Afforestation-Induced Shifts in Soil Bacterial Diversity and Community Structure in the Saihanba Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. DNA Extraction and Bioinformatic Analysis
2.3. Statistical Analyses
3. Results
3.1. Soil Chemical Properties across Four Vegetation Types
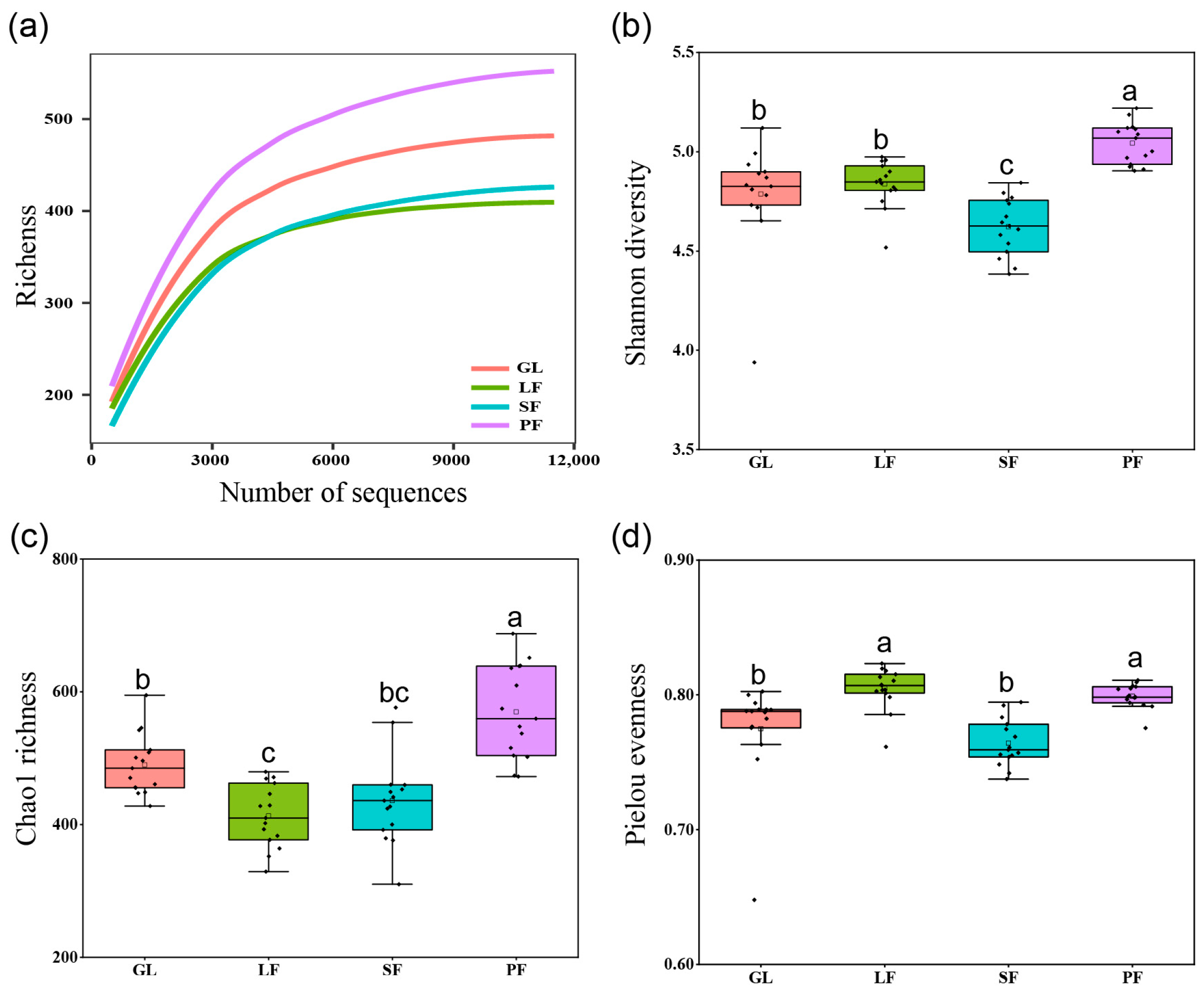
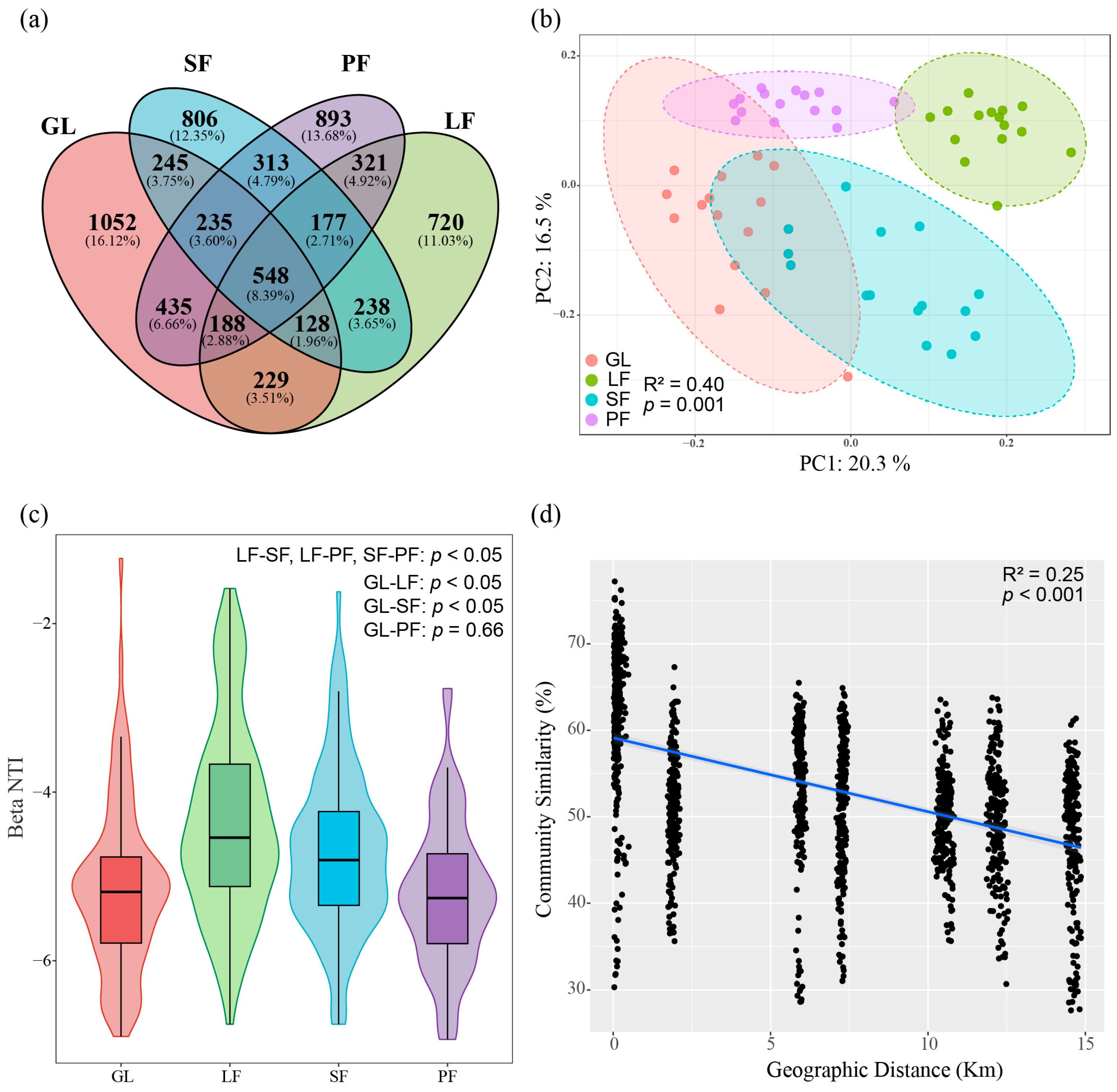
3.2. Bacterial Community Diversity and Structure across Four Vegetation Types
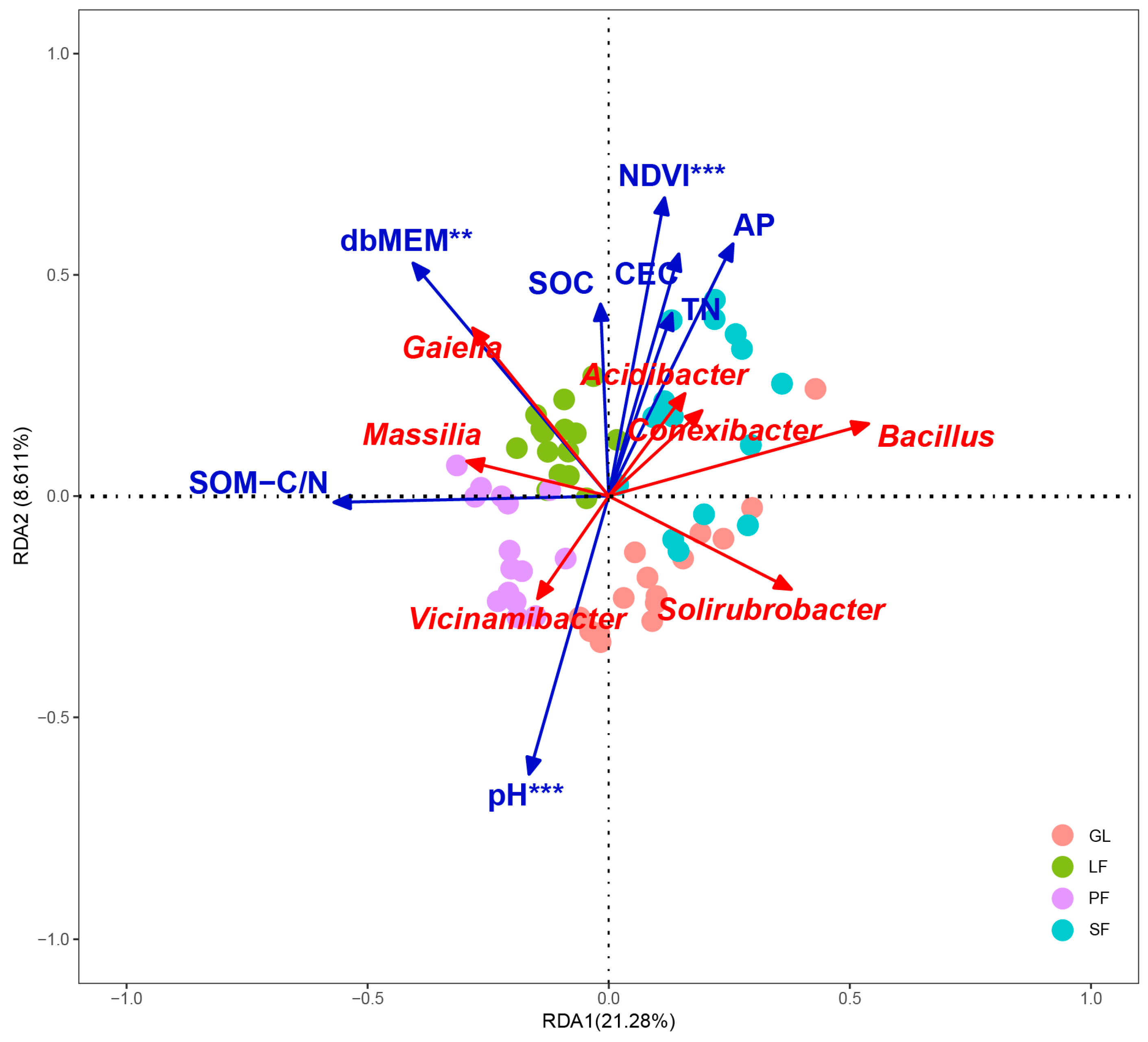
3.3. Bacterial Community Composition and Influencing Factors
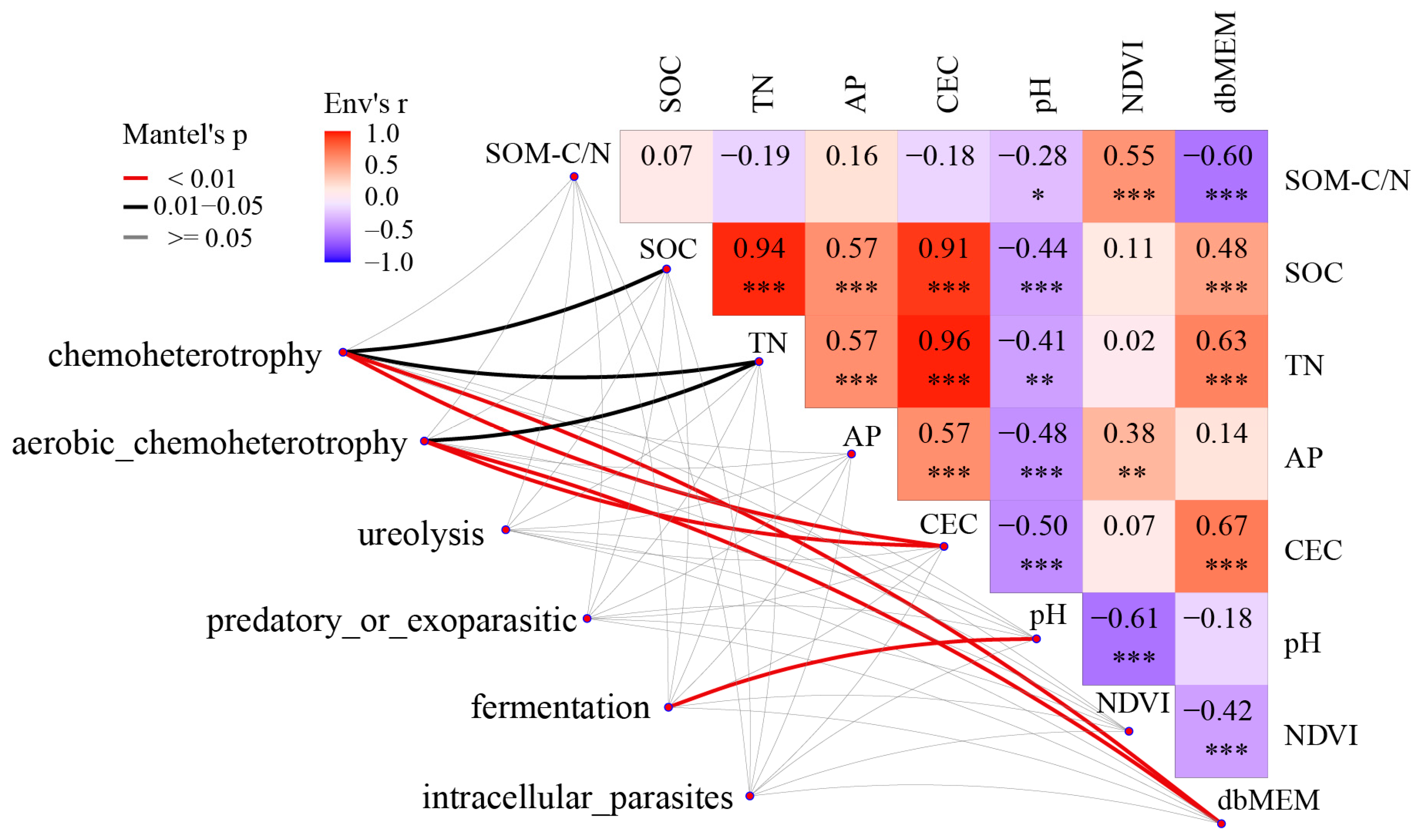
3.4. Functional Prediction of Bacterial Community in the Saihanba Area
3.5. Co-Occurrence Network Analysis of Bacterial Communities
4. Discussion
4.1. Influence of Soil Chemical Properties on Bacterial Alpha Diversity Post-Afforestation
4.2. Afforestation’s Impact on Bacterial Community Structure and Assembly Processes
4.3. Bacterial Community Composition and Functional Group Dynamics Post-Afforestation
4.4. Increased Complexity of Bacterial Co-Occurrence Networks Post-Afforestation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Dong, Y.; Agathokleous, E.; Liu, S.; Yu, Z. Demographic changes in China’s forests from 1998 to 2018. For. Ecosyst. 2023, 10, 100094. [Google Scholar] [CrossRef]
- Kou, M.; Jiao, J. Changes in vegetation and soil properties across 12 years after afforestation in the hilly-gully region of the Loess Plateau. Glob. Ecol. Conserv. 2022, 33, e01989. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Li, K.; Han, X.; Ni, R.; Shi, G.; de-Miguel, S.; Li, C.; Shen, W.; Zhang, Y.; Zhang, X. Impact of Robinia pseudoacacia stand conversion on soil properties and bacterial community composition in Mount Tai, China. For. Ecosyst. 2021, 8, 19. [Google Scholar] [CrossRef]
