Diversity and Biological Characteristics of Seed-Borne Bacteria of Achnatherum splendens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Methods
2.2.1. Isolation of Bacteria
2.2.2. 16S rDNA Molecular Identification of Bacteria
2.2.3. Determination of Swimming Motility
2.2.4. Determination of Biofilm Formation Ability
2.2.5. Determination of Antibiotic Resistance
2.3. Statistical Analysis
3. Results
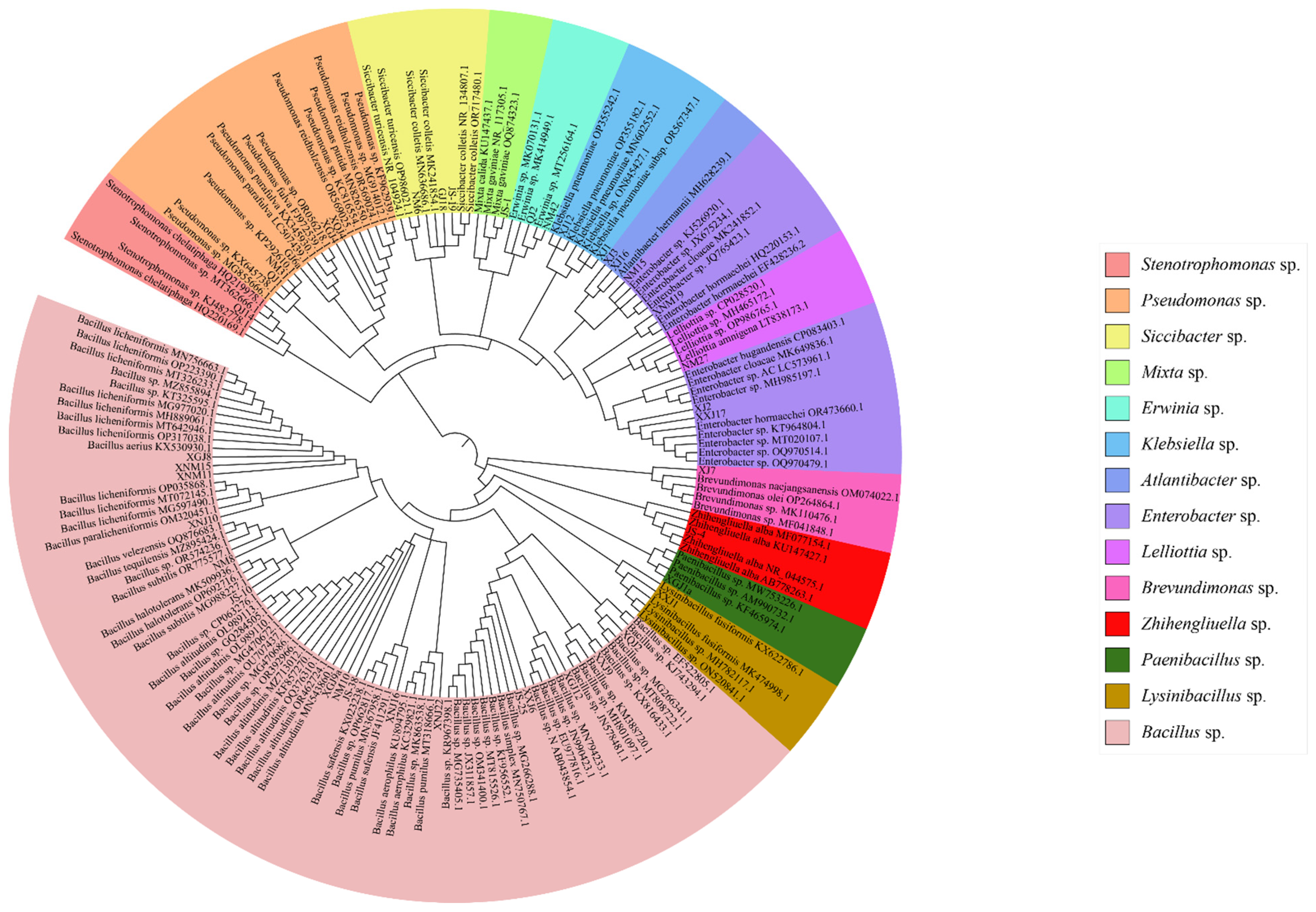
3.1. Bacterial Diversity of Seed-Borne Bacteria of A. splendens
3.1.1. Taxonomic Status of Bacteria
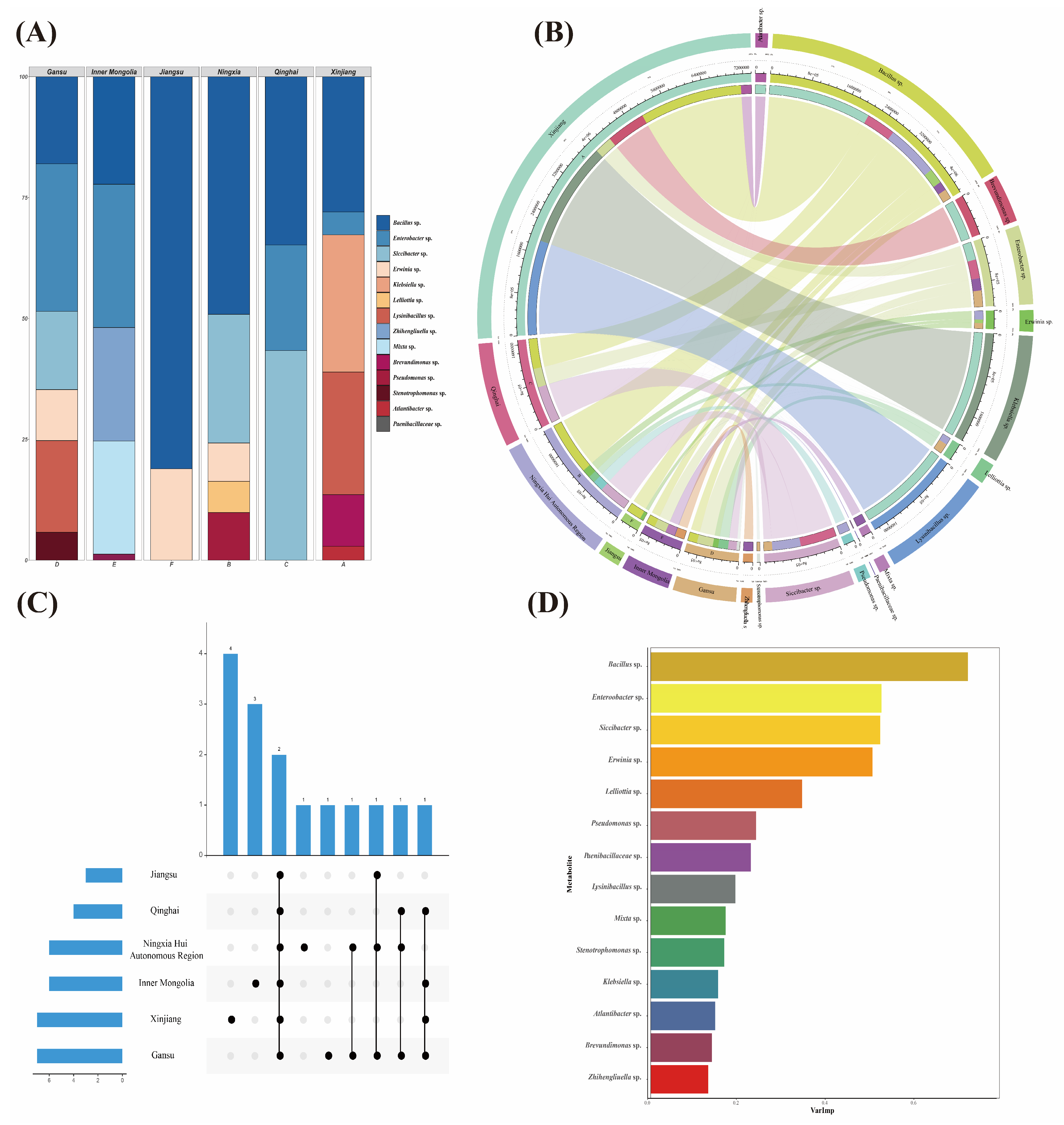
3.1.2. Bacterial Diversity Analysis of Seed-Borne Bacteria of A. splendens
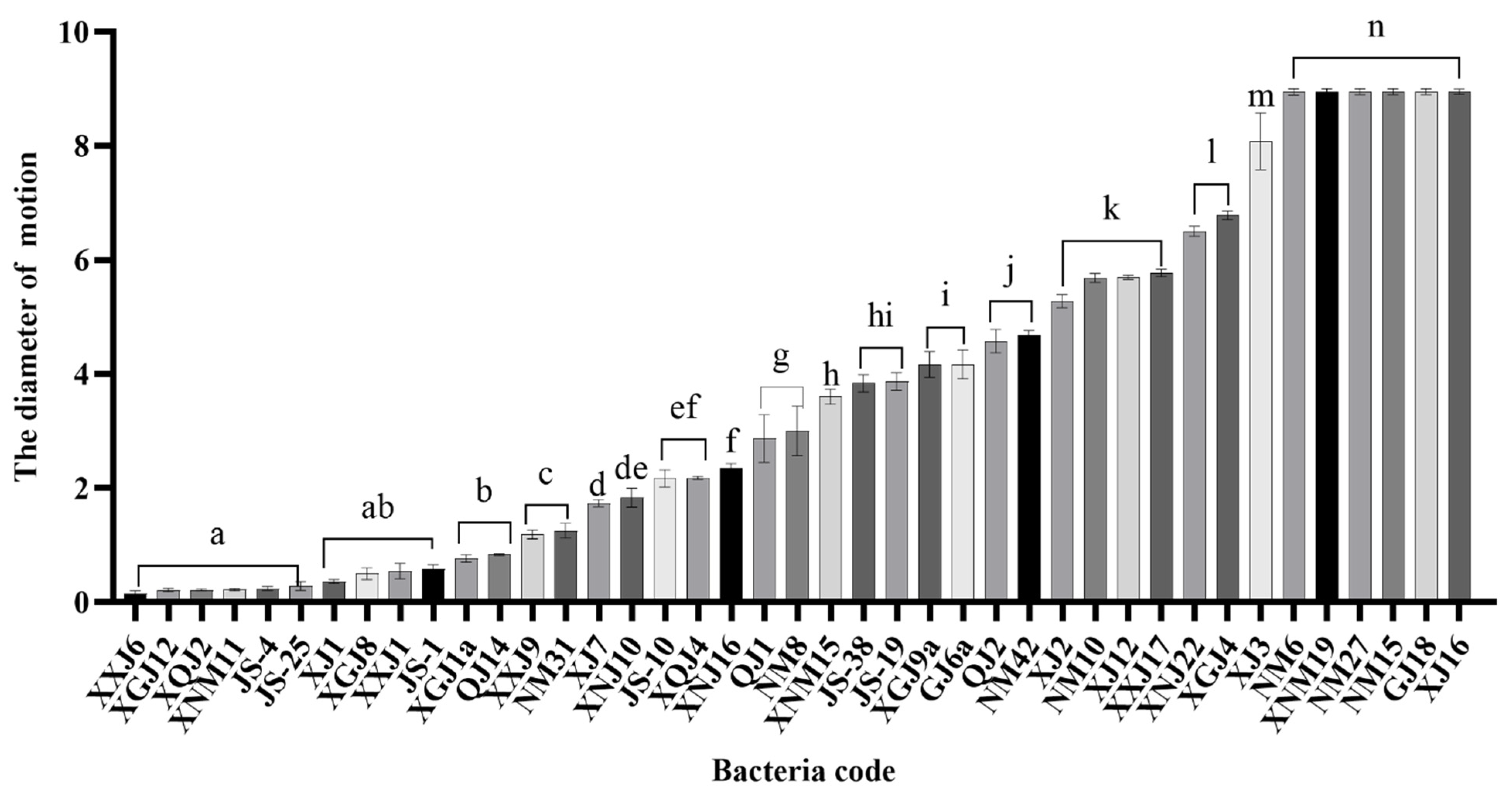
3.2. Mobility Analysis
3.3. Analysis of Biofilm Formation Ability
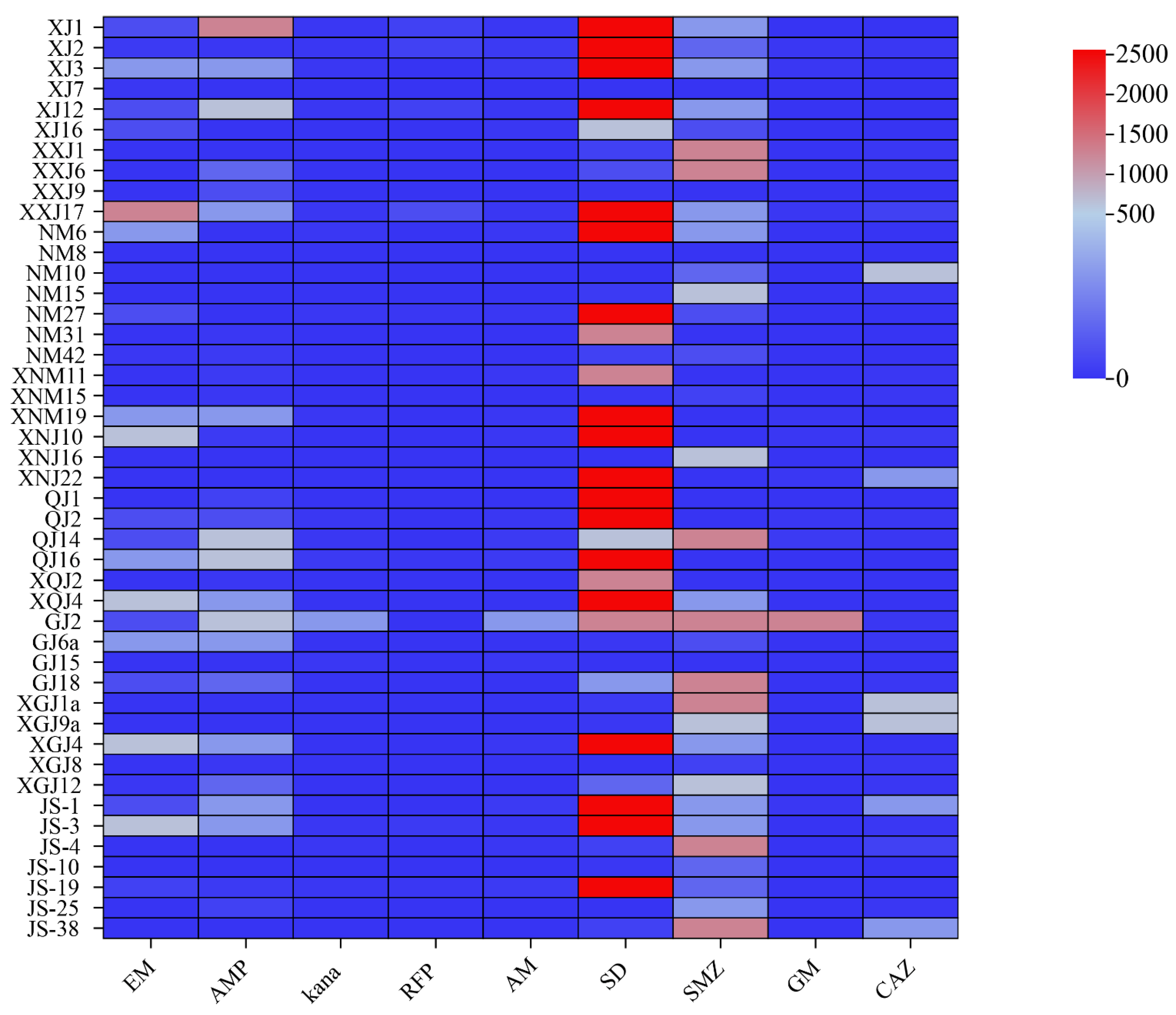
3.4. Antibiotic Resistance Analysis
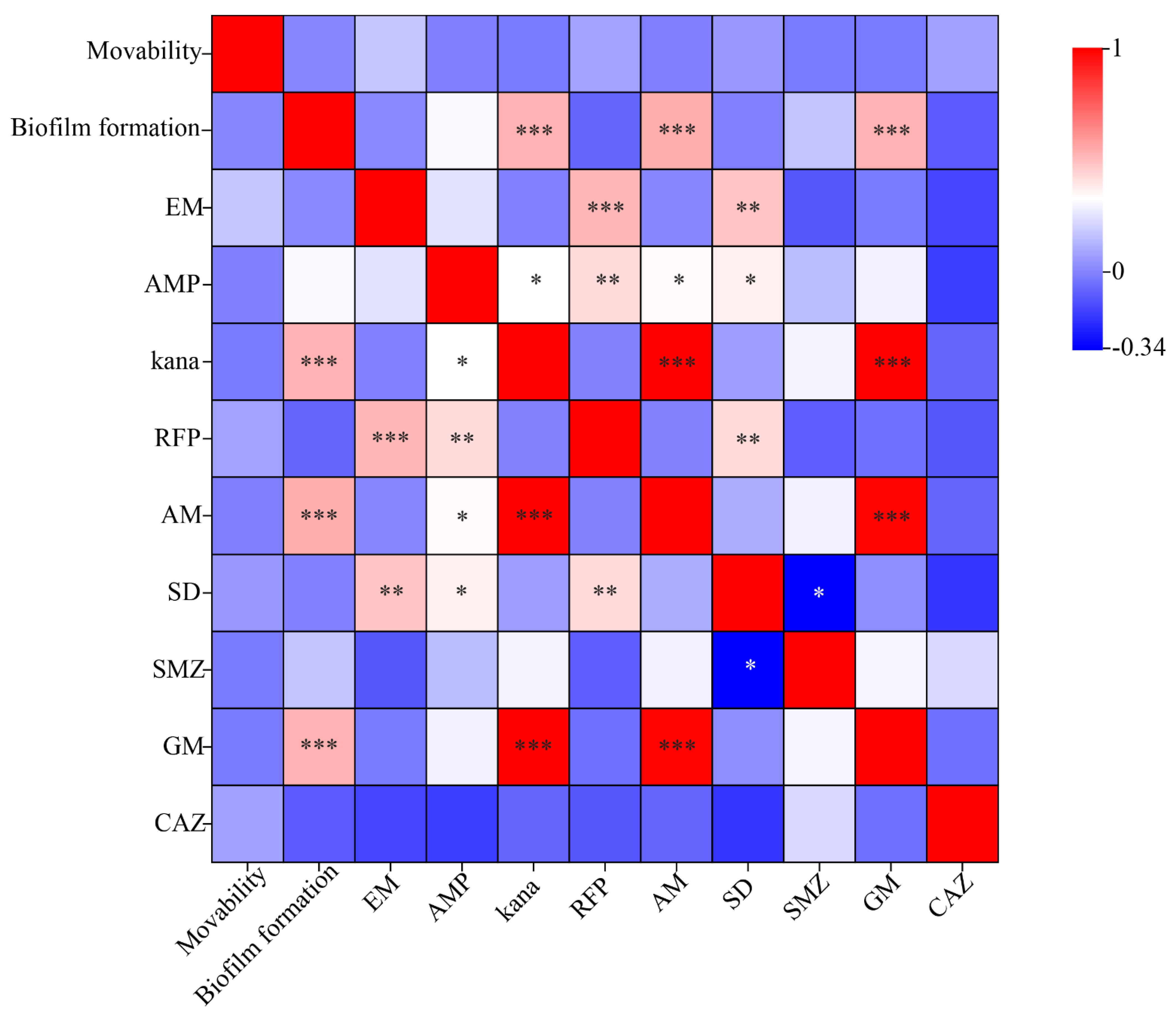
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Irfan, M.; Chen, Q.; Yue, Y.; Pang, R.; Lin, Q.; Zhao, X.; Chen, H. Co-Production of Biochar, Bio-Oil and Syngas from Halophyte Grass (Achnatherum splendens L.) under Three Different Pyrolysis Temperatures. Bioresour. Technol. 2016, 211, 457–463. [Google Scholar] [CrossRef]
- Liu, B.; Ju, Y.; Xia, C.; Zhong, R.; Christensen, M.J.; Zhang, X.; Nan, Z. The Effect of Epichloë Endophyte on Phyllosphere Microbes and Leaf Metabolites in Achnatherum Inebrians. iScience 2022, 25, 104144. [Google Scholar] [CrossRef]
- Geisen, S.; Kostenko, O.; Cnossen, M.C.; Ten Hooven, F.C.; Vreš, B.; Van Der Putten, W.H. Seed and Root Endophytic Fungi in a Range Expanding and a Related Plant Species. Front. Microbiol. 2017, 8, 1645. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Xu, M.; Sheng, J.; Chen, L.; Men, Y.; Gan, L.; Guo, S.; Shen, L. Bacterial Community Compositions of Tomato (Lycopersicum esculentum Mill.) Seeds and Plant Growth Promoting Activity of ACC Deaminase Producing Bacillus subtilis (HYT-12-1) on Tomato Seedlings. World J. Microbiol. Biotechnol. 