Abstract
The Chlamydiae phylum is comprised of obligate intracellular bacteria including human pathogens such as Chlamydia trachomatis and lesser-known Chlamydia-related bacteria like Waddlia chondrophila or Simkania negevensis. Despite broad differences, these bacteria share a similar development including a persistent state induced using stressors such as immune responses, nutrient starvation, or penicillin introduction. In microbiology, this persistent state is identified by enlarged bacteria, called aberrant bodies, which are unable to divide but are able to survive and resume the developmental cycle upon clearance of the stressor. Clinically, chlamydial persistence is thought to be linked to chronic disease and long-term infections with pathogenic strains. This review aims to share and discuss the latest discoveries made on the little-known mechanisms that take place during stress response. The results indicate that an inter-linked homeostasis between iron and tryptophan is required for effective bacterial proliferation. During stress, Chlamydiae attempt to compensate by inducing tight regulations of the tryptophan and iron acquisition operons. These compensations allow bacterial survival but result in the halting of cell division. As cell division is tightly linked to peptidoglycan synthesis and regulation, treatment with β-lactamase inhibitors can also exhibit an aberrant body phenotype.
1. Introduction
The bacterial phylum of the Chlamydiae is comprised of strictly intracellular bacteria including the well-known human pathogens Chlamydia trachomatis, Chlamydia pneumoniae and Chlamydia psittaci, which are part of the Chlamydiaceae family. C. trachomatis is one of the most common bacterial-induced sexual infections [1]. While genital infections with C. trachomatis (genovariants D to K) are commonly asymptomatic (up to 80% in women), they can lead to genital tract inflammation. In males, complications are limited to urethritis, while in females the more serious infections can lead to pelvic inflammatory diseases, ectopic pregnancy, miscarriage, or tubal infertility [2,3,4]. Ocular infection with C. trachomatis genovariants A, B, or C can cause trachoma, a disease which may lead to irreversible blindness over time [5]. Other pathogens, such as C. psittaci, C. pneumoniae, or C. abortus, can provoke potentially deadly lung infections in humans. C. pneumoniae is spread through small respiratory droplets; C. psittaci and C. abortus are less common human pathogens that are zoonotic agents transmitted by birds and ruminants, respectively [6,7].
Also, part of the Chlamydiae phylum are the recently discovered families referred to as the Chlamydia-related bacteria, which include the Parachlamydiaceae, Waddliaceae, and Simkaniaceae. The Chlamydia-like species have been observed in mammalian cells, amoeba, and protists [8]. Chlamydia-related bacteria are adaptable to a broader range of hosts than the Chlamydiaceae, which are limited complex hosts such as mammals, birds, and reptiles [9]. The genomes of Chlamydia-related bacteria are approximately twice as large as those of the Chlamydiaceae family, resulting in increased metabolic potential, possibly explaining the broader range of hosts available [10]. Despite these differences, all Chlamydiae members share a common biphasic developmental cycle.
The cycle initiates when elementary bodies (EBs), the infectious and non-replicative form of the bacteria (~0.3 µm in diameter), adhere to and enter the host cell via endocytosis. Following entry, EBs surround themselves with a protective endosomal vacuole known as the inclusion. As the developmental cycle progresses, EBs transition to a slightly larger (~1 µm), non-infectious and proliferative form named reticulate bodies (RBs). By hijacking the host cell metabolic pathways, RBs acquire the necessary components for replication. During the mid-cycle of infection, RBs begin to re-differentiate into infectious EBs which are then released into the extracellular matrix either by extrusion of the inclusion, or by host cell lysis [10,11].
During the developmental cycle, RBs may be exposed to stress conditions such as nutrient starvation, antibiotic introduction, or host immune responses. If these stressors are introduced, the bacteria enter into a persistent state of infection marked by the presence of a third form of bacteria called aberrant bodies (ABs). During persistence, ABs stop dividing, become enlarged, and no longer produce infectious progeny [12,13,14,15]. However, this phenomenon can be reversed with the clearance of the stress condition, after which ABs can transition back into RBs and resume the developmental cycle. It is hypothesized that the transition to persistent ABs is linked to the chronic physiopathology caused by chlamydial infections in humans [16]. In this article, we will give a summary on the history of aberrant bodies and updates on chlamydial response to different aberrance-inducing conditions, both in terms of biological persistence but also in terms of morphological changes associated with enlarged non-dividing bacteria with or without DNA replication.
2. Historical Overview on the Discovery of Aberrant Bodies
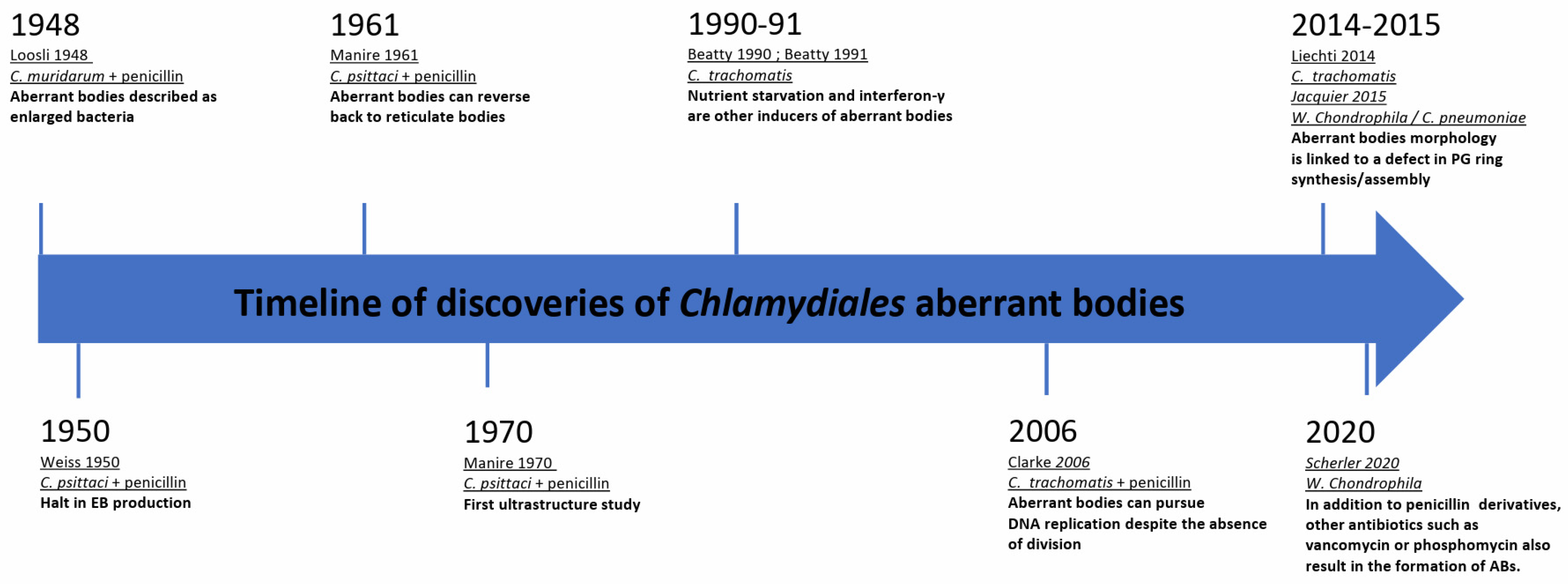
Some of the earliest morphological observations of ABs date back to 1948. In vivo, in mice, Loosli et al. observed aberrant growth of C. muridarum, which at the time was thought to be a virus, upon introduction of penicillin treatments [17]. Two years later, Weiss et al. made a similar observation during infection of chick embryo cells with C. psittaci followed by penicillin treatment. Weiss et al. described the so-called “granules” as greatly enlarged and noted that they could not undergo binary fission after exposure to the antibiotic. Furthermore, it was observed that the RBs on the periphery of the inclusion, which actively divide and closely associate with the inner face of the inclusion membrane, were affected more quickly by the penicillin treatment than those in the center of the inclusion. Ultimately, the bacteria lost their characteristic spherical shape and became irregular “plaques” or ABs [18]. Additionally, Weiss et al. discovered that this persistent state resulted in a halt of EB production. Later investigations of other members of the Chlamydiaceae showed that upon penicillin treatment, a similar division and differentiation defect was detected [19,20]. A timeline of these discoveries is presented in Figure 1.