- Huang, H.; Tian, D.; Zhou, L.; Su, H.; Ma, S.; Feng, Y.; Tang, Z.; Zhu, J.; Ji, C.; Fang, J. Effects of afforestation on soil microbial diversity and enzyme activity: A meta-analysis. Geoderma 2022, 423, 115961. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Singh, B.K.; Maestre, F.T. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Franks, A.E.; Huang, X.; Yang, Q.; Cheng, Z.; Liu, Y.; Ma, B.; Xu, J.; He, Y. Loss of microbial diversity weakens specific soil functions, but increases soil ecosystem stability. Soil Biol. Biochem. 2023, 177, 108916. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Zeng, Q.; Man, X.; Lebreton, A.; Dai, Y.; Martin, F.M. The bacterial and fungal microbiomes of ectomycorrhizal roots from stone oaks and Yunnan pines in the subtropical forests of the Ailao Mountains of Yunnan. Front. Microbiol. 2022, 13, 916337. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Zhao, W.; Wang, L.; Xie, D.; Huo, W.; Wu, Y.; Zhang, J. Comparative effects of sulfuric and nitric acid rain on litter decomposition and soil microbial community in subtropical plantation of Yangtze River Delta region. Sci. Total Environ. 2017, 601–602, 669–678. [Google Scholar] [CrossRef]
- Wan, P.; He, R. Soil microbial community characteristics under different vegetation types at the national nature reserve of Xiaolongshan Mountains, Northwest China. Ecol. Inform. 2020, 55, 101020. [Google Scholar] [CrossRef]
