CRISPR/Cas9-Mediated Knockout of the HuR Gene in U251 Cell Inhibits Japanese Encephalitis Virus Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus
2.2. Selection and Design of sgRNA
2.3. Construction of LentiCRISPR-V2 Recombinant Plasmid
2.4. Lentivirus Packaging and Infection
2.5. Acquisition of Monoclonal Cells
2.6. Identification of HuR Gene Knockout U251 Cell Lines
2.7. Counting Kit-8 (CCK-8) Assay
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Construction of the LentiCRISPR V2-sgRNA Recombinant Plasmid
3.2. Screening and Sequencing of HuR Gene Knockout Monoclonal Cells
3.3. Western Blot Analysis of HuR Gene Knockout Monoclonal Cells
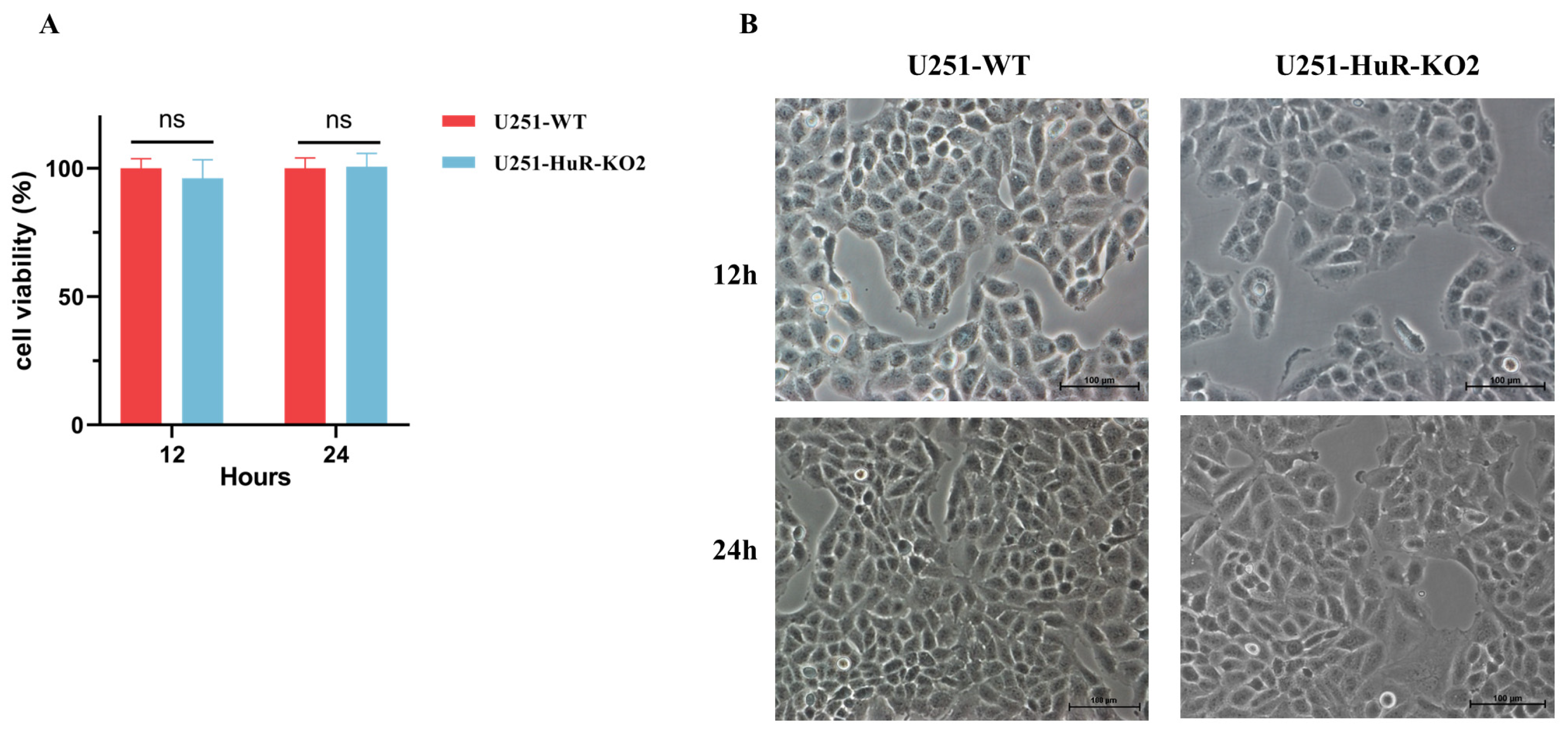
3.4. Effect of HuR Gene Knockout on Cell Viability in U251 Cell Lines
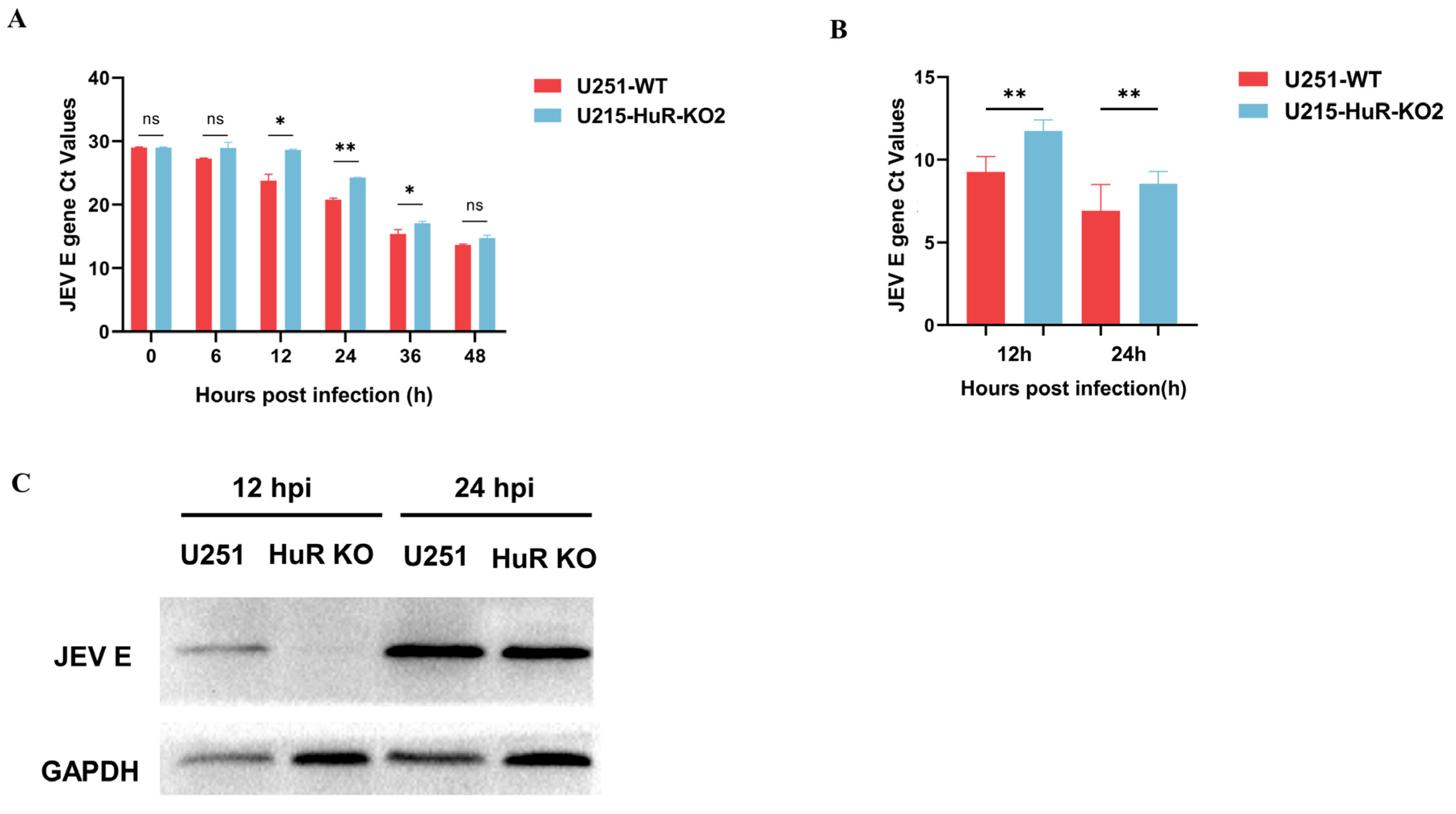
3.5. Effect of Knockout of HuR Gene on JEV Replication
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, W.L.; Wang, W.C.; Chang, W.C.; Tseng, J.T. The RNA-binding protein HuR stabilizes cytosolic phospholipase A2alpha mRNA under interleukin-1beta treatment in non-small cell lung cancer A549 Cells. J. Biol. Chem. 2011, 286, 35499–35508. [Google Scholar] [CrossRef]
- Holmes, B.; Benavides-Serrato, A.; Freeman, R.S.; Landon, K.A.; Bashir, T.; Nishimura, R.N.; Gera, J. mTORC2/AKT/HSF1/HuR constitute a feed-forward loop regulating Rictor expression and tumor growth in glioblastoma. Oncogene 2018, 37, 732–743. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, A.; Zhang, A.; Zhou, C. HuR promotes breast cancer cell proliferation and survival via binding to CDK3 mRNA. Biomed. Pharmacother. 2017, 91, 788–795. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Hu, R.; Xu, R.; Xu, W. LncRNA B4GALT1-AS1 recruits HuR to promote osteosarcoma cells stemness and migration via enhancing YAP transcriptional activity. Cell Prolif. 2018, 51, e12504. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Ding, Y.; Zhang, H.; Ding, N.; Lu, M. HuR Promotes Ovarian Cancer Cell Proliferation by Regulating TIMM44 mRNA Stability. Cell Biochem. Biophys. 