Denitrification Characteristics of the Low-Temperature Tolerant Denitrification Strain Achromobacter spiritinus HS2 and Its Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Strain
2.2. Molecular Identification
2.3. Nitrification Performance under Different Temperature
2.4. Nitrification Performance under Different Culture Conditions
2.5. Nitrogen Balance Analysis
2.6. The Nitrogen Removal Pathway of Strain HS2
2.7. The Strain HS2 Applied in Sewage
2.8. Analytical Analysis
3. Results
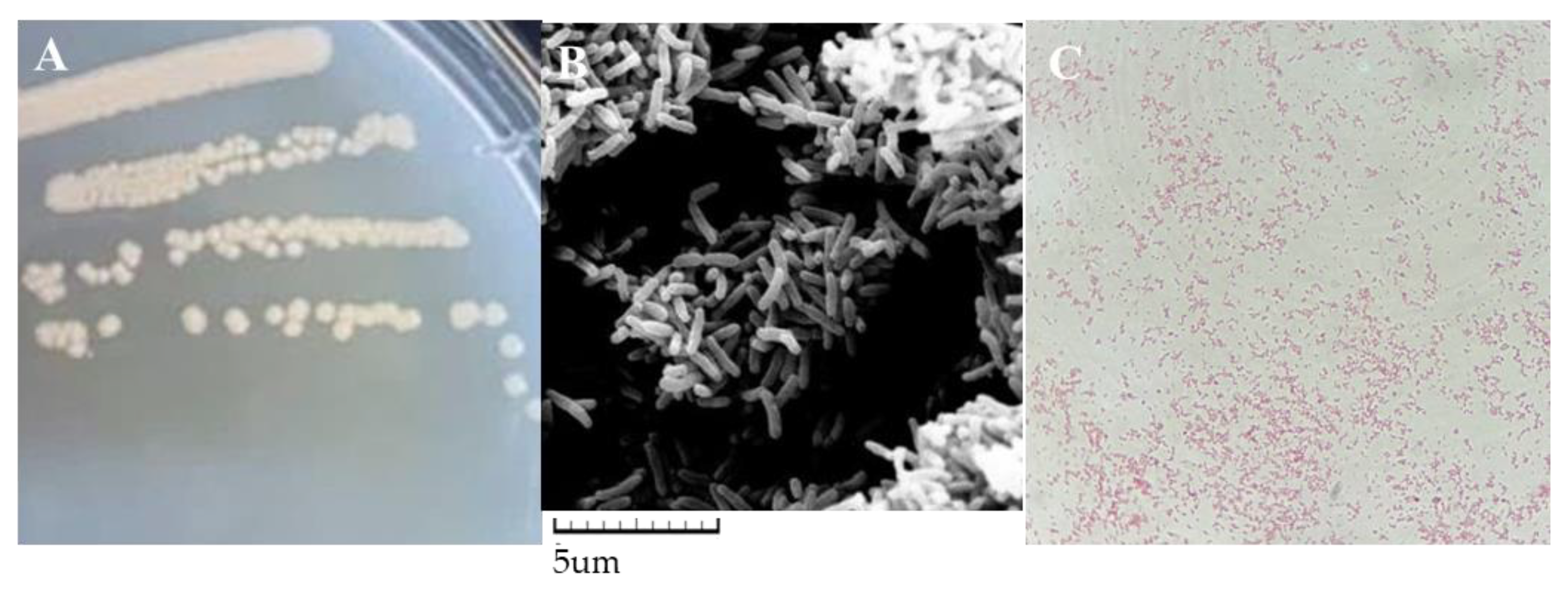
3.1. Isolation and Identification of Cold-Tolerant Strain
3.2. Nitrification Performance of Strain HS2 under Different Temperature
3.3. Nitrification Performance under Different Culture Conditions
3.4. Nitrogen Balance Analysis
3.5. The Nitrogen Removal Pathway of Strain HS2
3.6. The Application of Strain HS2 in Sewage Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yang, M.; Lu, D.W.; Qin, B.D.; Liu, Q.L.; Zhao, Y.M.; Liu, H.L.; Ma, J. Highly efficient nitrogen removal of a coldness-resistant and low nutrient needed bacterium, Janthinobacterium sp. M-11. Bioresour. Technol. 2018, 256, 366–373. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.L.; Li, J.; Wu, X.Y.; Feng, H.X.; Dong, W.Y. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Yao, S.; Ni, J.R.; Chen, Q.; Borthwick, A.G.L. Enrichment and characterization of a bacteria consortium capable of heterotrophic nitrification and aerobic denitrification at low temperature. Bioresour. Technol. 2013, 127, 151–157. [Google Scholar] [CrossRef]
- Zhao, T.T.; Chen, P.P.; Zhang, L.J.; Zhang, L.; Gao, Y.H.; Ai, S.; Liu, H.; Liu, X.Y. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. TAC-1 at low temperature and high ammonia nitrogen. Bioresour. Technol. 2021, 339, 125620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, G.M.; Wu, M.R.; Li, S.; Miao, L.; Liu, Z. Photobacterium sp. NNA4, an efficient hydroxylamine-transforming heterotrophic nitrifier/aerobic denitrifier. J. Biosci. Bioeng. 2019, 128, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Mishra, A.K. Nitrogen removal and metabolic profiling of a cold-adaptive and biofilm producing paddy soil bacterium Cupriavidus sp. PDN31. Arch. Agron. Soil Sci. 2018, 64, 1608–1621. [Google Scholar] [CrossRef]
- Xu, Y.; He, T.X.; Li, Z.L.; Ye, Q.; Chen, Y.L.; Xie, E.Y.; Zhang, X. Nitrogen Removal Characteristics of Pseudomonas putida Y-9 Capable of Heterotrophic Nitrification and Aerobic Denitrification at Low Temperature. BioMed Res. Int. 2017, 2017, 1429018. [Google Scholar] [CrossRef]
- Dos Santos, P.R.; Daniel, L.A. A review: Organic matter and ammonia removal by biological activated carbon filtration for water and wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 591–606. [Google Scholar] [CrossRef]
- Kou, L.Q.; Huang, T.L.; Zhang, H.H.; Wen, G.; Li, N.; Wang, C.X.; Lu, L.C. Mix-cultured aerobic denitrifying bacterial communities reduce nitrate: Novel insights in micro-polluted water treatment at lower temperature. Sci. Total Environ. 2021, 796, 148910. [Google Scholar] [CrossRef]
- Yang, J.R.; Wang, Y.; Chen, H.; Lyu, Y.K. Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2019, 274, 56–64. [Google Scholar] [CrossRef]