- Yu, Z.; Liang, K.; Huang, G.; Wang, X.; Lin, M.; Chen, Y.; Zhou, Z. Soil bacterial community shifts are driven by soil nutrient availability along a teak plantation chronosequence in tropical forests in China. Biology 2021, 10, 1329. [Google Scholar] [CrossRef]
- Singavarapu, B.; Du, J.; Beugnon, R.; Cesarz, S.; Eisenhauer, N.; Xue, K.; Wang, Y.; Bruelheide, H.; Wubet, T. Functional Potential of Soil Microbial Communities and Their Subcommunities Varies with Tree Mycorrhizal Type and Tree Diversity. Microbiol. Spectr. 2023, 11, e0457822. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.P.; Geisen, S. Trophic regulations of the soil microbiome. Trends Microbiol. 2019, 27, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Xie, L.; Yin, C. Interspecific plant-plant interactions increase the soil microbial network stability, shift keystone microbial taxa, and enhance their functions in mixed stands. For. Ecol. Manag. 2023, 533, 120851. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.; Yrjälä, K.; Tong, Z.; Zhang, J. The introduction of Phoebe bournei into Cunninghamia lanceolata monoculture plantations increased microbial network complexity and shifted keystone taxa. For. Ecol. Manag. 2022, 509, 120072. [Google Scholar] [CrossRef]
- Khan, M.A.W.; Bohannan, B.J.M.; Nüsslein, K.; Tiedje, J.M.; Tringe, S.G.; Parlade, E.; Barberán, A.; Rodrigues, J.L.M. Deforestation impacts network co-occurrence patterns of microbial communities in Amazon soils. FEMS Microbiol. Ecol. 2019, 95, fiy230. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Guo, Z.; Zhao, W.; Song, C.; Wang, H.; Li, J.; Mumin, R.; Sun, Y.; Cui, B. Response of fungal communities to afforestation and its indication for forest restoration. For. Ecosyst. 2023, 10, 100125. [Google Scholar] [CrossRef]
