Abstract
Xylella fastidiosa is an aerobic, Gram-negative bacterium that is responsible for many plant diseases. The bacterium is the causal agent of Pierce’s disease in grapes and is also responsible for citrus variegated chlorosis, peach phony disease, olive quick decline syndrome and leaf scorches of various species. The production of biofilm is intrinsically linked with persistence and transmission in X. fastidiosa. Biofilm formation is regulated by members of the Diffusible Signal Factor (DSF) quorum sensing signalling family which are comprised of a series of long chain cis-unsaturated fatty acids. This article describes the evaluation of a library of N-acyl sulfonamide bioisosteric analogues of BDSF, XfDSF1 and XfDSF2 for their ability to control biofilm production in X. fastidiosa. The compounds were screened against both the wild-type strain Temecula and an rpfF* mutant which can perceive but not produce XfDSF. Planktonic cell abundance was measured via OD600 while standard crystal violet assays were used to determine biofilm biomass. Several compounds were found to be effective biofilm inhibitors depending on the nature of the sulfonamide substituent. The findings reported here may provide future opportunities for biocontrol of this important plant pathogen.
1. Introduction
Xylella fastidiosa is an aerobic, Gram-negative bacterium that is responsible for many plant diseases. According to the European Commission, X. fastidiosa “is one of the most dangerous plant bacteria worldwide” [1]. It was first reported by Wells et al. as the causal agent of Pierce’s disease in grapes [2]. The bacterium is also responsible for citrus variegated chlorosis, peach phony disease, olive quick decline syndrome (OQDS) and leaf scorches of almond, Japanese plum, elm, sycamore, oak, mulberry and maple. Common symptoms exhibited by plants infected by X. fastidiosa include scorching and wilting of foliage and stunted growth in leaves, fruit and plant height, followed by the eventual death of the plant.
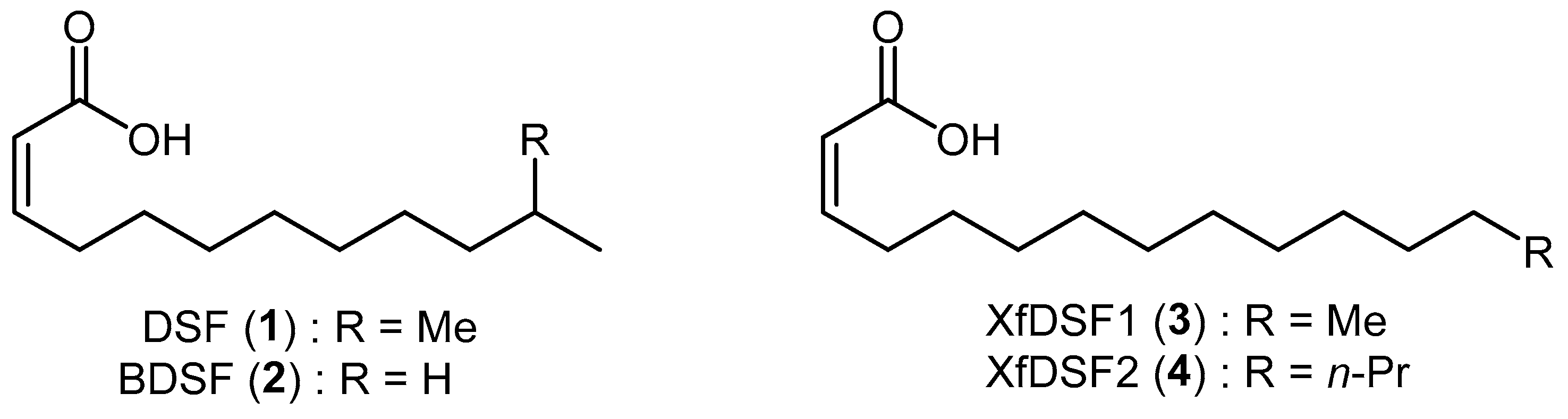
Biofilm formation in X. fastidiosa is a group behaviour regulated by members of the Diffusible Signal Factor family of signalling molecules. These messenger molecules are comprised of cis-unsaturated fatty acids of which cis-11-methyl-2-dodecenoic acid (1), known as Diffusible Signal Factor or DSF, and cis-2-dodecenoic acid (2), known as Burkholderia Diffusible Signal Factor or BDSF, are commonly employed by a wide range of bacteria for the regulation of diverse biological functions including biofilm formation, antibiotic tolerance and virulence (Figure 1) [3,4]. Although X. fastidiosa in principle is capable of producing many long chain saturated and unsaturated fatty acids, only two biologically active DSF derivatives have been isolated from the bacterium, namely 14-carbon XfDSF1 (3) and 16-carbon XfDSF2 (4), where Xf refers to Xylella fastidiosa [5,6]. The biosynthesis and perception of XfDSF signalling molecules in X. fastidiosa is very similar to that of DSF in X. campestris. RpfF is the key protein involved in the biosynthetic pathway, using 3-hydroxyacyl-acyl carrier protein dehydratase activity to introduce the cis-unsaturated double bond at the α,β-position. Additionally, thioesterase activity hydrolyses the thioester bond to release the carboxylic acid [7]. RpfC is the main sensor protein. Experiments have shown that, unlike X. campestris, RpfF also plays a role in signal perception in X. fastidiosa but this is probably a secondary pathway [8]. Signal detection activates cyclic di-GMP phosphodiesterase activity in RpfG which, in turn, triggers the expression of target genes such as hxfA and hxfB which are responsible for cell–cell aggregation and biofilm formation [9,10]. Interestingly, the introduction of rpfF mutations has an opposite effect on virulence in X. fastidiosa versus X. campestris [11]. rpfF mutants, which were incapable of DSF production and subsequently biofilm formation, caused early onset, severe scorching/wilting symptomatic of hypervirulence compared to the WT strain. The enhanced virulence of mutants incapable of DSF-mediated gene regulation was associated with reduced adhesiveness of the cells to surfaces, thus facilitating movement between xylem vessels to more extensively colonise infected plants. By contrast, in X. campestris, the absence of DSF by way of rpfF mutation results in hypovirulence. It is, therefore, reasonable to suggest that X. fastidiosa uses quorum sensing to partition its populations into plants on the basis of local DSF concentrations to enable to coexistence of hyperadhesive cells capable of acquisition and transmission by insect vectors as well as cells of low adhesiveness that are capable of rapid spread throughout the plant [11].
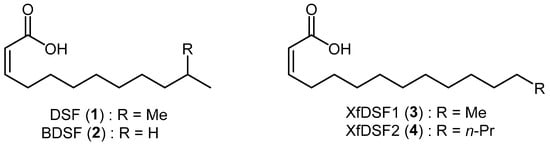
Figure 1.
Structures of DSF/BDSF and XfDSF1/2 messenger molecules.
While X. campestris is most responsive to DSF molecules with chains between 10 and 14 carbons [3], X. fastidiosa, by contrast, responds to molecules with chain lengths of 12 to 18 carbons [6]. X. fastidiosa also responds to BDSF, albeit at concentrations 3 and 20 times higher than the native signals XfDSF1 and XfDSF2, respectively. Of the two biologically active DSF species produced by X. fastidiosa, 16-carbon XfDSF2 is more active as a signalling molecule. X. fastidiosa responds to XfDSF2 at concentrations six times lower than XfDSF1. Prior research also demonstrated that when an X. fastidiosa rpfF deletion mutant was exposed to XfDSF2, expression of the hxfB gene was approximately 30-fold higher when compared to exposure to XfDSF1. Both XfDSF1 and XfDSF2 stimulated biofilm formation and increased the attachment of cells to a liquid–air interface of shaken cultures in glass tubes. After 24 h of incubation, the number of cells attached to the glass was 2.8- or 1.7-fold higher in the presence of XfDSF2 or XfDSF1, respectively, than in the control, indicating that XfDSF2 more effectively induces adhesion. This may account in part for the association between increased exposure to XfDSF and increased transmission.
There are limited examples in the literature of successful inhibition of biofilm formation in X. fastidiosa. Given that DSF-derived molecules play a major role in controlling biofilm formation in this bacterium, which, in turn, affects the adhesiveness of cells that is central to both virulence and transmission, we wondered if synthetic analogues of DSF might have an inhibitory effect. We have previously established that N-acyl sulfonamide derivatives of BDSF can successfully interfere with biofilm formation and quorum sensing in a variety of DSF-sensitive bacteria such as P. aeruginosa, B. cepacia and A. baumannii [12,13,14]. In this article, we describe the evaluation of these BDSF bioisosteres for their ability to inhibit X. fastidiosa biofilm production for the first time. In addition, this paper also describes the preparation of a series of novel 14- and 16-carbons analogues of XfDSF1 and XfDSF2 to elucidate the importance of the carbon chain. The inhibitory activity of these longer chain analogues is also measured, allowing for comparison with the corresponding 12-carbon BDSF derivatives.
2. Materials and Methods
2.1. Synthesis
1H NMR and 13C NMR spectra were recorded on Bruker Avance 300/400 MHz NMR spectrometers. Compounds were purified by flash chromatography using Kieselgel 60/0.040–0.063 mm silica gel. 1-Tridecyne and 1-undecyne were sourced from Fisher Scientific, Ballycoolen, Dublin, Ireland. Sulfonamides were sourced from Fluorochem Ltd., Glossop, Derbyshire, UK. Triethylamine, platinum (IV) oxide, copper iodide, bis(triphenylphosphine)palladium(II) dichloride and reaction solvents were sourced from Sigma-Aldrich, Gillingham, Dorset, UK. All commercial reagents/solvents were used without additional purification unless otherwise stated.
2.1.1. Synthesis of Methyl 2-(Undec-1-yn-1-yl)benzoate (29)
CuI (5 mg, 0.030 mmol, 5 mol%) and bis(triphenylphosphine)palladium(II) dichloride (12 mg, 0.018 mmol, 3 mol%) were added to an oven-dried reaction tube. Distilled Et3N (0.8 mL, 5.703 mmol, 9.50 eq.) and 11 (156 mg, 0.595 mmol, 1.00 eq.) in degassed anhydrous MeCN (6 mL) were subsequently added before addition of 1-undecyne (0.14 mL, 0.709 mmol, 1.20 eq.). The reaction mixture was heated via microwave irradiation to 100 °C (120 W) for 1 h. The reaction mixture was filtered through a celite pad, quenched with water and extracted with diethyl ether (3 × 30 mL). The organic layers were combined and washed with water (20 mL), brine (20 mL) and dried with MgSO4. The mixture was then filtered and the solvent removed in vacuo. The crude residue was subjected to flash chromatography using hexane-diethyl ether (100:0–98:2) to afford 29 as a colourless oil (143 mg, 0.50 mmol, 84%).
1H NMR (400 MHz, CDCl3) δ 0.79 (t, 3H, J = 6.9 Hz, C11′), 1.11–1.29 (m, 10H, C6′–C10′), 1.33–1.44 (m, 2H, C5′), 1.54 (tt, 2H, J = 7.6 Hz, C4′), 2.38 (t, 2H, J = 7.1 Hz, C3′), 3.81 (s, 3H, C8), 7.20 (ddd, 1H, J = 7.6, 1.3 Hz, C5), 7.31 (ddd, 1H, J = 7.6, 1.4 Hz, C4), 7.41 (dd, 1H, J = 7.8, 1.1 Hz, C3), 7.78 (dd, 1H, J = 7.9, 1.3 Hz, C6).