- Kucera, I.; Laucik, J.; Dadak, V. The function of cytoplasmic membrane of paracoccus-denitrificans in controlling the rate of reduction of terminal acceptors. Eur. J. Biochem. 1983, 136, 135–140. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef]
- Gu, X.; Leng, J.; Zhu, J.; Zhang, K.; Zhao, J.; Wu, P.; Xing, Q.; Tang, K.; Li, X.; Hu, B. Influence mechanism of C/N ratio on heterotrophic nitrification- aerobic denitrification process. Bioresour. Technol. 2022, 343, 126116. [Google Scholar] [CrossRef]
- Joo, H.S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef]
- Huang, T.; Guo, L.; Zhang, H.; Su, J.; Wen, G.; Zhang, K. Nitrogen-removal efficiency of a novel aerobic denitrifying bacterium, Pseudomonas stutzeri strain ZF31, isolated from a drinking-water reservoir. Bioresour. Technol. 2015, 196, 209–216. [Google Scholar] [CrossRef]
- Wu, S.; Lv, N.; Zhou, Y.; Li, X. Simultaneous nitrogen removal via heterotrophic nitrification and aerobic denitrification by a novel Lysinibacillus fusiformis B301. Water Environ. Res. 2023, 95, e10850. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examinations of Water and Wastewater; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Dong, L.; Ge, Z.; Qu, W.; Fan, Y.; Dai, Q.; Wang, J. Characteristics and mechanism of heterotrophic nitrification/aerobic denitrification in a novel Halomonas piezotolerans strain. J. Basic Microbiol. 2022, 62, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Pramanik, A.; Dasgupta, A.; Mukherjee, S.; Mukherjee, J. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by Chryseobacterium sp. R31 Isolated from Abattoir Wastewater. BioMed Res. Int. 2014, 2014, 436056. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Zhao, B.H.; Wu, Y.M.; Huang, J.; Wang, H.Z.; Sun, X.Y.; Li, S.J. Characterization of Alcaligenes aquatilis as a novel member of heterotrophic nitrifier-aerobic denitrifier and its performance in treating piggery wastewater. Bioresour. Technol. 2022, 354, 127176. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.K.; Maiti, N.K. Molecular insight into the dynamic central metabolic pathways of Achromobacter xylosoxidans CF-S36 during heterotrophic nitrogen removal processes. J. Biosci. Bioeng. 2017, 123, 46–55. [Google Scholar] [CrossRef]
- Gupta, R.K.; Poddar, B.J.; Nakhate, S.P.; Chavan, A.R.; Singh, A.K.; Purohit, H.J.; Khardenavis, A.A. Role of heterotrophic nitrifiers and aerobic denitrifiers in simultaneous nitrification and denitrification process: A nonconventional nitrogen removal pathway in wastewater treatment. Lett. Appl. Microbiol. 2022, 74, 159–184. [Google Scholar] [CrossRef]
- Wei, R.; Hui, C.; Zhang, Y.P.; Jiang, Y.H.; Zhao, Y.H.; Du, L.N. Nitrogen removal characteristics and predicted conversion pathways of a heterotrophic nitrification-aerobic denitrification bacterium, Pseudomonas aeruginosa P-1. Environ. Sci. Pollut. Res. Int. 2021, 28, 7503–7514. [Google Scholar] [CrossRef]