- Wang, W.; Peng, S.; Fang, J. Root respiration and its relation to nutrient contents in soil and root and EVI among 8 ecosystems, northern China. Plant Soil 2010, 333, 391–401. [Google Scholar] [CrossRef]
- Bao, S.D. Agriculture Chemical Analysis of Soil; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Guo, Z.; Liu, J.; Wu, J.; Yang, D.; Mei, K.; Li, H.; Lu, H.; Yan, C. Spatial heterogeneity in chemical composition and stability of glomalin-related soil protein in the coastal wetlands. Sci Total Environ. 2022, 835, 155351. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Zhao, W.; Reyila, M.; Huang, K.C.; Liu, S.; Cui, B.K. Diversity of microbial communities of Pinus sylvestris var. mongolica at spatial scale. Microorganisms 2022, 10, 371. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, D.; Reyila, M.; Huang, K.; Liu, S.; Cui, B. Soil microbial community structure of Larix gmelinii forest in the Aershan area. Biodivers. Sci. 2023, 31, 22258. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2, high-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhan, L.; Tang, W.; Wang, Q.; Dai, Z.; Zhou, L.; Feng, T.; Chen, M.; Wu, T.; Hu, E.; et al. MicrobiotaProcess: A comprehensive R package for deep mining microbiome. Innovation 2023, 4, 100388. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. R package, Version 2.5–7; Vegan: Community Ecology Package; 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 19 April 2023).