2020, 78, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Muralidharan, R.; Srivastava, A.; Johnston, S.E.; Zhao, Y.D. Molecular Targeting of HuR Oncoprotein Suppresses MITF and Induces Apoptosis in Melanoma Cells. Cancers 2021, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Diosa-Toro, M.; Prasanth, K.R.; Bradrick, S.S.; Garcia Blanco, M.A. Role of RNA-binding proteins during the late stages of Flavivirus replication cycle. Virol. J. 2020, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Dave, P.; Rani, P.; Behera, P.; Das, S. Cellular Protein HuR Regulates the Switching of Genomic RNA Templates for Differential Functions during the Coxsackievirus B3 Life Cycle. J. Virol. 2021, 95, e0091521. [Google Scholar] [CrossRef] [PubMed]
- Lemay, J.; Maidou-Peindara, P.; Bader, T.; Ennifar, E.; Rain, J.C.; Benarous, R.; Liu, L.X. HuR interacts with human immunodeficiency virus type 1 reverse transcriptase, and modulates reverse transcription in infected cells. Retrovirology 2008, 5, 47. [Google Scholar] [CrossRef]
- Barnhart, M.D.; Moon, S.L.; Emch, A.W.; Wilusz, C.J.; Wilusz, J. Changes in cellular mRNA stability, splicing, and polyadenylation through HuR protein sequestration by a cytoplasmic RNA virus. Cell Rep. 2013, 5, 909–917. [Google Scholar] [CrossRef]
- Sokoloski, K.J.; Dickson, A.M.; Chaskey, E.L.; Garneau, N.L.; Wilusz, C.J.; Wilusz, J. Sindbis virus usurps the cellular HuR protein to stabilize its transcripts and promote productive infections in mammalian and mosquito cells. Cell Host Microbe 2010, 8, 196–207. [Google Scholar] [CrossRef]
- Lin, J.Y.; Brewer, G.; Li, M.L. HuR and Ago2 Bind the Internal Ribosome Entry Site of Enterovirus 71 and Promote Virus Translation and Replication. PLoS ONE 2015, 10, e0140291. [Google Scholar] [CrossRef]
- Schwerk, J.; Jarret, A.P.; Joslyn, R.C.; Savan, R. Landscape of post-transcriptional gene regulation during hepatitis C virus infection. Curr. Opin. Virol. 2015, 12, 75–84. [Google Scholar] [CrossRef]
- Gao, H.; Lin, Y.; Huang, C.; Li, X.; Diamond, M.S.; Liu, C.; Zhang, R.; Zhang, P. A genome-wide CRISPR screen identifies HuR as a regulator of apoptosis induced by dsRNA and virus. J. Cell Sci. 2022, 135, jcs258855. [Google Scholar] [CrossRef]
- Nadar, M.; Chan, M.Y.; Huang, S.W.; Huang, C.C.; Tseng, J.T.; Tsai, C.H. HuR binding to AU-rich elements present in the 3′ untranslated region of Classical swine fever virus. Virol. J. 2011, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, G.; Williams, N.; Netzband, R.; Schwarz, M.C.; Evans, M.J.; Pager, C.T. Zika Virus Subverts Stress Granules To Promote and Restrict Viral Gene Expression. J. Virol. 2019, 93, e00520-19. [Google Scholar] [CrossRef] [PubMed]
