid bacteria without genetic modification: Strategies and applications. Appl. Microbiol. Biotechnol. 2016, 100, 9407–9421. [Google Scholar] [CrossRef]
- Kaselitz, T.B.; Hariadi, N.I.; LiPuma, J.J.; Weinberg, J.B. Rhizobium radiobacter bacteremia in a neonate. Infection 2011, 40, 437–439. [Google Scholar] [CrossRef]
- Detrait, M.; D’Hondt, L.; Andre, M.; Lonchay, C.; Holemans, X.; Maton, J.P.; Canon, J.L. Agrobacterium radiobacter bacteremia in oncologic and geriatric patients: Presentation of two cases and review of the literature. Int. J. Infect. Dis. 2008, 12, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hansen, K.S.; Hansen, L.K. Rhizobium radiobacter as an opportunistic pathogen in central venous catheter-associated bloodstream infection: Case report and review. J. Hosp. Infect. 2008, 68, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Aujoulat, F.; Marchandin, H.; Zorgniotti, I.; Masnou, A.; Jumas-Bilak, E. Rhizobium pusense is the main human pathogen in the genus Agrobacterium/Rhizobium. Clin. Microbiol. Infect. 2015, 21, 472.e1–472.e5. [Google Scholar] [CrossRef] [PubMed]
- Chewapreecha, C.; Holden, M.T.G.; Vehkala, M.; Välimäki, N.; Yang, Z.; Harris, S.R.; Mather, A.E.; Tuanyok, A.; De Smet, B.; Le Hello, S.; et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat. Microbiol. 2017, 2, 16263. [Google Scholar] [CrossRef]
- Seng, R.; Chomkatekaew, C.; Tandhavanant, S.; Saiprom, N.; Phunpang, R.; Thaipadungpanit, J.; Batty, E.M.; Day, N.P.J.; Chantratita, W.; West, T.E.; et al. Genetic diversity, determinants, and dissemination of Burkholderia pseudomallei lineages implicated in melioidosis in Northeast Thailand. Nat. Commun. 2024, 15, 5699. [Google Scholar] [CrossRef]
- Syed, I.; Wooten, R.M. Interactions Between Pathogenic Burkholderia and the Complement System: A Review of Potential Immune Evasion Mechanisms. Front. Cell Infect. Microbiol. 2021, 11, 701362. [Google Scholar] [CrossRef]
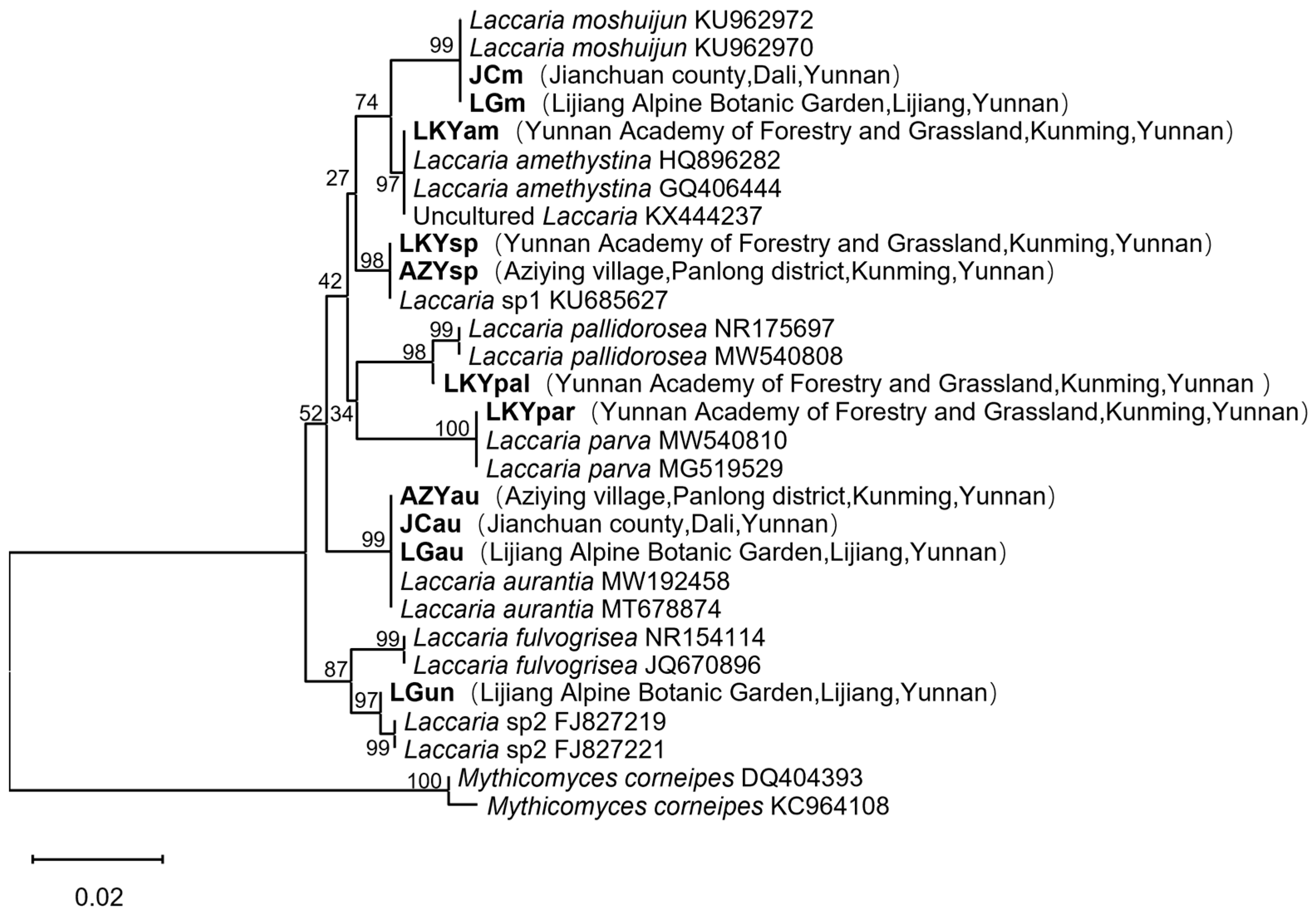

| Sample | Species | Location | Origins | Symbiosis Tree |
|---|---|---|---|---|
| LKYam | Laccaria amethystina | Yunnan Academy of Forestry and Grassland, Kunming | soil | Quercus variabilis |
