Genome Sequencing and Characterization of Bacillus velezensis N23 as Biocontrol Agent against Plant Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. In Vitro Antifungal Activity
2.3. Biofilm Formation and Swarming Motility Assay
2.4. Plant Material and Growth Conditions
2.5. Antagonism Testing of B. velezensis N23 in Planta
2.6. Genome Sequencing, Assembly and Annotation of B. velezensis N23
2.7. Phylogenetic Identification of Strain N23 Based on 16S rDNA, gyrA Gene Sequence, and Genome Sequence
2.8. PCR Detection of Antibiotic Biosynthesis Genes
2.9. Isolation and Characterization of Antifungal Lipopeptides
2.10. Statistical Analysis
3. Results
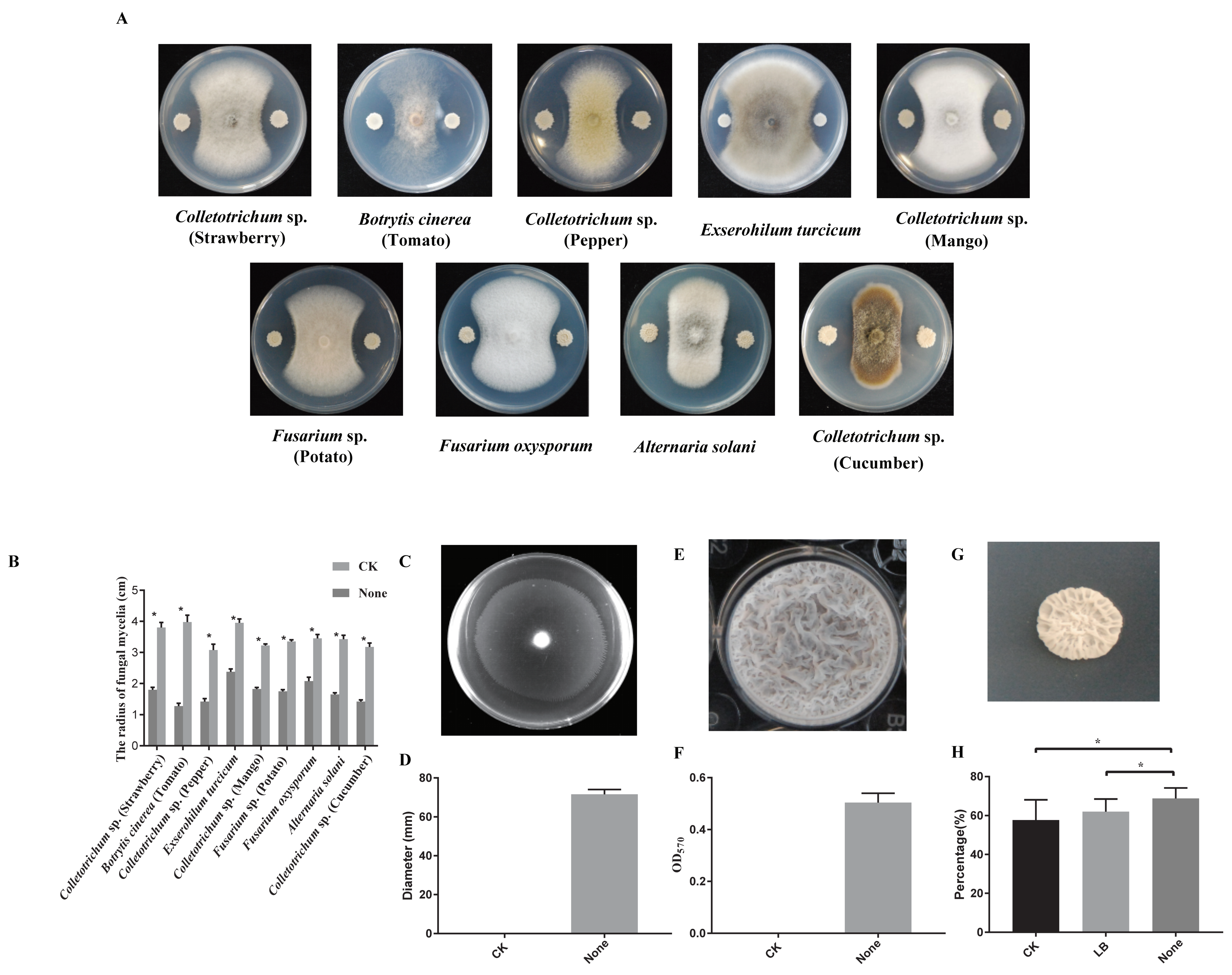
3.1. Biocontrol and Plant Growth Promoting Activity of Strain N23
3.2. Bioassay against Colletotrichum sp. in Pepper
3.3. The Genome Structure of Strain N23
3.4. Identification of Biocontrol Strain N23
3.5. Effect of B. velezensis N23 Lipopeptide Crude Extracts on Plant Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Savary, S.; Laetitia, W.; Sarah, J.P.; Paul, E.; Neil, M.; Andy, N. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.J.; Qiao, J.Q.; Gao, X.W.; Rainer, B. Novel Routes for Improving Biocontrol Activity of Bacillus Based Bioinoculants. Front. Microbiol. 2015, 6, 1395. [Google Scholar] [CrossRef]
- Yang, T.; Kadambot, H.M.S.; Liu, K. Cropping Systems in Agriculture and Their Impact on Soil Health—A Review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- He, D.C.; Zhan, J.S.; Xie, L.H. Problems, Challenges and Future of Plant Disease Management: From an Ecological Point of View. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Qiao, J.Q.; Wu, H.J.; Huo, R.; Gao, X.W.; Rainer, B. Stimulation of Plant Growth and Biocontrol by Bacillus amyloliquefaciens subsp. plantarum Fzb42 Engineered for Improved Action. Chem. Biol. Technol. Agric. 2014, 1, 12. [Google Scholar]
- Paterson, J.; Ghazaleh, J.; Li, Y.; Wang, Q.; Samina, M.; Harald, G.; Gerard, M. The Contribution of Genome Mining Strategies to the Understanding of Active Principles of PGPR Strains. FEMS Microbiol. Ecol. 2017, 93, fiw249. [Google Scholar] [CrossRef] [PubMed]
- Prisa, D.; Roberto, F.; Damiano, S. Microbial Biofertilisers in Plant Production and Resistance: A Review. Agriculture 2023, 13, 1666. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Ahmed, M.S.; Soliman, M.S.; Heba, M.S.; Alshaymaa, I.A.; Mohsin, M.; Amira, M.E.; Alia, A.M.E.; Taia, A.A.E.; Shaimaa, H.N.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Borriss, R. Bacillus, a Plant-Beneficial Bacterium. In Principles of Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 2015; pp. 379–391. [Google Scholar]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D.W. Bacillus subtilis: A Universal Cell Factory for Industry, Agriculture, Biomaterials and Medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Ali, R.; Yin, C. Bacillus amyloliquefaciens as an Excellent Agent for Biofertilizer and Biocontrol in Agriculture: An Overview for Its Mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef] [PubMed]