2014, 30, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Fleming, A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Danaher, M.; Radeck, W.; Kolar, L.; Keegan, J.; Cerkvenik-Flajs, V. Recent Developments in the Analysis of Avermectin and Milbemycin Residues in Food Safety and the Environment. Curr. Pharm. Biotechnol. 2011, 13, 936–951. [Google Scholar] [CrossRef]
- Litskas, V.D.; Karamanlis, X.N.; Batzias, G.C.; Tsiouris, S.E. Are the Parasiticidal Avermectins Resistant to Dissipation in the Environment? The Case of Eprinomectin. Environ. Int. 2013, 60, 48–55. [Google Scholar] [CrossRef]
- Bryan, A.; Shapir, N.; Sadowsky, M.J. Frequency and Distribution of Tetracycline Resistance Genes in Genetically Diverse, Nonselected, and Nonclinical Escherichia Coli Strains Isolated from Diverse Human and Animal Sources. Appl. Environ. Microbiol. 2004, 70, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Humphry, R.W.; Scott, K.P.; Hillman, K.; Fenlon, D.R.; Low, J.C. Influence of tetracycline exposure on tetracycline resistance and the carriage of tetracycline resistance genes within commensal Escherichia coli populations. J. Appl. Microbiol. 2003, 94, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, J. Detection and Diversity Evaluation of Tetracycline Resistance Genes in Grassland-Based Production Systems in Colombia, South America. Front. Microbiol. 2011, 2, 252. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; May, H.E.; AbuOun, M.; Stubberfield, E.; Gilson, D.; Chau, K.K.; Crook, D.W.; Shaw, L.P.; Read, D.S.; Stoesser, N.; et al. A Longitudinal Study Reveals Persistence of Antimicrobial Resistance on Livestock Farms Is Not Due to Antimicrobial Usage Alone. Front. Microbiol. 2023, 14, 1070340. [Google Scholar] [CrossRef] [PubMed]