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Deng, J.; Zhang, Y.; Yin, Y.; Zhu, X.; Zhu, W.; Zhou, Y. Comparison of soil bacterial community and functional characteristics following afforestation in the semi-arid areas. PeerJ 2019, 7, e7141. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; Ranger, J.; Dan, B.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Kim, S.; Zang, H.; Mortimer, P.; Shi, L.L.; Li, Y.J.; Xu, J.C.; Ostermann, A. Tree species and recovery time drives soil restoration after mining: A Chronosequence Study. Land Degrad. Dev. 2018, 29, 1738–1747. [Google Scholar] [CrossRef]
- Mcgroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- O’Brien, F.J.M.; Almaraz, M.; Foster, M.A.; Hill, A.F.; Huber, D.P.; King, E.K.; Langford, H.; Lowe, M.-A.; Mickan, B.S.; Miller, V.S.; et al. Soil Salinity and pH Drive Soil Bacterial Community Composition and Diversity Along a Lateritic Slope in the Avon River Critical Zone Observatory, Western Australia. Front. Microbiol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Rousk, J. Linking microbial community structure to trait distributions and functions using salinity as an environmental filter. Mbio 2019, 10, e01607-19. [Google Scholar] [CrossRef]
- Song, D.; Cui, Y.; Ma, D.; Li, X.; Liu, L. Spatial variation of microbial community structure and its driving environmental factors in two forest types in permafrost region of Greater Xing’an Mountains. Sustainability 2022, 14, 9284. [Google Scholar] [CrossRef]
- Xiao, R.; Man, X.; Duan, B.; Cai, T.; Ge, Z.; Li, X.; Vesala, T. Changes in soil bacterial communities and nitrogen mineralization with understory vegetation in boreal larch forests. Soil Biol. Biochem. 2022, 166, 108572. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-Fungal Interactions: Hyphens between Agricultural, Clinical, Environmental, and Food Microbiologists. Microbiol. Mol. Biol. R 2011, 75, 583–609. [Google Scholar] [CrossRef]
- Nazir, R.; Warmink, J.A.; Boersma, H.; Van Elsas, J.D. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol. Ecol. 2009, 71, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Courty, P.E.; Varoquaux, N.; Cole, B.; Montoya, L.; Xu, L.; Purdom, E.; Vogel, J.; Hutmacher, R.B.; Dahlberg, J.A.; et al. Successional adaptive strategies revealed by correlating arbuscular mycorrhizal fungal abundance with host plant gene expression. Mol. Ecol. 2023, 32, 2674–2687. [Google Scholar] [CrossRef]
- Deng, J.; Yin, Y.; Zhu, W.; Zhou, Y. Variations in soil bacterial community diversity and structures among different revegetation types in the Baishilazi nature reserve. Front. Microbiol. 2018, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, X.; Tai, X.; Sun, L.; Wu, M.; Zhang, W.; Chen, X.; Zhang, G.; Chen, T.; Liu, G.; et al. Variation in actinobacterial community composition and potential function in different soil ecosystems belonging to the arid Heihe River basin of Northwest China. Front. Microbiol. 2019, 10, 2209. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Qin, T.; Yan, D.; Lv, X.; Wang, J.; Huang, Y.; Lv, Z.; Liu, S.; Liu, F. How do tree species characteristics affect the bacterial community structure of subtropical natural mixed forests? Sci. Total Environ. 2021, 764, 144633. [Google Scholar] [CrossRef]
