Sebum Components Dampen the Efficacy of Skin Disinfectants against Cutibacterium acnes Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Media and Reagents
2.3. Phylotyping C. acnes PC Isolates
2.4. Preliminary Assessment of C. acnes Biofilm Formation with Oleic Acid Supplementation
2.5. Impact of Sebum-like Components on C. acnes Biofilm Formation
2.6. Confocal Microscopy
2.7. C. acnes and S. aureus Dual-Species Biofilm Formation in the Presence of Sebum-like Components
2.8. Disinfectant Efficacy Assays
2.8.1. Mono-Species Biofilms
2.8.2. Disinfectant Efficacy against Dual-Species Biofilms
2.9. Biofilm Matrix Disruption Assay
2.10. Statistical Analyses
3. Results
3.1. C. acnes PC Isolates Predominantly Belong to Phylotypes IB and II
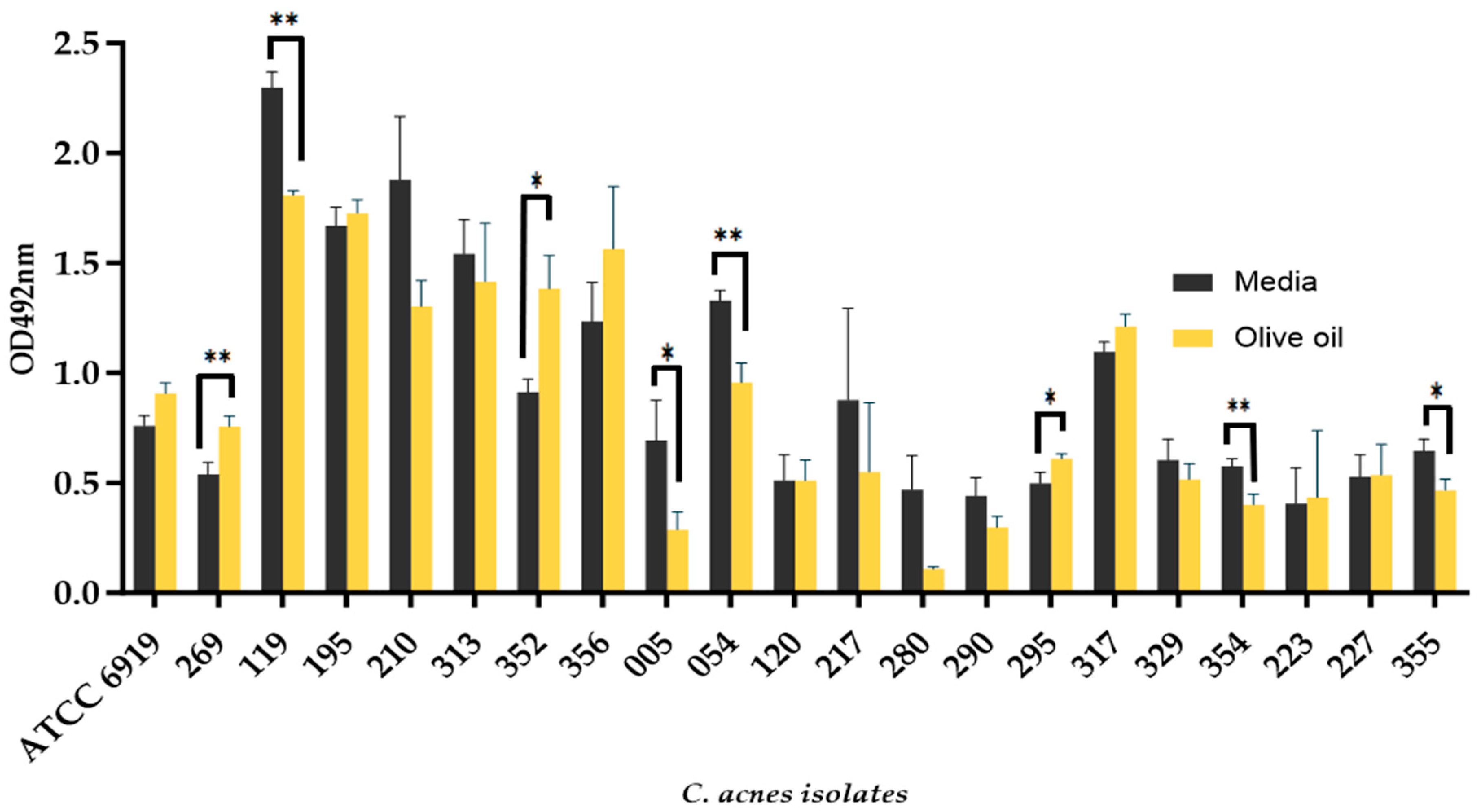
3.2. The Addition of Olive Oil as a Source of Oleic Acid Does Not Enhance the Biofilm Formation of all C. acnes Isolates
3.3. C. acnes Biofilm Formation Is Impacted by Different Sebum-like Components in an Isolate-Dependent Manner
3.4. Denser C. acnes Biofilms Are Formed in the Presence of Sebum-like Components
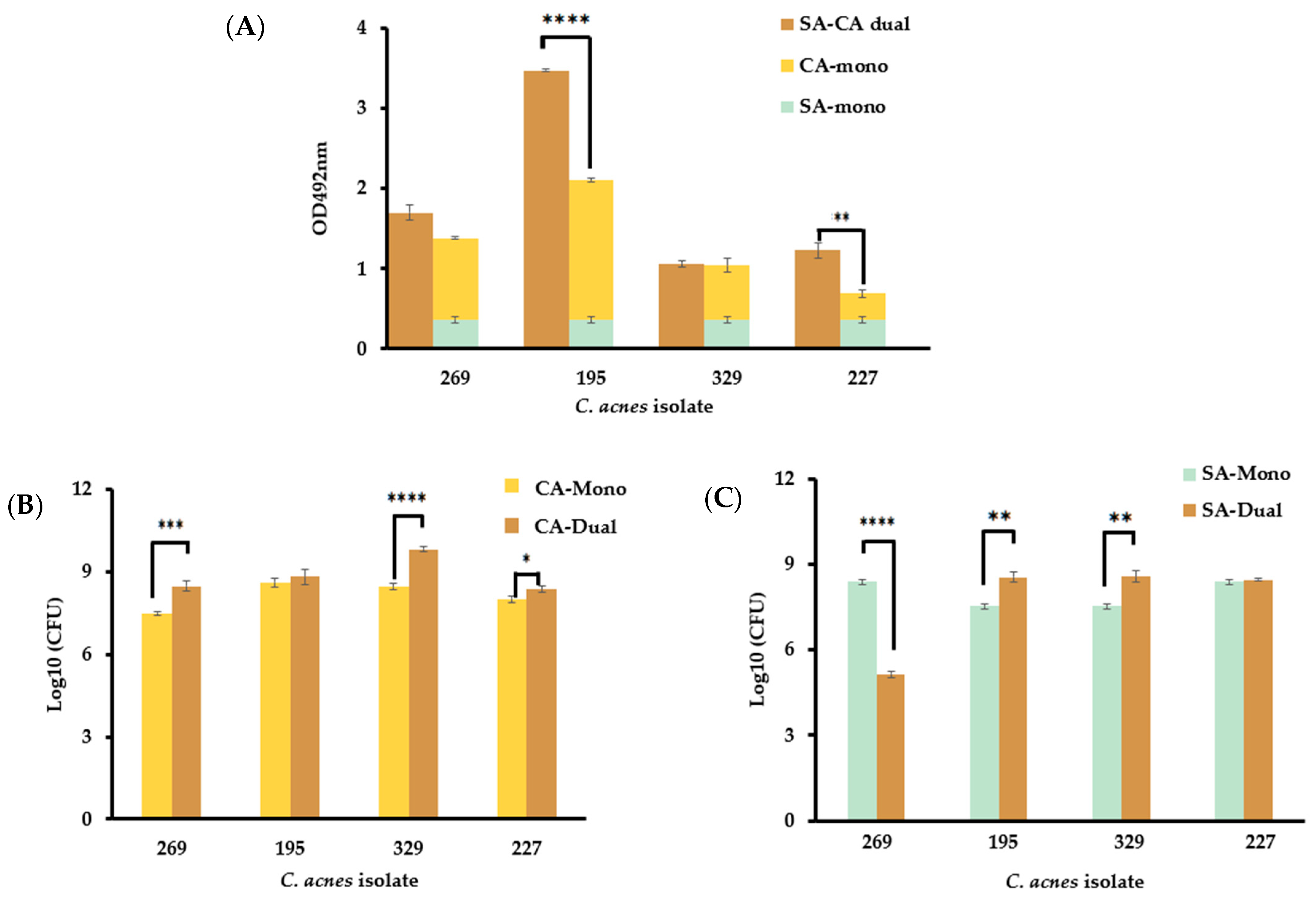
3.5. The Presence of S. aureus in Dual-Species Biofilms Affect C. acnes Growth in a Strain-Dependent Manner
3.6. Sebum-like Components Negatively Impact the Efficacy of the Standard Donor Skin Disinfectant and a Hypochlorous Acid-Based Skin Disinfectant
