Histopathology and Phylogeny of the Dinoflagellate Hematodinium perezi and the Epibiotic Peritrich Ciliate Epistylis sp. Infecting the Blue Crab Callinectes sapidus in the Eastern Mediterranean
Abstract
:1. Introduction
2. Materials and Methods
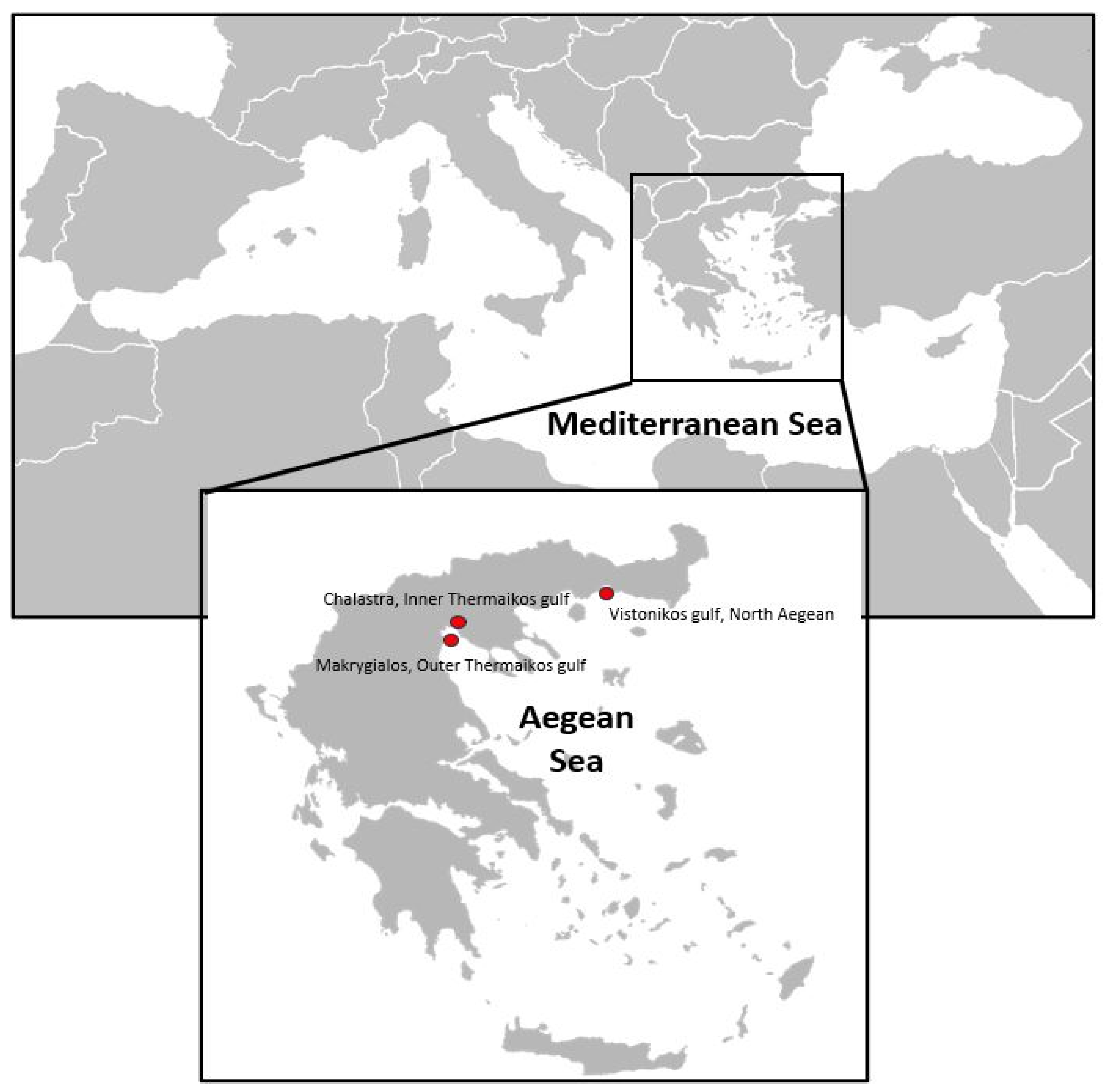
2.1. Animal Collection
2.2. Tissue Sampling
2.3. Histopathology
2.4. Molecular Detection and Identification of Hematodinium sp. and Ciliates
3. Results
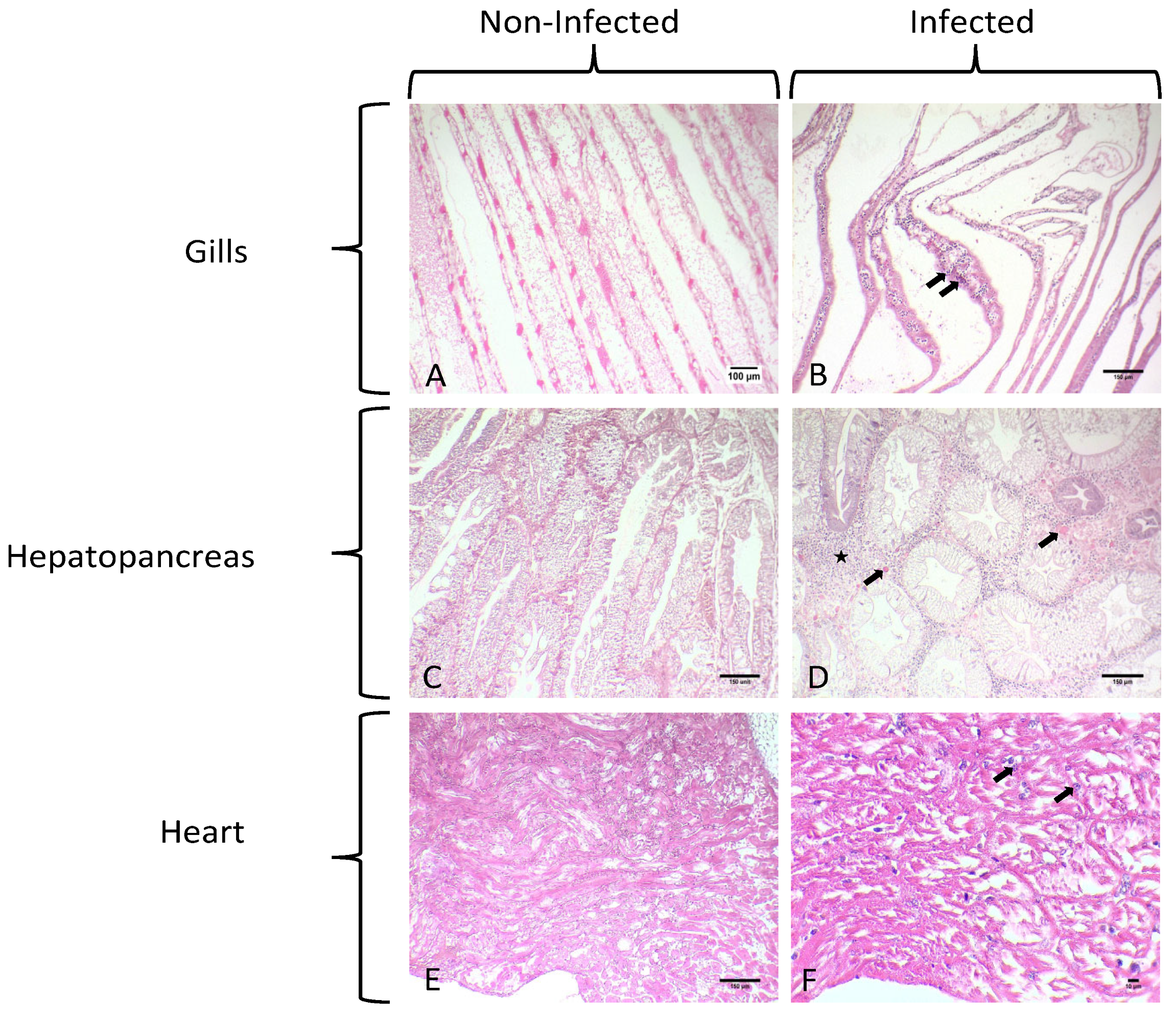
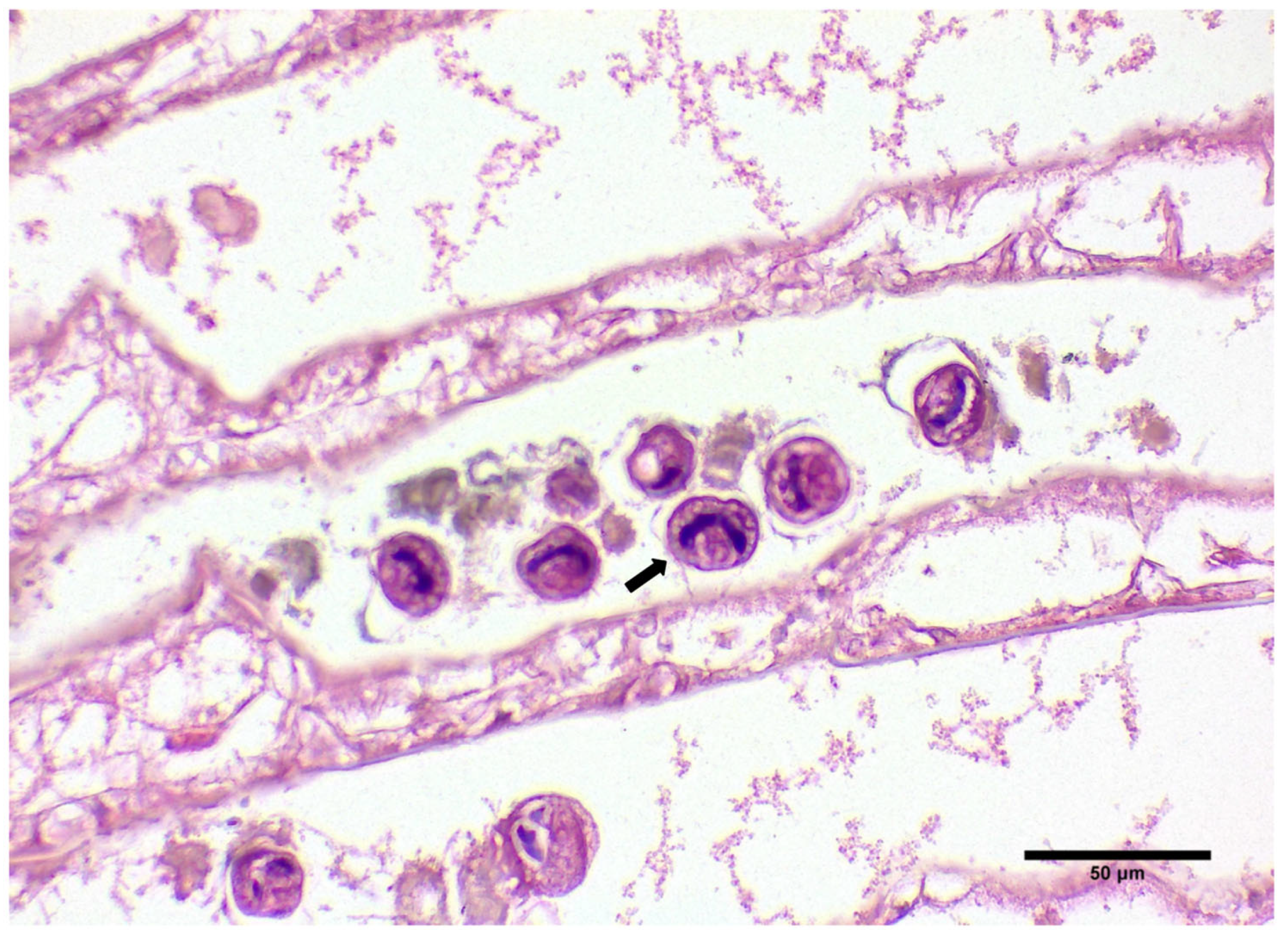
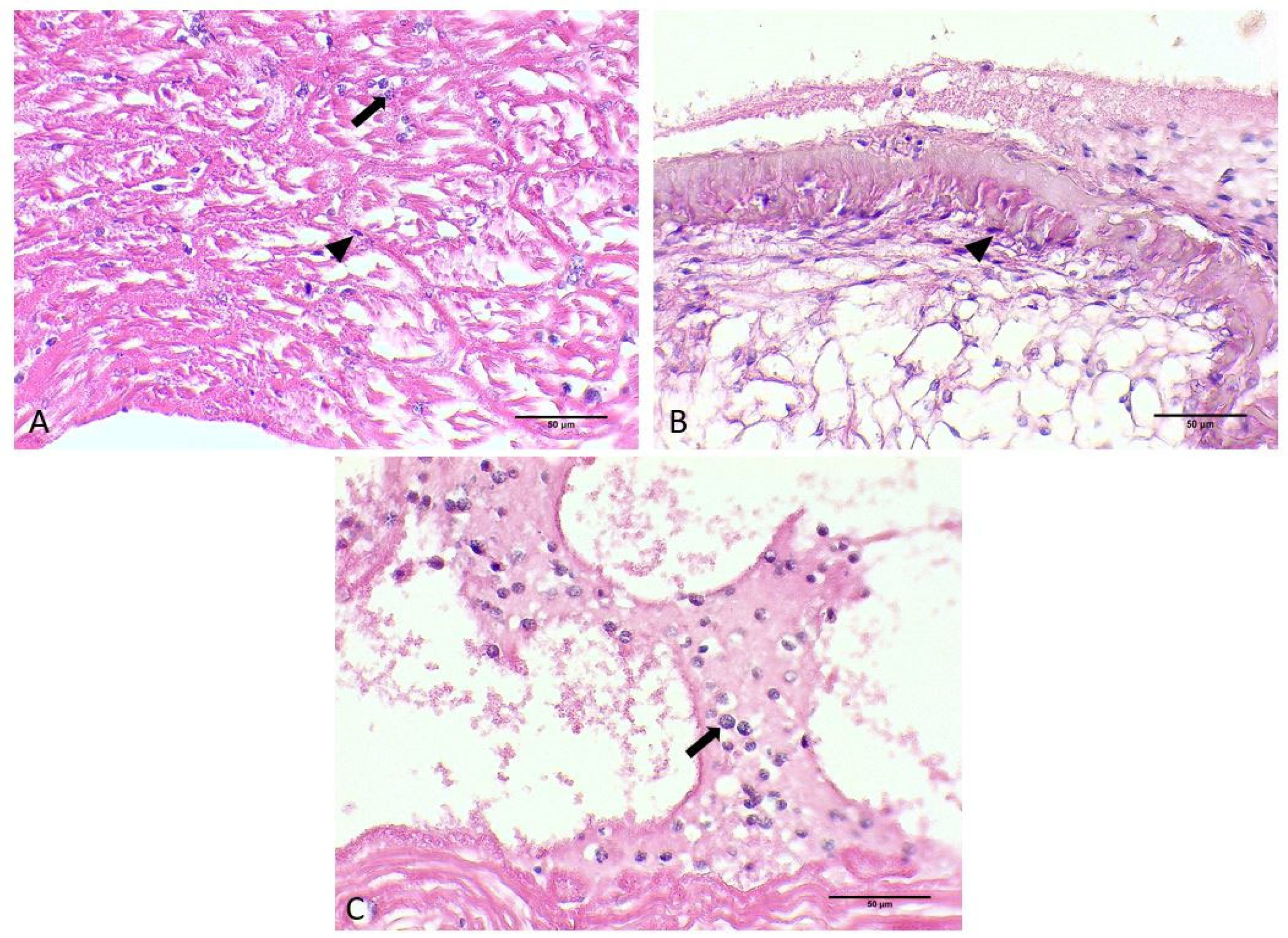
3.1. Gross Signs and Histopathological Results
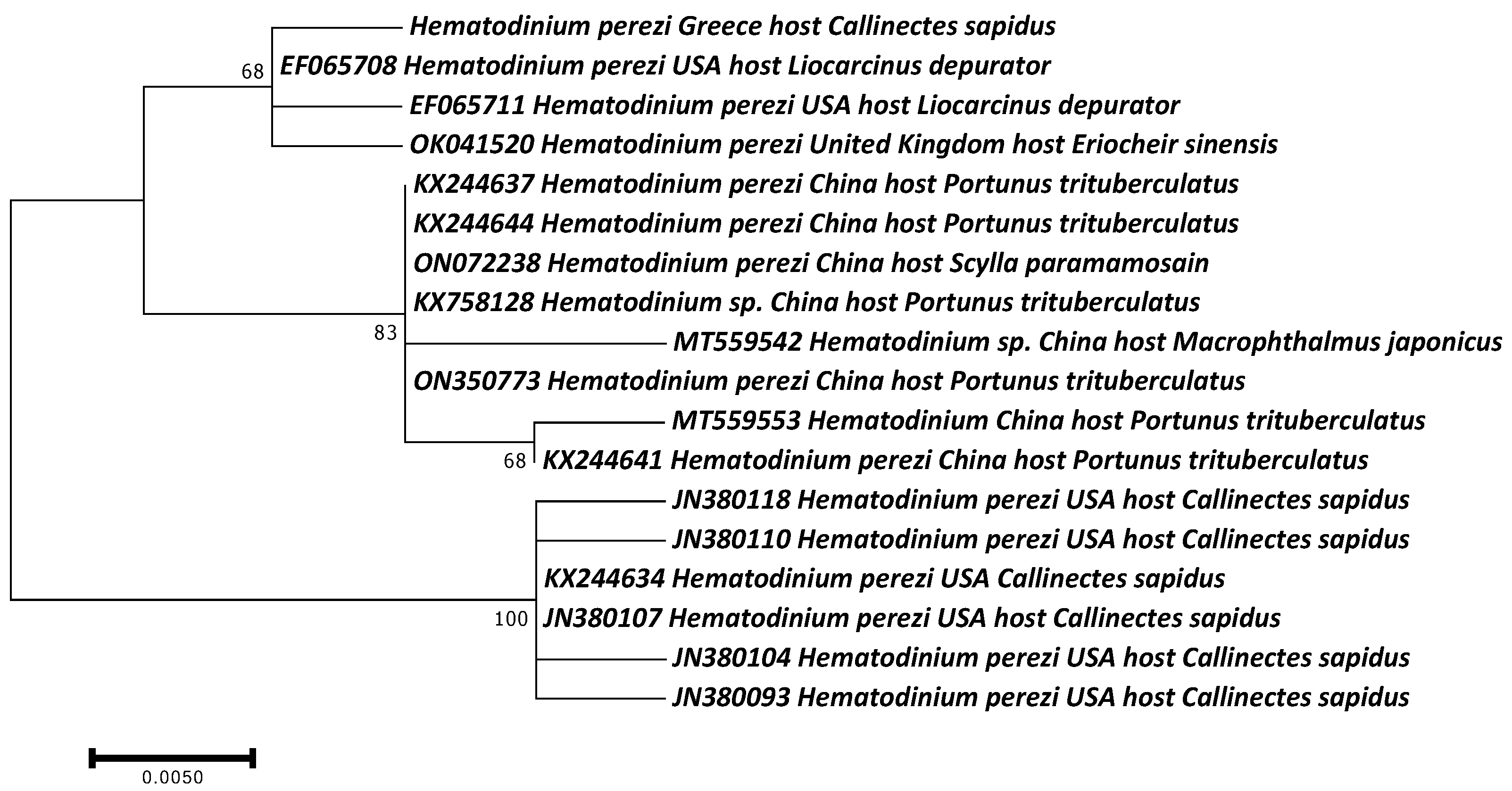
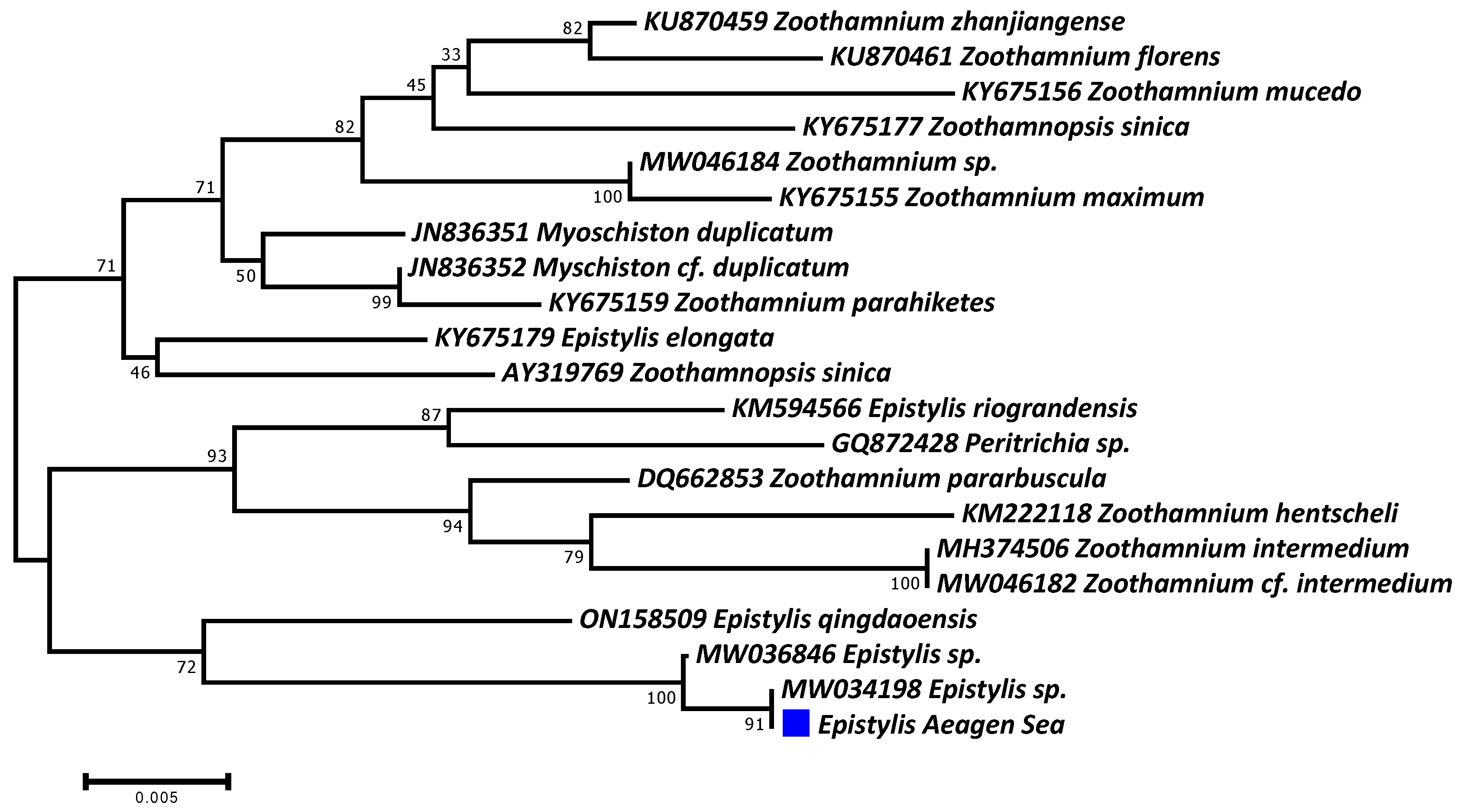
3.2. Molecular Identification of Parasites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lattos, A.; Chaligiannis, I.; Papadopoulos, D.; Giantsis, I.A.; Petridou, E.I.; Vafeas, G.; Staikou, A.; Michaelidis, B. How Safe to Eat Are Raw Bivalves? Host Pathogenic and Public Health Concern Microbes within Mussels, Oysters, and Clams in Greek Markets. Foods 2021, 10, 2793. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Facon, B.; Genton, B.J.; Shykoff, J.; Jarne, P.; Estoup, A.; David, P. A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol. Evol. 2006, 21, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.A.; Doblin, M.A.; Dobbs, F.C. Potential microbial bioinvasions via ships’ ballast water, sediment, and biofilm. Mar. Pollut. Bull. 2007, 55, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.E.; Gardner, T.; Vives, S.P.; Gilligan, M.R.; Courtenay, W.R.; Ray, G.C.; Hare, J.A. Biological invasion of the Indo-Pacific lionfish Pterois volitans along the Atlantic coast of North America. Mar. Ecol. Prog. Ser. 2002, 235, 289–297. [Google Scholar] [CrossRef]
- Ojaveer, H.; Galil, B.S.; Carlton, J.T.; Alleway, H.; Goulletquer, P.; Lehtiniemi, M.; Marchini, A.; Miller, W.; Occhipinti-Ambrogi, A.; Peharda, M.; et al. Historical baselines in marine bioinvasions: Implications for policy and management. PLoS ONE 2018, 13, e0202383. [Google Scholar] [CrossRef]
- Seebens, H.; Gastner, M.T.; Blasius, B. The risk of marine bioinvasion caused by global shipping. Ecol. Lett. 2013, 16, 782–790. [Google Scholar] [CrossRef]
- Giantsis, I.A.; Castells Sierra, J.; Chaskopoulou, A. The distribution of the invasive pest, rice water weevil Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae), is expanding in Europe: First record in the Balkans, confirmed by CO1 DNA barcoding. Phytoparasitica 2017, 45, 147–149. [Google Scholar] [CrossRef]