- Monciardini, P.; Cavaletti, L.; Schumann, P.; Rohde, M.; Donadio, S. Conexibacter woesei gen. nov., sp. nov., a novel representative of a deep evolutionary line of descent within the class Actinobacteria. Int. J. Syst. Evol. Microbiol. 2003, 53, 569–576. [Google Scholar] [CrossRef]
- Seki, T.; Matsumoto, A.; Shimada, R.; Inahashi, Y.; Ōmura, S.; Takahashi, Y. Conexibacter arvalis sp. nov., isolated from a cultivated field soil sample. Int. J. Syst. Evol. Microbiol. 2012, 62, 2400–2404. [Google Scholar] [CrossRef]
- Pukall, R.; Lapidus, A.; Glavina Del Rio, T.; Copeland, A.; Tice, H.; Cheng, J.F.; Lucas, S.; Chen, F.; Nolan, M.; Bruce, D.; et al. Complete genome sequence of Conexibacter woesei type strain (ID131577T). Stand Genom. Sci. 2010, 2, 212–219. [Google Scholar] [CrossRef]
- Tiepo, A.N.; Constantino, L.V.; Madeira, T.B.; Gonçalves, L.S.A.; Pimenta, J.A.; Bianchini, E.; de Oliveira, A.L.M.; Oliveira, H.C.; Stolf-Moreira, R. Plant growth-promoting bacteria improve leaf antioxidant metabolism of drought-stressed Neotropical trees. Planta 2020, 251, 83. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Liao, X.; Tan, X.; Yue, J.; Zhang, W.; Wu, J.; Willison, J.H.M.; Tian, Q.; Liu, Y. Soil nutrients, enzyme activities, and bacterial communities in varied plant communities in karst rocky desertification regions in Wushan County, Southwest China. Front. Microbiol. 2023, 14, 1180562. [Google Scholar] [CrossRef]
- Huber, K.J.; Overmann, J. Vicinamibacteraceae fam. Nov., the first described family within the subdivision 6 Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 2331–2334. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, X.; Dong, R.; Wang, X.; Liu, G.; Li, Q. Natural vegetation regeneration facilitated soil organic carbon sequestration and microbial community stability in the degraded karst ecosystem. Catena 2022, 222, 106856. [Google Scholar] [CrossRef]
- Huang, X.; Cui, C.; Hou, E.; Li, F.; Liu, W.; Jiang, L.; Luo, Y.; Xu, X. Acidification of soil due to forestation at the global scale. For. Ecol. Manag. 2022, 505, 119951. [Google Scholar] [CrossRef]
- Xu, T.; Chen, X.; Hou, Y.; Zhu, B. Changes in microbial biomass, community composition and diversity, and functioning with soil depth in two alpine ecosystems on the Tibetan plateau. Plant Soil 2021, 459, 137–153. [Google Scholar] [CrossRef]
- Liang, S.; Deng, J.; Jiang, Y.; Wu, S.; Zhou, Y.; Zhu, W. Functional Distribution of Bacterial Community under Different Land Use Patterns Based on FaProTax Function Prediction. Pol. J. Environ. Stud. 2020, 29, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Brackin, R.; Robinson, N.; Lakshmanan, P.; Schmidt, S. Microbial function in adjacent subtropical forest and agricultural soil. Soil Biol. Biochem. 2013, 57, 68–77. [Google Scholar] [CrossRef]
- Zhao, Z.B.; He, J.Z.; Quan, Z.; Wu, C.F.; Sheng, R.; Zhang, L.M.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Ran, Y.; Li, W.; Shao, M.; Zhang, Y.; Ji, Q.; Ding, X. Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 2023, 150, 110224. [Google Scholar] [CrossRef]
