Application of Acetate as a Substrate for the Production of Value-Added Chemicals in Escherichia coli
Abstract
1. Introduction
2. Metabolism of Acetate in E. coli
3. Acetate Transport in E. coli
4. Acetate Tolerance of E. coli
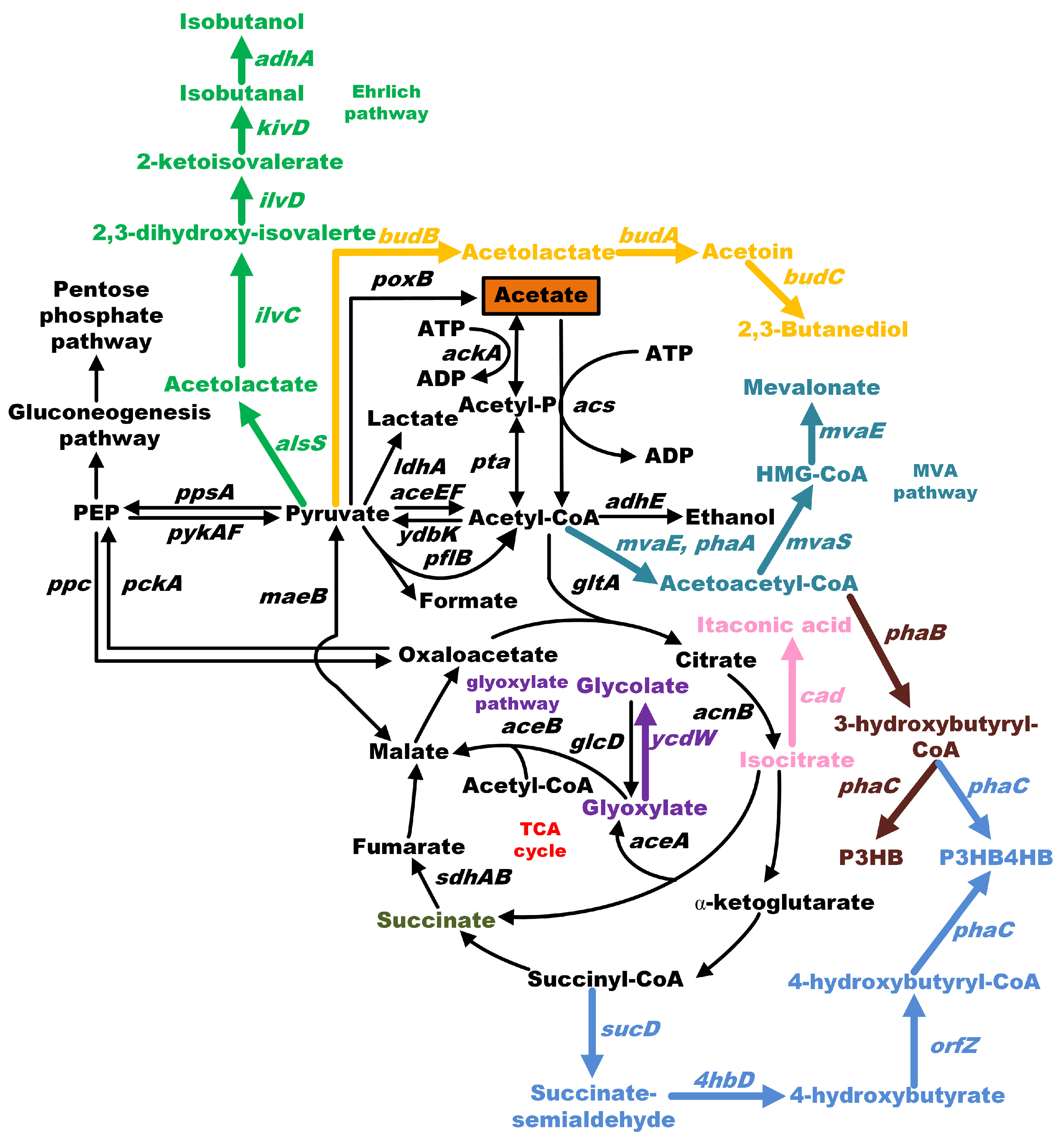
5. Using Acetate as a Substrate for the Production of Value-Added Chemicals in E. coli
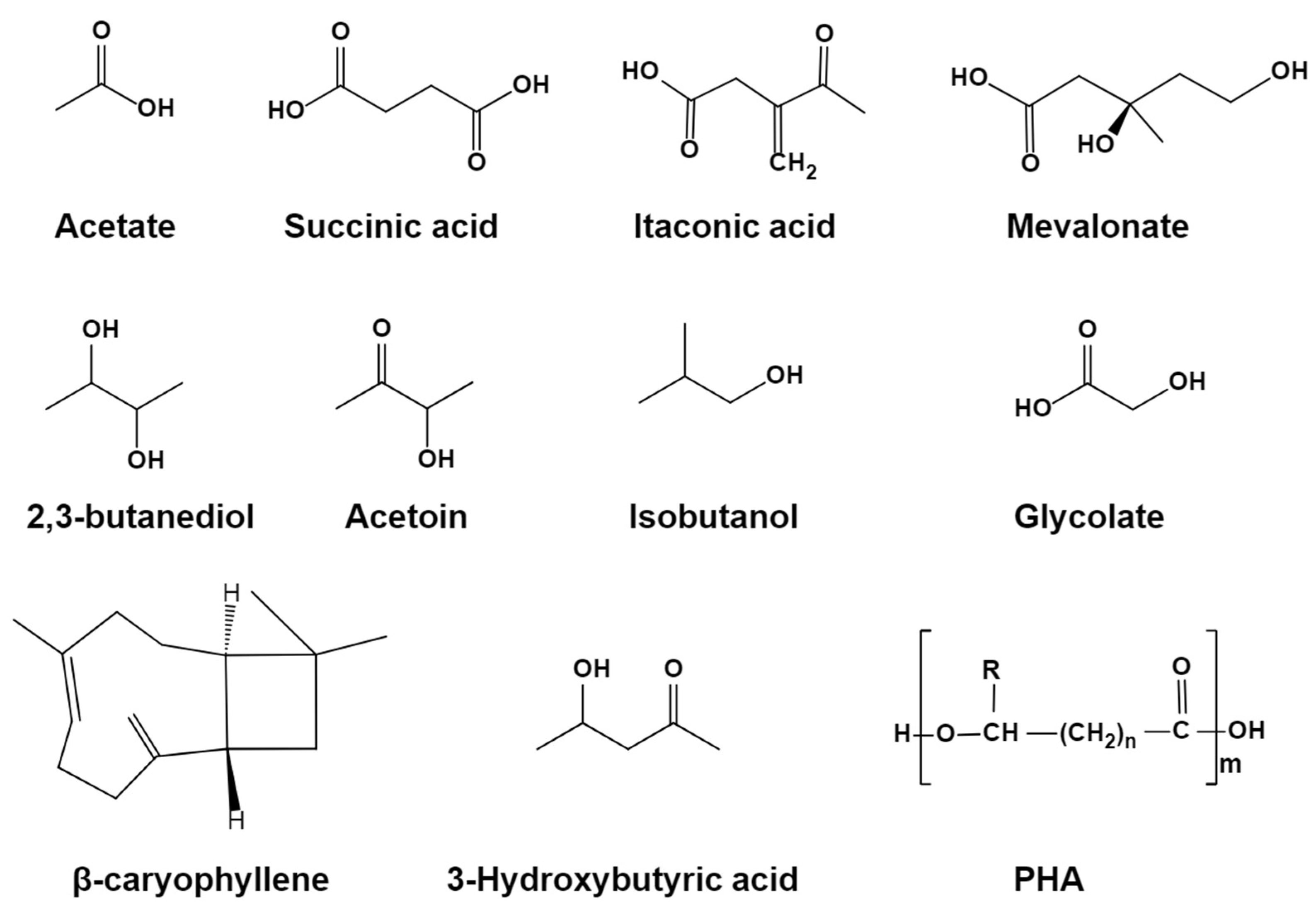
5.1. Succinic Acid
5.2. Itaconic Acid
5.3. Mevalonate (MA)
5.4. Isobutanol
5.5. 2,3-Butanediol and Acetoin
5.6. Glycolate
5.7. β-Caryophyllene
5.8. 3-Hydroxybutyric Acid and Polyhydroxyalkanoates
5.9. Sweet Protein
| Strain | Relevant Characteristics | Products | Titer | Acetate Conversion Rate | Fermentation Conditions | References |
|---|---|---|---|---|---|---|
| MG03(pTrc99a-gltA) | MG1655 (ΔsdhABΔiclRΔmaeBΔpoxB) with overexpressed gltA | succinate | 16.45 mM | 0.46 mol/mol | Batch fermentation in 25 mL flask at 37 °C and 220 rpm, SMAC medium | [35] |
| HB03(pTrc99a-gltA, pBAD33-Trc-fdh) | MG1655(ΔsdhABΔiclRΔmaeBΔpckAPtrc-m2-ackA-pta) with overexpressed fdh from Candida boidinii | succinate | 30.9 mM | 0.50 mol/mol | Batch fermentation in 250 mL flask containing 50 mL of NH4Cl-free M9 medium at 37 °C and 220 rpm | [36] |
| WCY-7 | MG1655 (ΔiclRΔsdhABΔmaeB) with overexpressed acs, gltA and acnB | succinate | 11.23 mM | 0.22 mol/mol | Batch fermentation in 300 mL flask containing SMAC medium at 37 °C and 250 rpm | [21] |
| WCIAG4 | Acetate-tolerant E. coli W with deletion of iclR and overexpression of cad, acs, gltA, and aceA | itaconic acid | 3.57 g/L | 0.161 g/g | Fed-batch in 5 L bioreactor containing 1.5 L of modified minimal acetate medium at 30 °C and 500 rpm | [38] |
| XU143 | BL21(DE3) with overexpressed acs and Enterococcus faecalis-derived mvaE and mvaS | mevalonate | 1.06 g/L | 0.30 g/g | Fed-batch in 5 L bioreactor containing 2 L of minimal medium | [44] |
| HM501::MAP | MG1655(DE3) (ΔfrdAΔptaΔldhAΔadhE) with overexpressed alsS, kivD, ilvC, ilvD, yqhD, acs, pckA, and maeB | isobutanol | 0.125 g/L | 0.042 g/g | Batch fermentation in 250 mL screw-cap flask containing 30 mL of M9 minimal medium at 30 °C and 200 rpm | [46] |
| WY002 | BW25113 (ΔpflBΔpoxBΔadhEΔldhA) with overexpressed adhA, kivD, alsS, pckA, maeB, alsS, ilvC, ilvD, acs, pntA, and yfjB | isobutanol | 0.157 g/L | 0.052 g/g | Batch fermentation in 300 mL flask containing 50 mL of medium at 30 °C and 200 rpm | [47] |
