A New Isolated Fungus and Its Pathogenicity for Apis mellifera Brood in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Morphological Characterization, and Culture Conditions of R. oryzae
2.2. DNA Extraction and PCR Amplification
2.3. Phylogenetic Analysis
2.4. Bioassay of Pathogenicity
2.5. Statistical Analysis
3. Results
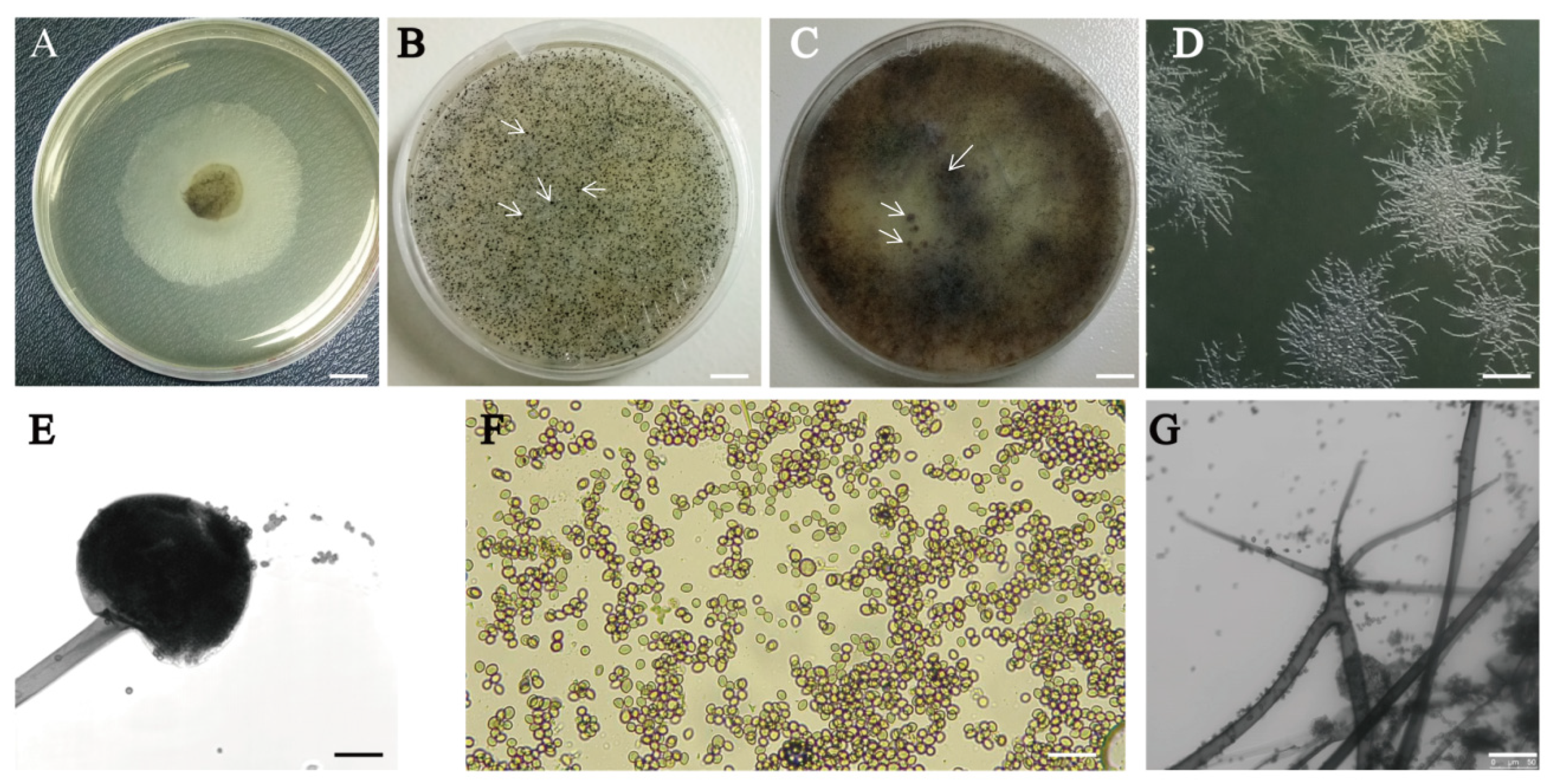
3.1. Isolation and Morphological Characterization of the New Fungal Isolate
3.2. Internal Transcribed Spacer (ITS) Sequence Analysis
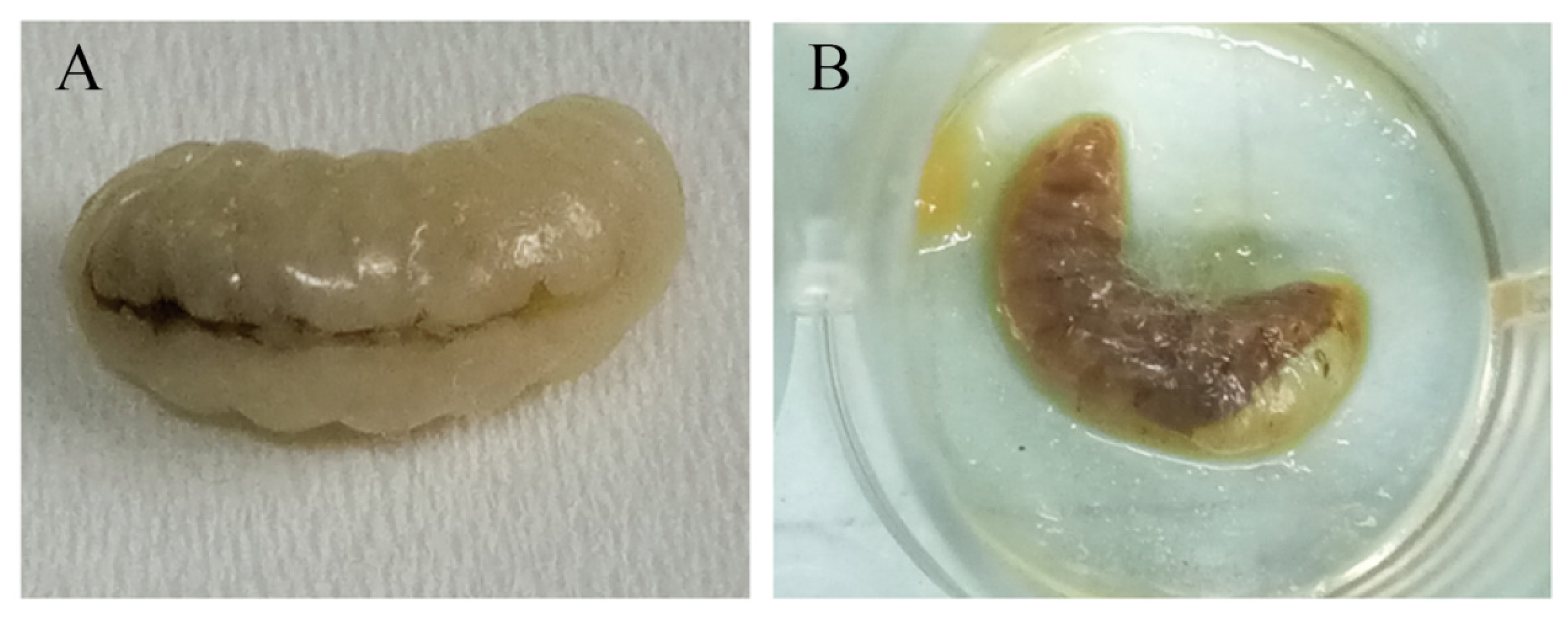
3.3. Pathogenicity Evaluation
4. Discussion
4.1. R. oryzae, a New Isolated Fungus That Infects Honeybee Larvae in Artificial Culture
4.2. The Virulence of R. oryzae for Honeybee Larvae Is Time- and Dosage-Dependent
4.3. The Potential Influence of R. oryzae on Honeybee Colonies Requires Further Investigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, A.; Brown, L.; Davies, G.; Whyatt, J.D.; Potts, S.G. Honeybee pollination benefits could inform solar park business cases, planning decisions and environmental sustainability targets. Biol. Conserv. 2021, 263, 109332. [Google Scholar] [CrossRef]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.; Brown, M.; Wilkins, S.; Hart, A.; Smith, G.C. Evaluating european food safety authority protection goals for honeybees (Apis mellifera): What do they mean for pollination? Integr. Environ. Assess. Manag. 2018, 14, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Stabentheiner, A.; Kovac, H. Honeybee economics: Optimisation of foraging in a variable world. Sci. Rep. 2016, 6, 28339. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, K.; Dziechciarz, P.; Trytek, M.; Borsuk, G. A scientific note on the strategy of wax collection as rare behavior of Apis mellifera. Apidologie 2022, 53, 40. [Google Scholar] [CrossRef]
- Bobis, O. Plants: Sources of diversity in propolis properties. Plants 2022, 11, 2298. [Google Scholar] [CrossRef] [PubMed]
- Mair, B.; Wolf, M. Studies on the botanical origin and the residues of pesticides in corbicular pollen loads and bee bread of bee colonies in the proximity of apple orchards in South Tyrol. J. Kult. 2023, 75, 225–234. [Google Scholar] [CrossRef]
- Parish, J.B.; Scott, E.S.; Hogendoorn, K. Collection of conidia of by honey bee workers. Australas. Plant Pathol. 2020, 49, 245–247. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.S. Bees brought to their knees: Microbes affecting honey bee health. Trends Microbiol. 2011, 19, 614–620. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, W.J.; Zhang, Y.H.; Luo, J.; Hou, J.A.; Chen, L.C.; Yi, G.Q.; Li, H.H.; Huang, M.F.; Dong, L.X.; et al. S-dinotefuran affects the social behavior of honeybees (Apis mellifera) and increases their risk in the colony. Pestic. Biochem. Physiol. 2023, 196, 105594. [Google Scholar] [CrossRef]