- Dame, Z.T.; Mahfuz, R.; Tofazzal, I. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green Chem. Lett. Rev. 2021, 14, 246–271. [Google Scholar] [CrossRef]
- Losick, R.; Youngman, P.; Piggot, P.J. Genetics of Endospore Formation in Bacillus-subtilis. Annu. Rev. Genet. 1986, 20, 625–669. [Google Scholar] [CrossRef] [PubMed]
- Errington, J. Regulation of Endospore Formation in Bacillus subtilis. Nat. Rev. Microbiol. 2003, 1, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hamdache, A.; Azarken, R.; Lamarti, A.; Aleu, J.; Collado, I.G. Comparative Genome Analysis of Bacillus spp. and Its Relationship with Bioactive Nonribosomal Peptide Production. Phytochem. Rev. 2013, 12, 685–716. [Google Scholar] [CrossRef]
- Magno-Pérez-Bryan, M.C.; Martínez-García, P.M.; Hierrezuelo, J.; Rodríguez-Palenzuela, P.; Arrebola, E.; Ramos, C.; de Vicente, A.; Pérez-García, A.; Romero, D. Comparative Genomics within the Bacillus Genus Reveal the Singularities of Two Robust Bacillus amyloliquefaciens Biocontrol Strains. Mol. Plant-Microbe Interact. 2015, 28, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, Z.; Li, Y.; Zhang, X.; Duan, Y.; Wang, Q. Biocontrol of Bacterial Fruit Blotch by Bacillus subtilis 9407 Via Surfactin-Mediated Antibacterial Activity and Colonization. Front. Microbiol. 2017, 8, 1973. [Google Scholar] [CrossRef]
- Gao, T.T.; Ding, M.Z.; Yang, C.H.; Fan, H.Y.; Chai, Y.R.; Li, Y. The Phosphotransferase System Gene Ptsh Plays an Important Role in Mnsod Production, Biofilm Formation, Swarming Motility, and Root Colonization in Bacillus Cereus 905. Res. Microbiol. 2019, 170, 86–96. [Google Scholar] [CrossRef]
- Li, Y.; Heloir, M.C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.W.; Wang, Q.; et al. Surfactin and Fengycin Contribute to the Protection of a Bacillus subtilis Strain against Grape Downy Mildew by Both Direct Effect and Defence Stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef]
- Peterson, S.B.; Savannah, K.B.; Joseph, D.M. The Central Role of Interbacterial Antagonism in Bacterial Life. Curr. Biol. 2020, 30, R1203–R1214. [Google Scholar] [CrossRef]
- Rendueles, O.; Ghigo, J.M. Multi-Species Biofilms: How to Avoid Unfriendly Neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Alexandra, K.; Romy, S.; Andreas, E.; Kathrin, S.; Isabelle, H.; Burkhard, M.; Björn, V.; Wolfgang, R.H.; Oleg, R.; et al. Comparative Analysis of the Complete Genome Sequence of the Plant Growth–Promoting Bacterium Bacillus amyloliquefaciens Fzb42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Koumoutsi, A.; Chen, X.H.; Anke, H.; Heiko, L.; Gabriele, H.; Peter, F.; Joachim, V.; Rainer, B. Structural and Functional Characterization of Gene Clusters Directing Nonribosomal Synthesis of Bioactive Cyclic Lipopeptides in Bacillus amyloliquefaciens Strain FZB 42. J. Bacteriol. 