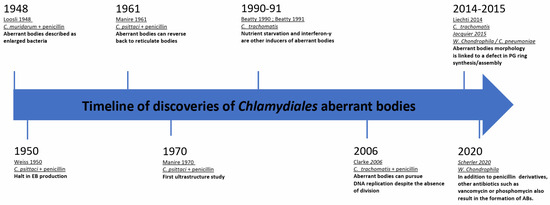
Figure 1.
Milestones of chlamydial aberrant bodies research.
The ability of ABs to reverse the persistent state was unknown until the 1960–1970s. In 1961, Galasso et al. showed that new chlamydial progeny originating from penicillin-induced aberrant bodies of C. psittaci-infected HeLa cells were viable following removal of the antibiotic. They found that even if aberrance was induced continuously for over 3 months, drug clearance led to a rapid increase in bacterial titer [21]. Electron microscopy was later used to visualize the morphological structure of the ABs and the bacteria were found to have large, flat sheets of membranes [22].
It was not until the 1990s that nutrient starvation and interferon-γ (IFNγ) treatment were described as additional inducers of persistence in Chlamydiaceae [23,24]. Approximately 20 years later, the first publications describing ABs of Chlamydia-related bacteria appeared. These studies included Estrella lausannensis [25], Waddlia chondrophila [26], Simkania negevensis [27], Protochlamydia amoebophila [28], and Rhabdochlamydia porcellionis [29]. Indeed, ABs have been observed in most families in the order Chlamydiales. Interestingly, iron starvation and alteration of the peptidoglycan synthesis pathway lead to the observation of aberrantly shaped bacteria in many members of the Chlamydiae phylum, as seen in Table 1. Interestingly, in the case of peptidoglycan synthesis disruption, they do not share the same response to drugs. More recently, Scherler et al. demonstrated, using W. chondrophila, that not only penicillin derivatives act on peptidoglycan biosynthesis. Other antibiotics targeting the peptidoglycan biosynthesis pathways, including vancomycin (targeting the D-ala/D-ala transpeptidase) and phosphomycin, a phosphonic acid antibiotic which targets MurA to inhibit bacterial cell wall biogenesis [27], also yield similar results. While many Chlamydiaceae are susceptible to β-lactam antibiotics, some other species, such as Simkania negevensis, are completely resistant to them. Even so, Simkania negevensis ABs were observed following a high dose of phosphomycin [27].

Table 1.
Table referencing research papers describing the presence of aberrant bodies in members of the Chlamydiae phylum with different types of stressors.
3. The Metabolic Shutdown Induced by Tryptophan Depletion Is Partially Rescued by Chlamydia’s Tryptophan Salvaging Pathway through the Preservation of Host Cell c-Myc
Chlamydial persistence originating from IFNγ is one of the most studied stressors due to its clinical relevance [24,57,58]. Interferons are secreted by Th1 cells, cytotoxic T lymphocytes, and NK cells as a part of the innate and adaptive immune response against a wide range of bacteria [59]. During infection of primate cell lines, IFNγ cytokines impact the biosynthesis of indoleamine 2,3 dioxygenase (IDO). IDO catabolizes tryptophan within host cells to reduce bioavailability by producing kynurenines [60]. C. trachomatis is auxotrophic for tryptophan synthesis, and without it, replication and re-differentiation are not possible. During infection, some RBs may succumb to a lack of tryptophan availability, but a fraction of them can enter the persistent state due to a mechanism of tryptophan-salvaging from indole [61]. IFNγ, in non-primate mammalian cell lines such as p47 GTPases in mice cells, rather than IDO, is responsible for the tryptophan loss by forcing the pathogen to export tryptophan-rich cytotoxic molecules that will target the p47 GTPases [62].
Survival from tryptophan starvation is possible in C. trachomatis genital strains due to the trpRBA operon encoded in their genome, with trpR being a tryptophan-dependent, auto-repressor of the operon. It is believed that the expression of genes from this operon allows for the conversion of exogenous indole produced by the genital tract microbiota into tryptophan, thereby evading IFNγ-mediated tryptophan starvation. Ocular strains of C. trachomatis are more sensitive to the effects of IFNγ, and if a neighboring biovar colonizes the eye, they do not produce indole. Indeed, the ocular strains of C. trachomatis have lost their tryptophan synthesis gene function [63]. Evolutionarily, the tryptophan synthesis pathway is weakly conserved among members of the Chlamydiae. Some species such as C. abortus, C. pneumoniae, C. psittaci, Protochlamydia amoebophila, and Waddlia chondrophila have lost all tryptophan-synthesizing genes. Simkania negevensis is the only Chlamydiales species that encodes the full tryptophan synthesis pathway. The remaining members encode partial operons.
It is interesting to note that despite this high variation in the presence of tryptophan metabolism genes amongst the Chlamydiae, there are similarities in the tryptophan content of some proteins. Proteins involved in tryptophan, nucleotide, S-adenosylmethionine, or dicarboxylate transport were shown to have a high percentage of tryptophan residues as do proteins involved in cell division and lipopolysaccharide (LPS) synthesis. A high percentage of tryptophan residues in essential proteins inherently increases Chlamydiae’s dependence to the amino acid [62]. However, in the event of tryptophan starvation, it is believed the degradation of previously synthesized tryptophan-rich proteins could help overcome the starvation by maintaining a pool of tryptophan for survival, as it is believed that IDO cannot degrade tryptophan in the bacterial inclusion [61,63].
Most recent studies suggest that IFNγ-induced persistence in Chlamydiae is not induced by the activity of IDO, but rather by host cell transcription factor c-Myc [39]. c-Myc is a major polyvalent regulator of cell growth and proliferation and is essential for successful C. trachomatis infection. By using a metabolite-uptake assay in an axenic culture, it was demonstrated that C. trachomatis’ peptidoglycan synthesis was linked to glutamine uptake. Glutamine plays a key role in EB to RB transition through the stimulation of c-Myc [64]. c-Myc stimulation increases production of glutamine transporters and glutaminases, which are required for chlamydial replication [64]. Upon IFNγ stress, c-Myc levels are depleted concomitantly with TCA cycle intermediary products, leading to chlamydial persistence. Persistence could be rescued with over-expression of c-Myc or by adding TCA intermediates despite the ongoing stress, in cell cultures as well as in organoids derived from human fallopian tubes [39].
In addition to the role of IFNγ on the depletion of c-Myc, tryptophan is involved in the inactivation of glycogen synthase kinase-3 (GSK3β) by phosphorylation. The inactivation of GSK3β prevents c-Myc phosphorylation at threonine 58, which initiates a cascade resulting in its degradation by the proteasome complex [65]. Overall, it seems that IDO allows not only the depletion of an essential tryptophan pool, but that its role also extends to the decrease in a broad range of host metabolism products taken up by the Chlamydiae through the indirect degradation of c-Myc [39].
Persistence has also been observed in a murine model of genital infection using the Chlamydia-like organism W. chondrophila. Fourteen days after infection of mice uterine horns with 109 bacteria, W. chondrophila DNA was detectable by RT-qPCR in the liver, spleen, lumbar lymph nodes, and muscles of the mice. Investigation of the IgG response in the mice concluded that IgG2a was the most abundant IgG present, suggesting a Th1-associated humoral response was prominent. Further attempts to re-infect McCoy cell cultures with infected spleen or liver homogenates showed no bacterial replication. While aberrant morphology was not observed, the bacteria were present in a persistent form in McCoy cells infected with liver/spleen homogenates, likely induced by the IFNγ secreted by the stimulated Th1 cells [15].