- Campos Calero, G.; Caballero Gómez, N.; Benomar, N.; Pérez Montoro, B.; Knapp, C.W.; Gálvez, A.; Abriouel, H. Deciphering Resistome and Virulome Diversity in a Porcine Slaughterhouse and Pork Products through Its Production Chain. Front. Microbiol. 2018, 9, 2099. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef]
- Tan, T.Q.; Mason, E.O., Jr.; Kaplan, S.L. Penicillin-Resistant Systemic Pneumococcal Infections in Children: A Retrospective Case-Control Study. Pediatrics 1993, 92, 761–767. [Google Scholar] [CrossRef]
- Bronzwaer, S.L.A.M.; Cars, O.; Buchholz, U.; Mölstad, S.; Goettsch, W.; Veldhuijzen, I.K.; Kool, J.L.; Sprenger, M.J.W.; Degener, J.E. The Relationship between Antimicrobial Use and Antimicrobial Resistance in Europe. Emerg. Infect. Dis. 2002, 8, 278–282. [Google Scholar] [CrossRef]
- Teschler, J.K.; Zamorano-Sánchez, D.; Utada, A.S.; Warner, C.J.A.; Wong, G.C.L.; Linington, R.G.; Yildiz, F.H. Living in the Matrix: Assembly and Control of Vibrio Cholerae Biofilms. Nat. Rev. Microbiol. 2015, 13, 255–268. [Google Scholar] [CrossRef]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter Baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Cope-Selby, N.; Cookson, A.; Squance, M.; Donnison, I.; Flavell, R.; Farrar, K. Endophytic Bacteria in Miscanthus Seed: Implications for Germination, Vertical Inheritance of Endophytes, Plant Evolution and Breeding. GCB Bioenergy 2017, 9, 57–77. [Google Scholar] [CrossRef]
