Comparison of Neutralizing Activity between Vaccinated and Unvaccinated Hospitalized COVID-19 Patients Infected with Delta, Omicron BA.1, or Omicron BA.2 Variant
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Sources
2.2.1. Baseline Characteristics
2.2.2. Variant Identification
2.2.3. Surrogate Virus Neutralization Test
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Baseline Characteristics
3.2. SARS-CoV-2 Variant Identification
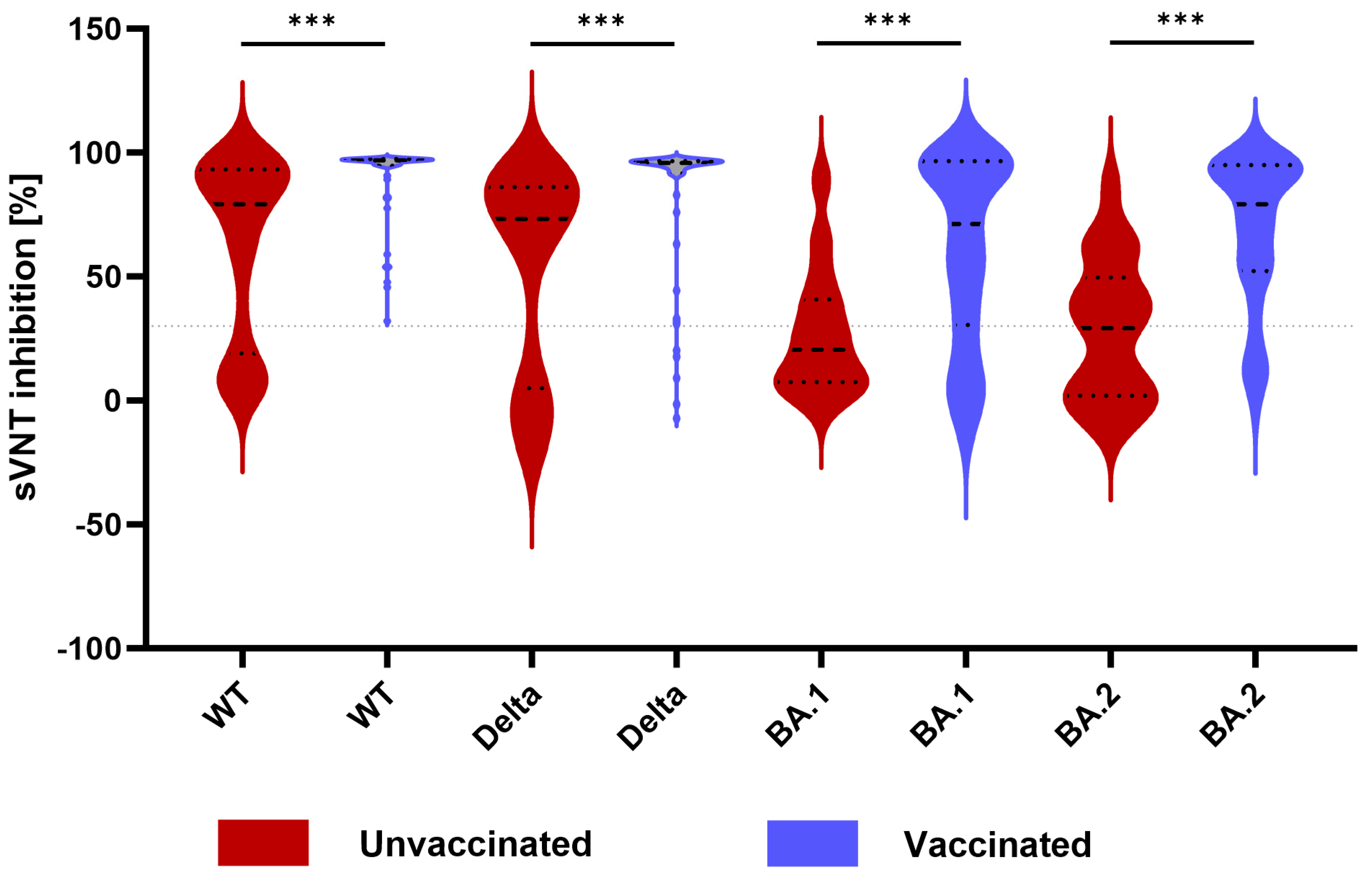
3.3. Neutralizing Activity between Unvaccinated and Vaccinated Patients and against Different SARS-CoV-2 Variants
3.4. Correlation between Ct Value and Neutralizing Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tylicki, L. Immunity after Vaccination against COVID-19. Vaccines 2023, 11, 1723. [Google Scholar] [CrossRef]
- Tay, J.H.; Porter, A.F.; Wirth, W.; Duchene, S. The Emergence of SARS-CoV-2 Variants of Concern Is Driven by Acceleration of the Substitution Rate. Mol. Biol. Evol. 2022, 39, msac013. [Google Scholar] [CrossRef]
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med. J. 2021, 62, 961–968. [Google Scholar] [CrossRef]
- Błaszczuk, A.; Michalski, A.; Sikora, D.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Antibody Response after SARS-CoV-2 Infection with the Delta and Omicron Variant. Vaccines 2022, 10, 1728. [Google Scholar] [CrossRef]
- Kamińska, D.; Dęborska-Materkowska, D.; Kościelska-Kasprzak, K.; Mazanowska, O.; Remiorz, A.; Poznański, P.; Durlik, M.; Krajewska, M. Immunity after COVID-19 Recovery and Vaccination: Similarities and Differences. Vaccines 2022, 10, 1068. [Google Scholar] [CrossRef]
- Castro Dopico, X.; Ols, S.; Loré, K.; Karlsson Hedestam, G.B. Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.S.; Siedner, M.J.; Boucau, J.; Lavine, C.L.; Ghantous, F.; Liew, M.Y.; Mathews, J.I.; Singh, A.; Marino, C.; Regan, J.; et al. Vaccine breakthrough infection leads to distinct profiles of neutralizing antibody responses by SARS-CoV-2 variant. JCI Insight 2022, 7, e159944. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Sprouse, K.R.; Bowen, J.E.; Joshi, A.; Franko, N.; Navarro, M.J.; Stewart, C.; Cameroni, E.; McCallum, M.; Goecker, E.A.; et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell 2022, 185, 872–880.e3. [Google Scholar] [CrossRef]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.Y.; et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell 2022, 185, 1539–1548.e5. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Stein, C.; Nassereldine, H.; Sorensen, R.J.; Amlag, J.O.; Bisignano, C.; Byrne, S.; Castro, E.; Coberly, K.; Collins, J.K.; Dalos, J.; et al. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- WHO. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 6 February 2024).