2004, 186, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The Iturin and Fengycin Families of Lipopeptides Are Key Factors in Antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant-Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, H.; Romero, D.; García-Gutiérrez, L.; Cazorla, F.M.; de Vicente, A.; Pérez-García, A. The Iturin-like Lipopeptides Are Essential Components in the Biological Control Arsenal of against Bacterial Diseases of Cucurbits. Mol. Plant-Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Shao, J.H.; Li, B.; Yan, X.; Shen, Q.R.; Zhang, R.F. Contribution of Bacillomycin D in Bacillus amyloliquefaciens SQR9 to Antifungal Activity and Biofilm Formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.P.; Zhou, H.F.; Zou, J.C.; Wang, X.Y.; Zhang, R.S.; Xiang, Y.P.; Chen, Z.Y. Bacillomycin L and Surfactin Contribute Synergistically to the Phenotypic Features of Bacillus subtilis 916 and the Biocontrol of Rice Sheath Blight Induced by Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2014, 99, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Younmi, L.; Wonsu, C.; Jungwook, P.; Hyeok-Tae, K.; Kotnala, B.; Jungyeon, K.; Yeo, J.Y.; Yongho, J. Characterization of Bacillus velezensis Ak-0 as a Biocontrol Agent against Apple Bitter Rot Caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef]
- Bordoh, P.K.; Asgar, A.; Matthew, D.; Yasmeen, S.; Gianfranco, R. A Review on the Management of Postharvest Anthracnose in Dragon Fruits Caused by Colletotrichum spp. Crop Prot. 2020, 130, 105067. [Google Scholar] [CrossRef]
- Yeimmy, P.R.; Chiara, R.; Carlos, D.G.; Clemencia, C. Green Management of Postharvest Anthracnose Caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar]
- Branda, S.S.; González-Pastor, J.E.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting Body Formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.Y.; Li, Y.; Fu, X.C.; Wang, Q. Screening and Characterization of Endophytic Bacillus for Biocontrol of Grapevine Downy Mildew. Crop Prot. 2017, 96, 173–179. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, Y.R.; Guo, J.H.; Richard, L. Evidence for Cyclic Di-Gmp-Mediated Signaling in Bacillus subtilis. J. Bacteriol. 2012, 194, 5080–5090. [Google Scholar] [CrossRef]
- Weng, J.; Wang, Y.; Li, J.; Shen, Q.R.; Zhang, R.F. Enhanced Root Colonization and Biocontrol Activity of Bacillus amyloliquefaciens SQR9 by Abrb Gene Disruption. Appl. Microbiol. Biotechnol. 2012, 97, 8823–8830. [Google Scholar] [CrossRef]
- Song, J.; Shang, L.; Wang, X.; Xing, Y.; Xu, W.; Zhang, Y.; Wang, T.; Li, H.; Zhang, J.; Ye, Z.; et al. MAPK11 Regulates Seed Germination and Aba Signaling in Tomato by Phosphorylating SNRKS. J. Exp. Bot. 2021, 72, 1677–1690. [Google Scholar] [CrossRef]
- Than, P.; Jeewon, P.; Hyde, R.K.; Pongsupasamit, D.S.; Mongkolporn, O.; Taylor, P.W.J. Characterization and Pathogenicity of Colletotrichum Species Associated with Anthracnose on Chilli (Capsicum spp.) in Thailand. Plant Pathol. 2008, 57, 562–572. [Google Scholar] [CrossRef]
