Parasitosis by Fasciola hepatica and Variations in Gut Microbiota in School-Aged Children from Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Sampling
2.2. Ethics Statement
2.3. Samples and Sample Processing
2.3.1. Samples
2.3.2. Detection of Fasciola hepatica by ELISA
2.3.3. DNA Extraction
2.3.4. PCR Amplification for Detection of Gut Microbiota
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cwiklinski, K.; O’Neill, S.M.; Donnelly, S.; Dalton, J.P. A prospective view of animal and human Fasciolosis. Parasite Immunol. 2016, 38, 558–568. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical ethods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef]
- Nyindo, M.; Lukambagire, A.H. Fascioliasis: An Ongoing Zoonotic Trematode Infection. Biomed. Res. Int. 2015, 2015, 786195. [Google Scholar] [CrossRef]
- Marcos, L.; Terashima Leguia, G.; Canales, M.; Espinoza, J.R.; Gotuzzo, E. La Infección por Fasciola hepática en el Perú: Una Enfermedad Emergente. Rev. Gastroenterol. Perú 2007, 27, 389–396. [Google Scholar]
- Espinoza, J.; Terashima, A.; Herrera-Velit, P.; Marcos, L.A. Fasciolosis humana y animal en el Perú: Impacto en la economía de las zonas endémicas. Rev. Peru Med. Exp. Salud Publica 2010, 27, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Gironènes, N.; Valero, M.A.; García-Bodelón, M.A.; Chico-Calero, I.; Punzón, C.; Presno, M.; Mas-Coma, S. Immune suppression in advanced chronic fascioliasis: An experimental study in a rat model. J. Infect. Dis. 2007, 195, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.P.; Robinson, M.W.; Mulcahy, G.; O’Neill, S.M.; Donnelly, S. Immunomodulatory molecules of Fasciola hepatica: Candidates for both vaccine and immunotherapeutic development. Vet. Parasitol. 2013, 195, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Drugs for Parasitic Infections, 3rd ed.; The Medical Letter: New Rochelle, NY, USA, 2013.
- Villegas, F.; Angles, R.; Barrientos, R.; Barrios, G.; Valero, M.A.; Hamed, K.; Grueninger, H.; Ault, S.K.; Montresor, A.; Engels, D.; et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl. Trop. Dis. 2012, 6, e1720. [Google Scholar] [CrossRef]
- Sezgin, O.; Altintas, E.; Disibeyaz, S.; Saritas, Ü.; Sahin, B. Hepatobiliary fascioliasis: Clinical and radiologic features and endoscopic management. J. Clin. Gastroenterol. 2004, 38, 285. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.; Schmitt, E.K.; Chen, C.W.; Samantray, S.; Venishetty, V.K.; Hughes, D. Triclabendazole in the treatment of human fascioliasis: A review. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Toro-Londono, M.A.; Bedoya-Urrego, K.; Garcia-Montoya, G.M.; Galvan-Diaz, A.L.; Alzate, J.F. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ 2019, 7, e6200. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Mutapi, F. The gut microbiome in the helminth infected host. Trends Parasitol. 