- Lavezzo, E.; Pacenti, M.; Manuto, L.; Boldrin, C.; Cattai, M.; Grazioli, M.; Bianca, F.; Sartori, M.; Caldart, F.; Castelli, G.; et al. Neutralising reactivity against SARS-CoV-2 Delta and Omicron variants by vaccination and infection history. Genome Med. 2022, 14, 61. [Google Scholar] [CrossRef]
- Oh, S.J.; O, S.W.; Choi, Y.J.; Kim, J.M.; Kim, D.; Kim, I.H.; Park, A.K.; Kim, H.M.; Rhee, J.E.; Jang, Y.R.; et al. Neutralizing antibody responses in vaccinated and unvaccinated individuals infected with Omicron BA.1 variant. J. Clin. Virol. 2022, 155, 105253. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Karim, F.; Cele, S.; Reedoy, K.; San, J.E.; Lustig, G.; Tegally, H.; Rosenberg, Y.; Bernstein, M.; Jule, Z.; et al. Omicron infection enhances Delta antibody immunity in vaccinated persons. Nature 2022, 607, 356–359. [Google Scholar] [CrossRef]
- Flacco, M.E.; Acuti Martellucci, C.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. COVID-19 vaccines reduce the risk of SARS-CoV-2 reinfection and hospitalization: Meta-analysis. Front. Med. 2022, 9, 1023507. [Google Scholar] [CrossRef]
- Ip, J.D.; Kok, K.H.; Chan, W.M.; Chu, A.W.; Wu, W.L.; Yip, C.C.; To, W.K.; Tsang, O.T.; Leung, W.S.; Chik, T.S.; et al. Intra-host non-synonymous diversity at a neutralizing antibody epitope of SARS-CoV-2 spike protein N-terminal domain. Clin. Microbiol. Infect. 2021, 27, 1350.e1–1350.e5. [Google Scholar] [CrossRef]
- Khong, K.W.; Zhang, R.; Hung, I.F. The Four Ws of the Fourth Dose COVID-19 Vaccines: Why, Who, When and What. Vaccines 2022, 10, 1924. [Google Scholar] [CrossRef]
- Hyams, C.; Challen, R.; Marlow, R.; Nguyen, J.; Begier, E.; Southern, J.; King, J.; Morley, A.; Kinney, J.; Clout, M.; et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom. Lancet Reg. Health Eur. 2023, 25, 100556. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Yuan, J.; Xu, Z.; Gu, Y.; Zhang, J.; Guan, Y.; Liang, J.; Lu, H.; Liu, Y. Viral and antibody dynamics of acute infection with SARS-CoV-2 omicron variant (B.1.1.529): A prospective cohort study from Shenzhen, China. Lancet Microbe 2023, 4, e632–e641. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef]