- Schallmey, M.; Ajay, S.; Owen, P.W. Developments in the Use of Bacillus species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Chen, Y.S.; Shi, C.M.; Huang, Z.B.; Zhang, Y.; Li, S.K.; Li, Y.; Ye, J.; Yu, C.; Zhuo, L.; et al. SOAPnuke: A Mapreduce Acceleration-Supported Software for Integrated Quality Control and Preprocessing of High-Throughput Sequencing Data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed]
- Phillippy, A.M.; Ryan, R.W.; Louise, M.J.; Claire, L.G.; Kathryn, E.H. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar]
- Zeng, Q.C.; Xie, J.B.; Li, Y.; Chen, X.Y.; Gu, X.F.; Yang, P.L.; Hu, G.C.; Wang, Q. Organization, Evolution and Function of Fengycin Biosynthesis Gene Clusters in the Bacillus amyloliquefaciens Group. Phytopathol. Res. 2021, 3, 26. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. Mafft Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Ramon, R.M.; Frank, O.G.; Jörg, P. Jspeciesws: A Web Server for Prokaryotic Species Circumscription Based on Pairwise Genome Comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Rosalie, A.M.; Laurence, T.; Frédéric, L.; Venkatachalam, L.; Lucier, J.F.; Daniel, G.; Larissa, C.; Hera, V.; Harsh, P.B.; Pascale, B.B.; et al. Bacillus subtilis Early Colonization of Arabidopsis thaliana Roots Involves Multiple Chemotaxis Receptors. Mbio 2016, 7, 10–1128. [Google Scholar]
- Cairns, L.S.; Laura, H.; Nicola, R.S.W. Biofilm Formation by Bacillus subtilis: New Insights into Regulatory Strategies and Assembly Mechanisms. Mol. Microbiol. 2014, 93, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.S.; Daniel, L. Molecular Mechanisms Involved in Bacillus subtilis Biofilm Formation. Environ. Microbiol. 2014, 17, 555–565. [Google Scholar]
- Chen, Y.; Fang, Y.; Chai, Y.R.; Liu, H.X.; Roberto, K.; Richard, L.; Guo, J.H. Biocontrol of Tomato Wilt Disease by Bacillus subtilis Isolates from Natural Environments Depends on Conserved Genes Mediating Biofilm Formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Bae, K.S. Phylogenetic Analysis of Bacillus subtilis and Related Taxa Based on Partial Gyra Gene Sequences Gene Sequences. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2000, 78, 123–127. [Google Scholar] [CrossRef]
- Zhao, H.B.; Shao, D.Y.; Jiang, C.M.; Shi, J.L.; Li, Q.; Huang, Q.S.; Muhammad, S.R.R.; Yang, H.; Jin, M.L. Biological Activity of Lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960. [Google Scholar] [CrossRef]
- Tanaka, K.; Yusuke, A.; Atsushi, I.; Hiromitsu, N. Synergistic Effects of [Ile7]Surfactin Homologues with Bacillomycin D in Suppression of Gray Mold Disease by Bacillus amyloliquefaciens Biocontrol Strain Sd-32. J. Agric. Food Chem. 2015, 63, 5344–5353. [Google Scholar] [CrossRef]
- Glaeser, S.P.; Peter, K. Multilocus Sequence Analysis (Mlsa) in Prokaryotic Taxonomy. Syst. Appl. Microbiol. 2015, 38, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.A.; Kim, S.J.; Kwon, S.W.; Alejandro, P.R. Bacillus velezensis Is Not a Later Heterotypic Synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. Plantarum and ‘Bacillus oryzicola’ Are Later Heterotypic Synonyms of Bacillus Velezensis Based on Phylogenomics. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar] [PubMed]