13C NMR (100 MHz, CDCl3) δ 14.1 (C11′, CH3), 19.8 (C3′, CH2), 22.7 (C10′, CH2), 28.7 (CH2), 29.0 (CH2), 29.2 (CH2), 29.3 (CH2), 29.5 (CH2), 31.9 (C9′, CH2), 52.0 (C8, CH3), 79.2 (C1′), 96.0 (C2′), 124.5 (C2), 127.1 (C5, CH), 130.1 (C6, CH), 131.4 (C4, CH), 131.9 (C1), 134.2 (C3, CH), 166.9 (C7).
IR (ATR) max cm−1 2925, 2854, 2226, 1735, 1718, 1484, 1447, 1292, 1249, 1128, 1083, 966, 756, 701.
HRMS (ESI) m/z: [M+H] Calcd for C19H26O2 287.2005; Found 287.2001.
2.1.2. Synthesis of Methyl 2-Undecylbenzoate (30)
29 (143 mg, 0.500 mmol, 1.00 eq.) in MeOH (4 mL) was added to an oven-dried RBF containing PtO2.H2O (6 mg, 0.025 mmol, 5 mol%). The RBF was next evacuated and then backfilled with H2. The reaction mixture was stirred for 1 h under a H2 atmosphere. Following filtration through a celite pad, the solvent was removed in vacuo to afford 30 as a yellow oil (139 mg, 0.479 mmol, 96%).
1H NMR (400 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.6 Hz, C11′), 1.14–1.44 (m, 16H, C3′-C10′), 1.49–1.67 (m, 2H, C2′), 2.93 (t, 2H, J = 7.8 Hz, C1′), 3.88 (s, 3H, C8), 7.15–7.29 (m, 2H, C3, C5), 7.40 (ddd, 1H, J = 1.3, 7.5 Hz, C4), 7.84 (dd, 1H, J = 1.1, 7.8 Hz, C6).
13C NMR (100 MHz, CDCl3) δ 14.1 (C11′, CH3), 22.7 (C10′, CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 29.8 (CH2), 31.8 (C2′, CH2), 31.9 (C9′, CH2), 34.5 (C1′, CH2), 51.8 (C8, CH3), 125.6 (C5, CH), 129.5 (C1), 130.5 (C6, CH), 130.9 (C3, CH), 131.8 (C4, CH), 144.7 (C2), 168.3 (C7).
IR (ATR) max cm−1 2924, 2854, 1726, 1601, 1575, 1464, 1433, 1256, 1189, 1104, 1081, 750, 710.
HRMS (ESI) m/z: [M+H] Calcd for C19H30O2 291.2318; Found 291.2313.
2.1.3. Synthesis of 2-Undecylbenzoic Acid (31)
LiOH (115 mg, 4.792 mmol, 10.00 eq.) was added to an RBF containing methyl 2-undecylbenzoate (30) (139 mg, 0.479 mmol, 1.00 eq.) in THF/MeOH/water 3:1:1 (15 mL). The reaction mixture was heated to reflux for 16 h and then allowed to cool to r.t. before the solvent was removed in vacuo. Following addition of water (15 mL), 2M aqueous HCl (15 mL) was added until the solution reached pH 2, before extraction with diethyl ether (5 × 30 mL). The organic layers were combined, washed with brine and dried over MgSO4. Following filtration, the solvent was removed in vacuo to afford 31 as a white solid (127 mg, 0.46 mmol, 96%), mp 37–39 °C.
1H NMR (300 MHz, CDCl3) δ 0.87 (t, 3H, J = 6.7 Hz, C11′), 1.16–1.48 (m, 16H, C3′–10′), 1.55–1.72 (m, 2H, C2′), 3.02 (t, 2H, J = 7.8 Hz, C1′), 7.20–7.33 (m, 2H, C3, C5), 7.46 (ddd, 1H, J = 7.5, 1.4 Hz, C4), 8.03 (dd, 1H, J = 8.5, 1.6 C6).
13C NMR (75 MHz, CDCl3) δ 14.1 (C11′, CH3), 22.7 (C10′, CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 29.7 (CH2), 31.8 (C2′, CH2), 31.9 (C9′, CH2), 34.6 (C1′, CH2), 125.8 (C5, CH), 128.2 (C1), 131.2 (C3, CH), 131.6 (C6, CH), 132.8 (C4, CH), 146.0 (C2), 173.5 (C7).
IR (ATR) max cm−1 2923, 2853, 2649, 1691, 1575, 1457, 1405, 1299, 1267, 929, 748, 723, 659, 560.
HRMS (ESI) m/z: [M+H] Calcd for C18H28O2 277.2162; Found 277.2159.
2.1.4. General Procedure for the Synthesis of N-(Sulfonyl)-2-undecylbenzamides
2-Undecylbenzoic acid (31) (25 mg, 0.090 mmol, 1.00 eq.) and DMAP (12 mg, 0.099 mmol, 1.10 eq.) were dissolved in dichloromethane (5 mL). The mixture was cooled to 0 °C before addition of the chosen sulfonamide reagent (0.085 mmol, 0.95 eq.). After 15 min, DCC (20 mg, 0.099 mmol, 1.10 eq.) was added, and stirring at r.t. was continued for 16 h. The urea byproduct was removed by celite pad filtration, and the solvent was removed in vacuo. The residue was dissolved in diethyl ether (10 mL), poured onto 2M aqueous HCl (20 mL) and extracted with diethyl ether (3 × 30 mL). The organic layers were combined, washed with brine and dried over MgSO4. Following filtration, the solvent was removed in vacuo, and the crude residue was subjected to flash chromatography using a suitable eluent.
2.1.5. N-(Tert-butylsulfonyl)-2-undecylbenzamide (32)
The title compound was synthesised using 2-undecylbenzoic acid (31) (25 mg, 0.090 mmol, 1.00 eq.), DMAP (12 mg, 0.099 mmol, 1.10 eq.), tert-butylsulfonamide (12 mg, 0.085 mmol, 0.95 eq.) and DCC (20 mg, 0.099 mmol, 1.10 eq.) in DCM (5 mL). The crude residue was subjected to flash chromatography using hexane-diethyl ether (70:30) to afford 32 as a white solid (24 mg, 0.060 mmol, 70%), mp 67–68 °C.
1H NMR (400 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.7 Hz, C11′), 1.16–1.39 (m, 16H, C3′-C10′), 1.53–1.68 (m, 2H, overlapped, C2′), 1.56 (s, 9H, C2″–C4″), 2.80 (t, 2H, J = 7.9 Hz, C1′), 7.20–7.33 (m, 2H, C3, C5), 7.36–7.49 (m, 2H, C4, C6).
13C NMR (100 MHz, CDCl3) δ 14.1 (C11′, CH3), 22.7 (C10′, CH2), 24.6 (C2″–C4″, 3 × CH3), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2), 29.6 (CH2), 29.6 (CH2), 29.6 (CH2), 31.9 (C2′, CH2), 32.0 (C9′, CH2), 33.4 (C1′, CH2), 62.5 (C1″), 126.0 (C5, CH), 126.8 (C6, CH), 130.9 (C3, CH), 131.5 (C4, CH), 133.3 (C1), 142.4 (C2), 166.6 (C7).
IR (ATR) max cm−1 3240, 2923, 2853, 1714, 1447, 1428, 1332, 1140, 1056, 844, 749, 649, 567, 511.
HRMS (ESI) m/z: [M+H] Calcd for C22H37NO3S 396.2567; Found 396.2563.
2.1.6. N-((4-Chlorophenyl)sulfonyl)-2-undecylbenzamide (33)
The title compound was synthesised using 2-undecylbenzoic acid (31) (25 mg, 0.090 mmol, 1.00 eq.), DMAP (12 mg, 0.099 mmol, 1.10 eq.), 4-chlorobenzenesulfonamide (16 mg, 0.085 mmol, 0.95 eq.) and DCC (20 mg, 0.099 mmol, 1.10 eq.) in DCM (5 mL). The crude residue was subjected to flash chromatography using hexane-diethyl ether (70:30) to afford 33 as a white solid (27 mg, 0.060 mmol, 70%), mp 70–72 °C.
1H NMR (400 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.9 Hz, C11′), 1.08–1.38 (m, 18H, C2′-C10′), 2.62 (t, 2H, J = 7.9 Hz, C1′), 7.17–7.25 (m, 2H, C3, C5), 7.32–7.44 (m, 2H, C4, C6), 7.49–7.59 (m, 2H, C3″, C5″), 8.04–8.14 (m, 2H, C2″, C6″), 8.59 (s, 1H, N–H).
13C NMR (100 MHz, CDCl3) δ 14.2 (C11′, CH3), 22.7 (C10′, CH2), 29.3 (CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (2 × CH2), 31.7 (C2′, CH2), 31.9 (C9′, CH2), 33.2 (C1′, CH2), 126.0 (C5, CH), 127.1 (C6, CH), 129.3 (C3″, C5″, 2 × CH), 130.1 (C2″, C6″, 2 × CH), 130.9 (C3, CH), 131.8 (C4, CH), 131.9 (C1), 136.8 (C1″), 140.8 (C4″), 142.8 (C2), 166.4 (C7).
IR (ATR) max cm−1 3254, 2925, 2854, 1703, 1587, 1476, 197, 1333, 1162, 1089, 1014, 823, 755, 624, 537, 485.
HRMS (ESI) m/z: [M+H] Calcd for C24H32ClNO3S 450.1864; Found 450.1860.
2.1.7. Synthesis of Methyl 2-(Tridec-1-yn-1-yl)benzoate (34)
CuI (5 mg, 0.030 mmol, 5 mol%) and bis(triphenylphosphine)palladium(II) dichloride (12 mg, 0.018 mmol, 3 mol%) were added to an oven-dried reaction tube. Distilled Et3N (0.8 mL, 5.703 mmol, 9.50 eq.) and 11 (156 mg, 0.595 mmol, 1.00 eq.) in degassed anhydrous MeCN (6 mL) were subsequently added before addition of 1-tridecyne (0.16 mL, 0.702 mmol, 1.20 eq.). The reaction mixture was heated via microwave irradiation at 100 °C (120 W) for 1 h. The reaction mixture was filtered through a celite pad, quenched with water and extracted with diethyl ether (3 × 30 mL). The organic layers were combined and washed with water (20 mL), brine (20 mL) and dried with MgSO4. The mixture was then filtered and the solvent removed in vacuo. The crude residue was subjected to flash chromatography using hexane-diethyl ether (100:0–98:2) to afford 34 as a yellow oil (150 mg, 0.478 mmol, 80%).
1H NMR (300 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.8 Hz, C13′), 1.17–1.39 (m, 14H, C6′-C12′), 1.39–1.53 (m, 2H, C5′), 1.53–1.69 (m, 2H, C4′), 2.47 (t, 2H, J = 7.1 Hz, C3′), 3.91 (s, 3H, C8), 7.30 (ddd, 1H, J = 7.6, 1.4 Hz, C5), 7.41 (ddd, 1H, J = 7.5, 1.5 Hz, C4), 7.50 (dd, 1H, J = 7.8, 1.2 Hz, C3), 7.87 (dd, 1H, J = 7.8, 1.4 Hz, C6).
13C NMR (75 MHz, CDCl3) δ 14.1 (C13′, CH3), 19.8 (C3′, CH2), 22.7 (C12′, CH2), 28.7 (C4′, CH2), 29.0 (C5′, CH2), 29.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2), 31.9 (C11′, CH2), 52.0 (C8, CH3), 79.2 (C1′), 96.1 (C2′), 124.5 (C2), 127.1 (C5, CH), 130.1 (C6, CH), 131.4 (C4, CH), 132.0 (C1), 134.2 (C3, CH), 167.0 (C7).
IR (ATR) max cm−1 2925, 2853, 2234, 1735, 1718, 1597, 1484, 1447, 1432, 1292, 1275, 1249, 1128, 1083, 756, 701.