- Nunes, A.L.; Katsanevakis, S.; Zenetos, A.; Cardoso, A.C. Gateways to alien invasions in the European seas. Aquat. Invasions 2014, 9, 133–144. [Google Scholar] [CrossRef]
- Small, H.J.; Huchin-Mian, J.P.; Reece, K.S.; Pagenkopp Lohan, K.M.; Butler, M.J.; Shields, J.D. Parasitic dinoflagellate Hematodinium perezi prevalence in larval and juvenile blue crabs Callinectes sapidus from coastal bays of Virginia. Dis. Aquat. Organ. 2019, 134, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Pollut. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kampouris, T.E.; Kouroupakis, E.; Batjakas, I.E. Morphometric relationships of the global invader Callinectes sapidus Rathbun, 1896 (Decapoda, brachyura, portunidae) from Papapouli lagoon, NW Aegean Sea, Greece. with notes on its ecological preferences. Fishes 2020, 5, 5. [Google Scholar] [CrossRef]
- Patrizia, P.; Giorgio, M. Parasites affect hemocyte functionality in the hemolymph of the invasive Atlantic blue crab Callinectes sapidus from a coastal habitat of the Salento Peninsula (SE Italy). Mediterr. Mar. Sci. 2018, 19, 193–200. [Google Scholar] [CrossRef]
- Nehring, S. Invasion history and success of the American blue crab Callinectes sapidus Rathbun, 1896 in European and adjacent waters. In In the Wrong Place–Alien Marine Crustaceans: Distribution, Biology and Impacts Invading Nature; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer Series in Invasion Ecology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 607–624. [Google Scholar]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish. Res. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Mizerek, T.; Regan, H.M.; Hovel, K.A. Seagrass habitat loss and fragmentation influence management strategies for a blue crab Callinectes sapidus fishery. Mar. Ecol. Prog. Ser. 2011, 427, 247–257. [Google Scholar] [CrossRef]
- Bilen, C.T.; Yesilyurt, I.N. Growth of blue crab, Callinectes sapidus, in the Yumurtalik Cove, Turkey: A molt process approach. Cent. Eur. J. Biol. 2014, 9, 49–57. [Google Scholar] [CrossRef]
- Manfrin, C.; Turolla, E.; Chung, J.S.; Giulianini, P.G. First occurrence of Callinectes sapidus (Rathbun, 1896) within the Sacca di Goro (Italy) and surroundings. Check List 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Abdel Razek, F.A.; Ismaiel, M.; Ameran, M.A.A. Occurrence of the blue crab Callinectes sapidus, Rathbun, 1896, and its fisheries biology in Bardawil Lagoon, Sinai Peninsula, Egypt. Egypt. J. Aquat. Res. 2016, 42, 223–229. [Google Scholar] [CrossRef]
