1. Introduction
The dietary composition of ruminant animals such as cattle, along with the microbial community structure of the rumen, influence animal productivity. The activity of the resident ruminal microbial consortium allows ruminant animals to occupy a specific ecological niche as consumers of cellulosic fiber [
1]. Because mammals do not produce fiber-degrading enzymes, ruminants are entirely dependent on the degradative capacity of the ruminal bacteria, fungi, and protozoa to ferment cellulose and hemicellulose into usable energy for the host [
2,
3,
4]. However, dependence on microbial fermentation means that ruminant animals exchange efficiency for substrate flexibility. The ruminal microbial fermentation of structural carbohydrates produces metabolizable energy for the host animal in the form of volatile fatty acids (VFA); however, fermentation byproducts, such as methane, can represent a loss of up to 12% of the dietary energy [
5,
6,
7].
Feed costs are the largest variable expense associated with producing beef cattle [
8], accounting for approximately 70% of the cost of producing finished cattle [
9]. Beef production in the U.S. utilizes feedlots at the end of the production chain, where a predominantly grain diet is fed to ensure efficient muscle and bone growth [
10]. When cattle are fed a grain-based diet, energy density is increased and therefore cattle production is optimized in a well-managed intensive feedlot system [
11]. However, when not well managed, pushing production efficiency can have negative impacts on animal health. The excessive consumption of fermentable carbohydrates by ruminant animals may lead to acidosis as acids accumulate in the rumen, causing a reduction in pH and potential damage to the gastrointestinal wall [
12]. To prevent acidosis via management practices, a moderate meal size and the addition of dietary roughage allow grains to be processed adequately [
12]. Cereal grains are a major energy source for cattle due to their high concentrations of starch [
11], a component that is well digested in the gastrointestinal tract when adequately processed [
13]. Previous studies have shown a close association between the ruminal microbiota composition, fermentation end products, and cattle feed efficiency [
14,
15,
16]. In addition to feed management and the gastrointestinal microbiome, feed efficiency can be influenced by breed, body composition, age, and the environment [
17]. Improving the cattle feed efficiency could decrease both feeding costs and environmental impacts, such as decreased the production of methane in beef production systems [
14].
The Residual Average Daily Gain (RADG) is a feed efficiency selection tool adopted by the American Angus Association in their breeding selection programs because it has a high degree (~0.3) of heritability [
18,
19]. Selection based upon RADG allows producers to improve the feedlot efficiency in finishing cattle by focusing on predictors of a sire’s genetic capacity for their future progeny to have good postweaning gain relative to other sires, assuming that a constant amount of feed is consumed. Therefore, the present study was designed to compare the composition and end products of the ruminal microbiome of beef cattle with different feed efficiencies at the beginning and end of an 82-day feedlot phase. We hypothesized that animals with different feed efficiencies would undergo differential changes in their microbial community compositions over the course of the finishing period.
4. Discussion
Feedlot cattle are fed high grain rations to maximize their rate of gain, to increase intramuscular marbling and improve carcass quality [
11,
12,
13,
30]. Grain diets are commonly high in starch, a readily fermentable substrate that can alter the microbial community composition of the rumen [
11,
12,
13,
31]. The present study’s findings showing that the most efficient steers (low RFI) had a lower average DMI and RFI than the least efficient (high RFI) steers were previously demonstrated in both dairy and beef cattle [
32].
Since the changes in the total VFA concentrations in the ruminal samples over the feedlot period were more pronounced in the less efficient steers, this may reflect their inability to quickly absorb these compounds. However, since the present study did not quantify VFA absorption, this hypothesis cannot be supported. Overall, the VFA concentrations were dominated by acetate regardless of the feed efficiency status. We had hypothesized that we would observe differences in the ruminal VFA concentrations between the efficiency groups that could be attributed to differences in the microbiome composition; however, the differences observed in the ruminal VFA concentrations were potentially masked by differences in absorption across the ruminal epithelium.
The number of observed OTUs increased numerically in both groups of steers during the 82 d feedlot period. Similarly, the Chao1 index, which estimates microbial richness while accounting for rare species [
33], increased in both groups of steers during the feedlot phase, especially in high-RFI steers. The microbial diversity, when assessed through the Shannon index, revealed a trend of increased diversity in the rumen of high-RFI steers over the course of the feedlot phase; however, when the microbial diversity was expressed using Faith’s Phylogenetic Diversity index, no differences were observed, indicating that the alpha-diversity metric used can affect the conclusions drawn. In the most efficient steers, no differences in the microbial diversity were observed during the feedlot finishing phase, regardless of the metrics used.