4. The Chlamydial Iron Uptake Operon Is Stimulated upon Iron Starvation and Is Tightly Regulated by Iron and Tryptophan Abundance
In all mammalian cells, free iron is stored in hemoprotein complexes. Building a pool of iron is essential because iron plays a major role in metabolic reactions that require electron exchanges [66]. Storing ferrous [Fe2+] and ferric [Fe3+] iron in protein complexes allows tight regulation to prevent iron acquisition by potential pathogens. Mammalian ferroxidases are able to convert ferrous iron in the serum to ferric iron. Ferric iron is then able to bind to transferrins with a high affinity. Extracellular iron-transferrin complexes can then bind to transferrin receptors, which are found on the surface of cells, and the entire complex will enter into a target cell through endocytosis [66]. The endocytosis cascade creates a destabilizing, acidic pH in the endosome that frees the ferric iron from the transferrin [66]. The reduction of ferrous iron occurs prior to ferric iron transport in the cytosol. There, it can be used for metabolic processes, such as the TCA cycle or the electron transport chain or it can be stored in a ferritin complex. The free apo-transferrin is then recycled through exocytosis where it can start a new cycle [66]. The current chlamydial iron acquisition model proposes multiple methods for the entry of free iron into the chlamydial inclusion.
One hypothesis is that a proportion of [Fe2+] diffuses through the inclusion membrane by passive transport. An active method is thought to happen through slow-recycling iron-transferrin endosomes. These inclusions are Rab11-transferrin-positive and have been observed to be in close vicinity to chlamydial inclusions [67]. These slow-recycling endosomes, which are not immediately sent to degradation by lysosomes and thus have a longer residence time, can transiently fuse to the inclusion membrane, releasing their iron content into the inclusion lumen [67,68]. To date, evidence of this kiss-and-run mechanism is lacking but a similar mechanism was hypothesized in 1998 for phospholipid acquisition [69]. Following fusion, the endosome detaches from the inclusion membrane and enters the classic endosomal recycling pathway. Once within the inclusion lumen, [Fe2+] and [Fe3+] can access the periplasm of bacteria by passive transport or via siderophore-like receptors, which have not yet been identified [67,68,70]. Transportation into the bacterial cytoplasm is thought to happen through the ABC transporter complex encoded by the ytgABCD operon [71]. YtgC has a C-terminal domain, YtgR, which has been found to be a DtxR-like iron-dependent repressor and is cleaved away from ytgC during infection [72]. This repressor is capable of binding to the promoter of its own operon and likely plays a role in iron homeostasis in all Chlamydiae as homologous genes can be found in the genomes of the previously described chlamydial species [73], including chlamydia-related bacteria.
Once iron levels are sufficiently high within the bacterial cell, YgtR also binds the intergenic region (IGR) of the tryptophan operon independently of the TrpR repressor. Thus, there is a need for YtgR to be additionally regulated by tryptophan so that the iron-regulated repression of the tryptophan operon does not occur during tryptophan starvation. Interestingly, by using 2,2-bipyridyl (BPDL), an iron chelator that can bind both [Fe2+] and [Fe3+], it has been demonstrated that the YtgR repressor also represses the trp operon in C. trachomatis in a trp-dependent manner [32]. Due to the rare triple tryptophan motif (WWW) present in the YtgC domain, YgtR levels can be regulated. Regulation occurs when ribosomes fail to read the WWW motif, (i) resulting in a Rho-independent translation termination and (ii) preventing repression of the tryptophan operon, even when iron levels are sufficiently high. YtgC therefore is important in sensing and in regulating both tryptophan and iron metabolism simultaneously [32].
5. The Aberrant Body Morphology Induced by Iron Starvation, IFNγ, and β-Lactamase Inhibitors Is Related either to a Defect in Peptidoglycan Ring Assembly or Its Regulation
Persistence induced by IFNγ, iron starvation, or penicillin treatment results in morphologically distinct ABs, which are characterized by their large size in comparison to EBs and RBs [13]. Although currently, there are no precisely defined quantitative parameters of ABs, neither of the AB morphology nor the inclusion size [13,30]. A common point of most of these triggers of persistence is that they affect the peptidoglycan biogenesis pathway. If a stressor is introduced during infection, peptidoglycan synthesis is believed to be down-regulated to limit the release of peptidoglycan compounds that can trigger host cell immunity [74].
Historically, the response to peptidoglycan-disrupting drugs in the Chlamydiaceae family remained a mystery, as the presence of a classical peptidoglycan sacculus could not be detected and the role of peptidoglycan was not yet documented. This was referred to as the “chlamydial anomaly” [75]. Later, peptidoglycan was found to be present in members of the Chlamydiaceae family [74,76,77]. In 2014, Jacquier et al. and Frandi et al. described in detail the proteins implicated in the division of members of the Chlamydiae phylum, including remodeling of peptidoglycan by the AmiA and NlpD amidases [47,78]. Co-temporally, Anthony T. Maurelli enriched the muropeptides belonging to peptidoglycan in C. trachomatis and identified their presence through mass spectrometry [77]. Georges Liechti and his team discovered peptidoglycan in small amounts in C. trachomatis, thanks to a new cell wall labeling method using d-amino acid dipeptide fluorescent probes, which are incorporated in newly synthetized peptidoglycan [74]. This work convincingly illustrates the polarized division as described in C. trachomatis by Abdelrahman in 2016 [79]. Detailed analysis of peptidoglycan degradation products by Greub et al. confirmed the importance of peptidoglycan during division [10,76,80,81]. Interestingly, not all Chlamydiae lack a peptidoglycan sacculus. In Protochlamydia amoebophila, peptidoglycan could be observed by electron microscopy surrounding the bacteria during replication [82].
In C. trachomatis, peptidoglycan has been observed at the divisional plane of the RB which forms the first septal disk. The septal disk expands to form a ring, which then constricts to finalize the division process. In C. trachomatis, penicillin binding protein 2 (PBP2) and PBP3 are two regulators which are independently responsible for the peptidoglycan ring expansion and constriction, respectively [83,84]. It has been observed that the volume and thickness of the ring decreases with the number of divisions that have occurred, resulting in smaller bacterial progeny size as infection time progresses. This study correlates well with the description made in 2018 that the transition from RB back to EB occurs after subsequent divisions reduce the chlamydial bacteria size below a certain threshold [85]. The enlarged phenotype of ABs is a result of the absence of division, despite continued bacterial growth. This occurs through the prevention of three different processes, peptidoglycan ring expansion, ring constriction, or peptidoglycan metabolism, as described by Cox et al. [84]. Peptidoglycan ring expansion can be blocked using a PBP2 inhibitor such as mecillinam. Alternatively, peptidoglycan ring constriction can be inhibited by PBP3 inhibitors such as piperacillin. Broad spectrum β-lactamase inhibitors, like penicillin G, are able to target both PBP2 and PBP3 [83,84]. It is likely that a similar mechanism is present in the other members of the Chlamydiae phylum, as they all have homologs of PBP2 and PBP3 [73].
Scherler conducted a comparative study of ABs of a distant relative of C. trachomatis: Waddlia chondrophila. ABs were induced by glycopeptides, penicillins, or iron chelators. There, a threshold of length and area was defined to classify a bacterium as an AB. It was found that treatment of Vero cells with phosphomycin (500 µg/mL), penicillin G (1000 µg/mL), clavulanic acid (900 µg/mL), piperacillin (500 µg/mL), mecillinam (200 µg/mL), deferoxamine (400 µM), and 2,2′ bi-pyridyl (100 µM) resulted in inclusions containing a great majority of ABs compared to RBs. Treatments with MP265 (100 µM), teicoplanin (250 µg/mL), and novobiocin (450 µM) led to more heterogenous mixes of RBs and ABs. The different treatment types also resulted in diversity in AB area, AB length, and number of ABs per inclusion. The treatments also differently impacted the ability of the ABs to replicate DNA. Mecillinam and phosphomycin treatment reduced DNA replication by less than 10-fold while iron-chelating drugs almost completely stopped the process. This study illustrates that variations in the formation of ABs in Chlamydia-like organisms results from different response mechanisms based on the stimulus present. This approach assessed the variations in AB size and showed that MP265 induces small aberrant bodies [13]. MP265 is a chemical compound, which binds to MreB to prevent filament formation and is thus critical in cell shaping. If MP265 is added early in the infection, the treated bacteria would exhibit aberrant bodies of similar morphology and size as compared to healthy RBs [13,86].