- Perdikaris, C.; Konstantinidis, E.; Gouva, E.; Ergolavou, A.; Klaoudatos, D.; Nathanailides, C.; Paschos, I. Occurrence of the invasive crab species Callinectes sapidus rathbun, 1896, in NW Greece. Walailak J. Sci. Technol. 2016, 13, 503–510. [Google Scholar]
- Kampouris, T.E.; Porter, J.S.; Sanderson, W.G. Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae): An assessment on its diet and foraging behaviour, Thermaikos Gulf, NW Aegean Sea, Greece: Evidence for ecological and economic impacts. Crustac. Res. 2019, 48, 23–37. [Google Scholar] [CrossRef]
- Salama, S.S.A.E.H.; Abouelatta, M.E.; Abd El Rahman, E.S.K.; Sherif, A.H. Bacterial pathogens causing the blue crab (Callinectes sapidus) mortality at Suez Canal (El-Temsah Lake) in Ismailia Governorate. Egypt. J. Aquat. Biol. Fish. 2022, 26, 151–168. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14. [Google Scholar]
- Shaw, B.L.; Battle, H.I. the Gross and Microscopic Anatomy of the Digestive Tract of the Oyster Crassostrea Virginica (Gmelin). Can. J. Zool. 1957, 35, 325–347. [Google Scholar] [CrossRef]
- Howard, D.W.; Smith, C.S. Histological Techniques for Marine Bivalve Mollusks; NOAA Technical Memorandum NMFS-F/NEC-25; National Oceanic and Atmospheric Administration: Woods Hole, MA, USA, 1983; p. 97. [Google Scholar]
- Small, H.J.; Shields, J.D.; Hudson, K.L.; Reece, K.S. Molecular detection of Hematodinium sp. infecting the blue crab, Callinectes sapidus. J. Shellfish Res. 2007, 26, 131–139. [Google Scholar] [CrossRef]
- Li, C.; Shields, J.D.; Miller, T.L.; Small, H.J.; Pagenkopp, K.M.; Reece, K.S. Detection and quantification of the free-living stage of the parasitic dinoflagellate Hematodinium sp. in laboratory and environmental samples. Harmful Algae 2010, 9, 515–521. [Google Scholar] [CrossRef]
- Lara, E.; Berney, C.; Harms, H.; Chatzinotas, A. Cultivation-independent analysis reveals a shift in ciliate 18S rRNA gene diversity in a polycyclic aromatic hydrocarbon-polluted soil. FEMS Microbiol. Ecol. 2007, 62, 365–373. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Ben Souissi, J.; Busoni, G.; Coco, S.; Chryssanthi, A.; Fanelli, E.; et al. Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: A large-scale survey based on local ecological knowledge. Glob. Change Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.H.; Peck, L.S.; Hughes, K.A.; Aldridge, D.C. Antarctica: The final frontier for marine biological invasions. Glob. Change Biol. 2019, 25, 2221–2241. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, L.; Shukla, S.P.; Vennila, A.; Gashaw, T.D. Bioinvasion in antarctic ecosystems. Proc. Natl. Acad. Sci. India Sect. B—Biol. Sci. 2012, 82, 353–359. [Google Scholar] [CrossRef]
- Lattos, A.; Feidantsis, K.; Georgoulis, I.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Staikou, A.; Michaelidis, B. Pathophysiological responses of Pinna nobilis individuals enlightens the etiology of mass mortality situation in the mediterranean populations. Cells 2021, 10, 2838. [Google Scholar] [CrossRef] [PubMed]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Karagiannis, D. Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia infected Mussels from Thermaikos gulf, Greece. Animals 2022, 12, 2805. [Google Scholar] [CrossRef] [PubMed]
- Lattos, A.; Feidantsis, K.; Giantsis, I.A.; Theodorou, J.A.; Michaelidis, B. Seasonality in Synergism with Multi-Pathogen Presence Leads to Mass Mortalities of the Highly Endangered Pinna nobilis in Greek Coastlines: A Pathophysiological Approach. Microorganisms 2023, 11, 1117. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Giantsis, I.A.; Feidantsis, K.; Georgoulis, I.; Karagiannis, D.; Carella, F.; Michaelidis, B. Investigation of the highly endangered Pinna nobilis’ mass mortalities: Seasonal and temperature patterns of health status, antioxidant and heat stress responses. Mar. Environ. Res. 2023, 188, 105977. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Giantsis, I.A.; Georgoulis, I.; Karagiannis, D.; Michaelidis, B. Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence. Fishes 2023, 8, 408. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Tsavea, E.; Kolygas, M.; Athanassopoulou, F.; Bitchava, K. Virulence Genes and In Vitro Antibiotic Profile of Photobacterium damselae Strains, Isolated from Fish Reared in Greek Aquaculture Facilities. Animals 2022, 12, 3133. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Lotze, H.K. Marine biodiversity conservation. Curr. Biol. 2021, 31, R1190–R1195. [Google Scholar] [CrossRef]