2015, 31, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, L.; Nausch, N.; Free, A.; Taylor, D.W.; Mutapi, F. The microbiota and helminths: Sharing the same niche in the human host. Parasitology 2014, 141, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Graham, A.L.; Knowles, S.C.L. Parasite-Microbiota Interactions With the Vertebrate Gut: Synthesis Through an Ecological Lens. Front. Microbiol. 2018, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Houlden, A.; Hayes, K.S.; Bancroft, A.J.; Worthington, J.J.; Wang, P.; Grencis, R.K.; Roberts, I.S. Chronic Trichuris muris Infection in C57BL/6 Mice Causes Significant Changes in Host Microbiota and Metabolome: Effects Reversed by Pathogen Clearance. PLoS ONE 2015, 10, e0125945. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, R.W.; Li, W.; Beshah, E.; Dawson, H.D.; Urban, J.F., Jr. Worm Burden-Dependent Disruption of the Porcine Colon Microbiota by Trichuris suis Infection. PLoS ONE 2012, 7, e35470. [Google Scholar] [CrossRef]
- Nourrisson, C.; Scanzi, J.; Pereira, B.; NkoudMongo, C.; Wawrzyniak, I.; Cian, A.; Viscogliosi, E.; Livrelli, V.; Delbac, F.; Dapoigny, M.; et al. Blastocystis Is Associated with Decrease of Fecal Microbiota Protective Bacteria: Comparative Analysis between Patients with Irritable Bowel Syndrome and Control Subjects. PLoS ONE 2014, 9, e111868. [Google Scholar] [CrossRef]
- Kay, G.L.; Millard, A.; Sergeant, M.J.; Midzi, N.; Gwisai, R.; Mduluza, T.; Ivens, A.; Nausch, N.; Mutapi, F.; Pallen, M. Differences in the faecal microbiome in Schistosoma haematobium infected children vs. uninfected children. PLoS Negl. Trop. Dis. 2015, 9, e0003861. [Google Scholar] [CrossRef]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef]
- Partida-Rodríguez, O.; Serrano-Vázquez, A.; Nieves-Ramírez, M.E.; Moran, P.; Rojas, L.; Portillo, T.; González, E.; Hernández, E.; Finlay, B.B.; Ximenez, C. Human Intestinal Microbiota: Interaction between Parasites and the Host Immune Response. Arch. Med. Res. 2017, 48, 690–700. [Google Scholar] [CrossRef]
- Valero, M.A.; Periago, M.V.; Pérez-Crespo, I.; Angles, R.; Villegas, F.; Aguirre, C.; Strauss, W.; Espinoza, J.R.; Herrera, P.; Terashima, A.; et al. Field evaluation of a coproantigen detection test for fascioliasis diagnosis and surveillance in human hyperendemic areas of Andean countries. PLoS Negl. Trop. Dis. 2012, 6, e1812. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut microbiota in children with type 1 diabetes difers from that in healthy children: A case–control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef]
- Benavides-Ward, A.; Vasquez-Achaya, F.; Silva-Caso, W.; Aguilar-Luis, M.A.; Mazulis, F.; Urteaga, N.; del Valle-Mendoza, J. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res. Notes. 2018, 11, 468. [Google Scholar] [CrossRef]