- Contreras, S.; Iftekhar, E.N.; Priesemann, V. From emergency response to long-term management: The many faces of the endemic state of COVID-19. Lancet Reg. Health Eur. 2023, 30, 100664. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; McBride, S.K.; Winders, B.; Schoen, D.; Trautmann, L.; Curlin, M.E.; Tafesse, F.G. Antibody Response and Variant Cross-Neutralization after SARS-CoV-2 Breakthrough Infection. JAMA 2022, 327, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Lee, D.X.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 2023, 8, e165265. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Arashiro, T.; Adachi, Y.; Moriyama, S.; Kinoshita, H.; Kanno, T.; Saito, S.; Katano, H.; Iida, S.; Ainai, A.; et al. Vaccination-infection interval determines cross-neutralization potency to SARS-CoV-2 Omicron after breakthrough infection by other variants. Med 2022, 3, 249–261.e4. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Ko, R.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.M.; Brackman, C.J.; To, E.M.W.; Yen, H.L.; Leung, K.; Cheng, S.M.S.; et al. Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera. J. Clin. Microbiol. 2021, 59, e02504-20. [Google Scholar] [CrossRef]


| All Patients (n = 97) | Unvaccinated (n = 45) | Vaccinated (n = 51) | p Value | |
|---|---|---|---|---|
| Symptom onset to hospital admission (days), median [IQR] | 1 [0–3] | 1 [0–3] | 1 [0–4] | 0.471 |
| Age, median [IQR] | 73 [65–82] | 71 [58–82.5] | 74 [67–81] | 0.646 |
| Female sex | 45 (46.4%) | 24 (53.3%) | 21 (38.2%) | 0.234 |
| Male sex | 52 (53.6%) | 21 (46.7%) | 30 (61.8%) | |
| Nationality | ||||
| Korean | 97 (100%) | |||
| Non-Korean | 0 (0%) | |||
| Charlson Comorbidity Index score | ||||
| 0 | 40 (41.2%) | 23 (51.1%) | 15 (29.4%) | 0.006 ** |
| 1–2 | 32 (33.0%) | 17 (37.8%) | 16 (31.4%) | |
| ≥3 | 25 (25.8%) | 5 (11.1%) | 20 (39.2%) | |
| Immunosuppression | ||||
| Any type | 16 (16.5%) | 6 (13.3%) | 10 (19.6%) | 0.410 |
| Cancer treatment (chemotherapy or radiation) | 10 (10.3%) | 4 (8.9%) | 6 (11.8%) | 0.746 |
| Organ transplantation | 5 (5.2%) | 1 (2.2%) | 4 (7.8%) | 0.367 |
| Corticosteroids | 4 (4.1%) | 1 (2.2%) | 3 (5.9%) | 0.620 |
| HIV/AIDS | 0 (0%) | |||
| PrEP/PEP | 0 (0%) | |||
| Vaccination status | ||||
| Unvaccinated | 45 (46.4%) | |||
| Partially vaccinated a | 1 (1.0%) | |||
| Fully vaccinated | 51 (52.6%) | |||
| BNT-BNT | 16 (31.4%) | |||
| ChAd-ChAd | 11 (21.6%) | |||
| ChAd-BNT | 1 (2.0%) | |||
| BNT-BNT-BNT | 12 (23.5%) | |||