HRMS (ESI) m/z: [M+H] Calcd for C21H30O2 315.2318; Found 315.2312.
2.1.8. Synthesis of Methyl 2-Tridecylbenzoate (35)
34 (142 mg, 0.452 mmol, 1.00 eq.) in MeOH (4 mL) was added to an oven-dried RBF containing PtO2.H2O (6 mg, 0.023 mmol, 5 mol%). The RBF was next evacuated and then backfilled with H2. The reaction mixture was stirred for 1 hr under a H2 atmosphere. Following filtration through a celite pad, the solvent was removed in vacuo to afford 35 as a yellow oil (136 mg, 0.428 mmol, 95%).
1H NMR (400 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.7 Hz, C13′), 1.16–1.43 (m, 20H, C3′–12′), 1.51–1.65 (m, 2H, C2′), 2.93 (t, 2H, J = 7.8 Hz, C1′), 3.87 (s, 3H, C8), 7.16–7.32 (m, 2H, C3, C5), 7.38 (ddd, 1H, J = 7.5, 1.5 Hz, C4), 7.84 (dd, 1H, J = 7.8, 1.3 Hz, C6).
13C NMR (100 MHz, CDCl3) δ 14.1 (C13′, CH3), 22.7 (C12′, CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 29.7 (CH2), 29.8 (CH2 × 2), 31.9 (C2′, CH2), 32.0 (C11′, CH2), 34.5 (C1′, CH2), 51.8 (C8, CH3), 125.6 (C5, CH), 129.5 (C1), 130.5 (C6, CH), 130.9 (C3, CH), 131.7 (C4, CH), 144.8 (C2), 168.2 (C7).
IR (ATR) max cm−1 3396, 3240, 2853, 1713, 1450, 1432, 1332, 1206, 1139, 1057, 844, 749, 649, 568, 511.
HRMS (ESI) m/z: [M+H] Calcd for C21H34O2 319.2631; Found 319.2624.
2.1.9. Synthesis of 2-Tridecylbenzoic Acid (36)
LiOH (103 mg, 4.280 mmol, 10.00 eq.) was added to an RBF containing methyl 2-tridecylbenzoate (35) (136 mg, 0.428 mmol, 1.00 eq.) in THF/MeOH/water 3:1:1 (15 mL). The reaction mixture was heated to reflux for 16 h and then allowed to cool to r.t. before the solvent was removed in vacuo. Following addition of water (15 mL), 2M aqueous HCl (15 mL) was added until the solution reached pH 2, before extraction with diethyl ether (5 × 30 mL). The organic layers were combined, washed with brine and dried over with MgSO4. Following filtration, the solvent was removed in vacuo to afford 36 as an off-white solid (123 mg, 0.405 mmol, 94%), mp 32–34 °C.
1H NMR (400 MHz, CDCl3) δ 0.87 (t, 3H, J = 6.7 Hz, C13′), 1.18–1.44 (m, 20H, C3′-C12′), 1.55–1.71 (m, 2H, C2′), 3.02 (t, 2H, J = 7.8 Hz, C1′), 7.20–7.33 (m, 2H, C3, C5), 7.46 (ddd, 1H, J = 7.5, 1.4 Hz, C4), 8.03 (dd, 1H, J = 8.2, 1.7 Hz, C6).
13C NMR (100 MHz, CDCl3) δ 14.1 (C13′, CH3), 22.7 (C12′, CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (2 × CH2), 29.7 (3 × CH2), 31.8 (C2′, CH2), 31.9 (C11′, CH2), 34.6 (C1′, CH2), 125.8 (C5, CH), 128.1 (C1), 131.2 (C3, CH), 131.6 (C6, CH), 132.8 (C4, CH), 146.0 (C2), 173.3 (C7).
IR (ATR) max cm−1 2921, 2852, 1689, 1602, 1574, 1456, 1404, 1297, 1266, 928, 747, 721, 657, 561.
HRMS (ESI) m/z: [M+H] Calcd for C20H32O2 305.2475; Found 305.2476.
2.1.10. General Procedure for the Synthesis of N-(Sulfonyl)-2-tridecylbenzamides
2-Tridecylbenzoic acid (36) (25 mg, 0.082 mmol, 1.00 eq.) and DMAP (11 mg, 0.090 mmol, 1.10 eq.) were dissolved in dichloromethane (5 mL). The mixture was cooled to 0 °C before addition of the chosen sulfonamide reagent (0.078 mmol, 0.95 eq.). After 15 min, DCC (18 mg, 0.090 mmol, 1.10 eq.) was added and stirring at r.t. was continued for 16 h. The urea byproduct was removed by celite pad filtration, and the solvent was removed in vacuo. The residue was dissolved in diethyl ether (10 mL), poured onto 2M aqueous HCl (20 mL) and extracted with diethyl ether (3 × 30 mL). The organic layers were combined, washed with brine and dried with MgSO4. Following filtration, the solvent was removed in vacuo, and the crude residue was subjected to flash chromatography using a suitable eluent.
2.1.11. N-(Tert-butylsulfonyl)-2-tridecylbenzamide (37)
The title compound was synthesised using 2-tridecylbenzoic acid (36) (25 mg, 0.082 mmol, 1.00 eq.), DMAP (11 mg, 0.090 mmol, 1.10 eq.), tert-butylsulfonamide (11 mg, 0.078 mmol, 0.95 eq.) and DCC (18 mg, 0.090 mmol, 1.10 eq.) in DCM (5 mL). The crude residue was subjected to flash chromatography using hexane-diethyl ether (70:30) to afford 37 as an off-white solid (23 mg, 0.053 mmol, 68%), mp 60–62 °C.
1H NMR (400 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.7 Hz, C13′), 1.16–1.38 (m, 20H, C3′-C12′), 1.48–1.68 (m, 2H, overlapped, C2′), 1.54 (s, 9H, C2″–C4″), 2.79 (t, 2H, J = 7.9 Hz, C1′), 7.19–7.32 (m, 2H, C3, C5), 7.35–7.49 (m, 2H, C4, C6), 8.11 (s, 1H, N–H).
13C NMR (100 MHz, CDCl3) δ 14.1 (C13′, CH3), 22.7 (C12′, CH2), 24.5 (C2″–C4″, 3 × CH3), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.6 (4 × CH2), 29.7 (CH2), 31.9 (C2′, CH2), 32.0 (C9′, CH2), 33.4 (C1′, CH2), 62.4 (C1″), 126.0 (C5, CH), 127.0 (C6, CH), 130.8 (C3, CH), 131.5 (C4, CH), 133.3 (C1), 142.4 (C2), 166.8 (C7).
IR (ATR) max cm−1 3238, 2922, 2852, 1712, 1447, 1429, 1331, 1238, 1138, 1055, 1041, 1019, 843, 749, 649, 567, 511.
HRMS (ESI) m/z: [M+H] Calcd for C24H41NO3S 424.2879; Found 424.2893.
2.1.12. N-((4-Chlorophenyl)sulfonyl)-2-tridecylbenzamide (38)
The title compound was synthesised using 2-tridecylbenzoic acid (36) (25 mg, 0.082 mmol, 1.00 eq.), DMAP (15 mg, 0.090 mmol, 1.10 eq.), 4-chlorobenzenesulfonamide (15 mg, 0.078 mmol, 0.95 eq.) and DCC (18 mg, 0.090 mmol, 1.10 eq.) in DCM (5 mL). The crude residue was subjected to flash chromatography using hexane-diethyl ether (70:30) to afford 38 as an off-white solid (33 mg, 0.069 mmol, 89%), mp 77–78 °C.
1H NMR (300 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.7 Hz, C13′), 1.07–1.41 (m, 20H, C3′-C12′), 2.61 (t, 2H, J = 7.8 Hz, C1′), 7.15–7.28 (m, 2H, C3, C5), 7.33–7.43 (m, 2H, C4, C6), 7.49–7.59 (m, 2H, C3″, C5″), 8.02–8.14 (m, 2H, C2″, C6″), 8.57 (s, 1H, N–H).
13C NMR (75 MHz, CDCl3) δ 14.1 (C13′, CH3), 22.7 (C12′, CH2), 29.4 (CH2), 29.4 (CH2), 26.5 (CH2), 29.6 (CH2), 29.7 (2 × CH2), 29.7 (2 × CH2), 31.7 (C2′, CH2), 31.9 (C7′, CH2), 33.2 (C1′, CH2), 126.0 (C5, CH), 127.1 (C6, CH), 129.3 (C3″, C5″, CH), 130.1 (C2″, C6″, CH), 130.9 (C3, CH), 131.8 (C4, CH), 132.0 (C1), 136.9 (C1″), 140.8 (C4″), 142.7 (C2), 166.4 (C7).
IR (ATR) max cm−1 3240, 2923, 2853, 1705, 1586, 1430, 1350, 1241, 1170, 1085, 1057, 890, 848, 753, 616, 568, 484.
HRMS (ESI) m/z: [M+H] Calcd for C26H36ClNO3S 478.2177; Found 478.2172.
2.2. Microbiology
Bacterial strains and culture media. X. fastidiosa Temecula1 was wild-type strain ATCC 700964. The XfDSF-biosensor strain (previously designated X. fastidiosa rpfF*-XfHA biosensor) consists of the rpfF* mutant (E141A E161A) that exhibits blocked DSF synthesis but can still sense externally applied DSF harbouring pXfHA (hxfA=::phoA) [6]. Inoculum of X. fastidiosa was grown on periwinkle wilt GelRite medium (PWG medium) plates for 5 to 7 days before transfer to PD3 broth. All cultures were grown at 28 °C.
Biofilm assay. The effects of individual compounds on the biofilm formation capacity of both the wild-type X. fastidiosa strain and the X. fastidiosa rpfF* mutant were determined in cells growing in PD3 broth cultures. Cells were grown on PWG plates for 7 days, resuspended in PD3 broth to an OD600 of 0.05 and added (2 mL) to PD3 broth cultures containing a final concentration of 10 µM of each compound or an equal volume of DMSO alone as a control. The glass tubes, containing 10 mL of a culture, were shaken (200 rpm) for 24 h at 28 °C, during which time a visible biofilm formed at the liquid–air interface that was attached to the tube. The medium, containing unattached cells, was then removed by aspiration, and the concentration of planktonic cells was assessed as OD600. The tubes from which planktonic cells had been removed were washed three times with tap water to remove any unattached cells, and the biomass attached to the glass wall was stained with 2 mL of 1% crystal violet (CV) for 10 min. Excess CV was removed by washing the tubes three times with tap water, and the retained CV was dissolved in 1 mL of 95% ethanol and quantified by measuring absorbance at 595 nm (Spectronic 21D spectrophotometer; Milton Roy, Harvey, LA, USA). The data reported are an average of three replicates for each treatment. The experiments were conducted twice with similar results.