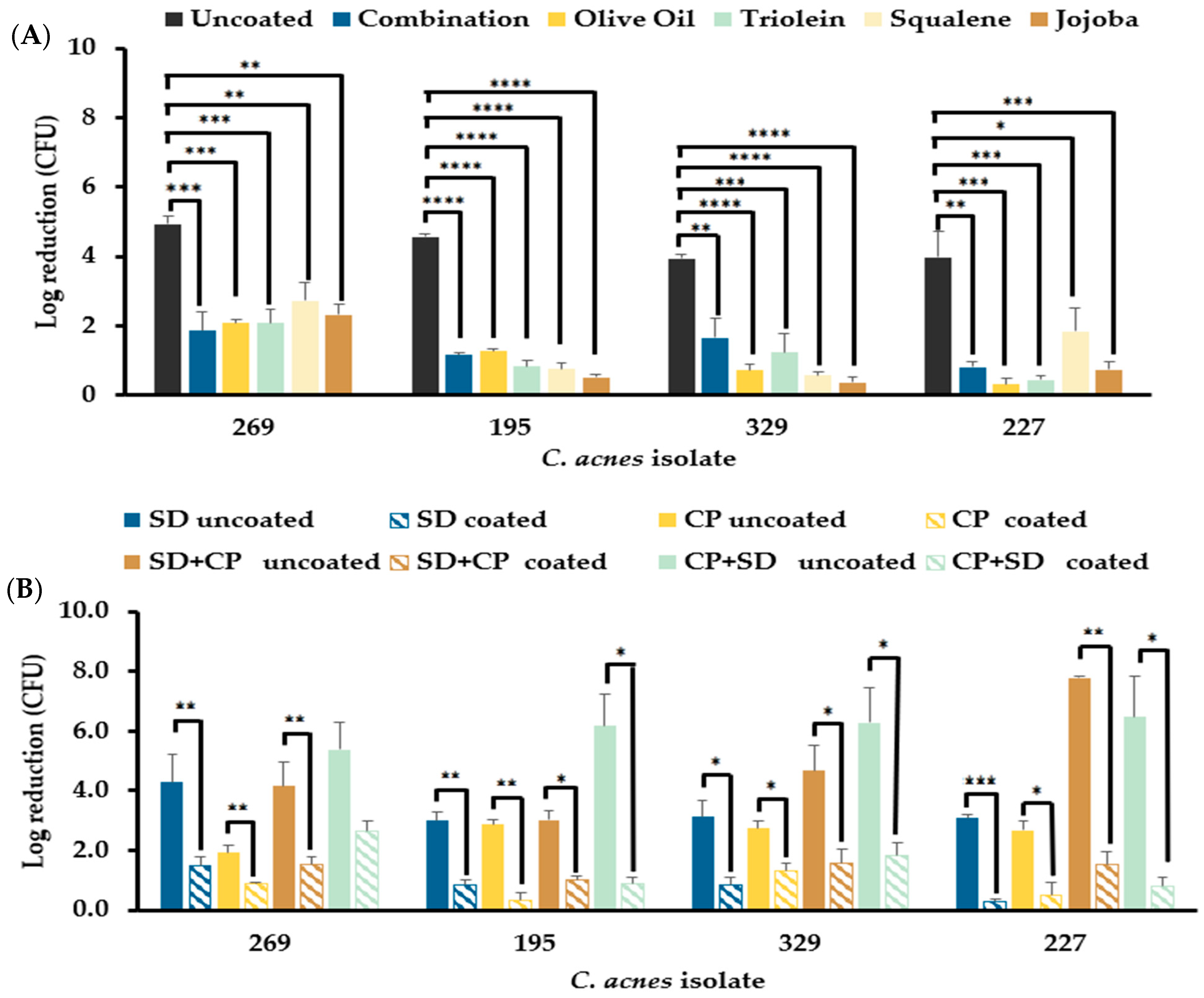
3.6.1. Mono-Species C. acnes Biofilms Resist Disinfection in the Presence of Sebum-like Components
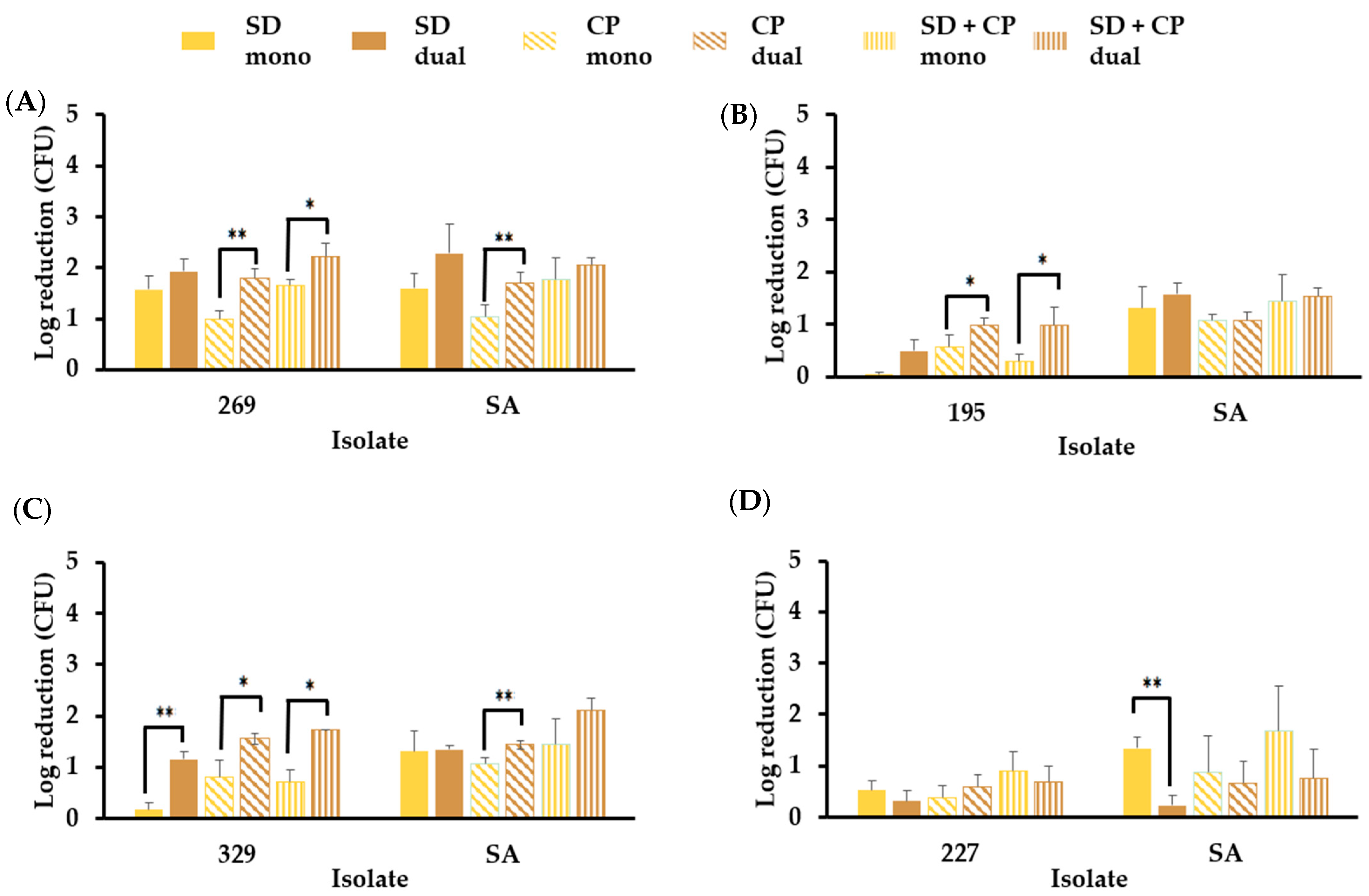
3.6.2. S. aureus in Mono- and Dual-Species Biofilm Models Resist Elimination by Skin Disinfectants in the Presence of Sebum-like Components
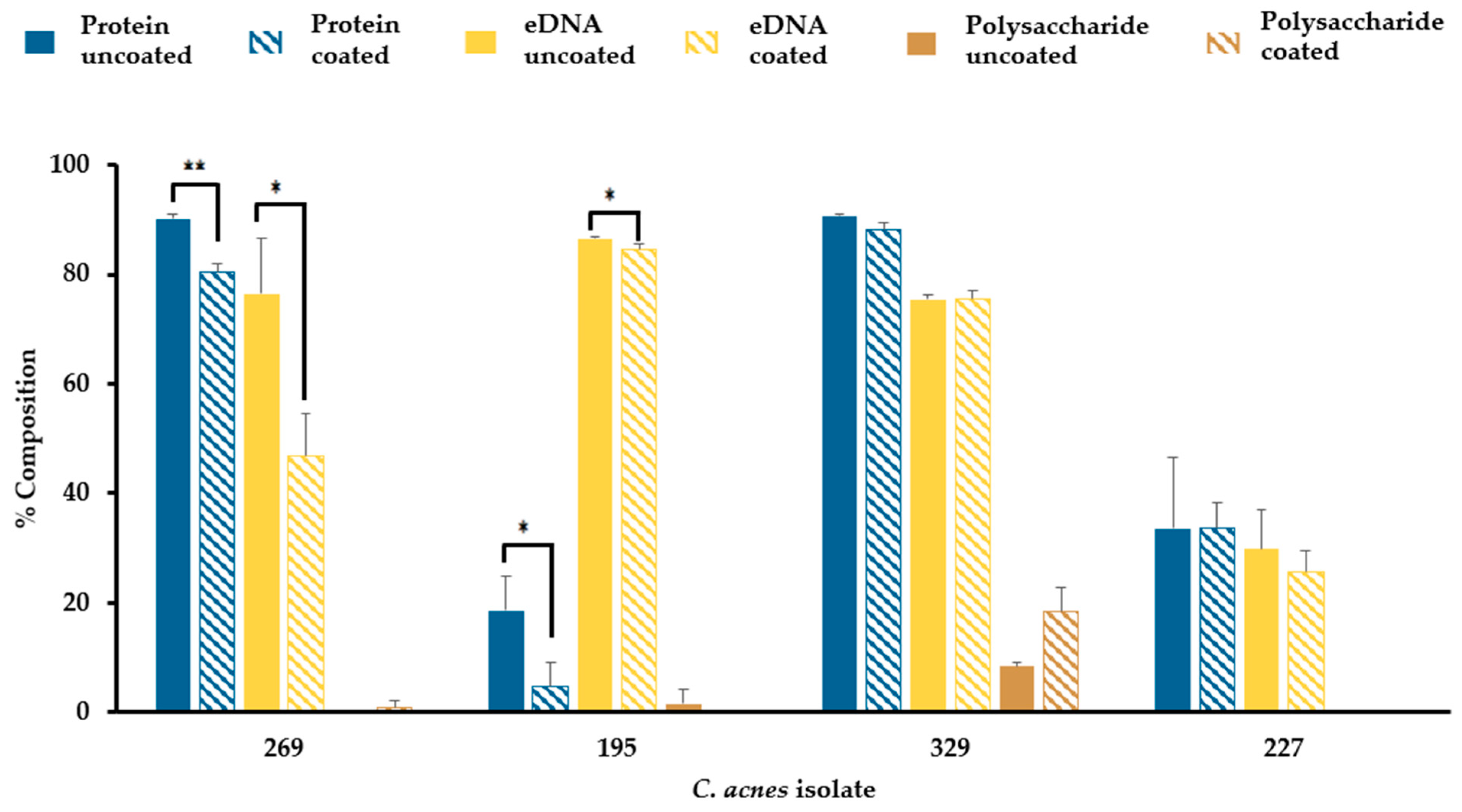
3.7. Sebum-like Components Impact the Composition of C. acnes Biofilm Matrix in a Strain-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ying, S.; Zeng, D.N.; Chi, L.; Tan, Y.; Galzote, C.; Cardona, C.; Lax, S.; Gilbert, J.; Quan, Z.X. The influence of age and gender on skin-associated microbial communities in urban and rural human populations. PLoS ONE 2015, 10, e0141842. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and its Interplay with the Environment. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 4–11. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Kallenbach, K.; Miller, I.M.; Prens, E.; Saunte, D.M.; Bjarnsholt, T.; Jemec, G.B. Normal skin microbiota is altered in pre-clinical hidradenitis suppurativa. Acta. Derma. Vener. 2017, 97, 208–213. [Google Scholar] [CrossRef]
- Jahns, A.C.; Alexeyev, O.A. Three-dimensional distribution of Propionibacterium acnes biofilms in human skin. Exp. Dermatol. 2014, 23, 687–689. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Vyas, T.; Rapalli, V.K.; Chellappan, D.K.; Dua, K.; Dubey, S.K.; Singhvi, G. Bacterial biofilms associated skin disorders: Pathogenesis, advanced pharmacotherapy and nanotechnology-based drug delivery systems as a treatment approach. Life Sci. 2021, 287, 120148. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial biofilms in urinary tract infections and prostatitis: Etiology, pathogenicity, and combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Urbán, E. Relevance of anaerobic bacteremia in adult patients: A never-ending story? Eur. J. Microbiol. Immunol. 2020, 10, 64–75. [Google Scholar] [CrossRef]
- Lodhi, S.H.; Abbasi, A.; Ahmed, T.; Chan, A. Acne on the valve: Two intriguing cases of Cutibacterium acnes endocarditis. Cureus 2020, 12, e8532. [Google Scholar] [CrossRef]
- Schmid, B.; Hausmann, O.; Hitzl, W.; Achermann, Y.; Wuertz-Kozak, K. The role of Cutibacterium acnes in intervertebral disc inflammation. Biomedicines 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Arcos, S.; Evans, S.; McIntyre, T.; Pang, C.; Yi, Q.L.; DiFranco, C.; Goldman, M. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020, 60, 2918–2928. [Google Scholar] [CrossRef]
- Ramirez-Arcos, S.; Goldman, M. Skin disinfection methods: Prospective evaluation and postimplementation results. Transfusion 2010, 50, 59–64. [Google Scholar] [CrossRef]