- Alías-Villegas, C.; Fuentes-Romero, F.; Cuéllar, V.; Navarro-Gómez, P.; Soto, M.J.; Vinardell, J.-M.; Acosta-Jurado, S. Surface Motility Regulation of Sinorhizobium Fredii HH103 by Plant Flavonoids and the NodD1, TtsI, NolR, and MucR1 Symbiotic Bacterial Regulators. Int. J. Mol. Sci. 2022, 23, 7698. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Lavallée, C.; Rouleau, D.; Gaudreau, C.; Roger, M.; Tsimiklis, C.; Locas, M.-C.; Gagnon, S.; Delorme, J.; Labbé, A.-C. Performance of an Agar Dilution Method and a Vitek 2 Card for Detection of Inducible Clindamycin Resistance in Staphylococcus spp. J. Clin. Microbiol. 2010, 48, 1354–1357. [Google Scholar] [CrossRef]
- Chen, X.; Krug, L.; Yang, H.; Li, H.; Yang, M.; Berg, G.; Cernava, T. Nicotiana Tabacum Seed Endophytic Communities Share a Common Core Structure and Genotype-Specific Signatures in Diverging Cultivars. Comput. Struct. Biotechnol. J. 2020, 18, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial Seed Endophytes: Genera, Vertical Transmission and Interaction with Plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Díaz Herrera, S.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat Seeds Harbour Bacterial Endophytes with Potential as Plant Growth Promoters and Biocontrol Agents of Fusarium Graminearum. Microbiol. Res. 2016, 186–187, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of Bacterial Endophytes from Germinated Maize Kernels. Can. J. Microbiol. 2007, 53, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Kaga, H.; Mano, H.; Tanaka, F.; Watanabe, A.; Kaneko, S.; Morisaki, H. Rice Seeds as Sources of Endophytic Bacteria. Microb. Environ. 2009, 24, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, R.; Yao, B.; Zhang, W.; Yang, C.; Chen, X. Culturable Seed-Borne Bacteria of Lucerne Imported from Europe and North America and Their Pathogenicity to Plants and Animals. Acta Prataculturae Sin. 2023, 32, 161. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, C.; Lei, Y.; Kuang, W. Analysis of Bacterial Communities in White Clover Seeds via High-Throughput Sequencing of 16S rRNA Gene. Curr. Microbiol. 