3. Results and Discussion
3.1. Chemistry
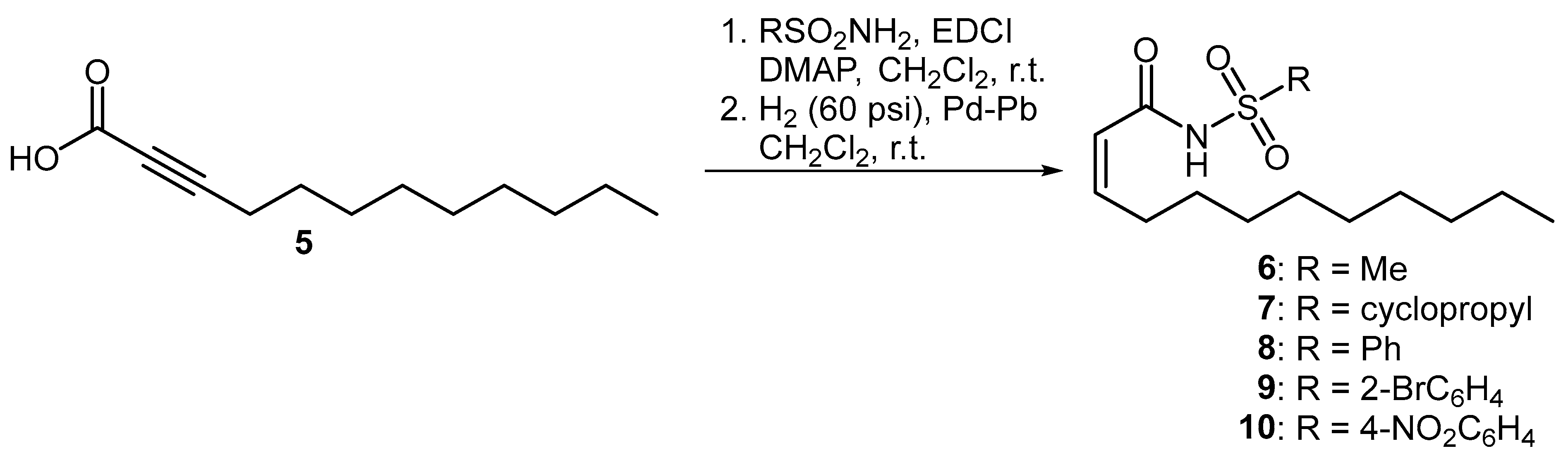
Three series of compounds were evaluated in this study. The first set of molecules were olefinic derivatives of BDSF where a bioisosteric sulfonamide is installed in place of the carboxylic acid functionality. The incorporation of bioisosteric groups in this manner is a well-established strategy for the development of biologically active analogues [15,16,17]. These compounds, which have been shown to disrupt intercellular communication in bacterial species such as Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Burkholderia cepacia, were prepared using a previously published methodology [12,13,18]. Accordingly, propargylic acid 5 was coupled to both alkyl- and aryl-substituted sulfonamides, followed by partial hydrogenation using Lindlar’s catalyst to afford the cis-unsaturated 12-carbon N-acyl sulfonamides 6–10 (Figure 2).
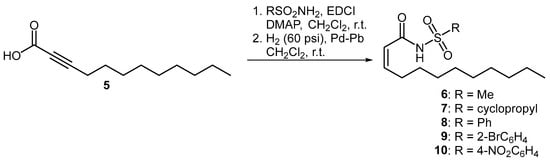
Figure 2.
Preparation of olefinic BDSF analogues.
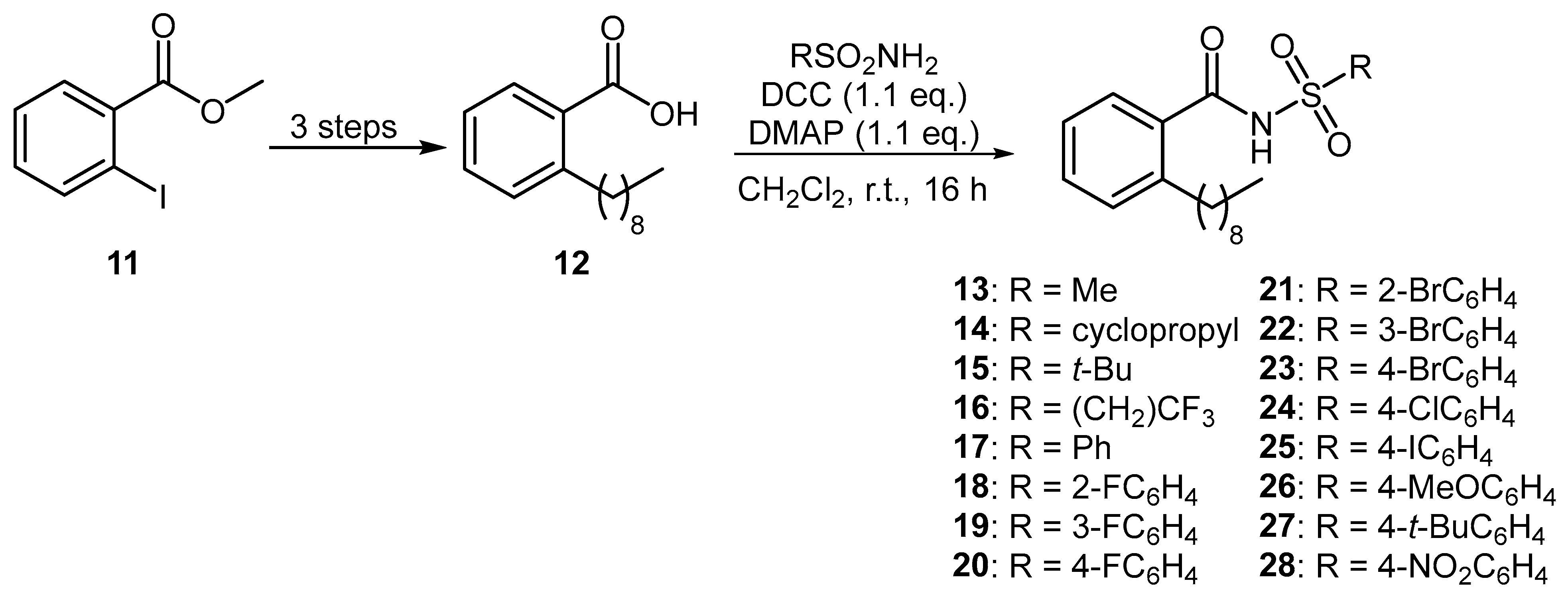
A series of aromatic BDSF analogues were also prepared using an established synthetic route [14]. Starting from methyl 2-iodobenzoate (11), common intermediate 12 was prepared over three steps by way of a Sonogashira coupling with 1-nonyne, followed by hydrogenation and subsequent basic hydrolysis (Figure 3). DCC-mediated coupling of carboxylic acid 12 with various sulfonamides produced a diverse range of 16 aromatic N-acyl sulfonamides 13–28.
Figure 3.
Preparation of aromatic BDSF analogues.
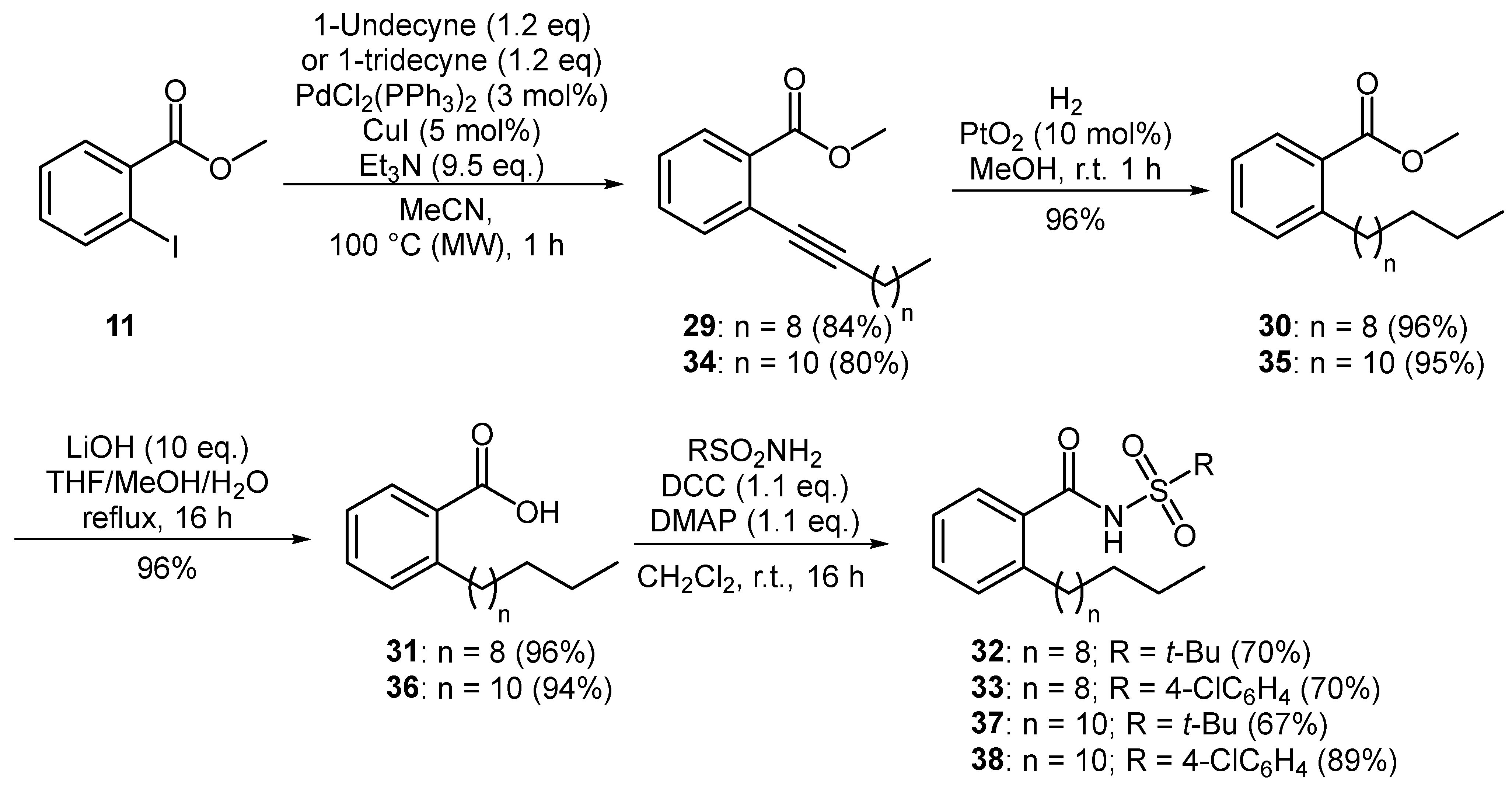
A similar synthetic strategy was employed for the preparation of novel, longer chain analogues of XfDSF1 and XfDSF2. Sonogashira coupling of aryl iodide 11 with 1-undecyne afforded alkyne 29 in a 84% yield (Figure 4). Based on previous work, it was expected that hydrogenation of 29 at atmospheric pressure for 24 h in the presence of Adam’s catalyst would furnish methyl 2-undecylbenzoate (30). However, 1H-NMR analysis of the crude reaction mixture revealed the presence of two products in a 34:66 ratio as measured by the respective integrations of the methoxy singlets occurring at 3.88 ppm and 3.65 ppm. While the signals for the minor component matched that of target 30, the signals for the major product suggested that both the aromatic ring and the alkyne had been reduced. In order to avoid over-reduction, we found that a reaction time of 1 h was sufficient to fully convert alkyne 29 to methyl 2-undecylbenzoate (30) in a 96% yield with no evidence of any unwanted side products. Removal of the methyl ester was achieved by treating 30 with excess lithium hydroxide in THF/MeOH/H2O at reflux for 16 h, producing pure 2-undecylbenzoic acid (31) as a white solid in a 96% yield. Carboxylic acid 31 was coupled to tert-butylsulfonamide and 4-chlorobenzenesulfonamide, respectively, using a combination of DCC and DMAP to produce novel N-acylsulfonamides 32 and 33 each in a 70% yield. Homologous analogues were produced in a similar manner to furnish XfDSF2 analogues 37 and 38 in yields of 48% and 64% over four steps from 1-tridecyne.