- Walther-Wenke, G.; Schrezenmeier, H.; Deitenbeck, R.; Geis, G.; Burkhart, J.; Höchsmann, B.; Sireis, W.; Schmidt, M.; Seifried, E.; Gebauer, W.; et al. Screening of platelet concentrates for bacterial contamination: Spectrum of bacteria detected, proportion of transfused units, and clinical follow-up. Ann. Hematol. 2010, 89, 83–91. [Google Scholar] [CrossRef]
- Freudenburg-de Graaf, W.; Spelmink, S.; Heijnen, J.; de Korte, D. Transfusion transmitted bacterial infections (TTBI) involving contaminated platelet concentrates: Residual risk despite intervention strategies. Ann. Blood 2022, 7, 26. [Google Scholar] [CrossRef]
- Loza-Correa, M.; Kou, Y.; Taha, M.; Kalab, M.; Ronholm, J.; Schlievert, P.M.; Cahill, M.P.; Skeate, R.; Cserti-Gazdewich, C.; Ramirez-Arcos, S. Septic transfusion case caused by a platelet pool with visible clotting due to contamination with Staphylococcus aureus. Transfusion 2017, 57, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Borrel, V.; Gannesen, A.V.; Barreau, M.; Gaviard, C.; Duclairoir-Poc, C.; Hardouin, J.; Konto-Ghiorghi, Y.; Lefeuvre, L.; Feuilloley, M.G. Adaptation of acneic and non acneic strains of Cutibacterium acnes to sebum-like environment. Microbiologyopen 2019, 8, e00841. [Google Scholar] [CrossRef] [PubMed]
- E1054–08; Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents. ASTM International: West Conshohocken, PA, USA, 2008.