While penicillins affect division by preventing peptidoglycan ring constriction or expansion, persistence inducers such as iron or tryptophan starvation may instead affect peptidoglycan metabolism by blocking the synthesis of necessary peptidoglycan precursors. Interestingly, bactoprenol, one such peptidoglycan precursor, is synthetized by the methylerythritol phosphate pathway (MEP) which is both iron and pyruvate dependent and widely found in bacteria [87]. The MEP pathway initiates with a pyruvate substrate that goes through several reactions to yield bactoprenol, a carrier module known to transport peptidoglycan monomers into existing peptidoglycan chains that are being synthesized. Pyruvate is also a key molecule during the TCA cycle. Therefore, tryptophan depletion may indirectly prevent the MEP pathway from functioning by downregulating c-Myc, ultimately preventing the synthesis of bactoprenol required for peptidoglycan synthesis. In the MEP pathway, the transition from methyl-D-erythritol-2,4-cyclodiphosphate (MEcPP) to isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) requires two reactions catalyzed by two enzymes, IspG and IspH, respectively. These enzymes contain iron–sulfur clusters which are obtained from free cytosolic iron. During iron starvation, these enzymes are non-functional, potentially halting peptidoglycan synthesis and thus chlamydial division [88].
6. Conclusions/Discussion
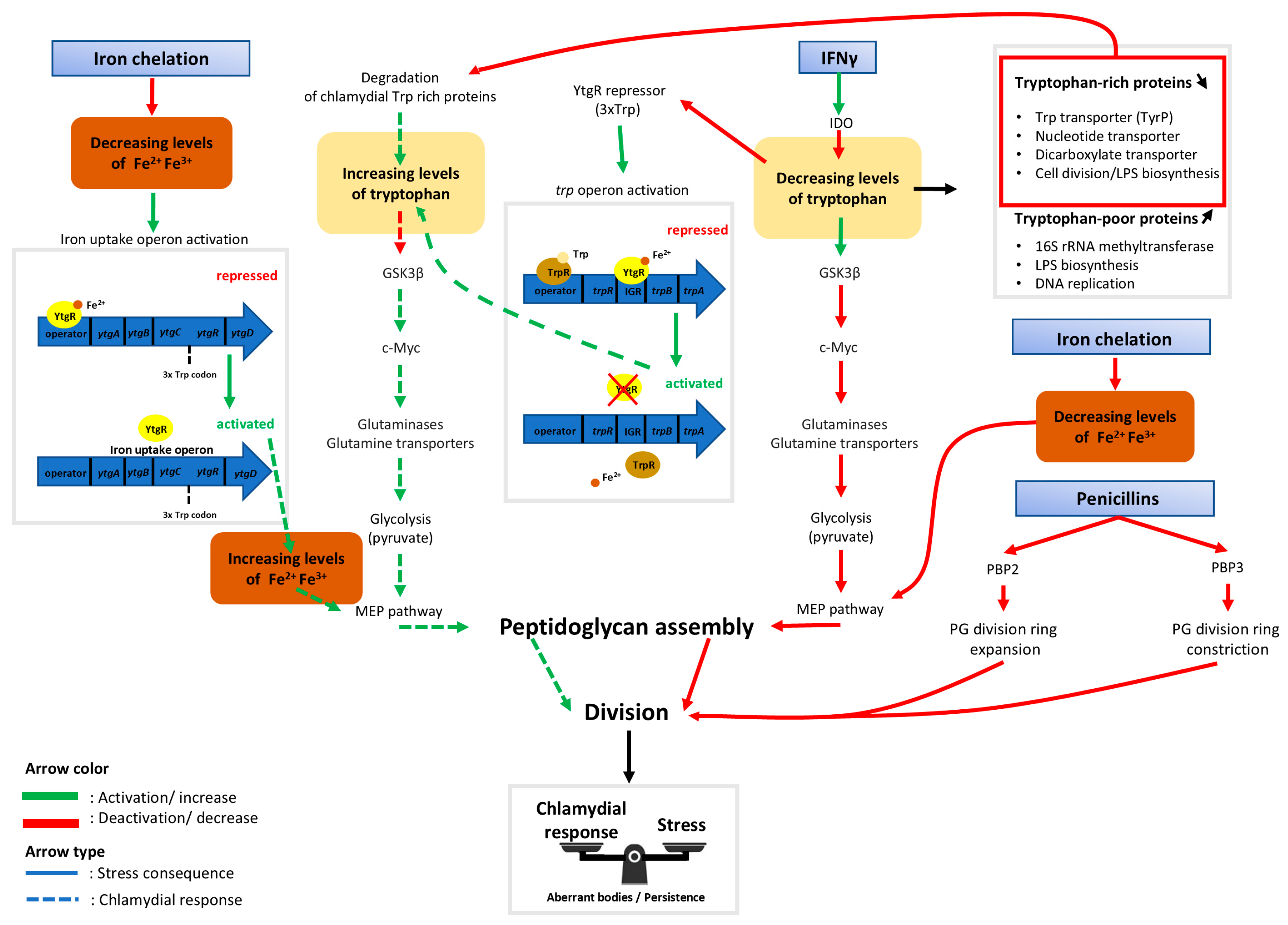
Throughout this review, progress in the understanding of ABs induced by tryptophan and iron starvation in the Chlamydiae phylum has been shown. The latest discoveries have unveiled the links between the two different types of stressors on the bi-sensitive ytgR repressor of the ytgABCD iron operon and the trp operon. Repression of these operons is both iron and tryptophan dependent and regulating mechanisms are enacted to prevent a self-sabotaging repression only when tryptophan is depleted. Details of the findings summarized here can be found in Figure 2.
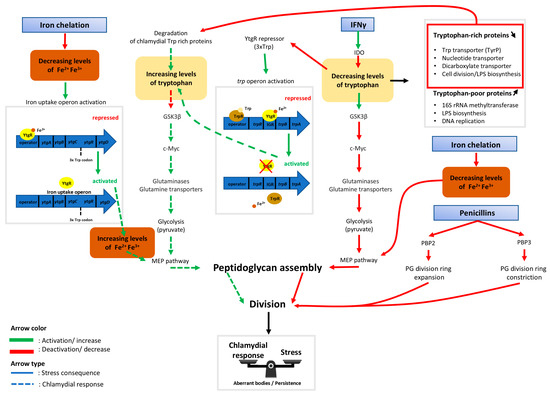
Figure 2.