| WΔ4 | E. coli W (ΔldhAΔadhEΔpta ΔfrdA) with overexpressed budA, budB, and budC from Enterobacter cloacae subsp. dissolvens | 2,3-butanediol and acetoin | 1.16 g/L | 0.09 g/g | Pulsed fed-batch fermentation in a bioreactor with a working volume of 200 mL at 30 °C and 800 rpm | [50] |
| K12 ΔA4 (pGAx4/pEn-gltA-pta-ackA) | MG1655(DE3) (ΔendA ΔrecAΔxylBΔgclΔaceBΔglcBΔglcD) with overexpressed ycd, aceA, aceK, gltA, pta, and ackA | glycolate | 2.75 g/L | 0.58 g/g | Batch fermentation in 500 mL shake flasks containing 50 mL minimal medium at 37 °C and 200 rpm | [53] |
| YJM67 | BL21(DE3) with overexpressed QHS1 from Artemisia annua, mvaE and mvaS from Enterococcus faecalis, GPPS2 from Abies grandis, ACSAP from Acetobacter pasteurianus, nphT7 from Streptomyces sp. strain CL190, and ERG12, ERG8, ERG19, and IDI1 from Saccharomyces cerevisiae | β-caryophyllene | 1.05 g/L | 0.021 g/g | Fed-batch in 5 L fermenter containing 2 L of M9 minimal medium at 30 °C | [55] |
| FP06 | BW25113 with overexpressed phaA and phaB from R. eutropha, and pct4543 from C. beijerinckii 8052 | (R)-3-hydroxybutyric acid | 0.86 g/L | 0.27 g/g | Batch fermentation in 500 mL shake flasks containing 50 mL minimal medium at 37 °C and 200 rpm | [57] |
| JM109 (pBHR68 + pMCS-acs) | JM109 containing phaCAB and acs | poly-3-hydroxybutyrate | 1.27 g/L | 0.254 g/g | Batch fermentation in 250 mL shake flasks containing 50 mL SMAC media at 37 °C and 220 rpm | [58] |
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wendisch, V.F.; Brito, L.F.; Gil Lopez, M.; Hennig, G.; Pfeifenschneider, J.; Sgobba, E.; Veldmann, K.H. The flexible feedstock concept in Industrial Biotechnology: Metabolic engineering of Escherichia coli, Corynebacterium glutamicum, Pseudomonas, Bacillus and yeast strains for access to alternative carbon sources. J. Biotechnol. 2016, 234, 139–157. [Google Scholar] [CrossRef]
- Kim, Y.; Lama, S.; Agrawal, D.; Kumar, V.; Park, S.H. Acetate as a potential feedstock for the production of value-added chemicals: Metabolism and applications. Biotechnol. Adv. 2021, 49, 107736. [Google Scholar] [CrossRef] [PubMed]
- Mutyala, S.; Kim, J.R. Recent advances and challenges in the bioconversion of acetate to value-added chemicals. Bioresour. Technol. 2022, 364, 128064. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.; Moss, K.; Henkel, M.; Hausmann, R. Biotechnological perspectives of pyrolysis oil for a bio-based economy. Trends Biotechnol. 2017, 35, 925–993. [Google Scholar] [CrossRef]