2019, 76, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, X.; Lou, K. Isolation, Characterization, and Insecticidal Activity of an Endophyte of Drunken Horse Grass, Achnatherum Inebrians. J. Insect Sci. 2013, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Yangzong, Z.; Yuan, Z.; Shi, R.; Feng, K.; Xin, P.; Song, T. The Microbial Communities and Natural Fermentation Quality of Ensiling Oat (Avena sativa L.) Harvest from Different Elevations on the Qinghai-Tibet Plateau. Front. Microbiol. 2023, 13, 1108890. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wu, Y.; Li, F.; Tang, W.; Gong, W.; Han, X.; White, J.F.; Ji, X.; Li, H. Seed Endophytes and Their Roles in Host Plant Stress Resistance. J. Soil Sci. Plant Nutr. 2023, 23, 2927–2937. [Google Scholar] [CrossRef]
- Hodgson, S.; De Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical Transmission of Fungal Endophytes Is Widespread in Forbs. Ecol. Evol. 2014, 4, 1199–1208. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-Borne Endophytic Bacillus Amyloliquefaciens RWL-1 Produces Gibberellins and Regulates Endogenous Phytohormones of Oryza Sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing Endophytic Competence and Plant Growth Promotion of Bacterial Endophytes Inhabiting the Seed Endosphere of Rice. BMC Microbiol. 2017, 17, 209. [Google Scholar] [CrossRef]
- Cankar, K.; Kraigher, H.; Ravnikar, M.; Rupnik, M. Bacterial Endophytes from Seeds of Norway Spruce (Picea abies L. Karst). FEMS Microbiol. Lett. 2005, 244, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Tank, D.C.; Boulanger, L.-A.; Bascom-Slack, C.A.; Eisenman, K.; Kingery, D.; Babbs, B.; Fenn, K.; Greene, J.S.; Hann, B.D.; et al. Bioactive Endophytes Warrant Intensified Exploration and Conservation. PLoS ONE 2008, 3, e3052. [Google Scholar] [CrossRef]