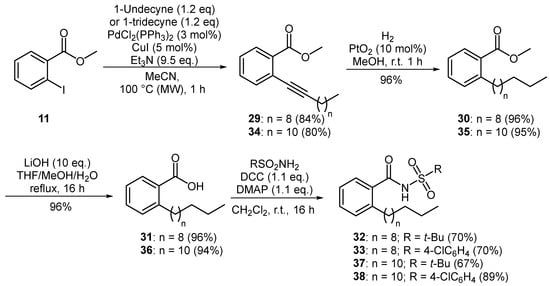
Figure 4.
Preparation of aromatic XfDSF analogues.
3.2. Biological Evaluation
Biofilms are ubiquitous in nature and constitute a key component of microbial survival mechanisms [19,20,21,22]. Quorum sensing-regulated biofilm production also plays a critical role in X. fastidiosa adhesion and transmission. The major vectors of X. fastidiosa are xylem-fluid-feeding insects such as sharpshooters and spittlebugs [23]. Once ingested, the bacteria multiply in the insect foregut and form a mat-like biofilm [24]. Following initial lateral attachment, the cells align themselves in a polar fashion as the biofilm matures in fully colonised insects [25]. This polar alignment generates a larger surface area which supports the uptake of nutrients [26]. The biofilm plays a vital role in persistence in the host insect. Not only does the matrix facilitate adhesion to the foregut, but it also provides protection from the turbulent environment of the digestive tract. Although extensive bacterial colonisation and biofilm formation is essential for persistence, very few live bacterial cells are required for transmission [27]. Vectors can inoculate plants very quickly after acquisition [28,29]. The insects transmit the pathogen via short hopping flights from plant to plant, which facilitates the rapid spread of disease [30]. Once inside the plant, X. fastidiosa begins to multiply and spreads throughout the xylem system from the site of inoculation [31]. The pathogen begins to form biofilm colonies which occupy the bordered pits [32]. In the later stages of infection, the biofilm completely occludes the xylem vessels, restricting water and nutrient transport [33].
Our library of compounds was tested against two different strains of X. fastidiosa: wild-type strain Temecula and an rpfF* mutant. The rpfF* mutant has a point mutation that prevents the RpfF protein from producing XfDSF but still permits the involvement of RpfF with RpfC in the perception of XfDSF. Given that the wild-type strain would produce XfDSF1 and XfDSF2 and their presence would have made it difficult to disentangle any agonistic effect that may have been observed with our compounds in such cultures, agonistic effects could instead be observed by examining the behaviour of the rpfF* mutant. By contrast, antagonism of XfDSF signalling in X. fastidiosa was discernible in the wild-type strain.
Bacterial cultures were grown on periwinkle wilt GelRite (PWG) agar as X. fastidiosa produces less XfDSF on this medium. Harvested cells were dispersed into a Pierce’s disease (PD3) broth and shaken vigorously for four days. Compounds were added at a final concentration of 10 µM. The amount of DMSO used to solubilise the compounds was kept to an absolute minimum, with a final concentration of DMSO in the various cultures of 0.05%. Pure DMSO was also included as a control. The initial cell concentration of each of the strains was approximately 107 cells/mL as determined by the optical density measured at a wavelength of 600 nm (OD600). After four days, substantial growth of most of the cultures had occurred. At this point, many of the cells had formed a biofilm ring at the air/medium interface.
The planktonic cells remaining in solution were carefully removed and their abundance determined by again measuring the OD600 of these cells. A crystal violet (CV) binding assay was used to determine the abundance of biofilm biomass in each culture. The optical density conferred by the bound crystal violet was measured which was proportional to the number of cells in the biofilm. Measures of the planktonic and biofilm cells (OD600 and CV, respectively) are shown separately for the wild-type strain (Figure 5) and the rpfF* mutant (Figure 6). It should be noted that the biofilms produced by X. fastidiosa are not particularly adhesive to glass surfaces, and there tends to be a relatively large variation in the proportion of the biofilm that ends up adhering to the glass tube surface (as opposed to that remaining as clumped cells in the culture)—leading to the relatively large variation in biofilm abundance seen in such experiments where crystal violet staining of wall-associated cells is assessed.
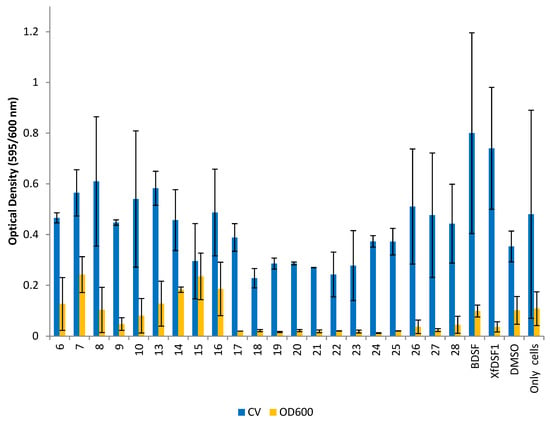
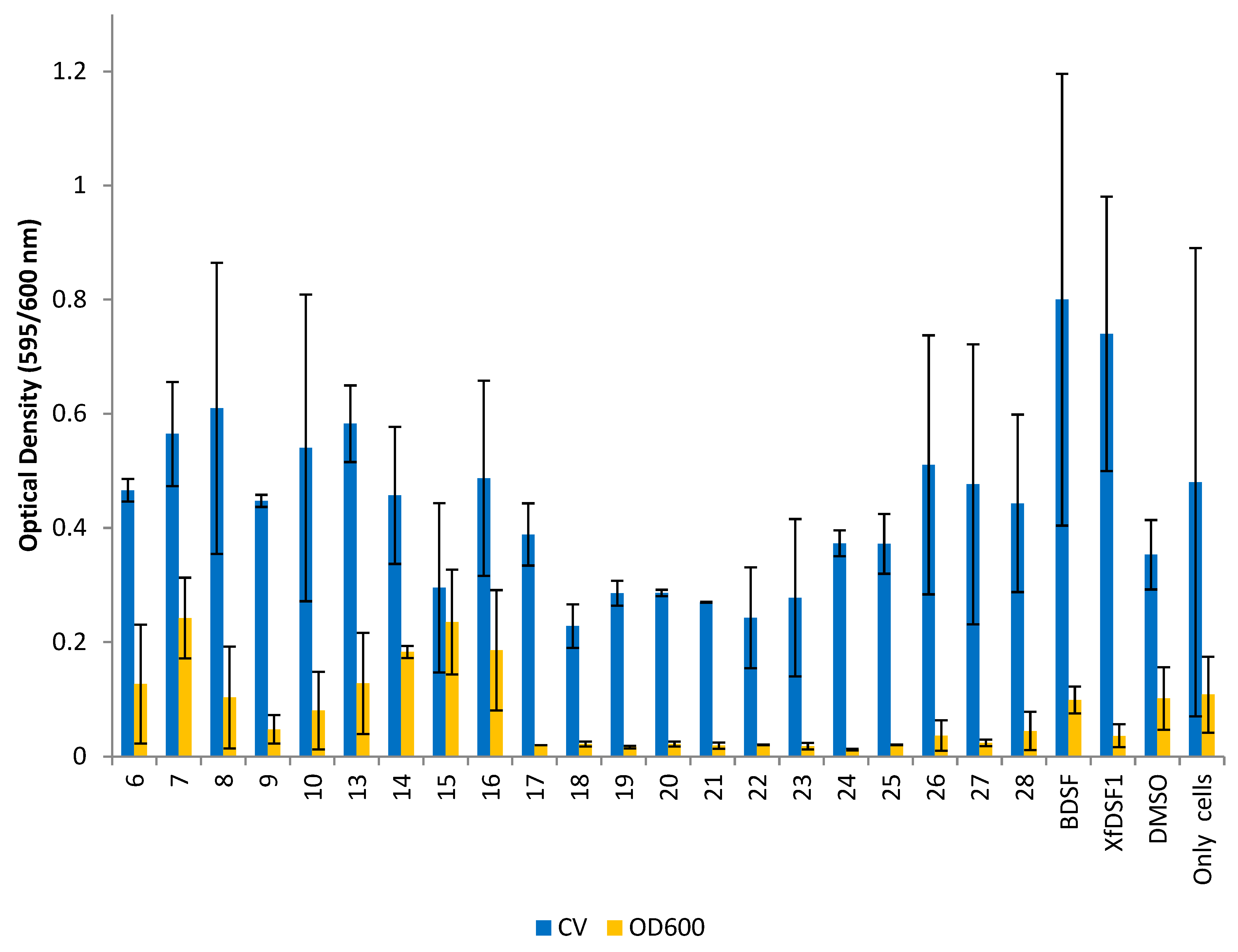
Figure 5.
Optical density readings for wild-type strain Temecula. OD595 abundance of crystal violet retained in biofilm cells attached to glass culture tubes after addition of BDSF analogues (blue bars). OD600 abundance of planktonic cells remaining in suspension after addition of BDSF analogues (yellow bars). The error bars represent the standard deviations of the means.
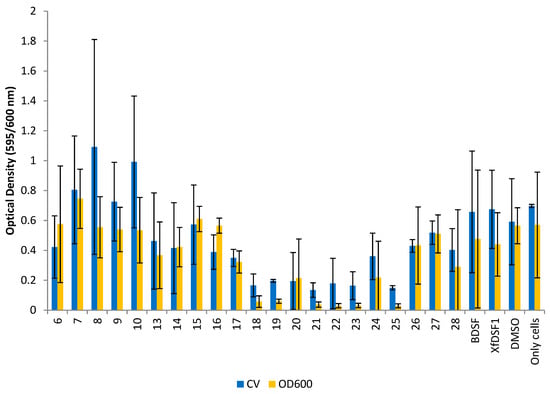
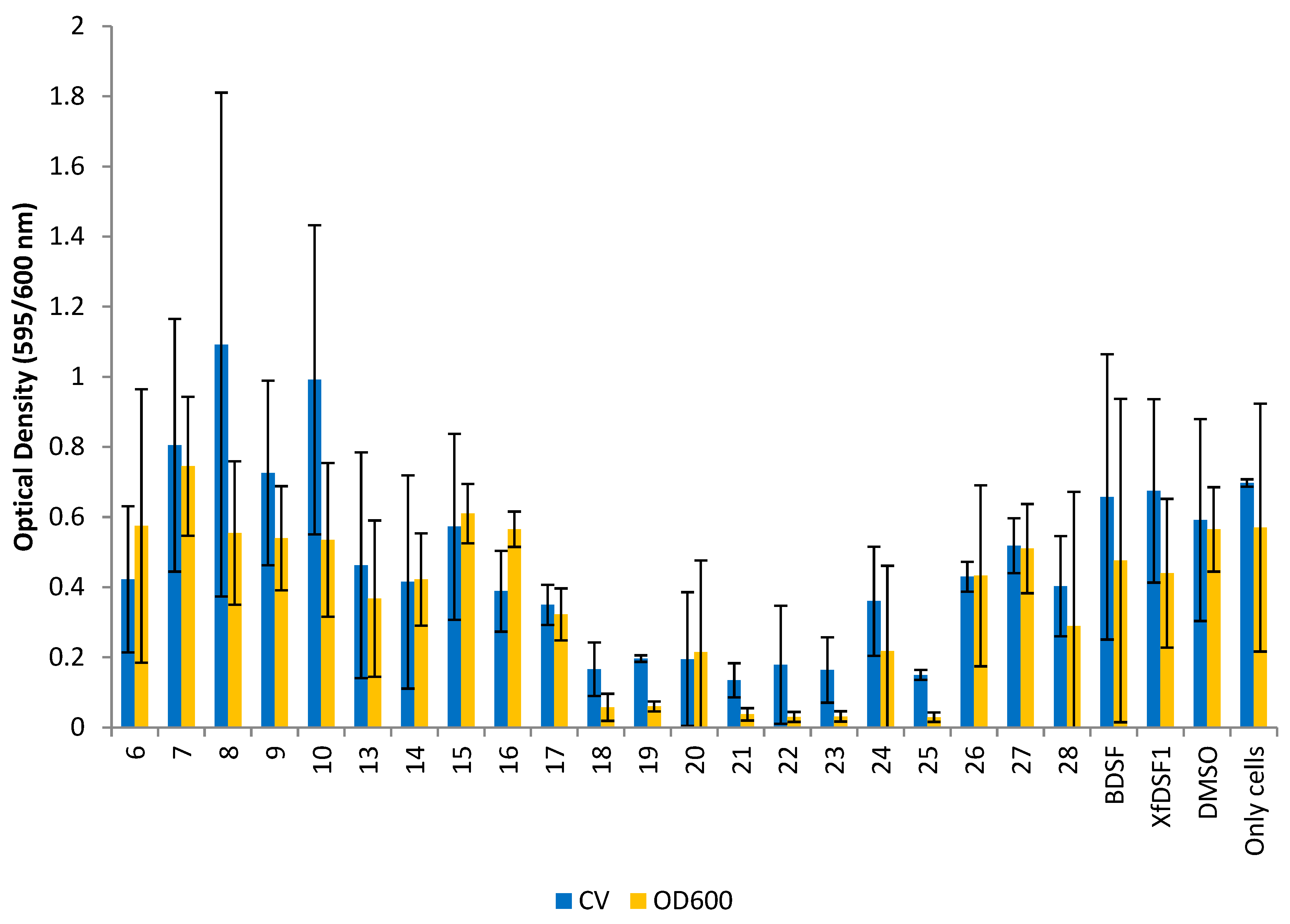
Figure 6.