- Huang, M.Q.; Cui, Y.W.; Huang, J.L.; Sun, F.L.; Chen, S. A novel Pseudomonas aeruginosa strain performs simultaneous heterotrophic nitrification-aerobic denitrification and aerobic phosphate removal. Water Res. 2022, 221, 118823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Xu, A.A.; Awasthi, M.K.; Kong, D.D.; Chen, J.S.; Wang, Y.F.; Xu, P. Aerobic denitrification performance and nitrate removal pathway analysis of a novel fungus Fusarium solani RADF-77. Bioresour. Technol. 2020, 295, 122250. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yan, J.B.; Wu, L.; Bao, Y.Z.; Yu, D.Q.; Li, J. Simultaneous nitrification and denitrification of hypersaline wastewater by a robust bacterium Halomonas salifodinae from a repeated-batch acclimation. Bioresour. Technol. 2021, 341, 125818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, G.M.; Miao, L.L.; Liu, Z.P. Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Bioresour. Technol. 2016, 206, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.T.; Bohu, T.; Wu, S.H.; Bai, Z.H.; Zhuang, X.L. Nitrogen Removal Characteristics and Constraints of an Alphaproteobacteria with Potential for High Nitrogen Content Heterotrophic Nitrification-Aerobic Denitrification. Microorganisms 2022, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Sha, C.Q.; Jiang, W.; Li, W.G.; Zhang, D.Y.; Li, J.; Meng, L.Q.; Piao, Y.J. Ammonium removal at low temperature by a newly isolated heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas fluorescens wsw-1001. Environ. Technol. 2015, 36, 2488–2494. [Google Scholar] [CrossRef]
- Liu, S.X.; Liu, Q.; Wu, H.; Jiang, W.Y.; Kahaer, A.; Tang, Q.; Hu, Z.Q.; Hong, C.; Liu, D.Q. Integrative chemical and omics analysis of the ammonia nitrogen removal characteristics and mechanism of a novel oligotrophic heterotrophic nitrification-aerobic denitrification bacterium. Sci. Total Environ. 2022, 852, 158519. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Dafale, N.A.; Hathi, Z.; Purohit, H. Genomic and functional potential of the immobilized microbial consortium MCSt-1 for wastewater treatment. Sci. Total Environ. 2021, 777, 146110. [Google Scholar] [CrossRef]
- Mupindu, P.; Zhao, Y.; Wang, X.; Hu, Y. Effect of sulfamethoxazole on nitrate removal by simultaneous heterotrophic aerobic denitrification. Water Environ. Res. 2022, 94, e10716. [Google Scholar] [CrossRef]
- Bucci, P.; Coppotelli, B.; Morelli, I.; Zaritzky, N.; Caravelli, A. Heterotrophic nitrification-aerobic denitrification performance in a granular sequencing batch reactor supported by next generation sequencing. Int. Biodeterior. Biodegrad. 2021, 160, 105210. [Google Scholar] [CrossRef]
- Zheng, M.; He, D.; Ma, T.; Chen, Q.; Liu, S.; Ahmad, M.; Gui, M.; Ni, J. Reducing NO and N2O emission during aerobic denitrification by newly isolated Pseudomonas stutzeri PCN-1. Bioresour. Technol. 2014, 162, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Zi, X.L.; Wang, X.F.; Zhang, X.; Gao, H.F.; Hu, N. Marinobacter hydrocarbonoclasticus NY-4, a novel denitrifying, moderately halophilic marine bacterium. Springerplus 2013, 2, 346. [Google Scholar] [CrossRef] [PubMed]