- Wu, S.H.; Huang, B.H.; Gao, J.; Wang, S.; Liao, P.C. The effects of afforestation on soil bacterial communities in temperate grassland are modulated by soil chemical properties. PeerJ 2019, 7, e6147. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, A.B.; Prendergast-Miller, M.T.; Richardson, A.E.; Toscas, P.; Farrell, M.; Macdonald, L.M.; Baker, G.; Wark, T.; Thrall, P.H. Network analysis reveals that bacteria and fungi form modules that correlate independently with soil parameters. Environ. Microbiol. 2015, 17, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; et al. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.J.; Gravel, D.; Guimarães, P.R., Jr.; Hembry, D.H.; Newman, E.A.; et al. Analysing ecological networks of species interactions. Biol. Rev. Camb. Philos. Soc. 2019, 94, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Jeanbille, M.; Buée, M.; Bach, C.; Cébron, A.; Frey-Klett, P.; Turpault, M.P.; Uroz, S. Soil Parameters Drive the Structure, Diversity and Metabolic Potentials of the Bacterial Communities Across Temperate Beech Forest Soil Sequences. Microb. Ecol. 2016, 71, 482–493. [Google Scholar] [CrossRef]
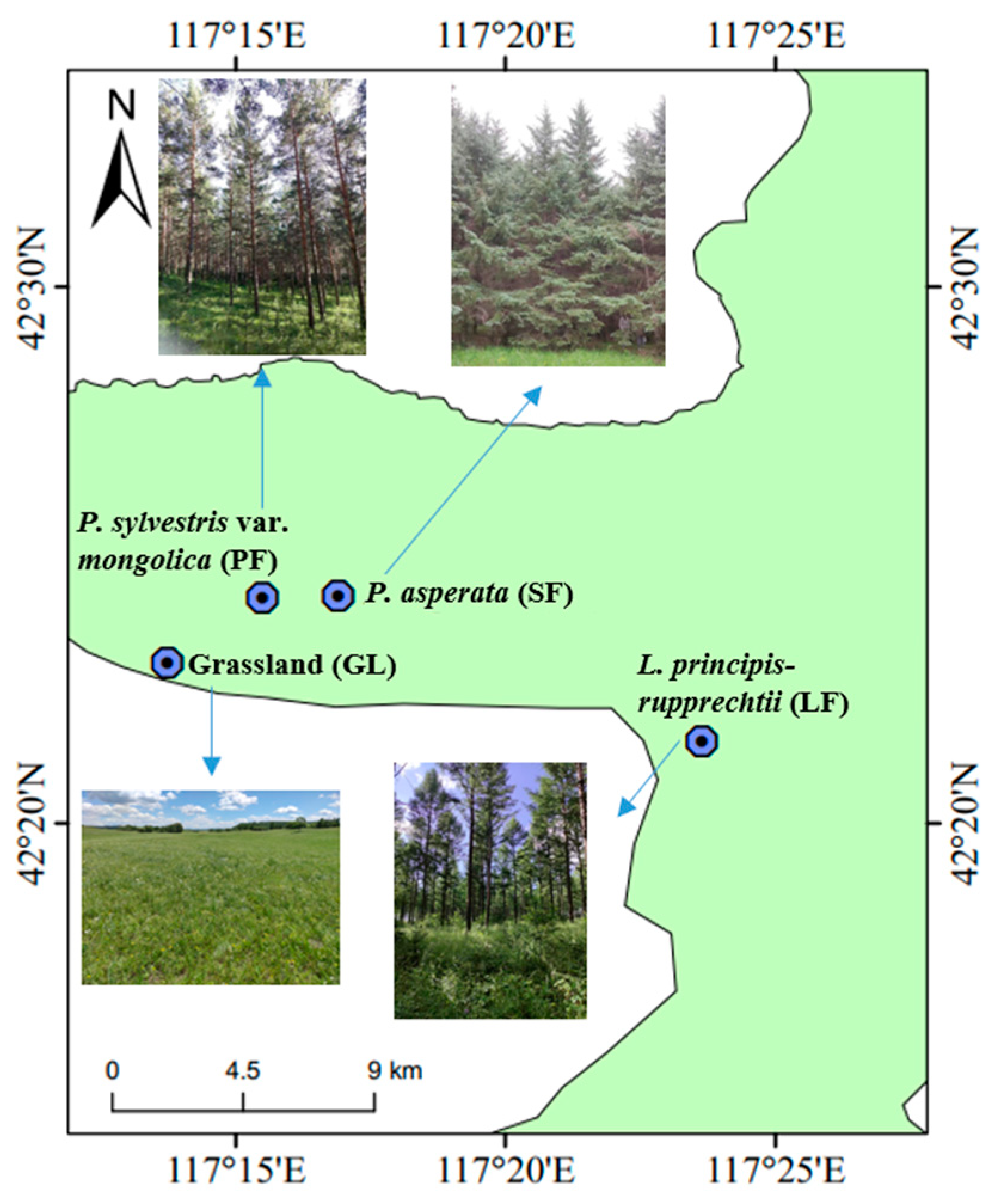

| Vegetation | GL | LF | SF | PF |
|---|---|---|---|---|
| Geographical coordinates | 42°21′35″ N, 117°13′2″ E | 42°21′32″ N, 117°23′39″ E | 42°24′17″ N, 117°16′53″ E | 42°24′14″ N, 117°15′31″ E |
| Stand age (a) | - | 39 | 38 | 36 |
| Altitude (m) | 1439.7 | 1724.2 | 1528.5 | 1522.2 |
| Average DBH (cm) | - | 29.32 ± 2.93 | 20.60 ± 4.08 | 28.21 ± 2.43 |
| Soil type | aeolian sandy soil | aeolian sandy soil | aeolian sandy soil | aeolian sandy soil |
| Soil Properties | GL | LF | SF | PF |
|---|---|---|---|---|
| pH | 6.68 ± 0.03 a | 5.97 ± 0.04 b | 5.90 ± 0.22 b | 6.38 ± 0.03 a |
| SOC (g/kg) | 13.10 ± 1.66 b | 20.16 ± 1.55 a | 14.66 ± 0.89 b | 12.16 ± 0.86 b |
| TN (g/kg) | 1.30 ± 0.15 b | 1.87 ± 0.12 a | 1.29 ± 0.07 b | 0.93 ± 0.07 c |
| SOM-C/N | 9.92 ± 0.17 d | 10.72 ± 0.16 c | 11.35 ± 0.21 b | 13.24 ± 0.28 a |
| AP (mg/kg) | 3.78 ± 0.20 b | 4.52 ± 0.27 a | 4.81 ± 0.19 a | 3.80 ± 0.14 b |
| CEC (cmol(+)/kg) | 6.80 ± 0.87 b | 11.94 ± 0.85 a | 7.68 ± 0.34 b | 4.91 ± 0.39 c |
| Diversity Indices | pH | TN | AP | CEC | SOC | SOM-C/N |
|---|---|---|---|---|---|---|
| Shannon | 0.297 * | −0.323 * | −0.361 ** | −0.359 ** | −0.234 | 0.313 * |
| Chao1 | 0.398 ** | −0.520 *** | −0.311 * | −0.568 *** | −0.425 *** | 0.237 |
| Pielou | 0.006 | 0.084 | −0.206 | 0.099 | 0.109 | 0.189 |
| PC1 | −0.159 | 0.213 | 0.225 | 0.233 | 0.127 | −0.321 * |
| PC2 | 0.042 | −0.075 | −0.188 | −0.061 | 0.060 | 0.436 *** |
| Alpha Index | Variable | Slope | Std. Error | t Value | Pr(>|t|) | Independent Contribution (%) | R2 | p |
|---|---|---|---|---|---|---|---|---|
| Shannon | AP | −0.08 | 0.03 | −2.93 | <0.01 | 37.8 | 0.27 | <0.001 |
| SOM-C/N | 0.08 | 0.02 | 4.76 | <0.001 | 62.2 | |||
| Chao1 | CEC | −15.78 | 7.34 | −2.15 | <0.05 | 34.78 | 0.43 | <0.001 |
| AP | −35.78 | 12.34 | −2.90 | <0.01 | 30.50 | |||