- Yu, Y.Y.; Jiang, C.H.; Wang, C.; Chen, L.J.; Li, H.Y.; Xu, Q.; Guo, J.H. An Improved Strategy for Stable Biocontrol Agents Selecting to Control Rice Sheath Blight Caused by Rhizoctonia solani. Microbiol. Res. 2017, 203, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Zhang, Y.Y.; Wang, T.; Huang, T.X.; Tang, S.Y.; Jin, Y.; Mi, D.D.; Zheng, Y.; Niu, D.D.; Guo, J.H.; et al. Kurstakin Triggers Multicellular Behaviors in AR156 and Enhances Disease Control Efficacy against Rice Sheath Blight. Plant Dis. 2023, 107, 1463–1470. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.J.; You, M.P.; Martin, J.B.; Chen, Y.L. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M.; Tarique, H.A. Factors Affecting Success of Biological Agents Used in Controlling the Plant-Parasitic Nematodes. Egypt. J. Biol. Pest Control 2020, 30, 17. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Paul, D.V.P.; Kromann, S.D.; Barbara, D.P. Biological Control Agents: From Field to Market, Problems, and Challenges. Trends Biotechnol. 2014, 32, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Philippe, J. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Niharika, R.; Rajni, S. Microbial Lipopeptides: Properties, Mechanics and Engineering for Novel Lipopeptides. Microbiol. Res. 2023, 271, 127363. [Google Scholar] [CrossRef]
- Fazle, R.M.; Baek, K.H. Antimicrobial Activities of Lipopeptides and Polyketides of Bacillus velezensis for Agricultural Applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Jenny, U.; Rita, G.; Sylvia, A.; Sabrina, P.; Kristin, D.; Philippe, S.K.; Rainer, B.; Anton, H. Cyclic Lipopeptides of Bacillus amyloliquefaciens subsp. Plantarum Colonizing the Lettuce Rhizosphere Enhance Plant Defense Responses toward the Bottom Rot Pathogen Rhizoctonia solani. Mol. Plant-Microbe Interact. 2015, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]



| Attribute | B. velezensis N23 |
|---|---|
| Genome size (bp) | 4,014,251 |
| G+C (%) | 46.5 |
| Protein-coding genes | 3883 |
| rRNA | 27 |
| tRNA | 86 |
| Protein-coding total length (bp) | 3,551,679 |
| Average length of protein-coding genes (bp) | 915 |
| DSM 7 | LL3 | TA208 | CAU B946 | N23 | NAU-B3 | SQR9 | FZB42 | CC09 | GUAL210 | SCSIO 05746 | YB-1631 | B28 | GQJK17 | BA59 | UCMB5075 | PS17 | BSD-2 | BAB-1 | NCIB 3610 | 168 | MG-1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSM 7 | 100 | |||||||||||||||||||||
| LL3 | 99.47 | 100 | ||||||||||||||||||||
| TA208 | 99.28 | 99.5 | 100 | |||||||||||||||||||
| CAU B946 | 93.39 | 93.26 | 93.27 | 100 | ||||||||||||||||||
| N23 | 93.4 | 93.27 | 93.27 | 100 | 100 | |||||||||||||||||
| NAU-B3 | 93.4 | 93.28 | 93.31 | 97.52 | 97.52 | 100 | ||||||||||||||||
| SQR9 | 93.09 | 92.99 | 93.02 | 97.18 | 97.18 | 97.82 | 100 | |||||||||||||||
| FZB42 | 93.37 | 93.3 | 93.3 | 97.58 | 97.58 | 98.26 | 98.39 | 100 | ||||||||||||||
| CC09 | 93.22 | 93.13 | 93.15 | 97.34 | 97.34 | 97.81 | 97.77 | 98.37 | 100 | |||||||||||||