- Andersen, J.H.; Halpern, B.S.; Korpinen, S.; Murray, C.; Reker, J. Baltic Sea biodiversity status vs. cumulative human pressures. Estuar. Coast. Shelf Sci. 2015, 161, 88–92. [Google Scholar] [CrossRef]
- Ribeiro, F.; Veríssimo, A. A new record of Callinectes sapidus in a western European estuary (Portuguese coast). Mar. Biodivers. Rec. 2014, 7, e36. [Google Scholar] [CrossRef]
- Morais, P.; Gaspar, M.; Garel, E.; Baptista, V.; Cruz, J.; Cerveira, I.; Leitão, F.; Teodósio, M.A. The Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its non-native distribution into the Ria Formosa lagoon and the Guadiana estuary (SW-Iberian Peninsula, Europe). BioInvasions Rec. 2019, 8, 123–133. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y. The American Blue Crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae) is Rapidly Expanding Through the Mediterranean Coast of Morocco. Thalassas 2020, 36, 267–271. [Google Scholar] [CrossRef]
- Marchessaux, G.; Mangano, M.C.; Bizzarri, S.; M’Rabet, C.; Principato, E.; Lago, N.; Veyssiere, D.; Garrido, M.; Scyphers, S.B.; Sarà, G. Invasive blue crabs and small-scale fisheries in the Mediterranean sea: Local ecological knowledge, impacts and future management. Mar. Policy 2023, 148, 105461. [Google Scholar] [CrossRef]
- Sharov, A.F.; Vølstad, J.H.; Davis, G.R.; Davis, B.K.; Lipcius, R.N.; Montane, M.M. Abundance and exploitation rate of the blue crab (Callinectes sapidus) in Chesapeake Bay. Bull. Mar. Sci. 2003, 72, 543–565. [Google Scholar]
- Walters, E.A.; Bojko, J.; Crowley, C.E.; Gandy, R.L.; Martin, C.W.; Shea, C.P.; Bateman, K.S.; Stentiford, G.D.; Behringer, D.C. Salinity and temperature affect the symbiont profile and host condition of Florida USA blue crabs Callinectes sapidus. J. Invertebr. Pathol. 2023, 198, 107930. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Dunn, A.M. Microsporidia in Aquatic Invertebrates. Microsporidia Pathog. Oppor. First Ed. 2014, 2014, 579–604. [Google Scholar] [CrossRef]
- Small, H.J. Advances in our understanding of the global diversity and distribution of Hematodinium spp.—Significant pathogens of commercially exploited crustaceans. J. Invertebr. Pathol. 2012, 110, 234–246. [Google Scholar] [CrossRef]
- Huchin-Mian, J.P.; Small, H.J.; Shields, J.D. The influence of temperature and salinity on mortality of recently recruited blue crabs, Callinectes sapidus, naturally infected with Hematodinium perezi (Dinoflagellata). J. Invertebr. Pathol. 2018, 152, 8–16. [Google Scholar] [CrossRef]