- Mazloum, A.; Karagyaur, M.; Chernyshev, R.; van Schalkwyk, A.; Jun, M.; Qiang, F.; Sprygin, A. Post-genomic era in agriculture and veterinary science: Successful and proposed application of genetic targeting technologies. Front. Vet. Sci. 2023, 10, 1180621. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Su, R.; Wang, W.; Liang, Y.; Zeng, X.; Shereen, M.A.; Bashir, N.; Zhang, Q.; Zhao, L.; Wu, K.; et al. EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathog. 2019, 15, e1008142. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Chen, J.; Liu, W.; Xiang, Q.; Luo, Z.; Wang, W.; Xu, W.; Wu, K.; Zhang, Q.; Liu, Y.; et al. Enterovirus 71 induces neural cell apoptosis and autophagy through promoting ACOX1 downregulation and ROS generation. Virulence 2020, 11, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Bashir, N.; Su, R.; Liu, F.; Wu, K.; Luo, Z.; Wu, J. Zika virus dysregulates the expression of astrocytic genes involved in neurodevelopment. PLoS Negl. Trop. Dis. 2021, 15, e0009362. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhao, Z.; Anees, A.; Li, Y.; Ashraf, U.; Chen, Z.; Song, Y.; Chen, H.; Cao, S.; Ye, J. p21-Activated Kinase 4 Signaling Promotes Japanese Encephalitis Virus-Mediated Inflammation in Astrocytes. Front. Cell Infect. Microbiol. 2017, 7, 271. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Cheng, M.; Ge, N.; Shu, J.; Xu, Z.; Su, X.; Kou, Z.; Tong, Y.; Qin, C.; et al. A single nonsynonymous mutation on ZIKV E protein-coding sequences leads to markedly increased neurovirulence in vivo. Virol. Sin. 2022, 37, 115–126. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Ye, J.; Ashraf, U.; Chen, Z.; Zhu, B.; He, W.; Xu, Q.; Wei, Y.; Chen, H.; et al. Quantitative Label-Free Phosphoproteomics Reveals Differentially Regulated Protein Phosphorylation Involved in West Nile Virus-Induced Host Inflammatory Response. J. Proteome Res. 2015, 14, 5157–5168. [Google Scholar] [CrossRef]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, H.; Zhao, F.; Yan, Q.; Li, Y.; Niu, X.; Zeng, W.; Wu, K.; Ling, B.; Fan, S.; et al. Genomic Characteristics and E Protein Bioinformatics Analysis of JEV Isolates from South China from 2011 to 2018. Vaccines 2022, 10, 1303. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nain, M.; Abdin, M.Z.; Kalia, M.; Vrati, S. Japanese encephalitis virus invasion of cell: Allies and alleys. Rev. Med. Virol. 