- Barnard, E.; Nagy, I.; Hunyadkürti, J.; Patrick, S.; McDowell, A. Multiplex touchdown PCR for rapid typing of the opportunistic pathogen Propionibacterium acnes. J. Clin. Microbiol. 2015, 53, 1149–1155. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet. Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- McDonald, C.P. Interventions implemented to reduce the risk of transmission of bacteria by transfusion in the English National Blood Service. Transfus. Med. Hemotherapy 2011, 38, 255–258. [Google Scholar] [CrossRef]
- Wong, P.Y.; Colville, V.L.; White, V.; Walker, H.M.; Morris, R.A.; Microbial Contamination and Infection Control Subcommittee, Australian Red Cross Blood Service. Validation and assessment of a blood-donor arm disinfectant containing chlorhexidine and alcohol. Transfusion 2004, 44, 1238–1242. [Google Scholar] [CrossRef]
- Karpanen, T.J.; Worthington, T.; Conway, B.R.; Hilton, A.C.; Elliott, T.S.; Lambert, P.A. Permeation of chlorhexidine from alcoholic and aqueous solutions within excised human skin. Antimicrob. Agents. Chemother. 2009, 53, 1717–1719. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Biswas, S.M.; Chakraborty, N. Shedded Artocarpus leaves-good plant sources of natural squalene with potent antioxidant and antimicrobial activity-alternative to marine animals. J. Nat. Pharm. 2013, 4, 21–27. [Google Scholar] [CrossRef]