Optical density readings for rpfF* mutant. OD595 abundance of crystal violet retained in biofilm cells attached to glass culture tubes after addition of BDSF analogues (blue bars). OD600 abundance of planktonic cells remaining in suspension after addition of BDSF analogues (yellow bars). The error bars represent the standard deviations of the means.
Analogues 6–10 and 13–28 were initially evaluated. Compounds 6–10 all feature a 12-carbon chain, in addition to a cis-unsaturated double bond, and can be considered first generation, direct mimics of BDSF (2). The original carboxylic acid is replaced with either an aliphatic (6–7) or aromatic (8–10) N-acyl sulfonamide bioisostere. As cis-unsaturated fatty acids are prone to isomerisation, molecules 13–28 represent a second generation series of BDSF analogues whereby a rigid benzene ring is installed to permanently lock the molecular geometry [34]. This series incorporates a wider range of aliphatic (13–16) and aromatic (17–28) sulfonamide substituents. The data in Figure 5 suggest that many of our compounds exhibit an inhibitory effect on both planktonic cell growth and biofilm biomass accumulation in the wild-type Temecula strain. In the CV assay, ortho-fluorophenylsulfonamide 18 (OD 0.228), ortho-bromophenylsulfonamide 21 (OD 0.269) and meta-bromophenylsulfonamide 22 (OD 0.243) produced the strongest inhibitory effect on biofilm biomass accumulation compared to the DMSO control (OD 0.353). A clear structure activity trend emerged with other haloaryl-substituted sulfonamides also inhibiting biofilm production, including meta-fluorosulfonamide 19 (OD 0.286), para-fluorosulfonamide 20 (OD 0.286) and para-bromosulfonamide 23 (OD 0.278). By contrast, all of the olefinic analogues 6–10, as well as signalling molecules BDSF (2) and XfDSF1 (3), produced OD readings greater than the DMSO control. Among the aromatic analogues, methylsulfonamide 13, cyclopropylsulfonamide 14 and 3,3,3-trifluoropropylsulfonamide 16, as well as para-methoxyphenylsulfonamide 26, para-tert-butylsulfonamide 27 and para-nitrophenylsulfonamide 28, were among the strongest promoters of biofilm cell accumulation. These latter results highlight how modifying the phenyl ring substituents can switch activity from biofilm inhibition to biofilm promotion.
The OD600 readings measuring the planktonic cell growth in Figure 5 revealed that all of our aromatic analogues containing an arylsulfonamide (17–28) displayed strong growth inhibition compared to the control. Of these, para-chlorophenylsulfonamide 24 was the most active with an OD reading of 0.012, an 8-fold reduction compared to the DMSO control (OD 0.102). Only a small negative effect of DMSO on cell growth was noted. The arylsulfonamides together had a mean OD reading of 0.022, representing a 4-fold average reduction in planktonic cell growth. While para-methoxyphenylsulfonamide 26 (OD 0.037) and para-nitrophenylsulfonamide 28 (OD 0.045) were slightly less active, they were still comparable to XfDSF1 (3) (OD 0.036). The alkyl-substituted sulfonamides 13–16 did not display an inhibitory effect. Of the olefinic analogues, ortho-bromophenylsulfonamide 9 (OD 0.048) was the only candidate to inhibit growth to a notable level.
The rpfF* mutant is often used as a measure of agonistic effects on cell growth since endogenous DSF is not present. The use of the rpfF* mutant was especially important in this study as the effects of particular DSF analogues on phenotypes of Xylella fastidiosa were being assessed. The addition of such analogues to strains that were capable of producing their own native DSF would have complicated interpretation of the assessment of the direct effects of the compounds on X. fastidiosa phenotypes such as biofilm formation. The behaviour of our compounds towards the rpfF* mutant strain was similar to the wild-type strain, with a reduction in cell growth observed in most cultures (Figure 6). The CV and OD600 readings for the DMSO control were recorded as 0.592 and 0.565, respectively—reflecting the minor inhibitory effect of DMSO on cell growth. The optical density readings from the CV assay revealed that 17 of the 21 compounds reduced biofilm biomass accumulation. Of the aromatic analogues, ortho-bromophenylsulfonamide 21 was the most potent inhibitor, producing an OD reading of 0.135, a 4-fold reduction compared to the control. Other haloaryl-substituted sulfonamides, including ortho-fluorosulfonamide 18 (OD 0.166), para-bromosulfonamide 23 (OD 0.164) and para-iodosulfonamide 25 (OD 0.150), were almost equally effective, reflecting the same trend observed with Temecula. By contrast, alkyl-substituted sulfonamides 13–16 and non-halogenated arylsulfonamides 26–28 exhibited poor biofilm inhibition in the rpfF* mutant. Methylsulfonamide 6 (OD 0.423) was the only olefinic analogue to reduce biofilm biomass accumulation, while olefinic phenylsulfonamide 8 (OD 1.092) produced the largest growth enhancement effect with a 1.8-fold increase. Signalling molecules BDSF (2) (OD 0.658) and XfDSF1 (3) (OD 0.675) also increased biofilm biomass accumulation in the rpfF* strain.
The most potent compound in the OD600 assay was para-iodophenylsulfonamide 25 (OD 0.029) which was responsible for an almost 19-fold reduction in planktonic cell growth. Once again, the halogen-substituted sulfonamides were among the most potent inhibitors with an average OD reading of 0.072, representing a 7-fold growth reduction, observed in the cultures containing fluoro- and bromo-substituted phenylsulfonamides 18–20 and 21–23, respectively. While the olefinic analogues 6–10 were ineffective inhibitors, only cyclopropylsulfonamide 7 (OD 0.745) actually increased planktonic cell growth in this strain. In line with expectations, BDSF (2) and XfDSF1 (3) increased biofilm biomass accumulation in the CV assay but reduced planktonic cell growth with OD600 readings of 0.476 and 0.440, respectively. It is worth noting that BDSF analogues 6–9 and 13–28 have been previously assessed using a combination of MTT assays [13] and the Galleria mellonella in vivo model [14] and no compound in this series was found to display significant cytotoxic effects.
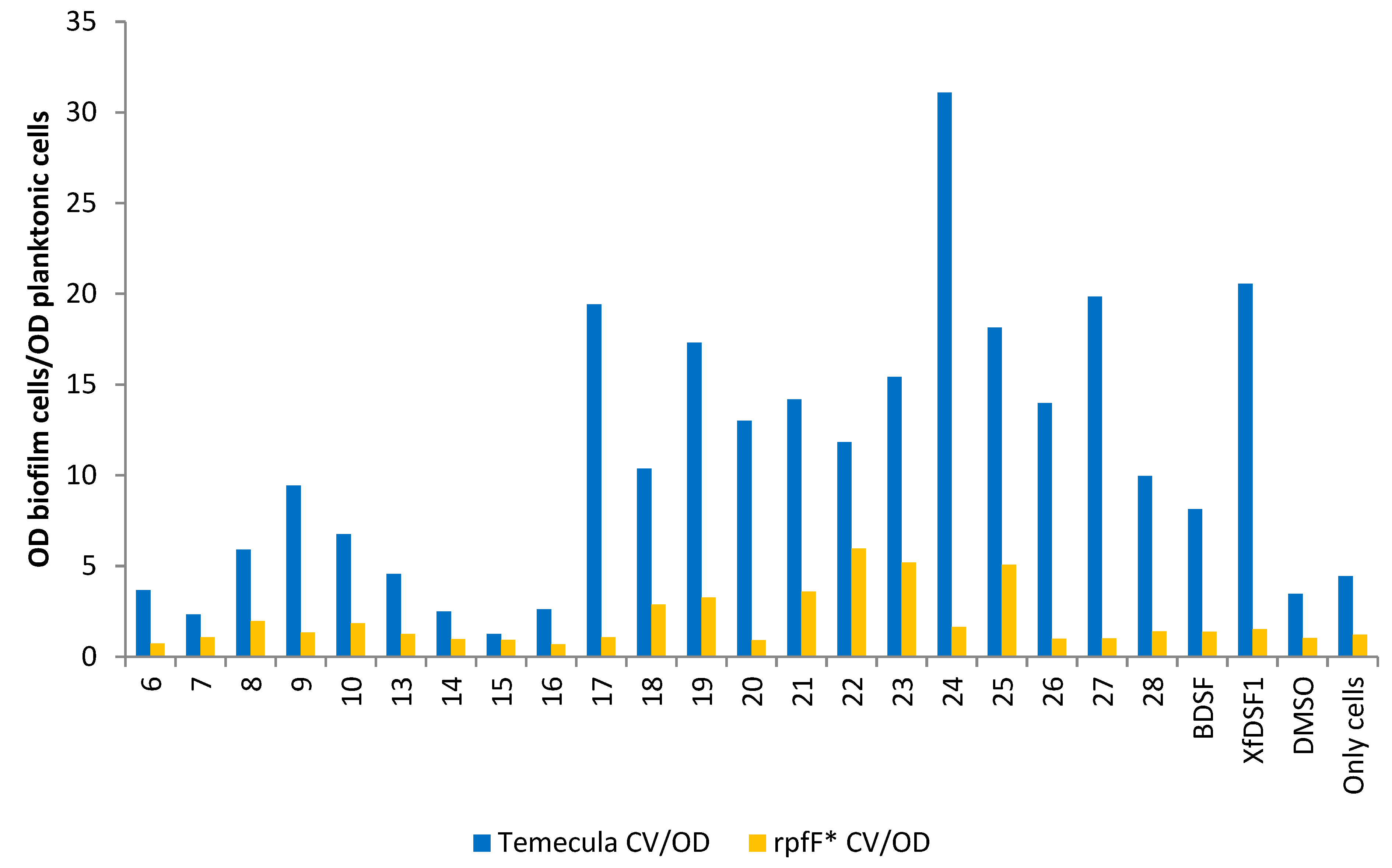
One means of identifying agonistic and antagonistic effects in X. fastidiosa is to examine the ratio of the biofilm biomass to planktonic cells in each culture, i.e., the ratio of CV to OD600 optical densities (Figure 7). For almost all of our compounds, including for the positive controls of BDSF (2) and XfDSF1 (3) signalling molecules, stronger biofilm formation was observed in the wild-type cultures compared to rpfF*. This most likely reflects the accumulation of native DSF that facilitates biofilm formation and which is made only by the WT strain and not the rpfF* mutant. The stronger induction of biofilm by XfDSF1 (3) in the wild-type strain compared to rpfF* mutant suggested that the assay was working as expected, but also suggested that the endogenous (intracellular) production of DSF by the cells results in higher concentrations of this signal molecule than when supplied extracellularly. That is, there may be limits to the solubility of these molecules or their ability to be taken up from the extracellular milieu. In the presence of phenylsulfonamide 17, para-chlorophenylsulfonamide 24 and tert-butylphenylsulfonamide 27, there was an approximately 18-fold difference between the CV/OD ratios in the wild-type Temecula and rpfF* strains. The smallest difference in CV/OD ratios for the Temecula and rpfF* strains was recorded for tert-butylsulfonamide 15.