- Neissi, A.; Rafiee, G.; Farahmand, H.; Rahimi, S.; Mijakovic, L. Cold-Resistant Heterotrophic Ammonium and Nitrite-Removing Bacteria Improve Aquaculture Conditions of Rainbow Trout (Oncorhynchus mykiss). Microb. Ecol. 2020, 80, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Wehrfritz, J.M.; Reilly, A.; Spiro, S.; Richardson, D.J. Purification of hydroxylamine oxidase from thiosphaera pantotropha: Identification of electron acceptors that couple heterotrophic nitrification to aerobic denitrification. Fed. Eur. Biochem. Soc. 1993, 335, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Mpongwana, N.; Ntwampe, S.K.O.; Mekuto, L.; Akinpelu, E.A.; Dyantyi, S.; Mpentshu, Y. Isolation of high-salinity-tolerant bacterial strains, Enterobacter sp., Serratia sp., Yersinia sp., for nitrification and aerobic denitrification under cyanogenic conditions. Water Sci. Technol. 2016, 73, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, J.; He, L.; He, X.; Pan, Z.; Wang, Y.; He, Q. High-temperature biofilm system based on heterotrophic nitrification and aerobic denitrification treating high-strength ammonia wastewater: Nitrogen removal performances and temperature-regulated metabolic pathways. Bioresour. Technol. 2022, 344 Pt A, 126184. [Google Scholar] [CrossRef]
- Mendoza, L.F.D.; Quimi Mujica, J.G.; Risco Cunayque, J.M.; Aroni Lucana, G.W.; Intriago Angulo, J.J.; De la Cruz, V.I.S.; Escobar, V.A.C.; Matonnier, E.M. Assessment of Heterotrophic Nitrification Capacity in Bacillus spp. and its Potential Application in the Removal of Nitrogen from Aquaculture Water. J. Pure Appl. Microbiol. 2019, 13, 1893–1908. [Google Scholar] [CrossRef]
- Shukla, S.; Rajta, A.; Setia, H.; Bhatia, R. Simultaneous nitrification–denitrification by phosphate accumulating microorganisms. World J. Microbiol. Biotechnol. 2020, 36, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.G.; Chen, L.; Zhou, Y.J.; Meng, L.Q.; Zhang, S.M. Isolation and application of a thermotolerant nitrifying bacterium Gordonia paraffinivorans N52 in sewage sludge composting for reducing nitrogen loss. Bioresour. Technol. 2022, 363, 127959. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, S.; Uematsu, C.; Dohra, H.; Fujiwara, T. Pyruvic oxime dioxygenase from heterotrophic nitrifier Alcaligenes faecalis is a nonheme Fe(II)-dependent enzyme homologous to class II aldolase. Sci. Rep. 2017, 7, 39991. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.K.; Tripathy, S.; Mohanty, S.; Maiti, N.K. Aerobic and heterotrophic nitrogen removal by Enterobacter cloacae CF-S27 with efficient utilization of hydroxylamine. Bioresour. Technol. 2017, 232, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Hu, Y.Y.; Liang, D.H.; Wang, G.B.; Xie, J.Y.; Zhu, X.Q. Enhanced denitrification of sewage via bio-microcapsules embedding heterotrophic nitrification-aerobic denitrification bacteria Acinetobacter pittii SY9 and corn cob. Bioresour. Technol. 2022, 358, 127260. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Shi, J. Micro-Polluted Surface Water Treated by Yeast-Chitosan Bio-Microcapsules. Materials 2020, 13, 3519. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.Y.; Zhao, M.; Yuan, B.H.; Yao, J.C.; Chen, J.; Hrynshpan, D.; Savitskaya, T. A fungus-bacterium co-culture synergistically promoted nitrogen removal by enhancing enzyme activity and electron transfer. Sci. Total Environ. 2021, 754, 142109. [Google Scholar] [CrossRef]






| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| 16S | 27F: AGAGTTTGATCMTGGCTCAG |
| 1492R: 5′-TACGGYTACCTTGTTACGACTT | |
| napA | Nap1: TCTGGACCATGGGCTTCAACCA |
| Nap2: ACGACGACCGGCCAGCGCAG | |
| nirK | Nir-F: CTACTTCTCCCATCATAC |
| Nir-R: CACAGGTTGTTGTTCACT | |
| norB | Nor-F: CGNGARTTYCTSGARCARCC |
| Nor-R: CRTADGCVCCRWAGAAVGC | |
| nosZ | Nos-F: CGYTGTTCMTCGACAGCCAG |
| Nos-R: CGSACCTTSTTGCCSTYGCG |
| Initial TN (mg/L) | Final N (mg/L) | Intracellular-N (mg/L) | N Lose (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| NH4+–N | NH2OH | NO3−–N | NO2−–N | Organic N | |||
| 198.96 ± 0.86 | 0 | 0.52 ± 0.12 | 0 | 0 | 32.18 ± 1.71 | 116.12 ± 7.52 | 50.22 ± 6.01 |
| Gene | Gene ID | Function |
|---|---|---|
| Denitrogen | ||
| NarI | gene 2675 | respiratory nitrate reductase subunit gamma |
| NarH | gene 2677 | nitrate reductase subunit beta |
| NarG | gene 2678 | nitrate reductase subunit alpha |
| Nar | gene 5329 | nitrate reductase |
| MoaA | gene 2673 | molybdenum cofactor biosynthesis protein MoaA |
| MoaD | gene 199 | molybdenum cofactor biosynthesis protein |
| MoaE | gene 615 | molybdenum cofactor biosynthesis protein |
| Mob B | gene 616 | molybdenum cofactor biosynthesis protein B |
| MoeA | gene 617 | molybdenumtransferase |
| MoeB | gene 3185 | molybdopterin-synthase adenylyltransferase |
| Assimilatory nitrite reduction | ||
| NirD | gene 776 | nitrite reductase (NAD(P)H) small subunit |
| NirB | gene 777 | nitrite reductase large subunit |
| Nitrification process | ||
| AMO | gene 3664 | ammonia monooxygenase |
| Amino acid metabolism | ||
| GlnA | gene 1683 | glutamine synthetase |
| GlnH | gene 4575 | glutamine ABC transporter substrate-binding protein |
| GlnP | gene 4576 | glutamine ABC transporter permease |
| Gud | gene 3273 | NADP-specific glutamate dehydrogenase |
| GltA | gene 4215 | glutamate synthase subunit alpha |
| GltB | gene 4216 | glutamate synthase subunit beta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.-J.; Zhang, T.; Hu, L.-K.; Liu, S.-Y.; Li, C.-C.; Jin, Y.-S.; Liu, H.-B. Denitrification Characteristics of the Low-Temperature Tolerant Denitrification Strain Achromobacter spiritinus HS2 and Its Application. Microorganisms 2024, 12, 451. https://doi.org/10.3390/microorganisms12030451
Gao Y-J, Zhang T, Hu L-K, Liu S-Y, Li C-C, Jin Y-S, Liu H-B. Denitrification Characteristics of the Low-Temperature Tolerant Denitrification Strain Achromobacter spiritinus HS2 and Its Application. Microorganisms. 2024; 12(3):451. https://doi.org/10.3390/microorganisms12030451
Chicago/Turabian StyleGao, Ya-Juan, Ting Zhang, Ling-Kang Hu, Shi-Yuan Liu, Chen-Chen Li, Yong-Sheng Jin, and Hong-Bin Liu. 2024. "Denitrification Characteristics of the Low-Temperature Tolerant Denitrification Strain Achromobacter spiritinus HS2 and Its Application" Microorganisms 12, no. 3: 451. https://doi.org/10.3390/microorganisms12030451
APA StyleGao, Y.-J., Zhang, T., Hu, L.-K., Liu, S.-Y., Li, C.-C., Jin, Y.-S., & Liu, H.-B. (2024). Denitrification Characteristics of the Low-Temperature Tolerant Denitrification Strain Achromobacter spiritinus HS2 and Its Application. Microorganisms, 12(3), 451. https://doi.org/10.3390/microorganisms12030451