The relative abundance of
Firmicutes was higher at the end of the finishing period compared to the beginning in the most efficient animals (i.e., low RFI). A similar trend was observed in the inefficient animals (high-RFI), although the increase was not as significant. Conversely, ruminal
Bacteroidetes tended to decrease in abundance in the least efficient animals during the feedlot period. However, the most noteworthy difference was seen in an archaeal phylum with low ruminal abundance, namely
Euryarchaeota. It significantly increased at the end of the finishing period in the least efficient steers. The phylum
Euryarchaeota contains a diverse group of obligate anaerobic methanogens [
34]. Therefore, an increase in the abundance of
Euryarchaeota subsequently resulted in an increase in the methanogenic genera
Methanobrevibacter and
Methanosphaera within this phylum.
A classic study by Turnbaugh and collaborators identified a link between the
Firmicutes/
Bacteroidetes ratio and obesity in mice [
35], indicating that this ratio affected the efficiency with which those animals harvested energy from their diets. It should be noted that the importance of this ratio in terms of microbiome activity and host physiology is still poorly understood [
36]. However, the increase in the
Firmicutes/
Bacteroidetes ratio during the 82 d feedlot period suggests that in both efficiency groups the ruminal microbial community structure changed to harvest more energy from the readily fermentable starch-rich diet.
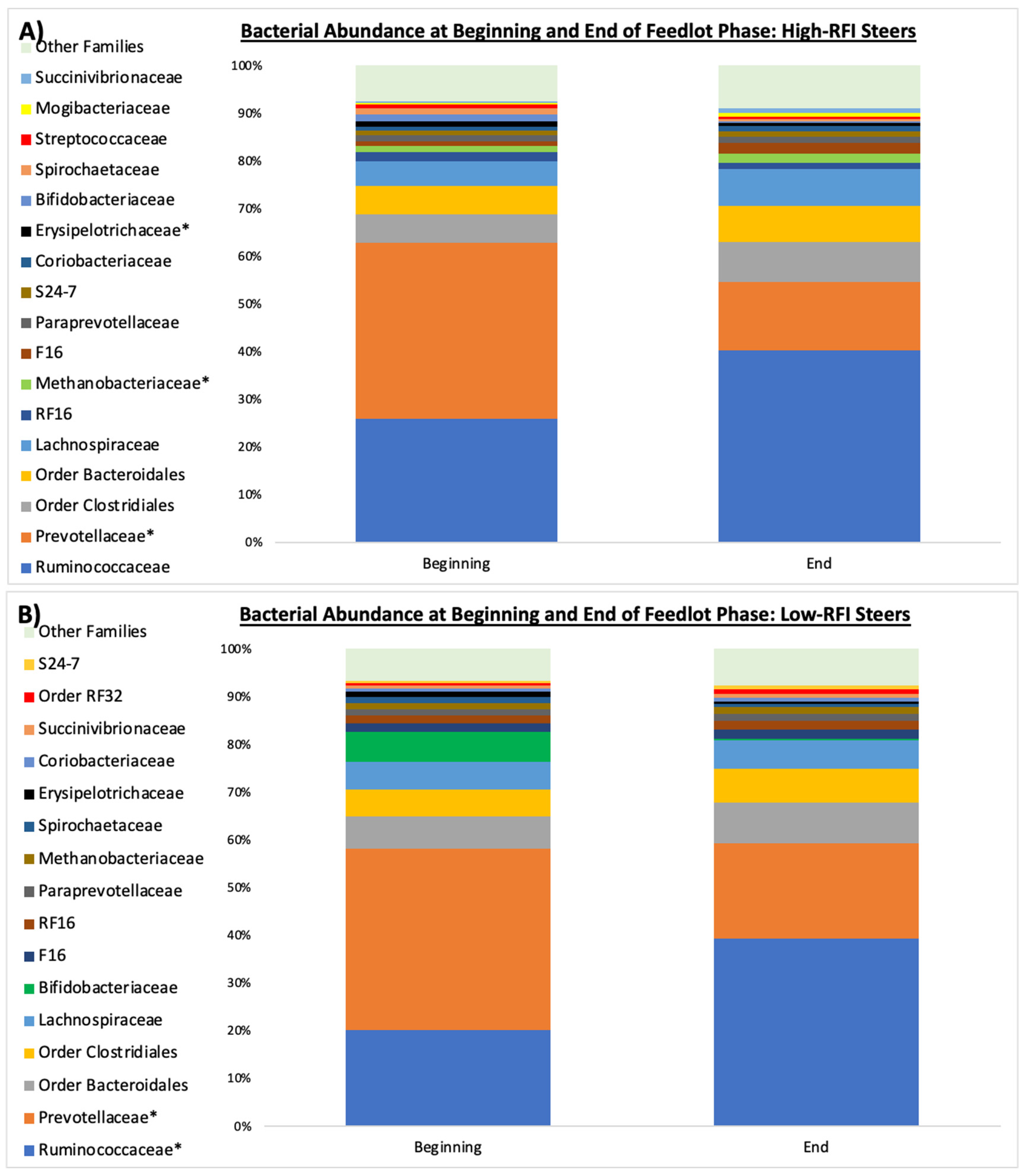
The family
Prevotellaceae consists of amylolytic Gram-negative bacteria that proliferate rapidly in the presence of high-starch diets, such as feedlot diets [
37,
38,
39]. Thus, the decrease in
Prevotellaceae observed in both efficiency groups during the feedlot phase was unexpected. However, the
Ruminococcaceae family contains bacteria involved in the degradation of complex carbohydrates, including neutral detergent fiber (NDF) degradation [
40,
41], so the increased abundance of
Ruminococcaceae in both groups of steers could be partially explained by environmental niche-filling caused by the decrease in
Prevotellaceae. Moreover, the increase in
Ruminococaceae could potentially mitigate the effects of the low ruminal pH associated with the starch-containing feedlot diet by fermenting dietary carbohydrate to VFA rather than lactate [
42,
43].
While more changes occurred at the family level throughout the feedlot period, these changes occurred in bacterial families with smaller relative abundances and only occurred in the least efficient steers. For example, the abundance of
Erysipelotrichaceae decreased, whereas the family
Methanobacteriaceae increased in abundance in the least efficient steers. Previous studies have shown a greater abundance of the