| ChAd-BNT-BNT | 2 (3.9%) | |||
| ChAd-ChAd-BNT | 5 (9.8%) | |||
| ChAd-ChAd-mRNA | 4 (7.8%) | |||
| Last vaccination dose to infection (days), median [IQR] | 96.5 [62.8–149.5] | |||
| Ct value of first PCR on admission, median [IQR] | 18.5 [15.5–22.6] | 20.3 [15.4–22.4] | 17.9 [15.5–23.2] | 0.791 |
| Symptom onset to first PCR (days), median [IQR] | 2 [0–5] | 2 [0–5] | 1 [0–5] | 0.184 |
| SARS-CoV-2 variant | ||||
| Delta | 51 (52.6%) | 28 (62.2%) | 23 (45.1%) | 0.093 |
| Omicron | 46 (47.4%) | 17 (37.8%) | 28 (54.9%) | |
| Omicron BA.1 | 37 (38.1%) | 12 (26.7%) | 24 (47.1%) | |
| Omicron BA.2 | 9 (9.3%) | 5 (11.1%) | 4 (7.8%) | |
| Surrogate virus neutralization test Positivity (≥30% signal inhibition) | ||||
| WT | 84 (86.6%) | 32 (71.1%) | 51 (100%) | <0.001 *** |
| Delta | 77 (79.4%) | 31 (68.9%) | 46 (90.2%) | 0.009 ** |
| BA.1 | 55 (56.7%) | 16 (35.6%) | 39 (76.5%) | <0.001 *** |
| BA.2 | 65 (67.0%) | 22 (48.9%] | 43 (84.3%) | <0.001 *** |
| Neutralizing antibody titer (% signal inhibition), median [IQR] | ||||
| WT | 94.3 [62.8–96.9] | 79.2 [19.0–93.3] | 96.8 [94.7–97.4] | <0.001 *** |
| Delta | 88.6 [51.9–96.0] | 73.3 [4.9–86.1] | 95.8 [91.6–96.6] | <0.001 *** |
| BA.1 | 36.9 [9.6–83.4] | 20.5 [7.3–40.8] | 71.2 [30.4–96.5] | <0.001 *** |
| BA.2 | 52.2 [13.3–86.2] | 29.2 [1.9–49.5] | 79.1 [52.2–95.0] | <0.001 *** |
| Symptom onset to serologic tests (days), median [IQR] | 7 [3–16.0] | 6 [3–17.5] | 8.5 [4.8–14.0] | 0.462 |
| Delta-Infected Patients | BA.1-Infected Patients | BA.2-Infected Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surrogate Virus Neutralization Test | Unvac (n = 28) | Vac (n = 23) | p Value | Unvac (n = 12) | Vac (n = 24) | p Value | Unvac (n = 5) | Vac (n = 4) | p Value |
| Positivity (≥30% signal inhibition) | |||||||||
| WT | 26 (92.9%) | 23 (100%) | 0.495 | 5 (41.7%) | 24 (100%) | <0.001 *** | 1 (20%) | 4 (100%) | 0.048 * |
| Delta | 24 (85.7%) | 22 (95.7%) | 0.362 | 4 (33.3%) | 20 (83.3%) | 0.007 ** | 3 (60%) | 4 (100%) | 0.444 |
| BA.1 | 9 (32.1%) | 21 (91.3%) | <0.001 *** | 7 (58.3%) | 16 (66.7%) | 0.720 | 1 (20%) | 2 (50%) | 0.524 |
| BA.2 | 13 (46.4%) | 23 (100%) | <0.001 *** | 8 (66.7%) | 18 (75.0%) | 0.700 | 1 (20%) | 2 (50%) | 0.524 |
| Neutralizing antibody titer (% signal inhibition), median [IQR] | |||||||||
| WT | 85.0 [66.5–93.6] | 96.7 [95.3–97.2] | <0.001 *** | 19.0 [8.7–92.1] | 96.9 [84.4–97.4] | <0.001 *** | 6.7 [5.6–50.8] | 87.2 [59.5–97.3] | 0.064 |