- Laomettachit, T.; Liangruksa, M.; Termsaithong, T.; Tangthanawatsakul, A.; Duangphakdee, O. A model of infection in honeybee colonies with social immunity. PLoS ONE 2021, 16, e0247294. [Google Scholar] [CrossRef] [PubMed]
- Santorelli, L.A.; Wilkinson, T.; Abdulmalik, R.; Rai, Y.; Creevey, C.J.; Huws, S.; Gutierrez-Merino, J. Beehives possess their own distinct microbiomes. Environ. Microbiome 2023, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Sinkeviciene, J.; Taraseviciene, Ä.; Tamutis, V. Fungi and mycotoxins in bee pollen collected in Lithuania. Appl. Sci. 2023, 13, 1571. [Google Scholar] [CrossRef]
- Cui, P.; Kong, K.; Yao, Y.; Huang, Z.D.; Shi, S.P.; Liu, P.; Huang, Y.C.; Abbas, N.; Yu, L.S.; Zhang, Y.L. Community composition, bacterial symbionts, antibacterial and antioxidant activities of honeybee-associated fungi. BMC Microbiol. 2022, 22, 168. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, L.; Luo, J.; Yang, S.; Deng, Y.C.; Li, J.H.; Hou, C.S. Two pathogenic fungi isolated from chalkbrood samples and honey bee viruses they carried. Front. Microbiol. 2022, 13, 843842. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.; Weston, M.; Vannette, R.L. Bees just wanna have fungi: A review of bee associations with nonpathogenic fungi. Fems Microbiol. Ecol. 2023, 99, fiad077. [Google Scholar] [CrossRef] [PubMed]
- Sevim, A.; Akpinar, R.; Karaoglu, S.A.; Bozdeveci, A.; Sevim, E. Prevalence and phylogenetic analysis of (Maassen ex Claussen) LS Olive & Spiltoir (1955) isolates from honeybee colonies in Turkey. Biologia 2022, 77, 2689–2699. [Google Scholar] [CrossRef]
- Getachew, A.; Wubie, A.J.; Wu, J.L.; Xu, J.; Wu, P.J.; Abejew, T.A.; Tu, Y.Y.; Zhou, T.; Xu, S.F. Molecular identification of pathogenicity associated genes in honeybee fungal pathogen, by restricted enzyme-mediated integration (REMI) constructed mutants. Int. J. Agric. Biol. 2018, 20, 2879–2890. [Google Scholar]
- Niu, G.D.; Johnson, R.M.; Berenbaum, M.R. Toxicity of mycotoxins to honeybees and its amelioration by propolis. Apidologie 2011, 42, 79–87. [Google Scholar] [CrossRef]
- Grupe, A.C.; Quandt, C.A. A growing pandemic: A review of Nosema parasites in globally distributed domesticated and native bees. PLoS Pathog. 2020, 16, e1008580. [Google Scholar] [CrossRef]