- Marcos, L.; Maco, V.; Samalvides, F.; Terashima, A.; Espinoza, J.R.; Gotuzzo, E. Risk factors for Fasciola hepatica infection in children: A case-control study. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 158–166. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Hewitson, J.P.; Maizels, R.M. Immunomodulation by helminth parasites: Defining mechanisms and mediators. Int. J. Parasitol. 2013, 43, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, R.; To, J.; Lund, M.E.; Pinar, A.; Mansell, A.; Robinson, M.W.; O’Brien, B.A.; Dalton, J.P.; Donnelly, S. The immune modulatory peptide FhHDM-1 secreted by the helminth Fasciola hepatica prevents NLRP3 inflammasome activation by inhibiting endolysosomal acidification in macrophages. FASEB J. 2017, 31, 85–95. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Thaiss, C.A.; Licona-Limon, P.; Flavell, R.A. Role of the intes-tinal microbiome in liver disease. J. Autoimmun. 2013, 46, 66–73. [Google Scholar] [CrossRef]
- Schnabl, B. Linking intestinal homeostasis and liver disease. Curr. Opin. Gastroenterol. 2013, 29, 264–270. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-liver axis in hepatic dis-ease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Nguyen Thu, H.; Dermauw, V.; Tran Huy, T.; Roucher, C.; Dorny, P.; Nguyen Thi, H.; Trung, K.H.; Dao Van, T.; Do Nhu, B.; Nguyen Kim, T. Diagnosing Human Fascioliasis Using ELISA Immunoassays at a Tertiary Referral Hospital in Hanoi: A Cross-Sectional Study. Trop. Med. Infect. Dis. 2022, 7, 76. [Google Scholar] [CrossRef]
- Harinasuta, T.; Pungpak, S.; Keystone, J.S. Trematode infections. Opisthorchiasis, clonorchiasis, fascioliasis, and paragonimiasis. Infect. Dis. Clin. N. Am. 1993, 7, 699. [Google Scholar] [CrossRef]
- Cruz YLópez, O.R.; Gómez de la Vega, E.; Cárdenas-Perea, M.E.; Gutiérrez-Dávila, A.; Tamariz-Cruz, O.J. Human fasciolosis diagnosed in the acute phase: A first clinical report in Mexico. Rev. Gastroenterol. Mex. 2016, 81, 111–113, (In English, Spanish). [Google Scholar] [CrossRef]
- Sezgın, O.; Altintaş, E.; Tombak, A.; Uçbılek, E. Fasciola hepatica-induced acute pancreatitis: Report of two cases and review of the literature. Turk. J. Gastroenterol. 2010, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Beştaş, R.; Cetin, S. Clinical presentation and management of Fasciola hepatica infection: Single-center experience. World J. Gastroenterol. 2011, 17, 4899. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Wexler, A.G.; Goodman, A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Azad, M.A.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P.I. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Hague, A.; Elder, D.J.; Hicks, D.J.; Paraskeva, C. Apoptosis in colorectal tumour cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer 1995, 60, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.L.; Herrera, G.; Muñoz, M.; Vega, L.; Cruz-Saavedra, L.; García-Corredor, D.; Pulido-Medellín, M.; Bulla-Castañeda, D.M.; Giraldo, J.C.; Bernal, M.C.; et al. Describing the intestinal microbiota of Holstein Fasciola-positive and -negative cattle from a hyperendemic area of fascioliasis in cen-tral Colombia. PLoS Negl. Trop. Dis. 2021, 15, e0009658. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Iorio, A.; Porcari, S.; Masucci, L.; Sanguinetti, M.; Perno, C.F.; Gasbarrini, A.; Putignani, L.; Cammarota, G. How the gut parasitome affects human health. Therap. Adv. Gastroenterol. 2022, 15, 17. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, Y.E.; Yıldız, M.R. Microbiota and parasite relationship. Diagn. Microbiol. Infect. Dis. 2023, 106, 115954. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | ELISA− | ELISA+ | Odds Ratio | Fisher Exact Test p | |
|---|---|---|---|---|---|---|
| Frequency N = 103 (%) | Frequency N = 87 (%) | Frequency N = 16 (%) | 95% CI | |||
| Age (range years) | ||||||
| 4–8 | 45 (43.7) | 38 (43.7) | 7 (43.8) | 23.1–66.8 | 1.003 | 1.000 |
| 9–14 | 58 (56.3) | 49 (56.3) | 9 (56.2) | 33.2–76.9 | 1.000 | 1.000 |
| Gender | ||||||
| Female | 48 (46.6) | 41 (47.1) | 7 (43.8) | 23.1–66.8 | 0.873 | 1.000 |
| Male | 55 (53.4) | 46 (52.9) | 9 (56.2) | 33.2–76.9 | 1.146 | 1.000 |
| Risk Factors | Total | ELISA− | ELISA+ | Odds Ratio | Fisher Exact Test p | ||
|---|---|---|---|---|---|---|---|
| Frequency N = 103 (%) | Frequency N = 87 (%) | Frequency N = 16 (%) | 95% CI | ||||
| Drinks | Boiled water | 80 (77.7) | 69 (79.3) | 11 (78.6) | 44.4–85.8 | 0.574 | 0.344 |
| Non-boiled water | 72 (69.9) | 58 (66.7) | 14 (87.5) | 64.0–96.5 | 3.500 | 0.139 | |
| Emollients/infusions | 33 (32.0) | 32 (36.8) | 1 (6.2) | 1.1–28.3 | 0.115 | 0.018 | |
| Ditch water | 18 (17.5) | 11 (12.6) | 7 (43.8) | 23.1–66.8 | 5.374 | 0.007 | |
| Meal | Andean lupin | 99 (96.1) | 84 (96.6) | 15 (93.8) | 71.7–98.9 | 0.536 | 0.497 |
| Raw lettuce | 96 (93.2) | 81 (93.1) | 15 (93.8) | 71.7–98.9 | 1.111 | 1.000 | |
| Raw salad | 84 (81.6) | 79 (90.8) | 15 (93.8) | 71.7–98.9 | 1.519 | 1.000 | |
| Watercress | 34 (33.0) | 31 (35.6) | 3 (18.8) | 6.6–43.0 | 0.417 | 0.253 | |
| Chews grass | 10 (9.7) | 10 (11.5) | 0 (0.0%) | 0.0–19.4 | 0.000 | 0.355 | |
| Livestock contact | Yes | 95 (92.2) | 80 (92.0) | 15 (93.8) | 71.7–98.9 | 1.312 | 1.000 |
| No | 8 (7.8) | 7 (8.0) | 1 (6.2) | 1.1–28.3 | 0.762 | 1.000 | |
| Clinical Symptoms | ELISA− | ELISA+ | Odds Ratio | Fisher Test p | |
|---|---|---|---|---|---|
| Frequency N = 87 (%) | Frequency N = 16 (%) | 95% CI | |||
| Abdominal pain in the last 3 months | 66 (75.9) | 11 (68.8) | 44.4–85.8 | 0.700 | 0.543 |
| Weight loss in the last 3 months | 47 (54.0) | 9 (56.2) | 33.2–76.9 | 1.094 | 1.000 |