Schematic indicating dynamics between host cell metabolism and the chlamydial response to iron starvation, IFNγ, and antibiotics acting on peptidoglycan biosynthesis. Rectangles with blue gradient represent aberrant body (AB) inducers. Following host cell immune response by interferon gamma (IFNγ), the cytoplasmic tryptophan pool is depleted by the indolamine 2,3-dioxygenase enzyme (IDO) [59]. The absence of tryptophan then activates glycogen synthase kinase-3 beta (GSK3β), an enzyme that initiates host cell c-Myc degradation [39]. The consequence of this is a decreased production of glutaminases and glutamine transporters necessary for the TCA cycle to function. In addition to this reduced function, glycolysis is slowed down resulting in a decrease in pyruvate production [39]. Therefore, there may not be sufficient pyruvate necessary for bactoprenol synthesis by the methylerythritol (MEP) pathway [87]. Bactoprenol is an essential compound of the bacterial peptidoglycan ring, so without it, division is halted, leading to the production of aberrant bodies. If there is a lack of tryptophan (Trp) available, the trpRBA operon, which is normally repressed by both TrpR and YtgR, is activated. TrpR repression is released due to the absence of the necessary tryptophan cofactor while YtgR repression stops because there is not enough tryptophan available to assemble the unstable 3xTrp motif in its sequence [32]. To counteract the effects of tryptophan deprivation, tryptophan-rich chlamydial proteins are degraded to refill the pool of bacterial tryptophan, which is sequestered from the host cell and isolated from IDO [62]. During iron stress, proliferation-favorable homeostasis is disrupted. The absence of free ferrous iron prevents the synthesis of peptidoglycan (PG) via the blockage of the MEP pathway due to the lack of two iron-dependent enzymes, IspH and IspG [88]. The ytgABCD operon then becomes activated due to the lack of a [Fe2+] cofactor which is required for YtgR-mediated repression [32]. While the chlamydial response allows an increase to the pools of tryptophan and of free iron, this compensation might not be sufficient to completely overcome the stress. Phosphomycin and glycopeptide antibiotics such as vancomycin and teicoplanin induce aberrant bodies by affecting directly the peptidoglycan synthesis pathway [13,76]. Penicillin derivatives target penicillin binding proteins (PBP) to prevent the peptidoglycan division ring from expanding or constricting [76]. Together, this may create a new metabolic equilibrium that allows bacterial survival at the cost of proliferation.
The current hypothesis indicates that, following host cell immune response by IFNγ, the host cell tryptophan pool is depleted by the enzyme IDO. The absence of tryptophan activates GSK3β, an enzyme known to initiate host cell c-Myc degradation. This results in a decrease in the production of glutaminases and glutamine transporters necessary for the TCA cycle to perform optimally. With the halting of the TCA cycle, pyruvate, which is necessary for the bactoprenol synthesis by the MEP pathway, is not produced in a sufficient concentration. Bactoprenol is an essential compound of the peptidoglycan ring and therefore, division is halted, leading to ABs. The persistence of ABs may be a result of their ability to counteract the consequences of stressors. When tryptophan is absent, the trpRBA operon, normally repressed by both TrpR and YtgR, is activated. TrpR repression ends due to the absence of a necessary tryptophan co-factor, while YtgR repression stops once there is an insufficient pool of tryptophan to assemble the 3xtrp motif in its sequence. Moreover, degradation of previously produced tryptophan-rich chlamydial proteins refills the tryptophan pool within the inclusion, which is inaccessible to IDO in the host cytoplasm. While the chlamydial response is not robust enough to completely overcome the immune response, it is able to create a metabolic equilibrium to allow bacterial survival.
During iron stress, proliferation-favorable homeostasis is also disrupted. The absence of free ferrous iron may prevent the synthesis of peptidoglycan through the inactivation of iron-dependent enzymes IspH and IspG, thereby blocking the MEP pathway. Peptidoglycan is necessary during division due to its presence in the peptidoglycan division ring, which must expand and then constrict to complete a round of replication. The ytgABCD operon is then activated due to the lack of [Fe2+] cofactor required for YtgR-mediated repression. Although iron uptake by Chlamydiae is high during infection, it is not sufficient to overcome once there is a lack of iron, leading to another inducer of persistence.
β-lactamase inhibitors also induce persistence in Chlamydiae. These inhibitors act on the peptidoglycan division ring in a different mechanism to iron stress detailed before. Rather than preventing the synthesis of peptidoglycan, β-lactamase inhibitors affect the regulation of the ring itself by either preventing the expansion of the ring during the initiation of division or the constriction that is necessary to complete division. In each scenario detailed here, the outcome is a termination of cell division characteristic of persistent ABs.
Proposing a general model that would encompass the effect of different stressors for all members of the family is an extremely tedious task due to multiple layers of complexity that occur. One layer is the genomic variation between Chlamydiaceae in the trp operon. Some Chlamydiae genomes, such as W. chondrophila, do not contain any known trp operon. Even so, by aligning the YtgR sequences of different Chlamydiae species, it was shown that W. chondrophila does indeed contain the triple-tryptophan motif in its YtgR amino acid sequence that is believed to regulate the repression of the trp operon in other Chlamydiae. Inversely, S. negevensis, which does encode a full trp operon, does not harbor this triple-tryptophan regulating sequence in its YtgR repressor [73]. Additionally, both W. chondrophila and S. negevensis are known to infect a broad range of host cells including protozoa, amoeba, and mammalian cells. Each host has a unique capacity for immune response and iron or tryptophan utilization, adding an additional layer of complexity to modeling the persistence response in these bacterial species.
To further investigate the global regulation of different Chlamydiae species, transcriptional studies have been performed under multiple conditions like heat shock, iron starvation, or tryptophan starvation [33,36,55,89,90]. However, comparing the results of the assays has proven to be difficult. For example, RNA sequencing data derived following treatment of cultured cells infected with W. chondrophila [55] or C. trachomatis [33] with iron chelator BPDL to induce persistence, resulted in dissimilar regulations for most genes. Strikingly, the bacterial stress operon regulated by HrcA was activated for W. chondrophila, but not for C. trachomatis. Conversely, this operon was activated in persistent C. trachomatis during heat shock [36]. Differential triggering of HrcA response between different chlamydial species in the same type of host cell may reflect different susceptibility. These results imply that Chlamydiae have a metabolic toolkit optimized for a specific target organism. This aligns with the fact that these intracellular bacteria have a greatly reduced genome, partially resulting from co-evolution with a specific host [91,92]. The importance of host cell compatibility is greatly illustrated in studies where spontaneous ABs were observed without any treatment, as infection in a non-optimal host will increase stress for those bacteria (Table 1).
Additionally, the parameters that have been historically used to characterize ABs separately from EBs or RBs have been described here. Recent evidence suggests that these historic classifications may not encompass all types of ABs which can arise. For example, following treatment of cell cultures infected with either C. trachomatis or W. chondrophila with MP265, an MreB inhibitor, the bacteria exhibited persistent behavior, but there was no significant increase in the area and length of the cells, depending on the timing of the treatment [13,30]. It would therefore appear that the enlarged bacterial size is not mandatory when peptidoglycan disruption co-occurs with cell wall synthesis inhibition. Given this new finding, it would be prudent to re-examine those previous studies that excluded a persistence phenotype based on size alone to potentially uncover novel insights.
In vitro studies of aberrant bodies generally focus on each stress individually, while their occurrence in vivo is more probably multifactorial. Genomic and metabolomic studies could help assess the level of synergy or incompatibility between chlamydial species and their potential host cells. Moving forward, examining the differential susceptibility of stress from one host cell to another can provide new understanding of chlamydial infection. These new insights could help provide an explanation as to why enlarged ABs have rarely been described in vivo, and why they can appear spontaneously.
Funding
This research was funded by the Swiss National Science Foundation for grant number 197768 to Gilbert Greub. This grant indeed partially supports the salary of Thomas Kozusnik during his research dedicated to the persistence of chlamydia and chlamydia-related organisms, as well as mechanisms at play in the formation of aberrant bodies.
Data Availability Statement
The unpublished data may be made available, upon reasonable request, from G. Greub, but you may explore more deeply the genomics pathways discussed using our chlamDB database available for free at the following website: https://chlamdb.ch (accessed on 24 February 2024).
Conflicts of Interest
The authors have no specific conflicts of interest related to the present article. G. Greub is a medical advisor at Resistell (Muttenz, Switzerland), a start-up developing a new approach to detect the antibiotic susceptibility of bacteria using nanomotion technology. G. Greub also has two ongoing research agreements, respectively, with Resistell and Becton-Dickinson (Baltimore, MD, USA). In addition, G. Greub is co-director of JeuPRO, a start-up editing and distributing games on microbes (i.e., the Krobs game and the MyKrobs game).