- Ayo Fasasi, K. Microbiota of honeybees, Apis mellifera Adansonii (Hymenoptera: Apidae) from selected ecozones, South West Nigeria. Pak. J. Biol. Sci. 2018, 21, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Vojvodic, S.; Jensen, A.B.; James, R.R.; Boomsma, J.J.; Eilenberg, J. Temperature dependent virulence of obligate and facultative fungal pathogens of honeybee brood. Vet. Microbiol. 2011, 149, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Klinger, E.G.; Vojvodic, S.; DeGrandi-Hoffman, G.; Welker, D.L.; James, R.R. Mixed infections reveal virulence differences between host-specific bee pathogens. J. Invertebr. Pathol. 2015, 129, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vojvodic, S.; Boomsma, J.J.; Eilenberg, J.; Jensen, A.B. Virulence of mixed fungal infections in honey bee brood. Front. Zool. 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.M.; Deveza, M.V.; Koshiyama, A.S.; Tassinari, S.; Barth, O.M.; Castro, R.N.; Lorenzon, M.C. Fungi infection in honeybee hives in regions affected by Brazilian sac brood. Arq. Bras. Med. Vet. E Zootec. 2014, 66, 1471–1478. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Grassl, J.; Carson, A.; Simmons, L.W.; Baer, B. Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite. Sci. Rep. 2016, 6, 36649. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, R.N.; Mei, Y.Z.; Andicoechea, J.; Manson, J.S.; Irwin, R.E. Consequences of a nectar yeast for pollinator preference and performance. Funct. Ecol. 2017, 31, 613–621. [Google Scholar] [CrossRef]
- Rering, C.C.; Rudolph, A.B.; Beck, J.J. Pollen and yeast change nectar aroma and nutritional content alone and together, but honey bee foraging reflects only the avoidance of yeast. Environ. Microbiol. 2021, 23, 4141–4150. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial activity of bee-collected pollen and beebread: State of the art and future perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Dharampal, P.S.; Carlson, C.; Currie, C.R.; Steffan, S.A. Pollen-borne microbes shape bee fitness. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182894. [Google Scholar] [CrossRef]
- Menezes, C.; Vollet-Neto, A.; Marsaioli, A.J.; Zampieri, D.; Fontoura, I.C.; Luchessi, A.D.; Imperatriz-Fonseca, V.L. A Brazilian social bee must cultivate fungus to survive. Curr. Biol. 2015, 25, 2851–2855. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.B.; Scott, E.S.; Hogendoorn, K. Nutritional benefit of fungal spores for honey bee workers. Sci. Rep. 2020, 10, 15671. [Google Scholar] [CrossRef]