- Samad, T.; Billings, N.; Birjiniuk, A.; Crouzier, T.; Doyle, P.S.; Ribbeck, K. Swimming Bacteria Promote Dispersal of Non-Motile Staphylococcal Species. ISME J 2017, 11, 1933–1937. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernández-Pascual, M.; Sanjuan, J.; Olivares, J. A fadD Mutant of Sinorhizobium Meliloti Shows Multicellular Swarming Migration and Is Impaired in Nodulation Efficiency on Alfalfa Roots. Mol. Microbiol. 2002, 43, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.G.; Seymour, J.; Kumbun, K.; Tyson, G.W. Diverse Populations of Lake Water Bacteria Exhibit Chemotaxis towards Inorganic Nutrients. ISME J. 2013, 7, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Seymour, J.R.; Samadani, A.; Hunt, D.E.; Polz, M.F. Rapid Chemotactic Response Enables Marine Bacteria to Exploit Ephemeral Microscale Nutrient Patches. Proc. Natl. Acad. Sci. USA 2008, 105, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Delaquis, P.J.; Korber, D.R.; Caldwell, D.E. Behavior of Pseudomonas fluorescens within the Hydrodynamic Boundary Layers of Surface Microenvironments. Microb. Ecol. 1987, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tomás, J.M. The Main Aeromonas Pathogenic Factors. ISRN Microbiol. 2012, 2012, 256261. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Flagellar and Twitching Motility Are Necessary for Pseudomonas aeruginosa Biofilm Development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Young, B.M.; Young, G.M. Effect of Flagellar Mutations on Yersinia enterocolitica Biofilm Formation. Appl. Environ. Microbiol. 2008, 74, 5466–5474. [Google Scholar] [CrossRef]