References
- PAHO/WHO|Chlamydia. Available online: https://www.paho.org/en/topics/chlamydia-infection (accessed on 17 November 2022).
- den Heijer, C.D.J.; Hoebe, C.J.P.A.; Driessen, J.H.M.; Wolffs, P.; van den Broek, I.V.F.; Hoenderboom, B.M.; Williams, R.; de Vries, F.; Dukers-Muijrers, N.H.T.M. Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease, Ectopic Pregnancy, and Female Infertility: A Retrospective Cohort Study among Primary Care Patients. Clin. Infect. Dis. 2019, 69, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Regan, L.; Greub, G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr. Opin. Infect. Dis. 2008, 21, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Goy, G.; Jaton, K.; Osterheld, M.-C.; Blumer, S.; Borel, N.; Vial, Y.; Hohlfeld, P.; Pospischil, A.; Greub, G. Role of Chlamydia trachomatis in miscarriage. Emerg. Infect. Dis. 2011, 17, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.N. Evolution of Chlamydia trachomatis. Ann. N. Y. Acad. Sci. 2011, 1230, E11–E18. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. The atypical pneumonias: Clinical diagnosis and importance. Clin. Microbiol. Infect. 2006, 12, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Pichon, N.; Guindre, L.; Laroucau, K.; Cantaloube, M.; Nallatamby, A.; Parreau, S. Chlamydia abortus in Pregnant Woman with Acute Respiratory Distress Syndrome. Emerg. Infect. Dis. 2020, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Greub, G. Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol. Rev. 2010, 34, 260–280. [Google Scholar] [CrossRef]
- Cheong, H.C.; Lee, C.Y.Q.; Cheok, Y.Y.; Tan, G.M.Y.; Looi, C.Y.; Wong, W.F. Chlamydiaceae: Diseases in Primary Hosts and Zoonosis. Microorganisms 2019, 7, 146. [Google Scholar] [CrossRef]
- Bayramova, F.; Jacquier, N.; Greub, G. Insight in the biology of Chlamydia-related bacteria. Microbes Infect. 2018, 20, 432–440. [Google Scholar] [CrossRef]
- AbdelRahman, Y.M.; Belland, R.J. The chlamydial developmental cycle. FEMS Microbiol. Rev. 2005, 29, 949–959. [Google Scholar] [CrossRef]
- Vouga, M.; Baud, D.; Greub, G. Simkania negevensis may produce long-lasting infections in human pneumocytes and endometrial cells. Pathog. Dis. 2017, 75, ftw115. [Google Scholar] [CrossRef] [PubMed]
- Scherler, A.; Jacquier, N.; Kebbi-Beghdadi, C.; Greub, G. Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila. Microorganisms 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.D.; Byrne, G.I. Differential Effects of Gamma Interferon on Chlamydia trachomatis Growth in Polarized and Nonpolarized Human Epithelial Cells in Culture. Infect. Immun. 1998, 66, 2349–2351. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.; Gyger, J.; Piersigilli, A.; Pilloux, L.; Greub, G.; Stojanov, M.; Baud, D. Waddlia chondrophila induces systemic infection, organ pathology, and elicits Th1-associated humoral immunity in a murine model of genital infection. Front. Cell. Infect. Microbiol. 2015, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Loosli, C.G.; Ritter, M.H. The pathogenesis and histopathology of air-borne pneumonitis virus infection in mice; the effect of penicillin G upon the developing lesion. J. Clin. Investig. 1948, 27, 547. [Google Scholar] [PubMed]
- Weiss, E. The effect of antibiotics on agents of the psittacosis-lymphogranuloma group. I. The effect of penicillin. J. Infect. Dis. 1950, 87, 249–263. [Google Scholar] [CrossRef]
- Hurst, E.W.; Landquist, J.K.; Melvin, P.; Peters, J.M.; Senior, N.; Silk, J.A.; Stacey, G.J. The therapy of experimental psittacosis and lymphogranuloma venereum (inguinale). Br. J. Pharmacol. Chemother. 1953, 8, 297–305. [Google Scholar] [CrossRef]
- Tamura, A.; Manire, G.P. Effect of Penicillin on the Multiplication of Meningopneumonitis Organisms (Chlamydia psittaci). J. Bacteriol. 1968, 96, 875–880. [Google Scholar] [CrossRef]
- Galasso, G.J.; Manire, G.P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J. Immunol. 1961, 86, 382–385. [Google Scholar] [CrossRef]
- Matsumoto, A.; Manire, G.P. Electron Microscopic Observations on the Effects of Penicillin on the Morphology of Chlamydia psittaci. J. Bacteriol. 1970, 101, 278–285. [Google Scholar] [CrossRef]
- Beatty, W.L.; Byrne, G.I.; Morrison, R.P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 1993, 90, 3998–4002. [Google Scholar] [CrossRef]
- Beatty, W.L.; Morrison, R.P.; Byrne, G.I. Persistent chlamydiae: From cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 1994, 58, 686–699. [Google Scholar] [CrossRef]
- De Barsy, M.; Bottinelli, L.; Greub, G. Antibiotic susceptibility of Estrella lausannensis, a potential emerging pathogen. Microbes Infect. 2014, 16, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Kebbi-Beghdadi, C.; Cisse, O.; Greub, G. Permissivity of Vero cells, human pneumocytes and human endometrial cells to Waddlia chondrophila. Microbes Infect. 2011, 13, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Vouga, M.; Baud, D.; Greub, G. Simkania negevensis, an Example of the Diversity of the Antimicrobial Susceptibility Pattern among Chlamydiales. Antimicrob. Agents Chemother. 2017, 61, e00638-17. [Google Scholar] [CrossRef] [PubMed]
- Sampo, A.; Matsuo, J.; Yamane, C.; Yagita, K.; Nakamura, S.; Shouji, N.; Hayashi, Y.; Yamazaki, T.; Yoshida, M.; Kobayashi, M.; et al. High-temperature adapted primitive Protochlamydia found in Acanthamoeba isolated from a hot spring can grow in immortalized human epithelial HEp-2 cells. Environ. Microbiol. 2014, 16, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Marquis, B.; Ardissone, S.; Greub, G. Temperature affects the host range of Rhabdochlamydia porcellionis and the infectivity of Waddlia chondrophila and Chlamydia trachomatis elementary bodies. bioRxiv 2022. [Google Scholar] [CrossRef]
- Brockett, M.R.; Liechti, G.W. Persistence Alters the Interaction between Chlamydia trachomatis and Its Host Cell. Infect. Immun. 2021, 89, e0068520. [Google Scholar] [CrossRef] [PubMed]
- Bowie, W.R. In vitro activity of clavulanic acid, amoxicillin, and ticarcillin against Chlamydia trachomatis. Antimicrob. Agents Chemother. 1986, 29, 713–715. [Google Scholar] [CrossRef]
- Pokorzynski, N.D.; Hatch, N.D.; Ouellette, S.P.; Carabeo, R.A. The iron-dependent repressor YtgR is a tryptophan-dependent attenuator of the trpRBA operon in Chlamydia trachomatis. Nat. Commun. 2020, 11, 6430. [Google Scholar] [CrossRef]
- Brinkworth, A.J.; Wildung, M.R.; Carabeo, R.A. Genomewide Transcriptional Responses of Iron-Starved Chlamydia trachomatis Reveal Prioritization of Metabolic Precursor Synthesis over Protein Translation. mSystems 2018, 3, e00184-17. [Google Scholar] [CrossRef]
- Thompson, C.C.; Carabeo, R.A. An Optimal Method of Iron Starvation of the Obligate Intracellular Pathogen, Chlamydia Trachomatis. Front. Microbiol. 2011, 2, 20. [Google Scholar] [CrossRef]