2016, 26, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Shen, B.; Cui, Y.; Chen, Y.; Wang, J.; Wang, L.; Kang, Y.; Zhao, X.; Si, W.; Li, W.; et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 2014, 156, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Uckeley, Z.M.; Moeller, R.; Kuhn, L.I.; Nilsson, E.; Robens, C.; Lasswitz, L.; Lindqvist, R.; Lenman, A.; Passos, V.; Voss, Y.; et al. Quantitative Proteomics of Uukuniemi Virus-host Cell Interactions Reveals GBF1 as Proviral Host Factor for Phleboviruses. Mol. Cell. Proteom. 2019, 18, 2401–2417. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, A.; Stieber, D.; Enger, P.O.; Golebiewska, A.; Molven, A.; Svendsen, A.; Westermark, B.; Niclou, S.P.; Olsen, T.K.; Chekenya Enger, M.; et al. U-251 revisited: Genetic drift and phenotypic consequences of long-term cultures of glioblastoma cells. Cancer Med. 2014, 3, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhen, Z.D.; Fan, D.Y.; Wang, P.G.; An, J. Axl Alleviates Neuroinflammation and Delays Japanese Encephalitis Progression in Mice. Virol. Sin. 2021, 36, 667–677. [Google Scholar] [CrossRef]
- Lang, M.; Berry, D.; Passecker, K.; Mesteri, I.; Bhuju, S.; Ebner, F.; Sedlyarov, V.; Evstatiev, R.; Dammann, K.; Loy, A.; et al. HuR Small-Molecule Inhibitor Elicits Differential Effects in Adenomatosis Polyposis and Colorectal Carcinogenesis. Cancer Res. 2017, 77, 2424–2438. [Google Scholar] [CrossRef]
- Lukosiute-Urboniene, A.; Jasukaitiene, A.; Silkuniene, G.; Barauskas, V.; Gulbinas, A.; Dambrauskas, Z. Human antigen R mediated post-transcriptional regulation of inhibitors of apoptosis proteins in pancreatic cancer. World J. Gastroenterol. 2019, 25, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.J.; Mubaid, S.; Busque, S.; de Los Santos, Y.L.; Ashour, K.; Sadek, J.; Lian, X.J.; Khattak, S.; Di Marco, S.; Gallouzi, I.E. The formation of HuR/YB1 complex is required for the stabilization of target mRNA to promote myogenesis. Nucleic Acids Res. 2023, 51, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lin, W.J.; Ren, M.; Qiu, J.; Yang, C.; Wang, X.; Li, N.; Zeng, T.; Sun, K.; You, L.; et al. m(6)A reader YTHDC1 modulates autophagy by targeting SQSTM1 in diabetic skin. Autophagy 2022, 18, 1318–1337. [Google Scholar] [CrossRef]