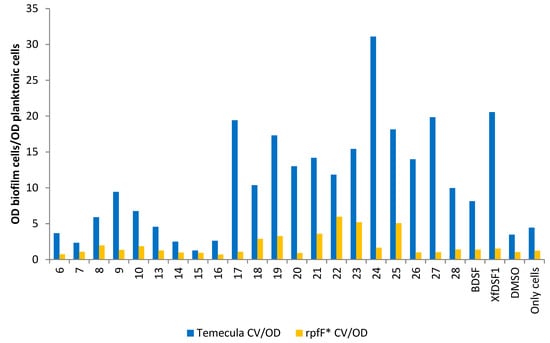
Figure 7.
Ratio of the biofilm cells to planktonic cells from cultures of wild-type strain Temecula and rpfF* mutant. Shown is the ratio of OD595 reflecting the abundance of crystal violet retained in biofilm cells attached to glass culture tubes relative to the OD600 of planktonic cells remaining in suspension after addition of DSF analogues to cultures of wild-type X. fastdiosa (blue bars) or an rpfF* mutant (yellow bars).
In general, X. fastidiosa is more sensitive to longer chain DSF signals compared to other bacterial species. These signalling molecules usually range from 12 to 18 carbons [6]. The most active of these quorum sensing signals is 16-carbon XfDSF2 (4) (Figure 1). We originally designed our aromatic N-acyl sulfonamides 13–28 as analogues of 12-carbon BDSF (2). As some of our aromatic analogues were active as either agonists or antagonists, we wondered if extending the alkyl chain at the C2 position would give rise to a more pronounced biological effect. This second series of compounds, containing 14-carbon or 16-carbon backbones, could serve as analogues of XfDSF1 (3) or XfDSF2 (4), respectively. The longer chain analogues, namely tert-butylsulfonamides 32 and 37 and para-chlorophenylsulfonamides 33 and 38, as well as our XfDSF1 (3) and XfDSF2 (4) reference samples, were evaluated using the same CV and OD600 assays described previously.
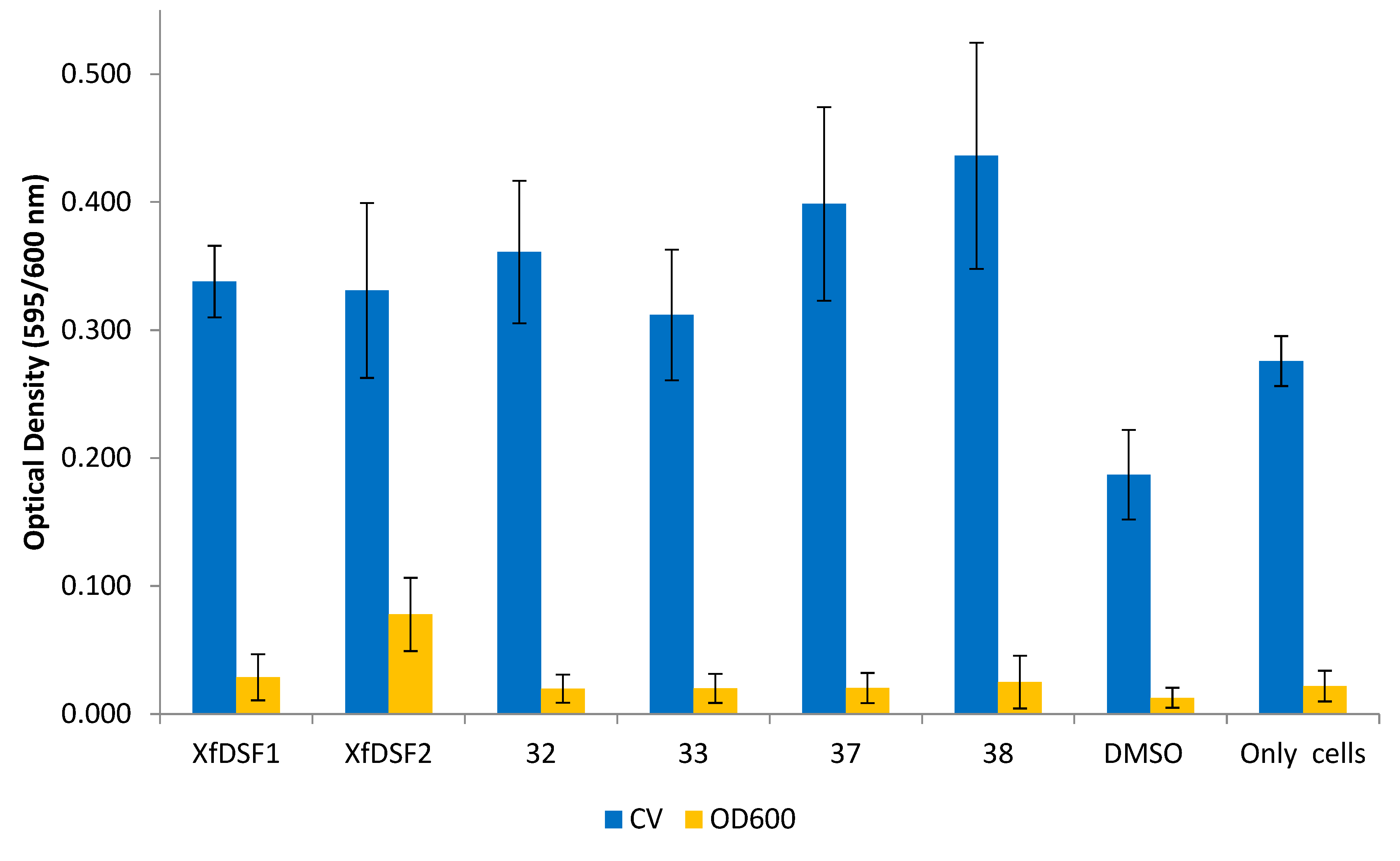
In contrast to the 2-nonyl-substituted analogues 13–28, the longer chain analogues appeared to increase both biofilm biomass and planktonic cell growth in the wild-type Temecula strain (Figure 8). In the CV assay, all of the bioisosteres 32–33, 37–38, in addition to signalling molecules XfDSF1 (3) and XfDSF2 (4), produced OD readings which were almost double that of the DMSO control. The 2-undecyl derivatives, namely tert-butylsulfonamide 32 (0.361) and para-chlorophenylsulfonamide 33 (0.312), were comparable to signalling molecules XfDSF1 (0.338) and XfDSF2 (0.331). The longer 2-tridecyl analogues were more active than their 2-undecyl counterparts—tert-butylsulfonamide 37 (0.399) and para-chlorophenylsulfonamide 38 (0.436) caused 2.2- and 2.4-fold increases in biofilm biomass growth, respectively. In the OD600 assay measuring planktonic cell growth, most compounds displayed comparable activity to the DMSO control (0.013). One molecule which did enhance cell growth was XfDSF2 (0.078). We had expected that extending the chain length at the C-2 position would enhance the inhibitory activity of our analogues. These results appear to contradict this hypothesis.
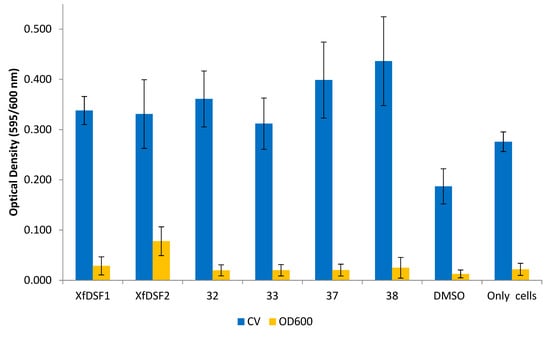
Figure 8.
Optical density readings of longer chain XfDSF analogues for wild-type strain Temecula. OD595 abundance of crystal violet retained in biofilm cells attached to glass culture tubes after addition of XfDSF analogues (blue bars). OD600 abundance of planktonic cells remaining in suspension after addition of XfDSF analogues (yellow bars). The error bars represent the standard deviations of the means.
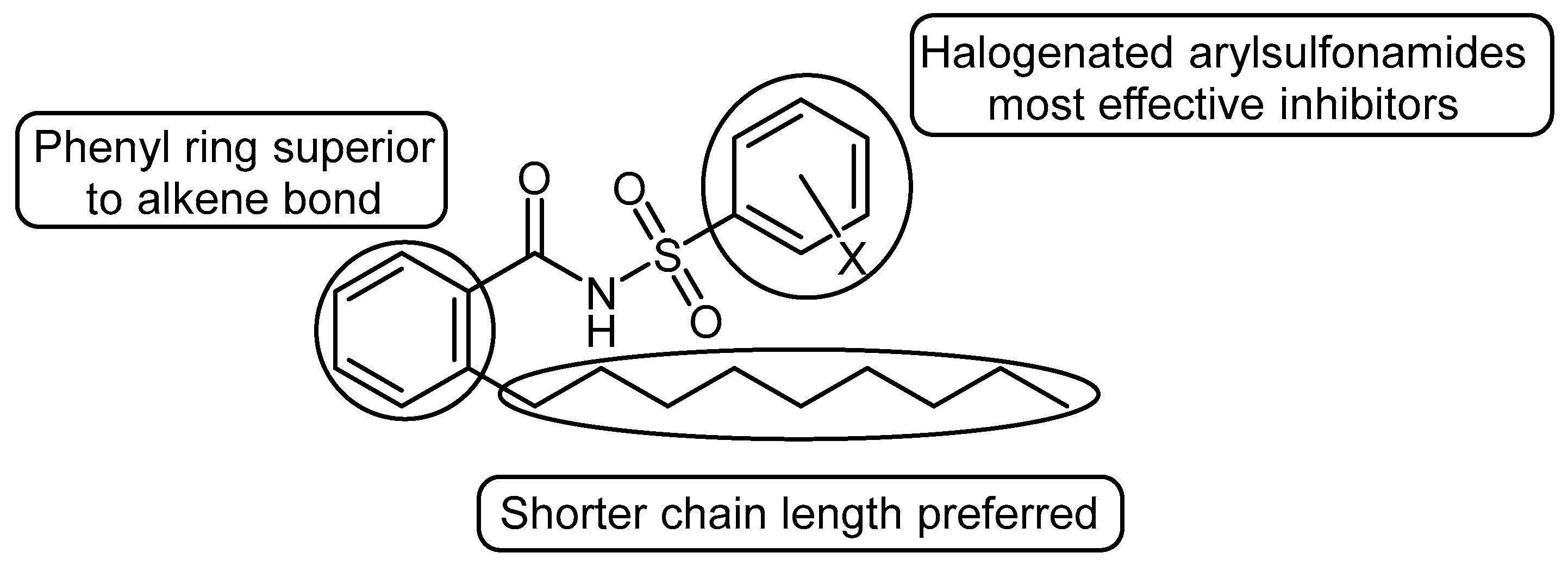
Figure 9 summarises the main structure–activity relationships identified in this study. The most effective biofilm inhibitors were found to be the aromatic BDSF analogues, in particular those incorporating haloaryl-substituted sulfonamides. The corresponding olefinic analogues tended to promote, rather than inhibit, biofilm production in X. fastidiosa. Extending the chain length in the equivalent aromatic XfDSF1 or XfDSF2 bioisosteres was not favourable, and resulted in significantly reduced biological activity, contrary to our initial expectations. Although determination of the mode-of-action was not a goal of this study, it seems likely that our bioisosteric analogues, acting as DSF mimics, behave like DSF signals and interact with a variety of downstream regulators such as RpfC and RpfG. It is known that XfDSF is required for de-repression of rpfC in the presence of RpfF [8]. Furthermore, RpfC is also involved in a signal cascade involving RpfG and other regulators that determine the intracellular concentration of second messengers such as cyclic di-GMP that ultimately determine phenotypes such as the expression of cellular adhesins important in the biofilm formation and virulence of this pathogen.