- Huchin-Mian, J.P.; Small, H.J.; Shields, J.D. Patterns in the natural transmission of the parasitic dinoflagellate Hematodinium perezi in American blue crabs, Callinectes sapidus from a highly endemic area. Mar. Biol. 2017, 164, 153. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Shields, J.D. A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis. Aquat. Organ. 2005, 66, 47–70. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Huang, Q. The parasitic dinoflagellate Hematodinium infects marine crustaceans. Mar. Life Sci. Technol. 2021, 3, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Pagenkopp Lohan, K.M.; Reece, K.S.; Miller, T.L.; Wheeler, K.N.; Small, H.J.; Shields, J.D. The role of alternate hosts in the ecology and life history of Hematodinium sp., a parasitic dinoflagellate of the blue crab (Callinectes sapidus). J. Parasitol. 2012, 98, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Xia, X.A.; Wu, Q.Y.; Liu, W.H.; Lin, Y.S. Infection with Hematodinium sp. in mud crabs Scylla serrata cultured in low salinity water in southern China. Dis. Aquat. Organ. 2008, 82, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, S.; Liu, Y.; Chen, T. Hematodinium infections in cultured chinese swimming crab, Portunus trituberculatus, in northern china. Aquaculture 2013, 396–399, 59–65. [Google Scholar] [CrossRef]
- Xu, W.; Xie, J.; Shi, H.; Li, C. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture 2010, 300, 25–31. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, M.; Xiao, J.; Xu, W.J.; Li, C.W. Hematodinium spp. Infections in wild and cultured populations of marine crustaceans along the coast of China. Dis. Aquat. Organ. 2017, 124, 181–191. [Google Scholar] [CrossRef]
- Ryazanova, T.V.; Eliseikina, M.G.; Kukhlevsky, A.D.; Kharlamenko, V.I. Hematodinium sp. infection of red Paralithodes camtschaticus and blue Paralithodes platypus king crabs from the Sea of Okhotsk, Russia. J. Invertebr. Pathol. 2010, 105, 329–334. [Google Scholar] [CrossRef]
- Ryazanova, T.V.; Eliseikina, M.G.; Kukhlevsky, A.D. First detection of Hematodinium sp. In spiny king crab Paralithodes brevipes, and new geographic areas for the parasite in tanner crab Chionoecetes bairdi, and red king crab Paralithodes camtschaticus. J. Invertebr. Pathol. 2021, 184, 107651. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Feist, S.W.; Bateman, K.S.; Hine, P.M. Haemolymph parasite of the shore crab Carcinus maenas: Pathology, ultrastructure and observations on crustacean haplosporidians. Dis. Aquat. Organ. 