- Mills, T.Y.; Sandoval, N.R.; Gill, R.T. Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol. Biofuels 2009, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic digestion for bioenergy production: Global status, environmental and techno-economic implications, and government policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef]
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food waste utilization for reducing carbon footprints towards sustainable and cleaner environment: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef]
- Straub, M.; Demler, M.; Weuster-Botz, D.; Durre, P. Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii. J. Biotechnol. 2014, 178, 67–72. [Google Scholar] [CrossRef]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef]
- Effendi, S.S.W.; Ng, I.S. Challenges and opportunities for engineered Escherichia coli as a pivotal chassis toward versatile tyrosine-derived chemicals production. Biotechnol. Adv. 2023, 69, 108270. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Castano-Cerezo, S.; Canovas, M. Acetate metabolism regulation in Escherichia coli: Carbon overflow, pathogenicity, and beyond. Appl. Microbiol. Biotechnol. 2016, 100, 8985–9001. [Google Scholar] [CrossRef]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; de Jong, H. Acetate metabolism and the inhibition of bacterial growth by acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef]
- Kiefer, D.; Merkel, M.; Lilge, L.; Henkel, M.; Hausmann, R. From acetate to bio-based products: Underexploited potential for industrial biotechnology. Trends Biotechnol. 2021, 39, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Schutze, A.; Benndorf, D.; Puttker, S.; Kohrs, F.; Bettenbrock, K. The impact of ackA, pta, and ackA-pta mutations on growth, gene expression and protein acetylation in Escherichia coli K-12. Front. Microbiol. 2020, 11, 233. [Google Scholar] [CrossRef]
- Nie, M.; Wang, J.; Zhang, K. A novel strategy for L-arginine production in engineered Escherichia coli. Microb. Cell Fact. 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Ma, Q.; Zhao, S.; Li, Q.; Gao, J. Alanine dehydrogenases from four different microorganisms: Characterization and their application in L-alanine production. Biotechnol. Biofuels Bioprod. 2023, 16, 12. [Google Scholar] [CrossRef]
- Wu, X.; Eiteman, M.A. Synthesis of citramalic acid from glycerol by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1483–1490. [Google Scholar] [CrossRef]
- Dolan, S.K.; Welch, M. The Glyoxylate Shunt, 60 Years On. Annu. Rev. Microbiol. 2018, 72, 309–330. [Google Scholar] [CrossRef]