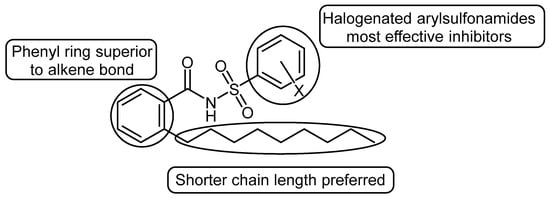
Figure 9.
Major structure–activity relationships.
4. Conclusions
In recent years, X. fastidiosa has been the subject of increased focus as a result of its significant economic impact. Losses of vines from Pierce’s disease and associated preventative measures cost the Californian wine-making industry USD 104 million per year [35], while, in Brazil, citrus variegated chlorosis has resulted in the loss of millions of citrus trees at a cost of USD 120 million per year [36]. In October 2013, scientists reported that X. fastidiosa was responsible for OQDS occurring in the southern Italian region of Apulia [37]. Over a three year period, OQDS was responsible for losses up to EUR 390 million in olive oil production in Italy [38]. The bacterium now threatens to become a significant plant pest throughout the Mediterranean basin. According to a JRC report, the microbe could cost the European Union up to EUR 5.5 billion per year, and consumers will likely be impacted in the form of higher prices [39,40].
DSF signalling molecules play a key role in regulating biofilm formation in X. fastidiosa. The production of biofilm is, in turn, intrinsically linked with cell adhesion and transmission. Accordingly, the development of DSF analogues may constitute a useful strategy for Xylella control by disrupting its innate ability to form adhesive biofilms. In this paper, we have demonstrated that N-acyl sulfonamide derivatives of BDSF can either inhibit or promote biofilm formation in X. fastidiosa depending on the nature of the sulfonamide substituent. In particular, haloaryl-substituted sulfonamides were found to be effective biofilm inhibitors. In general, shorter chain analogues of BDSF proved to be more potent, while longer chain derivatives of XfDSF1 and XfDSF2 prompted a reduced biological response. These results demonstrate that control of biofilm formation in X. fastidiosa can be influenced by the addition of appropriately designed DSF analogues. The strategy outlined here may provide opportunities for the control of this important plant pathogen.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12122496/s1, Compound Spectra.
Author Contributions
T.P.O. and S.E.L. designed the research. C.H. synthesised the compounds. C.B. conducted the biological evaluation. M.O. contributed to compound analysis and characterisation. C.H. and T.P.O. wrote the first draft. S.E.L. and C.B. reviewed the second draft. All authors have read and agreed to the published version of the manuscript.
Funding
Conor Horgan is grateful for funding by way of a Government of Ireland Postgraduate Research Scholarship (GOIPG/2017/1111) provided by the Irish Research Council. Michelle O’Driscoll thanks the Irish Research Council for a Government of Ireland Postgraduate Research Scholarship (GOIPG/2021/227). This publication has emanated from research supported in part by a Grant from the Science Foundation Ireland Research Infrastructure Programme under Grant number 21/RI/9705.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BDSF | Burkholderia diffusible signal factor |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DMAP | Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DSF | Diffusible signal factor |
| OD | Optical density |
| OQDS | Olive quick decline syndrome (OQDS) |
| QS | Quorum sensing |
| RBF | Round bottom flask |
| r.t. | Room temperature |
| WT | Wild-type |
| XfDSF | X. fastidiosa diffusible signal factor |
References
- European Commission. Xylella fastidiosa Fact Sheet. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/legislation/control-measures/xylella-fastidiosa_en (accessed on 8 October 2024).
- Wells, J.M.; Raju, B.C.; Hung, H.-Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Evol. Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- Wang, L.H.; He, Y.; Gao, Y.; Wu, J.E.; Dong, Y.H.; He, C.; Wang, S.X.; Weng, L.X.; Xu, J.L.; Tay, L.; et al. A Bacterial Cell-Cell Communication Signal with Cross-Kingdom Structural Analogues. Mol. Microbiol. 2004, 51, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Dow, J.M. Communication with a growing family: Diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol. 2011, 19, 145–152. [Google Scholar] [CrossRef]
- Beaulieu, E.D.; Ionescu, M.; Chatterjee, S.; Yokota, K.; Trauner, D.; Lindow, S. Characterization of a diffusible signaling factor from Xylella fastidiosa. MBio 2013, 4, e00539-12. [Google Scholar] [CrossRef]
- Ionescu, M.; Yokota, K.; Antonova, E.; Garcia, A.; Beaulieu, E.; Hayes, T.; Iavarone, A.T.; Lindow, S.E. Promiscuous Diffusible Signal Factor Production and Responsiveness of the Xylella fastidiosa Rpf System. mBio 2016, 7, e01054-16. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Christensen, Q.H.; Feng, Y.; Wang, H.; Cronan, J.E. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol. Microbiol. 2012, 83, 840–855. [Google Scholar] [CrossRef]
- Ionescu, M.; Baccari, C.; Da Silva, A.M.; Garcia, A.; Yokota, K.; Lindow, S.E. Diffusible signal factor (DSF) synthase RpfF of Xylella fastidiosa is a multifunction protein also required for response to DSF. J. Bacteriol. 2013, 195, 5273–5284. [Google Scholar] [CrossRef]
- Dow, J.M. Diffusible signal factor-dependent quorum sensing in pathogenic bacteria and its exploitation for disease control. J. Appl. Microbiol. 2017, 122, 2–11. [Google Scholar] [CrossRef]
- Guilhabert, M.R.; Kirkpatrick, B.C. Identification of Xylella fastidiosa antivirulence genes: Hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant-Microbe Interact. 2005, 18, 856–868. [Google Scholar] [CrossRef]
- Newman, K.L.; Almeida, R.P.; Purcell, A.H.; Lindow, S.E. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Appl. Environ. Microbiol. 2003, 69, 7319–7327. [Google Scholar] [CrossRef]
- An, S.-Q.; Murtagh, J.; Twomey, K.B.; Gupta, M.K.; O’Sullivan, T.P.; Ingram, R.; Valvano, M.A.; Tang, J.-l. Modulation of antibiotic sensitivity and biofilm formation in Pseudomonas aeruginosa by interspecies signal analogues. Nat. Commun. 2019, 10, 2334. [Google Scholar] [CrossRef]
- Huedo, P.; Kumar, V.P.; Horgan, C.; Yero, D.; Daura, X.; Gibert, I.; O’Sullivan, T.P. Sulfonamide-based diffusible signal factor analogs interfere with quorum sensing in Stenotrophomonas maltophilia and Burkholderia cepacia. Future Med. Chem. 2019, 11, 1565–1582. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.-C.; Horgan, C.; Yero, D.; Bravo, M.; Daura, X.; O’Driscoll, M.; Gibert, I.; O’Sullivan, T.P. Synthesis and evaluation of aromatic BDSF bioisosteres on biofilm formation and colistin sensitivity in pathogenic bacteria. Eur. J. Med. Chem. 2023, 261, 115819. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Huryn, D.M.; Smith, A.B. Carboxylic acid (bio)isosteres in drug design. ChemMedChem 2013, 8, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.; O’Sullivan, T.P. Recent Developments in the Practical Application of Novel Carboxylic Acid Bioisosteres. Curr. Med. Chem. 2022, 29, 2203–2234. [Google Scholar] [CrossRef]
- Kumar, V.P.; Gupta, M.K.; Horgan, C.; O’Sullivan, T.P. Synthesis of the quorum sensing molecule Diffusible Signal Factor using the alkyne zipper reaction. Tetrahedron Lett. 2018, 59, 2193–2195. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.; Blua, M.J.; Mizell Iii, R.F.; Andersen, P.C. The biology of xylem fluid–feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Purcell, A.H.; Finlay, A.H.; McLean, D.L. Pierce’s disease bacterium: Mechanism of transmission by leafhopper vectors. Science 1979, 206, 839–841. [Google Scholar] [CrossRef]
- Newman, K.L.; Almeida, R.P.; Purcell, A.H.; Lindow, S.E. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. USA 2004, 101, 1737–1742. [Google Scholar] [CrossRef]
- Killiny, N.; Almeida, R.P.P. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl. Environ. Microbiol. 2009, 75, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.; Purcell, A. Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata. Phytopathology 1995, 85, 209–212. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Purcell, A. Transmission of Xylella fastidiosa to grapevines by Homalodisca coagulata (Hemiptera: Cicadellidae). J. Econ. Entomol. 2003, 96, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.; Blua, M.J.; Lopes, J.R.; Purcell, A.H. Vector transmission of Xylella fastidiosa: Applying fundamental knowledge to generate disease management strategies. Ann. Entomol. 2005, 98, 775–786. [Google Scholar] [CrossRef]
- Blua, M.; Morgan, D. Dispersion of Homalodisca coagulata (Hemiptera: Cicadellidae), a vector of Xylella fastidiosa, into vineyards in southern California. J. Econ. Entomol. 2003, 96, 1369–1374. [Google Scholar] [CrossRef]
- Chatterjee, S.; Almeida, R.P.P.; Lindow, S. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M. Conducting units: Tracheids and vessels. In Xylem Structure and the Ascent of Sap; Springer: Berlin/Heidelberg, Germany, 2002; pp. 4–20. [Google Scholar]
- Baldi, P.; La Porta, N. Xylella fastidiosa: Host range and advance in molecular identification techniques. Front. Plant Sci. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Dugave, C.; Demange, L. cis−trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef] [PubMed]
- Tumber, K.P.; Alston, J.M.; Fuller, K. Pierce’s disease costs California $104 million per year. Calif. Agric. 2014, 68, 20–29. [Google Scholar] [CrossRef]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in Sao Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant. Pathol. 2013, 95, 668. [Google Scholar]
- Scala, V.; Pucci, N.; Salustri, M.; Modesti, V.; L’Aurora, A.; Scortichini, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; Loreti, S. Xylella fastidiosa subsp. pauca and olive produced lipids moderate the switch adhesive versus non-adhesive state and viceversa. PLoS ONE 2020, 15, e0233013. [Google Scholar] [CrossRef]
- Sanchez, B.; Barreiro-Hurle, J.; Soto Embodas, I.; Rodriguez-Cerezo, E. The Impact Indicator for Priority Pests (I2P2): A Tool for Ranking Pests According to Regulation (EU) 2016/2031; Europen Union: Maastricht, The Netherlands, 2019; p. 585182. [Google Scholar]
- Schneider, K.; Mourits, M.; van der Werf, W.; Lansink, A.O. On consumer impact from Xylella fastidiosa subspecies pauca. Ecol. Econ. 2021, 185, 107024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).