2004, 59, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.M.; Shaw, P.W.; Morritt, D. Prevalence and seasonality of Hematodinium (Alveolata: Syndinea) in a Scottish crustacean community. ICES J. Mar. Sci. 2009, 66, 1837–1845. [Google Scholar] [CrossRef]
- Bojko, J.; Stebbing, P.D.; Dunn, A.M.; Bateman, K.S.; Clark, F.; Kerr, R.C.; Stewart-Clark, S.; Johannesen, Á.; Stentiford, G.D. Green crab Carcinus maenas symbiont profiles along a North Atlantic invasion route. Dis. Aquat. Organ. 2018, 128, 147–168. [Google Scholar] [CrossRef]
- Davies, C.E.; Batista, F.M.; Malkin, S.H.; Thomas, J.E.; Bryan, C.C.; Crocombe, P.; Coates, C.J.; Rowley, A.F. Spatial and temporal disease dynamics of the parasite Hematodinium sp. In shore crabs, Carcinus maenas. Parasites Vectors 2019, 12, 472. [Google Scholar] [CrossRef]
- Eigemann, F.; Burmeister, A.; Skovgaard, A. Hematodinium sp. (Alveolata. Syndinea) detected in marine decapod crustaceans from waters of Denmark and Greenland. Dis. Aquat. Organ. 2010, 92, 59–68. [Google Scholar] [CrossRef]
- Lycett, K.A.; Pitula, J.S. Disease ecology of Hematodinium perezi in a high salinity estuary: Investigating seasonal trends in environmental detection. Dis. Aquat. Organ. 2017, 124, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Aldik, R.; Cengizler, I. The Investigation of Bacteria, Parasite and Fungi in Blue Crabs (Callinectes sapidus, Rathbun 1896) Caught From Akyatan Lagoon in East Mediterranean Sea. J. VetBio Sci. Tech. 2016, 2, 11–17. [Google Scholar] [CrossRef]
- Messick, G.A. Hematodinium perezi infections in adult and juvenile blue crabs Callinectes sapidus from coastal bays of Maryland and Virginia, USA. Dis. Aquat. Organ. 1994, 19, 77–82. [Google Scholar] [CrossRef]
- Shields, J.D.; Squyars, C.M. Mortality and hematology of blue crabs, Callinectes sapidus, experimentally infected with the parasitic dinoflagellate Hematodinium perezi. Fish. Bull. 2000, 98, 139–152. [Google Scholar]
- Shields, J.D.; Sullivan, S.E.; Small, H.J. Overwintering of the parasitic dinoflagellate Hematodinium perezi in dredged blue crabs (Callinectes sapidus) from Wachapreague Creek, Virginia. J. Invertebr. Pathol. 2015, 130, 124–132. [Google Scholar] [CrossRef]