- Ding, Z.; Fang, Y.; Zhu, L.; Wang, J.; Wang, X. Deletion of arcA, iclR, and tdcC in Escherichia coli to improve l-threonine production. Biotechnol. Appl. Biochem. 2019, 66, 794–807. [Google Scholar] [CrossRef]
- Niu, H.; Li, R.; Wu, J.; Cai, Z.; Yang, D.; Gu, P.; Li, Q. Production of succinate by recombinant Escherichia coli using acetate as the sole carbon source. 3 Biotech 2018, 8, 421. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 354–411. [Google Scholar] [CrossRef]
- Gimenez, R.; Nunez, M.F.; Badia, J.; Aguilar, J.; Baldoma, L. The gene yjcG, cotranscribed with the gene acs, encodes an acetate permease in Escherichia coli. J. Bacteriol. 2003, 185, 6448–6455. [Google Scholar] [CrossRef]
- Sa-Pessoa, J.; Paiva, S.; Ribas, D.; Silva, I.J.; Viegas, S.C.; Arraiano, C.M.; Casal, M. SATP (YaaH), a succinate-acetate transporter protein in Escherichia coli. Biochem. J. 2013, 454, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.M.; Takahashi, D.F.; Carvalhal, M.L.; Alterthum, F. Effects of acetate on the growth and fermentation performance of Escherichia coli KO11. Appl. Biochem. Biotechnol. 1999, 81, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sandoval, M.T.; Huerta-Beristain, G.; Trujillo-Martinez, B.; Bustos, P.; Gonzalez, V.; Bolivar, F.; Gosset, G.; Martinez, A. Laboratory metabolic evolution improves acetate tolerance and growth on acetate of ethanologenic Escherichia coli under non-aerated conditions in glucose-mineral medium. Appl. Microbiol. Biotechnol. 2012, 96, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Presser, K.A.; Ratkowsky, D.A.; Ross, T. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl. Environ. Microbiol. 1997, 63, 2355–2360. [Google Scholar] [CrossRef]
- Kim, M.; Im, E.; Jin Ahn, Y. Enhanced acetate tolerance and recombinant protein accumulation in Escherichia coli by transgenic expression of a heat shock protein from carrot (Daucus carota L.). Iran. J. Biotechnol. 2022, 20, e3177. [Google Scholar]
- Chong, H.; Yeow, J.; Wang, I.; Song, H.; Jiang, R. Improving acetate tolerance of Escherichia coli by rewiring its global regulator cAMP receptor protein (CRP). PLoS ONE 2013, 8, e7742. [Google Scholar] [CrossRef]
- Arnold, C.N.; McElhanon, J.; Lee, A.; Leonhart, R.; Siegele, D.A. Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J. Bacteriol. 2001, 183, 2178–2218. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, N.R.; Mills, T.Y.; Zhang, M.; Gill, R.T. Elucidating acetate tolerance in E. coli using a genome-wide approach. Metab. Eng. 2011, 13, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Jang, Y.S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Thakker, C.; Martinez, I.; San, K.Y.; Bennett, G.N. Succinate production in Escherichia coli. Biotechnol. J. 2012, 7, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, W.; Wang, Z.; Zhang, B.; Chen, K.; Ouyang, P. Enhanced succinic acid production from corncob hydrolysate by microbial electrolysis cells. Bioresour. Technol. 2016, 202, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, B.; Wu, H.; Li, Z.; Ye, Q.; Zhang, Y.P. Production of succinate from acetate by metabolically engineered Escherichia coli. ACS Synth. Biol. 2016, 5, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, H.; Fang, G.; Zhang, X.; Wu, H.; Li, Z.; Ye, Q. Central pathway engineering for enhanced succinate biosynthesis from acetate in Escherichia coli. Biotechnol. Bioeng. 2018, 115, 943–995. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, X.; Xie, H.; Wang, F.; Ren, Y.; Wei, D. Enhanced itaconic acid production by self-assembly of two biosynthetic enzymes in Escherichia coli. Biotechnol. Bioeng. 2017, 114, 457–462. [Google Scholar] [CrossRef]
- Noh, M.H.; Lim, H.G.; Woo, S.H.; Song, J.; Jung, G.Y. Production of itaconic acid from acetate by engineering acid-tolerant Escherichia coli W. Biotechnol. Bioeng. 2018, 115, 729–738. [Google Scholar] [CrossRef]
- Zhang, X.K.; Wang, D.N.; Chen, J.; Liu, Z.J.; Wei, L.J.; Hua, Q. Metabolic engineering of beta-carotene biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef]
- Hussain, M.H.; Hong, Q.; Zaman, W.Q.; Mohsin, A.; Wei, Y.; Zhang, N.; Fang, H.; Wang, Z.; Hang, H.; Zhuang, Y.; et al. Rationally optimized generation of integrated Escherichia coli with stable and high yield lycopene biosynthesis from heterologous mevalonate (MVA) and lycopene expression pathways. Synth. Syst. Biotechnol. 2021, 6, 85–94. [Google Scholar] [CrossRef]
- Marsafari, M.; Xu, P. Debottlenecking mevalonate pathway for antimalarial drug precursor amorphadiene biosynthesis in Yarrowia lipolytica. Metab. Eng. Commun. 2020, 10, e00121. [Google Scholar] [CrossRef]
- Satowa, D.; Fujiwara, R.; Uchio, S.; Nakano, M.; Otomo, C.; Hirata, Y.; Matsumoto, T.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of E. coli for improving mevalonate production to promote NADPH regeneration and enhance acetyl-CoA supply. Biotechnol. Bioeng. 2020, 117, 2153–2164. [Google Scholar] [CrossRef]