- Jumina, J.; Lavendi, W.; Singgih, T.; Triono, S.; Steven Kurniawan, Y.; Koketsu, M. Preparation of monoacylglycerol derivatives from Indonesian edible oil and their antimicrobial assay against Staphylococcus aureus and Escherichia coli. Sci. Rep. 2019, 9, 10941. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.J.; Mainwaring, D.E.; et al. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S. Chapter 3: Neutralizer Evaluation as Control Experiments for Antimicrobial Effectiveness Tests. In Handbook of Disinfectants and Antiseptics; Marcel-Dekker: New York, NY, USA, 1996; p. 300. [Google Scholar]
- Gordon, C.A.; Hodges, N.A.; Marriott, C. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1988, 22, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Ding, W.; Lin, J.; Tian, R.; Lu, L.; Liu, X.; Shen, X.; Qian, P.Y. Extracellular matrix-associated proteins form an integral and dynamic system during Pseudomonas aeruginosa biofilm development. Front. Cell. Infect. Microbiol. 2015, 5, 40. [Google Scholar] [CrossRef]
- Loza-Correa, M.; Ayala, J.A.; Perelman, I.; Hubbard, K.; Kalab, M.; Yi, Q.L.; Taha, M.; de Pedro, M.A.; Ramirez-Arcos, S. The peptidoglycan and biofilm matrix of Staphylococcus epidermidis undergo structural changes when exposed to human platelets. PLoS ONE 2019, 14, e0211132. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Brissonnet, F.; Trotier, E.; Briandet, R. The biofilm lifestyle involves an increase in bacterial membrane saturated fatty acids. Front. Microbiol. 2016, 7, 1673. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.; Grout, E.; Morris, T.; Brown, H.L. Cutibacterium acnes biofilm forming clinical isolates modify the formation and structure of Staphylococcus aureus biofilms, increasing their susceptibility to antibiotics. Anaerobe 2022, 76, 102580. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef] [PubMed]
- Bindu, B.S.C.; Mishra, D.P.; Narayan, B. Inhibition of virulence of Staphylococcus aureus—A food borne pathogen—By squalene, a functional lipid. J. Funct. Foods. 2015, 18, 224–234. [Google Scholar] [CrossRef]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef]
- Lunjani, N.; Ahearn-Ford, S.; Dube, F.S.; Hlela, C.; O’Mahony, L. Mechanisms of microbe-immune system dialogue within the skin. Genes. Immun. 2021, 22, 276–288. [Google Scholar] [CrossRef]
- EN 13727:2012+A1:2013; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity in the Medical Area—Test Method and Requirements (Phase 2, Step 1). European Committee for Electrotechnical Standardization: Brussels, Belgium, 2013. (In German)
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018, 56, 10–128. [Google Scholar] [CrossRef]


| C. acnes Isolate | Geographical Region (Canadian Province) |
|---|---|
| BPNBT-18005 | Alberta |
| BPNBT-18120 | Alberta |
| BPNBT-19210 | Alberta |
| BPNBT-19223 | Alberta |
| BPNBT-19269 | Alberta |
| BPNBT-19329 | Alberta |