| LKYpal | L. pallidorosea | Yunnan Academy of Forestry and Grassland, Kunming | soil | Quercus variabilis |
| LKYpar | L. parva | Yunnan Academy of Forestry and Grassland, Kunming | soil | Quercus variabilis |
| LKYsp * | L. sp1 | Yunnan Academy of Forestry and Grassland, Kunming | soil | Quercus variabilis |
| JCau | L. aurantia | Jianchuan county, Dali | humic substance | Pinus yunnanensis |
| JCm | L. moshuijun | Jianchuan county, Dali | humic substance | Pinus yunnanensis |
| LGm | L. moshuijun | Lijiang Alpine Botanic Garden, Lijiang | humic substance | Pinus yunnanensis |
| LGau | L. aurantia | Lijiang Alpine Botanic Garden, Lijiang | humic substance | Quercus guyavifolia |
| LGun * | L. sp2 | Lijiang Alpine Botanic Garden, Lijiang | humic substance | Quercus guyavifolia |
| AZYau | L. aurantia | Aziying village, Kunming | humic substance | Quercus variabilis |
| AZYsp * | L. sp1 | Aziying village, Kunming | humic substance | Quercus variabilis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Chen, X.; Shi, X.; Yang, Z.; Yang, L.; Liu, D.; Yu, F. Endophytic Bacterial Community, Core Taxa, and Functional Variations Within the Fruiting Bodies of Laccaria. Microorganisms 2024, 12, 2296. https://doi.org/10.3390/microorganisms12112296
Zhang K, Chen X, Shi X, Yang Z, Yang L, Liu D, Yu F. Endophytic Bacterial Community, Core Taxa, and Functional Variations Within the Fruiting Bodies of Laccaria. Microorganisms. 2024; 12(11):2296. https://doi.org/10.3390/microorganisms12112296
Chicago/Turabian StyleZhang, Kaixuan, Xin Chen, Xiaofei Shi, Zhenyan Yang, Lian Yang, Dong Liu, and Fuqiang Yu. 2024. "Endophytic Bacterial Community, Core Taxa, and Functional Variations Within the Fruiting Bodies of Laccaria" Microorganisms 12, no. 11: 2296. https://doi.org/10.3390/microorganisms12112296
APA StyleZhang, K., Chen, X., Shi, X., Yang, Z., Yang, L., Liu, D., & Yu, F. (2024). Endophytic Bacterial Community, Core Taxa, and Functional Variations Within the Fruiting Bodies of Laccaria. Microorganisms, 12(11), 2296. https://doi.org/10.3390/microorganisms12112296