- Jiang, Z.; Tuo, L.; Huang, D.; Osterman, I.A.; Tyurin, A.P.; Liu, S.; Lukyanov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Korshun, V.A.; et al. Diversity, Novelty, and Antimicrobial Activity of Endophytic Actinobacteria From Mangrove Plants in Beilun Estuary National Nature Reserve of Guangxi, China. Front. Microbiol. 2018, 9, 868. [Google Scholar] [CrossRef] [PubMed]

| Origin | Grass Species | Production Time | Source |
|---|---|---|---|
| Xinjiang Uygur Autonomous Region | Achnatherum splendens | 2020 | Seeds were purchased from Qinghai Womiao Ecological Technology Co., Ltd., Xining, China |
| Inner Mongolia Autonomous Region | Achnatherum splendens | 2021 | |
| Qinghai Province | Achnatherum splendens | 2022 | |
| Gansu Province | Achnatherum splendens | 2021 | |
| Ningxia Hui Autonomous Region | Achnatherum splendens | 2021 | |
| Jiangsu Province | Achnatherum splendens | 2020 |
| Antibiotic Name | Classification of Antibiotics | Screening Concentration (µg/mL) |
|---|---|---|
| EM | Macrolides antibiotics | 10 |
| ANP | β-lactams | 20 |
| Kana | Aminoglycosides | 40 |
| CAZ | Cephalosporins | 80 |
| GM | Aminoglycosides | 160 |
| SD | Sulfonamides | 320 |
| SMZ | Sulfonamides | 640 |
| AM | Aminoglycosides | 1280 |
| RFP | Rifamycins | 10, 20, 40, 80, 160, 320, 640, 1280, 2560 |
| Strain Code | Gram | 16S rDNA Identification | Accession Number |
|---|---|---|---|
| XJ1 | − | Klebsiella sp. | OR889436 |
| XJ2 | − | Enterobacter sp. | OR889437 |
| XJ3 | − | Atlantibacter sp. | OR889438 |
| XJ7 | − | Brevundimonas sp. | OR889439 |
| XJ12 | − | Klebsiella sp. | OR889440 |
| XJ16 | − | Enterobacter sp. | OR889441 |
| XXJ1 | − | Lysinibacillus sp. | OR889445 |
| XXJ6 | + | Bacillus sp. | OR889446 |
| XXJ9 | + | Bacillus sp. | OR889447 |
| XXJ17 | − | Enterobacter sp. | OR889448 |
| XNJ10 | + | Bacillus sp. | OR889455 |
| XNJ16 | + | Bacillus sp. | OR889456 |
| XNJ22 | + | Bacillus sp. | OR889466 |
| QJ1 | − | Pseudomonas sp. | OR889452 |
| QJ2 | − | Erwinia sp. | OR889453 |