- Disayathanoowat, T.; Li, H.; Supapimon, N.; Suwannarach, N.; Lumyong, S.; Chantawannakul, P.; Guo, J. Different dynamics of bacterial and fungal communities in hive-stored bee bread and their possible roles: A case study from two commercial honey bees in China. Microorganisms 2020, 8, 264. [Google Scholar] [CrossRef]
- Vocadlova, K.; Lüddecke, T.; Patras, M.A.; Marner, M.; Hartwig, C.; Benes, K.; Matha, V.; Mraz, P.; Schäberle, T.F.; Vilcinskas, A. Extracts of Talaromyces purpureogenus strains from Apis mellifera bee bread inhibit the growth of Paenibacillus spp. in vitro. Microorganisms 2023, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Skerl, M.I.S.; Gajger, I.T. Performance and Nosema spp. spore level in young honeybee (Apis mellifera carnica, Pollmann 1879) colonies supplemented with candies. Slov. Vet. Res. 2022, 59, 159–167. [Google Scholar] [CrossRef]
- Gryganskyi, A.P.; Lee, S.C.; Litvintseva, A.P.; Smith, M.E.; Bonito, G.; Porter, T.M.; Anishchenko, I.M.; Heitman, J.; Vilgalys, R. Structure, function, and phylogeny of the mating locus in the Rhizopus oryzae complex. PLoS ONE 2010, 5, e15273. [Google Scholar] [CrossRef] [PubMed]
- Gryganskyi, A.P.; Golan, J.; Dolatabadi, S.; Mondo, S.; Robb, S.; Idnurm, A.; Muszewska, A.; Steczkiewicz, K.; Masonjones, S.; Liao, H.L.; et al. Phylogenetic and phylogenomic definition of Rhizopus species. G3-Genes Genomes Genet. 2018, 8, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Jiang, Y.Y.; Hu, C.J.; Liu, G.A.; Li, Y.G.; Wang, S. Identification, pathogenic mechanism and control of causing postharvest fruit rot in pumpkin. Postharvest Biol. Technol. 2023, 204, 112460. [Google Scholar] [CrossRef]
- Febriani, R.; Sjamsuridzal, W.; Oetari, A.; Santoso, I.; Roosheroe, I.G. ITS regions of rDNA sequence and morphological analyses clarify five strains from tempeh as Rhizopus oryzae. In Proceedings of the 3rd International Symposium on Current Progress in Mathematics and Sciences 2017 (Iscpms2017), Bali, Indonesia, 26–27 July 2017; Volume 2023. [Google Scholar] [CrossRef]
- Khokhar, I.; Mukhtar, I.; Wang, J.H.; Jia, Y.; Yan, Y.C. A report of Rhizopus oryzae causing postharvest soft rot of apple fruit in China. Australas. Plant Dis. Notes 2019, 14, 7. [Google Scholar] [CrossRef]
- Cloutier, A.; Tran, S.; Avis, T.J. Suppressive effect of compost bacteria against grey mould and Rhizopus rot on strawberry fruit. Biocontrol Sci. Technol. 2020, 30, 143–159. [Google Scholar] [CrossRef]
- Wu, F.; Tong, X.Q.; Zhang, L.Q.; Mei, L.; Guo, Y.B.; Wang, Y.J. Suppression of Rhizopus fruit rot by volatile organic compounds produced by CF05. Biocontrol Sci. Technol. 2020, 30, 1351–1364. [Google Scholar] [CrossRef]