| GUAL210 | 93.34 | 93.27 | 93.3 | 97.6 | 97.6 | 98.14 | 98.08 | 98.42 | 99.07 | 100 | ||||||||||||
| SCSIO 05746 | 92.99 | 93.01 | 92.85 | 94.1 | 94.1 | 94.1 | 93.9 | 94.15 | 93.69 | 93.96 | 100 | |||||||||||
| YB-1631 | 93.29 | 93.28 | 93.06 | 93.95 | 93.95 | 93.91 | 93.76 | 93.91 | 93.99 | 93.83 | 97.52 | 100 | ||||||||||
| B28 | 93.55 | 93.54 | 93.49 | 93.91 | 93.92 | 93.88 | 93.78 | 93.83 | 93.83 | 93.89 | 97.39 | 98 | 100 | |||||||||
| GQJK17 | 76.63 | 76.63 | 76.67 | 76.62 | 76.61 | 76.73 | 76.67 | 76.68 | 76.64 | 76.65 | 76.66 | 76.67 | 76.89 | 100 | ||||||||
| BA59 | 76.91 | 76.92 | 76.93 | 76.93 | 76.93 | 77 | 77.1 | 76.98 | 77.1 | 76.94 | 76.87 | 76.98 | 76.89 | 98.63 | 100 | |||||||
| UCMB5075 | 76.67 | 76.68 | 76.7 | 76.76 | 76.76 | 76.76 | 76.82 | 76.78 | 76.78 | 76.74 | 76.7 | 76.83 | 76.75 | 80.37 | 80.27 | 100 | ||||||
| PS17 | 76.12 | 76.18 | 76.18 | 76.13 | 76.13 | 76.3 | 76.74 | 76.23 | 76.29 | 76.24 | 76.21 | 76.21 | 76.23 | 79.81 | 79.81 | 95.39 | 100 | |||||
| BSD-2 | 76.33 | 76.36 | 76.38 | 76.42 | 76.42 | 76.46 | 76.47 | 76.45 | 76.52 | 76.46 | 76.49 | 76.51 | 76.58 | 79.51 | 79.42 | 87.04 | 87.06 | 100 | ||||
| BAB-1 | 76.35 | 76.38 | 76.41 | 76.43 | 76.43 | 76.45 | 76.51 | 76.49 | 76.56 | 76.48 | 76.53 | 76.54 | 76.54 | 79.47 | 79.39 | 87 | 87.08 | 99.97 | 100 | |||
| NCIB 3610 | 76.32 | 76.41 | 76.34 | 76.39 | 76.38 | 76.5 | 76.49 | 76.47 | 76.5 | 76.38 | 76.41 | 76.46 | 76.48 | 79.43 | 79.36 | 86.84 | 87 | 97.92 | 97.9 | 100 | ||
| 168 | 76.28 | 76.39 | 76.34 | 76.34 | 76.33 | 76.48 | 76.49 | 76.49 | 76.5 | 76.38 | 76.39 | 76.43 | 76.44 | 79.41 | 79.35 | 86.84 | 86.97 | 97.93 | 97.91 | 100 | 100 | |
| MG-1 | 76.11 | 76.26 | 76.17 | 76.21 | 76.21 | 76.28 | 76.35 | 76.28 | 76.28 | 76.22 | 76.31 | 76.32 | 76.38 | 79.32 | 79.26 | 86.66 | 86.82 | 97.78 | 97.76 | 98.33 | 98.33 | 100 |
| Lipopeptide | Chromosomal Localization (from–to) | Similarity in Nucleotide Sequence (%) | Similarity in Amino Acid Sequence (%) |
|---|---|---|---|
| surfactin | 323,282 nt–349,440 nt | 97.7 | 98.0 |
| fengycin | 1,974,324 nt–2,011,993 nt | 96.6 | 97.0 |
| iturin | 1,914,164 nt–1,951,409 nt | 97.1 | 88.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Zeng, Q.; Jiang, W.; Wang, L.; Zhang, J.; Wang, Z.; Wang, Q.; Li, Y. Genome Sequencing and Characterization of Bacillus velezensis N23 as Biocontrol Agent against Plant Pathogens. Microorganisms 2024, 12, 294. https://doi.org/10.3390/microorganisms12020294
Yang P, Zeng Q, Jiang W, Wang L, Zhang J, Wang Z, Wang Q, Li Y. Genome Sequencing and Characterization of Bacillus velezensis N23 as Biocontrol Agent against Plant Pathogens. Microorganisms. 2024; 12(2):294. https://doi.org/10.3390/microorganisms12020294
Chicago/Turabian StyleYang, Panlei, Qingchao Zeng, Wenxiao Jiang, Luotao Wang, Jie Zhang, Zhenshuo Wang, Qi Wang, and Yan Li. 2024. "Genome Sequencing and Characterization of Bacillus velezensis N23 as Biocontrol Agent against Plant Pathogens" Microorganisms 12, no. 2: 294. https://doi.org/10.3390/microorganisms12020294