| XQJ2 | + | Bacillus sp. | OR915404 |
| QJ14 | − | Stenotrophomonas sp. | OR889451 |
| XQJ4 | − | Pseudomonas sp. | OR889467 |
| GJ6a | − | Pseudomonas sp. | OR889449 |
| GJ18 | − | Siccibacter sp. | OR889450 |
| XGJ1a | + | Paenibacillus sp. | OR889463 |
| XGJ9a | + | Bacillus sp. | OR889468 |
| XGJ4 | − | Pseudomonas sp. | OR889464 |
| XGJ8 | + | Bacillus sp. | OR889454 |
| XGJ12 | + | Bacillus sp. | OR889465 |
| NM6 | − | Siccibacter sp. | OR889429 |
| NM8 | + | Bacillus sp. | OR889430 |
| NM10 | + | Bacillus sp. | OR889431 |
| NM15 | − | Enterobacter sp. | OR889432 |
| NM27 | − | Lelliottia sp. | OR889433 |
| NM31 | − | Pseudomonas sp. | OR889434 |
| NM42 | − | Erwinia sp. | OR889435 |
| XNM11 | + | Bacillus sp. | OR889442 |
| XNM15 | + | Bacillus sp. | OR889443 |
| XNM19 | − | Enterobacter sp. | OR889444 |
| JS-1 | − | Mixta sp. | OR889457 |
| JS-4 | + | Zhihengliuella sp. | OR889458 |
| JS-10 | + | Bacillus sp. | OR889459 |
| JS-19 | − | Siccibacter sp. | OR889460 |
| JS-25 | + | Bacillus sp. | OR889461 |
| JS-38 | + | Bacillus sp. | OR889462 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xie, J.; Chen, H.; Zhu, S.; Hou, X.; Zhang, Z. Diversity and Biological Characteristics of Seed-Borne Bacteria of Achnatherum splendens. Microorganisms 2024, 12, 339. https://doi.org/10.3390/microorganisms12020339
Yang J, Xie J, Chen H, Zhu S, Hou X, Zhang Z. Diversity and Biological Characteristics of Seed-Borne Bacteria of Achnatherum splendens. Microorganisms. 2024; 12(2):339. https://doi.org/10.3390/microorganisms12020339
Chicago/Turabian StyleYang, Jie, Jinjing Xie, Haiyan Chen, Shaowei Zhu, Xuan Hou, and Zhenfen Zhang. 2024. "Diversity and Biological Characteristics of Seed-Borne Bacteria of Achnatherum splendens" Microorganisms 12, no. 2: 339. https://doi.org/10.3390/microorganisms12020339