- Kahane, S.; Friedman, M.G. Reversibility of heat shock in Chlamydia trachomatis. FEMS Microbiol. Lett. 1992, 97, 25–30. [Google Scholar] [CrossRef]
- Huang, Y.; Wurihan, W.; Lu, B.; Zou, Y.; Wang, Y.; Weldon, K.; Fondell, J.D.; Lai, Z.; Wu, X.; Fan, H. Robust Heat Shock Response in Chlamydia Lacking a Typical Heat Shock Sigma Factor. Front. Microbiol. 2022, 12, 812448. [Google Scholar] [CrossRef]
- Deka, S.; Vanover, J.; Sun, J.; Kintner, J.; Whittimore, J.; Schoborg, R.V. An early event in the herpes simplex virus type-2 replication cycle is sufficient to induce Chlamydia trachomatis persistence. Cell. Microbiol. 2007, 9, 725–737. [Google Scholar] [CrossRef]
- Deka, S.; Vanover, J.; Dessus-Babus, S.; Whittimore, J.; Howett, M.K.; Wyrick, P.B.; Schoborg, R.V. Chlamydia trachomatis enters a viable but non-cultivable (persistent) state within herpes simplex virus type 2 (HSV-2) co-infected host cells. Cell. Microbiol. 2006, 8, 149–162. [Google Scholar] [CrossRef]
- Vollmuth, N.; Schlicker, L.; Guo, Y.; Hovhannisyan, P.; Janaki-Raman, S.; Kurmasheva, N.; Schmitz, W.; Schulze, A.; Stelzner, K.; Rajeeve, K.; et al. c-Myc plays a key role in IFN-γ-induced persistence of Chlamydia trachomatis. eLife 2022, 11, e76721. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Campbell, R.; Kintner, J.; Whittimore, J.; Schoborg, R.V. Chlamydia muridarum Enters a Viable but Non-infectious State in Amoxicillin-treated BALB/c Mice. Microbes Infect. Inst. Pasteur 2012, 14, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Campbell, R.; Kintner, J.; Schoborg, R.V. Induction of the Chlamydia muridarum Stress/Persistence Response Increases Azithromycin Treatment Failure in a Murine Model of Infection. Antimicrob. Agents Chemother. 2014, 58, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Marti, H.; Borel, N.; Dean, D.; Leonard, C.A. Evaluating the Antibiotic Susceptibility of Chlamydia—New Approaches for in Vitro Assays. Front. Microbiol. 2018, 9, 1414. [Google Scholar] [CrossRef]
- Pospischil, A.; Borel, N.; Chowdhury, E.H.; Guscetti, F. Aberrant chlamydial developmental forms in the gastrointestinal tract of pigs spontaneously and experimentally infected with Chlamydia suis. Vet. Microbiol. 2009, 135, 147–156. [Google Scholar] [CrossRef]
- Goellner, S.; Schubert, E.; Liebler-Tenorio, E.; Hotzel, H.; Saluz, H.P.; Sachse, K. Transcriptional Response Patterns of Chlamydophila psittaci in Different In Vitro Models of Persistent Infection. Infect. Immun. 2006, 74, 4801–4808. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Wang, C.; Xie, Y.; Chen, Z.; Liu, L.; Su, Z.; Wu, Y. Transcriptional Analysis of 10 Selected Genes in a Model of Penicillin G Induced Persistence of Chlamydophila psittaci in HeLa Cells. J. Microbiol. Biotechnol. 2015, 25, 1246–1256. [Google Scholar] [CrossRef]
- Moulder, J.W.; Levy, N.J.; Schulman, L.P. Persistent Infection of Mouse Fibroblasts (L Cells) with Chlamydia psittaci: Evidence for a Cryptic Chlamydial Form. Infect. Immun. 1980, 30, 874–883. [Google Scholar] [CrossRef]
- Jacquier, N.; Frandi, A.; Pillonel, T.; Viollier, P.H.; Greub, G. Cell wall precursors are required to organize the chlamydial division septum. Nat. Commun. 2014, 5, 3578. [Google Scholar] [CrossRef] [PubMed]
- Al-Younes, H.M.; Rudel, T.; Brinkmann, V.; Szczepek, A.J.; Meyer, T.F. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell. Microbiol. 2001, 3, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Miller, R.D.; Sullivan, E.D.; Theodoropoulos, C.; Mathews, S.A.; Timms, P.; Summersgill, J.T. Protein Expression Profiles of Chlamydia pneumoniae in Models of Persistence versus Those of Heat Shock Stress Response. Infect. Immun. 2006, 74, 3853–3863. [Google Scholar] [CrossRef] [PubMed]
- Mannonen, L.; Kamping, E.; Penttilä, T.; Puolakkainen, M. IFN-γ induced persistent Chlamydia pneumoniae infection in HL and Mono Mac 6 cells: Characterization by real-time quantitative PCR and culture. Microb. Pathog. 2004, 36, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.A.; Dewez, F.; Borel, N. Penicillin G-Induced Chlamydial Stress Response in a Porcine Strain of Chlamydia pecorum. Int. J. Microbiol. 2016, 2016, e3832917. [Google Scholar] [CrossRef] [PubMed]
- Borel, N.; Dumrese, C.; Ziegler, U.; Schifferli, A.; Kaiser, C.; Pospischil, A. Mixed infections with Chlamydia and porcine epidemic diarrhea virus—A new in vitro model of chlamydial persistence. BMC Microbiol. 2010, 10, 201. [Google Scholar] [CrossRef]
- Philips, H.L.; Clarkson, M.J. Spontaneous change from overt to covert infection of Chlamydia pecorum in cycloheximide-treated mouse McCoy cells. Infect. Immun. 1995, 63, 3729–3730. [Google Scholar] [CrossRef]
- Rovero, A.; Kebbi-Beghdadi, C.; Greub, G. Spontaneous Aberrant Bodies Formation in Human Pneumocytes Infected with Estrella lausannensis. Microorganisms 2023, 11, 2368. [Google Scholar] [CrossRef]
- Ardissone, S.; Scherler, A.; Pillonel, T.; Martin, V.; Kebbi-Beghdadi, C.; Greub, G. Transcriptional Landscape of Waddlia chondrophila Aberrant Bodies Induced by Iron Starvation. Microorganisms 2020, 8, 1848. [Google Scholar] [CrossRef]
- Kahane, S.; Fruchter, D.; Dvoskin, B.; Friedman, M.G. Versatility of Simkania negevensis infection in vitro and induction of host cell inflammatory cytokine response. J. Infect. 2007, 55, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, M.E.; Gigliotti-Rothfuchs, A.; Wigzell, H. The role of IFN-γ in the outcome of chlamydial infection. Curr. Opin. Immunol. 2002, 14, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, L.G.; Miller, R.D.; Ramirez, J.A.; Molestina, R.E.; Summersgill, J.T. Characterization of Chlamydia pneumoniae Persistence in HEp-2 Cells Treated with Gamma Interferon. Infect. Immun. 2001, 69, 7927–7932. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Vidgen, M.E.; Taing, K.; Huston, W.M.; Timms, P. Dysbiosis of the Vaginal Microbiota and Higher Vaginal Kynurenine/Tryptophan Ratio Reveals an Association with Chlamydia trachomatis Genital Infections. Front. Cell. Infect. Microbiol. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Fehlner-Gardiner, C.; Roshick, C.; Carlson, J.H.; Hughes, S.; Belland, R.J.; Caldwell, H.D.; McClarty, G. Molecular Basis Defining Human Chlamydia trachomatis Tissue Tropism: A possible role for tryptophan synthase. J. Biol. Chem. 2002, 277, 26893–26903. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.; Byrne, G.; Jensen, R. Chlamydia exploit the mammalian tryptophan-depletion defense strategy as a counter-defensive cue to trigger a survival state of persistence. Front. Cell. Infect. Microbiol. 2014, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Nelson, D.E. How Chlamydia trachomatis conquered gut microbiome-derived antimicrobial compounds and found a new home in the eye. Proc. Natl. Acad. Sci. USA 2019, 116, 12136–12138. [Google Scholar] [CrossRef]