- Khokhar, I.; Jia, Y.; Mukhtar, I.; Wang, J.H.; Ruth, N.; Eltoukhy, A.; Fan, S.H.; Li, X.J.; Wang, J.Y.; Yan, Y.C. First report of causing postharvest fruit rot on pear in China. Plant Dis. 2019, 103, 1423. [Google Scholar] [CrossRef]
- Yoder, J.A.; Hedges, B.Z.; Heydinger, D.J.; Sammataro, D.; DeGrandi-Hoffman, G. Differences among fungicides targeting beneficial fungi associated with honey bee colonies. In Honey Bee Colony Health: Challenges and Sustainable Solutions; CRC Press: Boca Raton, FL, USA, 2012; pp. 181–192. [Google Scholar]
- Zhao, Y.Z.; Zhang, Z.F.; Cai, L.; Peng, W.J.; Liu, F. Four new filamentous fungal species from newly-collected and hive-stored bee pollen. Mycosphere 2018, 9, 1089–1116. [Google Scholar] [CrossRef]
- Gilliam, M.; Prest, D.B.; Lorenz, B.J. Microbiology of pollen and bee bread:taxonomy and enzymology of molds. Apidologie 1989, 20, 53–68. [Google Scholar] [CrossRef]
- Batra, L.R.; Batra, S.W.; Bohart, G.E. The mycoflora of domesticated and wild bees (Apoidea). Mycopathol. Mycol. Appl. 1973, 49, 13–44. [Google Scholar] [CrossRef]
- Gilliam, M. Fungi isolated from honey bees, Apis mellifera. J. Invertebr. Pathol. 1974, 24, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.A.; Heydinger, D.J.; Hedges, B.Z.; Sammataro, D.; Finley, J.; DeGrandi-Hoffman, G.; Croxall, T.J.; Christensen, B.S. Fungicides Reduce Symbiotic Fungi in Bee Bread and the Beneficial Fungi in Colonies. In Honey Bee Colony Health: Challenges and Sustainable Solutions; CRC Press: Boca Raton, FL, USA, 2012; pp. 193–214. [Google Scholar]
- Gilliam, M. Factors affecting development of chalkbrood disease in colonies of honey bees, Apis mellifera, fed pollen contaminated with Ascosphaera apis. J. Invertebr. Pathol. 1988, 52, 314–325. [Google Scholar] [CrossRef]
- Georg, L.K. A simple and rapid method for obtaining monospore cultures of fungi. Mycologia 1974, 39, 368–371. [Google Scholar] [CrossRef]
- Wubie, A.J.; Hu, Y.; Li, W.; Huang, J.; Guo, Z.; Xu, S.; Zhou, T. Factors analysis in protoplast isolation and regeneration from a chalkbrood fungus, Ascosphaera apis. Int. J. Agric. Biol. 2014, 16, 89–96. [Google Scholar]
- Jensen, A.B.; Aronstein, K.; Flores, J.M.; Vojvodic, S.; Palacio, M.A.; Spivak, M. Standard methods for fungal brood disease research. J. Apic. Res. 2013, 52, 1–20. [Google Scholar] [CrossRef]
- Crailsheim, K.; Brodschneider, R.; Aupinel, P.; Behrens, D.; Genersch, E.; Vollmann, J.; Riessberger-Gallé, U. Standard methods for artificial rearing of larvae. J. Apic. Res. 2013, 52, 1–16. [Google Scholar] [CrossRef]