| Delta | 77.5 [61.3–86.2] | 96.0 [94.0–96.6] | <0.001 *** | −2.4 [−13.0–71.1] | 94.9 [68.0–96.5] | <0.001 *** | 85.7 [−1.2–95.3] | 70.2 [35.8–96.7] | 0.730 |
| BA.1 | 19.9 [7.7–39.0] | 75.6 [41.1–96.6] | <0.001 *** | 29.0 [10.7–50.1] | 67.2 [8.0–96.4] | 0.112 | 5.7 [−0.6–25.6] | 17.4 [0.1–80.2] | 0.905 |
| BA.2 | 29.0 [7.7–50.7] | 79.8 [59.3–94.7] | <0.001 *** | 38.0 [1.4–55.3] | 81.9 [25.7–95.5] | 0.006 ** | 0.8 [−2.0–34.7] | 36.3 [10.2–86.0] | 0.111 |
| SO to serum sample collection (day), median [IQR] | 9 [3–19] | 7 [4.8–17.8] | 0.746 | 4 [0.3–8.0] | 9 [4–12.8] | 0.068 | 4 [1.5–12.5] | 5.5 [1.3–15.8] | 0.706 |
| Ct value, median [IQR] | 20.6 [15.4–22.7] | 19.4 [15.6–28.3] | 0.529 | 21.1 [16.4–25.3] | 17.3 [15.2–20.8] | 0.128 | 16.8 [14.1–19.4] | 20.2 [14.6–24.6] | 0.413 |
| SO to PCR (day), median [IQR] | 2 [1–7] | 2.5 [0–5.0] | 0.370 | 2.5 [0.3–7.8] | 2 [0–6.5] | 0.571 | 3 [0.5–3.0] | 0 [0–1.5] | 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.J.; Park, S.-J.; Yun, S.G.; Kim, S.W.; Nam, M.-H.; Shin, E.K.; Chang, E.-A.; Park, D.W.; Lee, C.K.; Yoon, Y.K.; et al. Comparison of Neutralizing Activity between Vaccinated and Unvaccinated Hospitalized COVID-19 Patients Infected with Delta, Omicron BA.1, or Omicron BA.2 Variant. Microorganisms 2024, 12, 509. https://doi.org/10.3390/microorganisms12030509
Kim KJ, Park S-J, Yun SG, Kim SW, Nam M-H, Shin EK, Chang E-A, Park DW, Lee CK, Yoon YK, et al. Comparison of Neutralizing Activity between Vaccinated and Unvaccinated Hospitalized COVID-19 Patients Infected with Delta, Omicron BA.1, or Omicron BA.2 Variant. Microorganisms. 2024; 12(3):509. https://doi.org/10.3390/microorganisms12030509
Chicago/Turabian StyleKim, Keun Ju, Seo-Jin Park, Seung Gyu Yun, Sang Wook Kim, Myung-Hyun Nam, Eun Kyong Shin, Eun-Ah Chang, Dae Won Park, Chang Kyu Lee, Young Kyung Yoon, and et al. 2024. "Comparison of Neutralizing Activity between Vaccinated and Unvaccinated Hospitalized COVID-19 Patients Infected with Delta, Omicron BA.1, or Omicron BA.2 Variant" Microorganisms 12, no. 3: 509. https://doi.org/10.3390/microorganisms12030509
APA StyleKim, K. J., Park, S.-J., Yun, S. G., Kim, S. W., Nam, M.-H., Shin, E. K., Chang, E.-A., Park, D. W., Lee, C. K., Yoon, Y. K., & Cho, Y. (2024). Comparison of Neutralizing Activity between Vaccinated and Unvaccinated Hospitalized COVID-19 Patients Infected with Delta, Omicron BA.1, or Omicron BA.2 Variant. Microorganisms, 12(3), 509. https://doi.org/10.3390/microorganisms12030509