| Diarrhea in the last 3 months | 17 (19.5) | 5 (31.2) | 14.2–55.6 | 1.872 | 0.325 |
| Headache | 8 (9.2) | 1 (6.2) | 1.1–28.3 | 0.658 | 1.000 |
| Nausea | 6 (6.9) | 2 (12.5) | 3.5–36.0 | 1.929 | 0.607 |
| Vomiting | 5 (5.7) | 3 (18.8) | 6.6–43.0 | 3.785 | 0.106 |
| Fever | 5 (5.7) | 2 (12.5) | 3.5–36.0 | 2.343 | 0.297 |
| Abdominal pain | 2 (2.3) | 1 (6.2) | 1.1–28.3 | 2.833 | 0.401 |
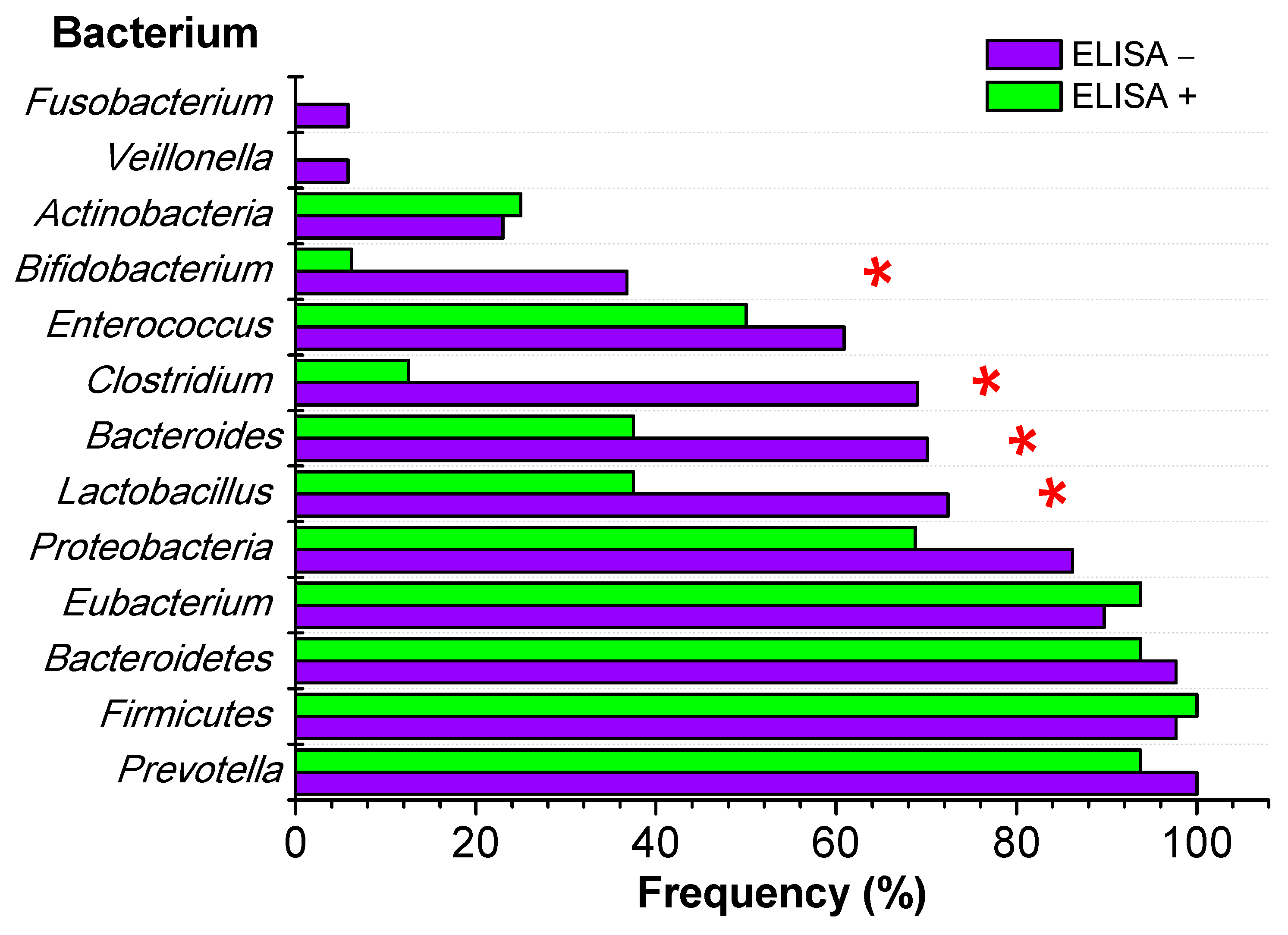
| Bacterium | ELISA− | ELISA+ | Fisher Test p | |
|---|---|---|---|---|
| Frequency N = 87 (%) | Frequency N = 16 (%) | 95% CI | ||
| Prevotella | 87 (100.0) | 15 (93.8) | 71.1–98.9 | 0.155 |
| Firmicutes | 85 (97.7) | 16 (100.0) | 80.6–100.0 | 1.000 |
| Bacteroidetes | 85 (97.7) | 15 (93.8) | 71.1–98.9 | 0.401 |
| Eubacterium | 78 (89.7) | 15 (93.8) | 71.7–98.9 | 1.000 |
| Proteobacteria | 75 (86.2) | 11 (68.8) | 44.4–85.8 | 0.135 |
| Lactobacillus | 63 (72.4) | 6 (37.5) | 18.5–61.4 | 0.010 |
| Bacteroides | 61 (70.1) | 6 (37.5) | 18.5–61.4 | 0.020 |
| Clostridium | 60 (69.0) | 2 (12.5) | 3.5–36.0 | <0.001 |
| Enterococcus | 53 (60.9) | 8 (50.0) | 2.8–72.0 | 0.423 |
| Bifidobacterium | 32 (36.8) | 1 (6.2) | 1.1–28.3 | 0.018 |
| Actinobacteria | 20 (23.0) | 4 (25.0) | 10.2–49.5 | 1.000 |
| Veillonella | 5 (5.8) | 0 (0.0) | 0.0–19.4 | 1.000 |
| Fusobacterium | 5 (5.8) | 0 (0.0) | 0.0–19.4 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Caso, W.; Carrillo-Ng, H.; Aguilar-Luis, M.A.; Tarazona-Castro, Y.; Valle, L.J.D.; Tinco-Valdez, C.; Palomares-Reyes, C.; Urteaga, N.; Bazán-Mayra, J.; Valle-Mendoza, J.d. Parasitosis by Fasciola hepatica and Variations in Gut Microbiota in School-Aged Children from Peru. Microorganisms 2024, 12, 371. https://doi.org/10.3390/microorganisms12020371
Silva-Caso W, Carrillo-Ng H, Aguilar-Luis MA, Tarazona-Castro Y, Valle LJD, Tinco-Valdez C, Palomares-Reyes C, Urteaga N, Bazán-Mayra J, Valle-Mendoza Jd. Parasitosis by Fasciola hepatica and Variations in Gut Microbiota in School-Aged Children from Peru. Microorganisms. 2024; 12(2):371. https://doi.org/10.3390/microorganisms12020371
Chicago/Turabian StyleSilva-Caso, Wilmer, Hugo Carrillo-Ng, Miguel Angel Aguilar-Luis, Yordi Tarazona-Castro, Luis J. Del Valle, Carmen Tinco-Valdez, Carlos Palomares-Reyes, Numan Urteaga, Jorge Bazán-Mayra, and Juana del Valle-Mendoza. 2024. "Parasitosis by Fasciola hepatica and Variations in Gut Microbiota in School-Aged Children from Peru" Microorganisms 12, no. 2: 371. https://doi.org/10.3390/microorganisms12020371
APA StyleSilva-Caso, W., Carrillo-Ng, H., Aguilar-Luis, M. A., Tarazona-Castro, Y., Valle, L. J. D., Tinco-Valdez, C., Palomares-Reyes, C., Urteaga, N., Bazán-Mayra, J., & Valle-Mendoza, J. d. (2024). Parasitosis by Fasciola hepatica and Variations in Gut Microbiota in School-Aged Children from Peru. Microorganisms, 12(2), 371. https://doi.org/10.3390/microorganisms12020371