- Rajeeve, K.; Vollmuth, N.; Janaki-Raman, S.; Wulff, T.F.; Baluapuri, A.; Dejure, F.R.; Huber, C.; Fink, J.; Schmalhofer, M.; Schmitz, W.; et al. Reprogramming of host glutamine metabolism during Chlamydia trachomatis infection and its key role in peptidoglycan synthesis. Nat. Microbiol. 2020, 5, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.; Urlbauer, B.; Kohlhuber, F.; Hammersen, B.; Eick, D. Ongoing mutations in the N-terminal domain of c-Myc affect transactivation in Burkitt’s lymphoma cell lines. Oncogene 1994, 9, 759–763. [Google Scholar] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Ouellette, S.; Carabeo, R. A Functional Slow Recycling Pathway of Transferrin is Required for Growth of Chlamydia. Front. Microbiol. 2010, 1, 112. [Google Scholar] [CrossRef]
- Pokorzynski, N.D.; Thompson, C.C.; Carabeo, R.A. Ironing Out the Unconventional Mechanisms of Iron Acquisition and Gene Regulation in Chlamydia. Front. Cell. Infect. Microbiol. 2017, 7, 394. [Google Scholar] [CrossRef]
- Hatch, G.M.; McClarty, G. Phospholipid Composition of Purified Chlamydia trachomatis Mimics That of the Eucaryotic Host Cell. Infect. Immun. 1998, 66, 3727–3735. [Google Scholar] [CrossRef]
- Ouellette, S.P.; Dorsey, F.C.; Moshiach, S.; Cleveland, J.L.; Carabeo, R.A. Chlamydia Species-Dependent Differences in the Growth Requirement for Lysosomes. PLoS ONE 2011, 6, e16783. [Google Scholar] [CrossRef]
- Gough, N.R. Two for One in Iron Homeostasis. Sci. Signal. 2012, 5, ec178. [Google Scholar] [CrossRef]
- Luo, Z.; Neville, S.L.; Campbell, R.; Morey, J.R.; Menon, S.; Thomas, M.; Eijkelkamp, B.A.; Ween, M.P.; Huston, W.M.; Kobe, B.; et al. Structure and Metal Binding Properties of Chlamydia trachomatis YtgA. J. Bacteriol. 2019, 202, e00580-19. [Google Scholar] [CrossRef]
- Pillonel, T.; Tagini, F.; Bertelli, C.; Greub, G. ChlamDB: A comparative genomics database of the phylum Chlamydiae and other members of the Planctomycetes-Verrucomicrobiae-Chlamydiae superphylum. Nucleic Acids Res. 2019, 48, D526–D534. [Google Scholar] [CrossRef]
- Liechti, G.; Kuru, E.; Hall, E.; Kalinda, A.; Brun, Y.V.; VanNieuwenhze, M.; Maurelli, A.T. A new metabolic cell wall labeling method reveals peptidoglycan in Chlamydia trachomatis. Nature 2014, 506, 507–510. [Google Scholar] [CrossRef]
- Moulder, J.W. Why is Chlamydia sensitive to penicillin in the absence of peptidoglycan. Infect. Agents Dis. 1993, 2, 87–99. [Google Scholar]
- Jacquier, N.; Viollier, P.H.; Greub, G. The role of peptidoglycan in chlamydial cell division: Towards resolving the chlamydial anomaly. FEMS Microbiol. Rev. 2015, 39, 262–275. [Google Scholar] [CrossRef]
- Packiam, M.; Weinrick, B.; Jacobs, W.R.; Maurelli, A.T. Structural characterization of muropeptides from Chlamydia trachomatis peptidoglycan by mass spectrometry resolves “chlamydial anomaly”. Proc. Natl. Acad. Sci. USA 2015, 112, 11660–11665. [Google Scholar] [CrossRef] [PubMed]
- Frandi, A.; Jacquier, N.; Théraulaz, L.; Greub, G.; Viollier, P.H. FtsZ-independent septal recruitment and function of cell wall remodelling enzymes in chlamydial pathogens. Nat. Commun. 2014, 5, 4200. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.; Ouellette, S.P.; Belland, R.J.; Cox, J.V. Polarized Cell Division of Chlamydia trachomatis. PLoS Pathog. 2016, 12, e1005822. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Yadav, A.K.; Pillonel, T.; Viollier, P.H.; Cava, F.; Greub, G. A SpoIID Homolog Cleaves Glycan Strands at the Chlamydial Division Septum. mBio 2019, 10, e01128-19. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Frandi, A.; Viollier, P.H.; Greub, G. Disassembly of a Medial Transenvelope Structure by Antibiotics during Intracellular Division. Chem. Biol. 2015, 22, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Pilhofer, M.; Aistleitner, K.; Biboy, J.; Gray, J.; Kuru, E.; Hall, E.; Brun, Y.V.; VanNieuwenhze, M.S.; Vollmer, W.; Horn, M.; et al. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat. Commun. 2013, 4, 2856. [Google Scholar] [CrossRef]
- Liechti, G.W. Localized Peptidoglycan Biosynthesis in Chlamydia trachomatis Conforms to the Polarized Division and Cell Size Reduction Developmental Models. Front. Microbiol. 2021, 12, 733850. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.V.; Abdelrahman, Y.M.; Ouellette, S.P. Penicillin-binding proteins regulate multiple steps in the polarized cell division process of Chlamydia. Sci. Rep. 2020, 10, 12588. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Enciso, G.A.; Boassa, D.; Chander, C.N.; Lou, T.H.; Pairawan, S.S.; Guo, M.C.; Wan, F.Y.M.; Ellisman, M.H.; Sütterlin, C.; et al. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nat. Commun. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.; Kuru, E.; Packiam, M.; Hsu, Y.-P.; Tekkam, S.; Hall, E.; Rittichier, J.T.; VanNieuwenhze, M.; Brun, Y.V.; Maurelli, A.T. Pathogenic Chlamydia Lack a Classical Sacculus but Synthesize a Narrow, Mid-cell Peptidoglycan Ring, Regulated by MreB, for Cell Division. PLoS Pathog. 2016, 12, e1005590. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.A.; Brown, M.J. The methylerythritol phosphate pathway and its significance as a novel drug target. Curr. Pharm. Biotechnol. 2003, 4, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Slade, J.A.; Brockett, M.; Singh, R.; Liechti, G.W.; Maurelli, A.T. Fosmidomycin, an inhibitor of isoprenoid synthesis, induces persistence in Chlamydia by inhibiting peptidoglycan assembly. PLoS Pathog. 2019, 15, e1008078. [Google Scholar] [CrossRef]
- Belland, R.J.; Nelson, D.E.; Virok, D.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Beatty, W.L.; Caldwell, H.D. Transcriptome analysis of chlamydial growth during IFN-γ-mediated persistence and reactivation. Proc. Natl. Acad. Sci. USA 2003, 100, 15971–15976. [Google Scholar] [CrossRef]
- Ouellette, S.P.; Hatch, T.P.; AbdelRahman, Y.M.; Rose, L.A.; Belland, R.J.; Byrne, G.I. Global transcriptional upregulation in the absence of increased translation in Chlamydia during IFNγ-mediated host cell tryptophan starvation. Mol. Microbiol. 2006, 62, 1387–1401. [Google Scholar] [CrossRef]
- Sigalova, O.M.; Chaplin, A.V.; Bochkareva, O.O.; Shelyakin, P.V.; Filaretov, V.A.; Akkuratov, E.E.; Burskaia, V.; Gelfand, M.S. Chlamydia Pan-Genomic Analysis Reveals Balance between Host Adaptation and Selective Pressure to Genome Reduction. BMC Genom. 2019, 20, 710. [Google Scholar] [CrossRef]
- Moran, N.A. Microbial minimalism: Genome reduction in bacterial pathogens. Cell 2002, 108, 583–586. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).