- Huang, Q.; Li, M.; Wang, F.; Li, C. The parasitic dinoflagellate Hematodinium perezi infecting mudflat crabs, Helice tientsinensis, in polyculture system in China. J. Invertebr. Pathol. 2019, 166, 107229. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Xie, G.; Wang, H.; Li, X.; Li, A.; Wan, X.; Huang, J.; Shi, C.; Zhang, Q.; Huang, J. Hematodinium perezi naturally infects Asian brush-clawed crab (Hemigrapsus takanoi). J. Fish Dis. 2023, 46, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, S.A.; Worm, B. Ecological role of large benthic decapods in marine ecosystems: A review. Mar. Ecol. Prog. Ser. 2012, 469, 195–213. [Google Scholar] [CrossRef]
- Yanti, D.; Tabalessy, R.; Simatauw, F.; Irwanto, I.; Inayah, I. Fishery Management for Crab Resources Using an Ecosystem Approach. In Proceedings of the First International Conference on Economics, Business and Social Humanities, ICONEBS 2020, Madiun, Indonesia, 4–5 November 2020. [Google Scholar] [CrossRef]
- Hungria, D.B.; dos Santos Tavares, C.P.; Pereira, L.Â.; de Assis Teixeira da Silva, U.; Ostrensky, A. Global status of production and commercialization of soft-shell crabs. Aquac. Int. 2017, 25, 2213–2226. [Google Scholar] [CrossRef]
- Sun, L.; Engle, C.; Kumar, G.; van Senten, J. Retail market trends for seafood in the United States. J. World Aquac. Soc. 2023, 54, 603–624. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattos, A.; Papadopoulos, D.K.; Giantsis, I.A.; Stamelos, A.; Karagiannis, D. Histopathology and Phylogeny of the Dinoflagellate Hematodinium perezi and the Epibiotic Peritrich Ciliate Epistylis sp. Infecting the Blue Crab Callinectes sapidus in the Eastern Mediterranean. Microorganisms 2024, 12, 456. https://doi.org/10.3390/microorganisms12030456
Lattos A, Papadopoulos DK, Giantsis IA, Stamelos A, Karagiannis D. Histopathology and Phylogeny of the Dinoflagellate Hematodinium perezi and the Epibiotic Peritrich Ciliate Epistylis sp. Infecting the Blue Crab Callinectes sapidus in the Eastern Mediterranean. Microorganisms. 2024; 12(3):456. https://doi.org/10.3390/microorganisms12030456
Chicago/Turabian StyleLattos, Athanasios, Dimitrios K. Papadopoulos, Ioannis A. Giantsis, Alexios Stamelos, and Dimitrios Karagiannis. 2024. "Histopathology and Phylogeny of the Dinoflagellate Hematodinium perezi and the Epibiotic Peritrich Ciliate Epistylis sp. Infecting the Blue Crab Callinectes sapidus in the Eastern Mediterranean" Microorganisms 12, no. 3: 456. https://doi.org/10.3390/microorganisms12030456