- Carlberg, D. Koch’s postulates revisited. Scientist 2000, 14, 6. [Google Scholar]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus. Aspergrillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Evison, S.E.F.; Jensen, A.B. The biology and prevalence of fungal diseases in managed and wild bees. Curr. Opin. Insect Sci. 2018, 26, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sinkeviciene, J.; Amsiejus, A. Mycobiota in bee pollen collected by different types of traps. Zemdirb. Agric. 2019, 106, 377–382. [Google Scholar] [CrossRef]
- Abe, A.; Oda, Y.; Asano, K.; Sone, T. The molecular phylogeny of the genus Rhizopus based on rDNA sequences. Biosci. Biotechnol. Biochem. 2006, 70, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Eltz, T.; Brühl, C.A.; Görke, C. Collection of mold (Rhizopus sp.) spores in lieu of pollen by the stingless bee Trigona collina. Insectes Sociaux 2002, 49, 28–30. [Google Scholar] [CrossRef]
- Shaw, D.E. Honeybees collecting Neurospora spores from steamed Pinus logs in Queensland. Mycologist 1993, 7, 182–185. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Morato, E.F. Stingless bees (Hymenoptera, Meliponini) feeding on stinkhorn spores (Fungi, Phallales): Robbery or dispersal? Revta Bras. Zool. 2000, 17, 881–884. [Google Scholar] [CrossRef]
- Maurer, E.; Hörtnagl, C.; Lackner, M.; Grässle, D.; Naschberger, V.; Moser, P.; Segal, E.; Semis, M.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a model system to study virulence potential of mucormycetes and evaluation of antifungal treatment. Med. Mycol. 2019, 57, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Uchida, R.; Koyama, N.; Tomoda, H. Anti-Rhizopus activity of tanzawaic acids produced by the hot spring-derived fungus Penicillium sp. BF-0005. J. Antibiot. 2018, 71, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kurakado, S.; Matsumoto, Y.; Sugita, T. Efficacy of posaconazole against infection in silkworm. Med. Mycol. J. 2021, 62, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, F.; Farmakiotis, D.; Yan, Y.; Albert, N.; Do, K.A.; Kontoyiannis, D.P. Diet modification and metformin have a beneficial effect in a fly model of obesity and mucormycosis. PLoS ONE 2014, 9, e108635. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Q.; Xia, Q.; Zhao, Z.Q.; Duan, Y.A.; Zhao, L.; Wang, H.Y.; Jiang, W.T.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Screening and identification of XERF-1 and its effect on apple replant disease. Sci. Hortic. 2022, 305, 111400. [Google Scholar] [CrossRef]