- Kim, Y.S.; Tang, P.W.; Welles, J.E.; Pan, W.; Javed, Z.; Elhaw, A.T.; Mythreye, K.; Kimball, S.R.; Hempel, N. HuR-dependent SOD2 protein synthesis is an early adaptation to anchorage-independence. Redox Biol. 2022, 53, 102329. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Chen, H.; Li, H.; Xu, P.; Liu, B.; Zhang, Q.; Lv, C.; Song, X. ATF3 -activated accelerating effect of LINC00941/lncIAPF on fibroblast-to-myofibroblast differentiation by blocking autophagy depending on ELAVL1/HuR in pulmonary fibrosis. Autophagy 2022, 18, 2636–2655. [Google Scholar] [CrossRef]
- Chellappan, R.; Guha, A.; Si, Y.; Kwan, T.; Nabors, L.B.; Filippova, N.; Yang, X.; Myneni, A.S.; Meesala, S.; Harms, A.S.; et al. SRI-42127, a novel small molecule inhibitor of the RNA regulator HuR, potently attenuates glial activation in a model of lipopolysaccharide-induced neuroinflammation. Glia 2022, 70, 155–172. [Google Scholar] [CrossRef]
- Chen, J.; Martindale, J.L.; Abdelmohsen, K.; Kumar, G.; Fortina, P.M.; Gorospe, M.; Rostami, A.; Yu, S. RNA-Binding Protein HuR Promotes Th17 Cell Differentiation and Can Be Targeted to Reduce Autoimmune Neuroinflammation. J. Immunol. 2020, 204, 2076–2087. [Google Scholar] [CrossRef]
- Yu, S.; Tripod, M.; Atasoy, U.; Chen, J. HuR Plays a Positive Role to Strengthen the Signaling Pathways of CD4+ T Cell Activation and Th17 Cell Differentiation. J. Immunol. Res. 2021, 2021, 9937243. [Google Scholar] [CrossRef]
- Kanzaki, H.; Chiba, T.; Kaneko, T.; Ao, J.; Kan, M.; Muroyama, R.; Nakamoto, S.; Kanda, T.; Maruyama, H.; Kato, J.; et al. The RNA-Binding Protein ELAVL1 Regulates Hepatitis B Virus Replication and Growth of Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2022, 23, 7878. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Zhang, Z.; Chaubey, B.; Pandey, V.N. Identification of cellular factors associated with the 3’-nontranslated region of the hepatitis C virus genome. Mol. Cell. Proteom. 2006, 5, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Korf, M.; Jarczak, D.; Beger, C.; Manns, M.P.; Kruger, M. Inhibition of hepatitis C virus translation and subgenomic replication by siRNAs directed against highly conserved HCV sequence and cellular HCV cofactors. J. Hepatol. 2005, 43, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, S.; Kumar, A.; Mullick, R.; Vasudevan, D.; Mukherjee, N.; Das, S. HuR Displaces Polypyrimidine Tract Binding Protein To Facilitate La Binding to the 3’ Untranslated Region and Enhances Hepatitis C Virus Replication. J. Virol. 