| BPNBT-18313 | British Columbia/Yukon |
| BPNBT-19119 | British Columbia/Yukon |
| BPNBT-19195 | British Columbia/Yukon |
| BPNBT-19280 | British Columbia/Yukon |
| BPNBT-19054 | Manitoba |
| BPNBT-19356 | Manitoba |
| BPNBT-18290 | Ontario |
| BPNBT-18317 | Ontario |
| BPNBT-19227 | Ontario |
| BPNBT-19295 | Ontario |
| BPNBT-19352 | Ontario |
| BPNBT-19354 | Ontario |
| BPNBT-19217 | Ontario |
| BPNBT-19355 | Ontario |
| C. acnes ID | Phylotype | % (n = 20) |
|---|---|---|
| BPNBT-19269 | IA | 5 |
| BPNBT-19119, 19195, 19210, 19313, 19352, and 19356 | IB | 30 |
| BPNBT-18290, 18317, 19005, 19054, 19120, 19217, 19280, 19295, 19329, and 19354 | II | 50 |
| BPNBT-19223, 19227, and 19355 | III | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaran, D.; Ramirez-Arcos, S. Sebum Components Dampen the Efficacy of Skin Disinfectants against Cutibacterium acnes Biofilms. Microorganisms 2024, 12, 271. https://doi.org/10.3390/microorganisms12020271
Kumaran D, Ramirez-Arcos S. Sebum Components Dampen the Efficacy of Skin Disinfectants against Cutibacterium acnes Biofilms. Microorganisms. 2024; 12(2):271. https://doi.org/10.3390/microorganisms12020271
Chicago/Turabian StyleKumaran, Dilini, and Sandra Ramirez-Arcos. 2024. "Sebum Components Dampen the Efficacy of Skin Disinfectants against Cutibacterium acnes Biofilms" Microorganisms 12, no. 2: 271. https://doi.org/10.3390/microorganisms12020271