- Popova, M.; Reyes, M.; Le Conte, Y.; Bankova, V. Propolis chemical composition and honeybee resistance against Varroa destructor. Nat. Prod. Res. 2014, 28, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Antonova, D.; Bankova, V. Chemical composition of propolis and American foulbrood: Is there any relationship? Bulg. Chem. Commun. 2017, 49, 171–175. [Google Scholar]
- Swanson, J.A.I.; Torto, B.; Kells, S.A.; Mesce, K.A.; Tumlinson, J.H.; Spivak, M. Odorants that induce hygienic behavior in honeybees: Identification of volatile compounds in chalkbrood-infected honeybee larvae. J. Chem. Ecol. 2009, 35, 1108–1116. [Google Scholar] [CrossRef]
- Foose, A.M.; Westwick, R.R.; Vengarai, M.; Rittschof, C.C. The survival consequences of grooming in the honey bee. Insectes Sociaux 2022, 69, 279–287. [Google Scholar] [CrossRef]
- Becher, M.A.; Hildenbrandt, H.; Hemelrijk, C.K.; Moritz, R.F.A. Brood temperature, task division and colony survival in honeybees: A model. Ecol. Model. 2010, 221, 769–776. [Google Scholar] [CrossRef]
- Medina-Flores, C.A.; Medina, L.A.M.; Guzmán-Novoa, E. Effect of hygienic behavior on resistance to chalkbrood disease (Ascosphaera apis) in Africanized bee colonies (Apis mellifera). Rev. Mex. Cienc. Pecu. 2022, 13, 225–239. [Google Scholar] [CrossRef]
- Pent, K.; Naudi, S.; Raimets, R.; Jürison, M.; Liiskmann, E.; Karise, R. Overlapping exposure effects of pathogen and dimethoate on honeybee (Apis mellifera Linnaeus) metabolic rate and longevity. Front. Physiol. 2023, 14, 1198070. [Google Scholar] [CrossRef]
- Jensen, A.B.; Welker, D.L.; Kryger, P.; James, R.R. Polymorphic DNA sequences of the fungal honey bee pathogen Ascosphaera apis. Fems Microbiol. Lett. 2012, 330, 17–22. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Holloway, B.A. Honey bee fungal pathogen, Ascosphaera apis; current understanding of host-pathogen interactions and host mechanisms of resistance. Microb. Pathog. 2013, 13, 402–410. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aynalem, T.; Meng, L.; Getachew, A.; Wu, J.; Yu, H.; Tan, J.; Li, N.; Xu, S. A New Isolated Fungus and Its Pathogenicity for Apis mellifera Brood in China. Microorganisms 2024, 12, 313. https://doi.org/10.3390/microorganisms12020313
Aynalem T, Meng L, Getachew A, Wu J, Yu H, Tan J, Li N, Xu S. A New Isolated Fungus and Its Pathogenicity for Apis mellifera Brood in China. Microorganisms. 2024; 12(2):313. https://doi.org/10.3390/microorganisms12020313
Chicago/Turabian StyleAynalem, Tessema, Lifeng Meng, Awraris Getachew, Jiangli Wu, Huimin Yu, Jing Tan, Nannan Li, and Shufa Xu. 2024. "A New Isolated Fungus and Its Pathogenicity for Apis mellifera Brood in China" Microorganisms 12, no. 2: 313. https://doi.org/10.3390/microorganisms12020313