2015, 89, 11356–11371. [Google Scholar] [CrossRef] [PubMed]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese Encephalitis Emergence in Australia: The Potential Population at Risk. Clin. Infect. Dis. 2023, 76, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774, 774A–774E. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Zhou, Y.; Li, F.; Deng, H.; Zhao, M.; Huang, Y.; Jiang, C.; Sun, X.; Xu, Z.; Zhu, L. Epidemiological investigation of swine Japanese encephalitis virus based on RT-RAA detection method. Sci. Rep. 2022, 12, 9392. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Hernandez-Triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 2017, 201, 85–92. [Google Scholar] [CrossRef]
- Mohsin, F.; Suleman, S.; Anzar, N.; Narang, J.; Wadhwa, S. A review on Japanese Encephalitis virus emergence, pathogenesis and detection: From conventional diagnostics to emerging rapid detection techniques. Int. J. Biol. Macromol. 2022, 217, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Sun, H.; Xie, M.; Yang, C.; Pan, Y.; Huang, D.; Cheng, L.; Chen, H.; Ma, J.; et al. Autoimmune encephalitis after Japanese encephalitis in children: A prospective study. J. Neurol. Sci. 2021, 424, 117394. [Google Scholar] [CrossRef] [PubMed]

| Target Names | Primer Sequences |
|---|---|
| HuR-sgRNA1 | F: CACCGCGGGCGAGCATACGACACCT |
| R: AAACAGGTGTCGTATGCTCGCCCGC | |
| HuR-sgRNA2 | F: CACCGTCCTCGTGGATCAGACTAC |
| R: AAACGTAGTCTGATCCACGAGGAC |
| Target Names | Primer Sequences |
|---|---|
| GAPDH-RT | F: AGAAGGCTGGGGCTCATTTG |
| R: GGGGCCATCCACAGTCTTC | |
| JEV-E-RT | F: TGGAGCCACTTGGGTG |
| R: TGGAGCCACTTGGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.-Q.; Cao, S.-J.; Zhao, Q. CRISPR/Cas9-Mediated Knockout of the HuR Gene in U251 Cell Inhibits Japanese Encephalitis Virus Replication. Microorganisms 2024, 12, 314. https://doi.org/10.3390/microorganisms12020314
Luo S-Q, Cao S-J, Zhao Q. CRISPR/Cas9-Mediated Knockout of the HuR Gene in U251 Cell Inhibits Japanese Encephalitis Virus Replication. Microorganisms. 2024; 12(2):314. https://doi.org/10.3390/microorganisms12020314
Chicago/Turabian StyleLuo, Sai-Qi, San-Jie Cao, and Qin Zhao. 2024. "CRISPR/Cas9-Mediated Knockout of the HuR Gene in U251 Cell Inhibits Japanese Encephalitis Virus Replication" Microorganisms 12, no. 2: 314. https://doi.org/10.3390/microorganisms12020314