- Liu, C.L.; Dong, H.G.; Xue, K.; Sun, L.; Yang, Y.; Liu, X.; Li, Y.; Bai, Z.; Tan, T.W. Metabolic engineering mevalonate pathway mediated by RNA scaffolds for mevalonate and isoprene production in Escherichia coli. ACS Synth. Biol. 2022, 11, 3305–3317. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, M.; Zhao, Q.; Xian, M.; Liu, H. Microbial production of mevalonate by recombinant Escherichia coli using acetic acid as a carbon source. Bioengineered 2018, 9, 116–123. [Google Scholar] [CrossRef]
- Deb, S.S.; Reshamwala, S.M.S.; Lali, A.M. Activation of alternative metabolic pathways diverts carbon flux away from isobutanol formation in an engineered Escherichia coli strain. Biotechnol. Lett. 2019, 41, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Seo, H.M.; Jeon, J.M.; Moon, Y.M.; Hong, J.W.; Hong, Y.G.; Bhatia, S.K.; Ahn, J.; Lee, H.; Kim, W.; et al. Enhanced isobutanol production from acetate by combinatorial overexpression of acetyl-CoA synthetase and anaplerotic enzymes in engineered Escherichia coli. Biotechnol. Bioeng. 2018, 115, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Zhao, S.; Niu, H.; Li, C.; Jiang, S.; Zhou, H.; Li, Q. Synthesis of isobutanol using acetate as sole carbon source in Escherichia coli. Microb. Cell Fact. 2023, 22, 196. [Google Scholar] [CrossRef]
- Mitsui, R.; Yamada, R.; Matsumoto, T.; Ogino, H. Bioengineering for the industrial production of 2,3-butanediol by the yeast, Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2022, 38, 3. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- Novak, K.; Kutscha, R.; Pflugl, S. Microbial upgrading of acetate into 2,3-butanediol and acetoin by E. coli W. Biotechnol. Biofuels 2020, 13, 177. [Google Scholar] [CrossRef]
- Kataoka, M.; Sasaki, M.; Hidalgo, A.R.; Nakano, M.; Shimizu, S. Glycolic acid production using ethylene glycol-oxidizing microorganisms. Biosci. Biotechnol. Biochem. 2001, 65, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Cabulong, R.B.; Lee, W.K.; Banares, A.B.; Ramos, K.R.M.; Nisola, G.M.; Valdehuesa, K.N.G.; Chung, W.J. Engineering Escherichia coli for glycolic acid production from D-xylose through the Dahms pathway and glyoxylate bypass. Appl. Microbiol. Biotechnol. 2018, 102, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Liu, C.X.; Yuan, Q.P.; Li, Z.J. Microbial production of glycolate from acetate by metabolically engineered Escherichia coli. J. Biotechnol. 2019, 291, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Meylemans, H.A.; Gough, R.V.; Quintana, R.L.; Garrison, M.D.; Bruno, T.J. High-density biosynthetic fuels: The intersection of heterogeneous catalysis and metabolic engineering. Phys. Chem. Chem. Phys. 2014, 16, 9448–9457. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Q. Engineering Escherichia coli to convert acetic acid to beta-caryophyllene. Microb. Cell Fact. 2016, 15, 74. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, H.J.; Hwang, J.H.; Kim, H.J.; Shin, N.; Lee, S.H.; Seo, S.O.; Bhatia, S.K.; Yang, Y.H. Validating a xylose regulator to increase polyhydroxybutyrate production for utilizing mixed sugars from lignocellulosic biomass using Escherichia coli. J. Microbiol. Biotechnol. 2023, 34, 1–10. [Google Scholar] [CrossRef]
- Fei, P.; Luo, Y.; Lai, N.; Wu, H. Biosynthesis of (R)-3-hydroxybutyric acid from syngas-derived acetate in engineered Escherichia coli. Bioresour. Technol. 2021, 336, 125323. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhang, Z.Z.; Tan, T.W.; Li, Z.J. Metabolic engineering of Escherichia coli for the synthesis of polyhydroxyalkanoates using acetate as a main carbon source. Microb. Cell Fact. 2018, 17, 10. [Google Scholar] [CrossRef]
- Leone, S.; Sannino, F.; Tutino, M.L.; Parrilli, E.; Picone, D. Acetate: Friend or foe? Efficient production of a sweet protein in Escherichia coli BL21 using acetate as a carbon source. Microb. Cell Fact. 2015, 14, 106. [Google Scholar] [CrossRef]
- Sen, A.; Bakshi, B.R. Techno-economic and life cycle analysis of circular phosphorus systems in agriculture. Sci. Total Environ. 2023, 872, 162016. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.; Han, J. Techno-economic analysis and life cycle assessment of poly (Butylene succinate) production using food waste. Waste Manag. 2023, 156, 168–176. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, P.; Li, F.; Huang, Z.; Gao, J. Application of Acetate as a Substrate for the Production of Value-Added Chemicals in Escherichia coli. Microorganisms 2024, 12, 309. https://doi.org/10.3390/microorganisms12020309
Gu P, Li F, Huang Z, Gao J. Application of Acetate as a Substrate for the Production of Value-Added Chemicals in Escherichia coli. Microorganisms. 2024; 12(2):309. https://doi.org/10.3390/microorganisms12020309
Chicago/Turabian StyleGu, Pengfei, Fangfang Li, Zhaosong Huang, and Juan Gao. 2024. "Application of Acetate as a Substrate for the Production of Value-Added Chemicals in Escherichia coli" Microorganisms 12, no. 2: 309. https://doi.org/10.3390/microorganisms12020309
APA StyleGu, P., Li, F., Huang, Z., & Gao, J. (2024). Application of Acetate as a Substrate for the Production of Value-Added Chemicals in Escherichia coli. Microorganisms, 12(2), 309. https://doi.org/10.3390/microorganisms12020309