| SOM-C/N | 15.43 | 7.06 | 2.18 | <0.05 | 34.68 | |||
| Pielou | CEC | 0.003 | 0.001 | 2.99 | <0.001 | 38.35 | 0.19 | <0.01 |
| AP | −0.01 | 0.005 | −2.28 | <0.005 | 17.22 | |||
| SOM-C/N | 0.007 | 0.002 | 2.95 | <0.001 | 44.64 |
| pH | TN | SOC | AP | CEC | SOM-C/N | dbMEM | NDVI | Total | |
|---|---|---|---|---|---|---|---|---|---|
| r | 0.230 | −0.057 | −0.066 | 0.012 | −0.078 | 0.003 | 0.123 | 0.271 | −0.07 |
| p | 0.001 *** | 0.857 | 0.915 | 0.385 | 0.948 | 0.433 | 0.005 ** | 0.001 *** | 0.908 |
| Vegetation | Nodes | Edges | Average Degree | Average Path Length | Network Diameter | Network Density | Clustering Coefficient | Modularity |
|---|---|---|---|---|---|---|---|---|
| GL | 165 | 116 | 1.406 | 1.600 | 5.945 | 0.009 | 0.357 | 0.969 |
| LF | 144 | 102 | 1.417 | 2.838 | 6.845 | 0.010 | 0.340 | 0.932 |
| SF | 175 | 164 | 1.874 | 3.582 | 8.505 | 0.011 | 0.260 | 0.835 |
| PF | 207 | 163 | 1.575 | 4.267 | 11.913 | 0.008 | 0.273 | 0.924 |
| Vegetation | OTU ID | Phylum | Class | Degree | PageRank | Modularity Class |
|---|---|---|---|---|---|---|
| GL | OTU175 | Acidobacteriota | Vicinamibacteria | 4 | 0.011 | 1 |
| GL | OTU351 | Acidobacteriota | Vicinamibacteria | 4 | 0.009 | 2 |
| GL | OTU966 | Verrucomicrobiota | Chlamydiae | 4 | 0.009 | 2 |
| LF | OTU31 | Actinobacteriota | Thermoleophilia | 6 | 0.015 | 1 |
| LF | OTU36 | Actinobacteriota | Thermoleophilia | 5 | 0.011 | 2 |
| LF | OTU99 | Acidobacteriota | Vicinamibacteria | 5 | 0.011 | 2 |
| LF | OTU40 | Methylomirabilota | Methylomirabilia | 5 | 0.011 | 2 |
| SF | OTU8 | Actinobacteriota | Thermoleophilia | 10 | 0.016 | 1 |
| SF | OTU53 | Pseudomonadota | Gammaproteobacteria | 8 | 0.013 | 1 |
| SF | OTU111 | Acidobacteriota | Vicinamibacteria | 7 | 0.012 | 2 |
| PF | OTU8 | Actinobacteriota | Thermoleophilia | 6 | 0.010 | 4 |
| PF | OTU576 | Acidobacteriota | Vicinamibacteria | 5 | 0.009 | 1 |
| PF | OTU411 | Acidobacteriota | Vicinamibacteria | 5 | 0.009 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-C.; Zhao, W.; Li, J.-N.; Mumin, R.; Song, C.-G.; Wang, H.; Sun, Y.-F.; Cui, B.-K. Afforestation-Induced Shifts in Soil Bacterial Diversity and Community Structure in the Saihanba Region. Microorganisms 2024, 12, 479. https://doi.org/10.3390/microorganisms12030479
Huang K-C, Zhao W, Li J-N, Mumin R, Song C-G, Wang H, Sun Y-F, Cui B-K. Afforestation-Induced Shifts in Soil Bacterial Diversity and Community Structure in the Saihanba Region. Microorganisms. 2024; 12(3):479. https://doi.org/10.3390/microorganisms12030479
Chicago/Turabian StyleHuang, Kai-Chuan, Wen Zhao, Jun-Ning Li, Reyila Mumin, Chang-Ge Song, Hao Wang, Yi-Fei Sun, and Bao-Kai Cui. 2024. "Afforestation-Induced Shifts in Soil Bacterial Diversity and Community Structure in the Saihanba Region" Microorganisms 12, no. 3: 479. https://doi.org/10.3390/microorganisms12030479
APA StyleHuang, K.-C., Zhao, W., Li, J.-N., Mumin, R., Song, C.-G., Wang, H., Sun, Y.-F., & Cui, B.-K. (2024). Afforestation-Induced Shifts in Soil Bacterial Diversity and Community Structure in the Saihanba Region. Microorganisms, 12(3), 479. https://doi.org/10.3390/microorganisms12030479