Erysipelotrichaceae family in low-methane-producing animals, with less hydrogen being produced; this leads to less interspecies hydrogen transfer and less methane production [
44]. It was hypothesized that the enrichment of the
Erysipelotrichaceae family was associated with the higher ruminal turnover rates found in low-methane-emitting ruminants, and that the more rapid ruminal liquid dilution rate favored rapid homofermentative starch-degrading microorganisms (e.g.,
Streptococcus bovis,
Sharpea azabuensis) [
45,
46], which might have been the case in our study.
In addition to VFA, the ruminal microbial fermentation process produces hydrogen, carbon dioxide, and methane, which are not utilized by the host [
1,
47,
48]. Methane is a potent greenhouse gas produced by ruminal archaea and can represent a loss of up to 12% of the dietary energy provided to ruminant animals [
5,
49,
50]. Archaea are metabolically diverse organisms that in the rumen are often associated with bacteria, fungi, and protozoa [
51], and that often serve as a reducing equivalent sink for NADH disposal under highly reduced ruminal environment conditions [
52,
53]. Interspecies hydrogen transfer was demonstrated using co-cultures of
Ruminococcus albus and
Methanobacterium ruminantium, which caused the fermentation end products to shift from ethanol to acetate, enabling
R.
albus to receive a higher ATP yield [
54,
55,
56]. In our study, the increase in ruminal
Methanobrevibacter and
Methanosphaera abundances in the least efficient steers (high-RFI) suggests that there was potentially a greater waste of dietary energy in the form of methane in these steers, contributing to their reduced feed efficiency.
Shifts in the relative abundances of
Methanobrevibacter and
Methanosphaera have been correlated with a decrease in the abundance of
Thermoplasmata [
57], which are methanogens that can utilize methanol and methylamine as a substrate [
58]. In the current study, the abundance of
Thermoplasmata was negligible (less than 0.00002% on average), and many animals did not even have OTUs assigned to this taxa, limiting our ability to run similar correlations. Although this relatively small microbial community of archaea might not be the only factor that underlies shifts in cattle efficiency and methane production, further examinations should be conducted to assess the impacts of
Thermoplasmata on rumen fermentation.
Changes in ruminal microbial communities have been associated with feed efficiency differences in beef cattle [
15,
59,
60]. More recently, the linkages between rumen microbiome activities and feed efficiencies in beef cattle have been studied by utilizing advanced metagenomics and metabolomics approaches [
61,
62]. While the RFI was used to define feed efficiency in the present study, a potential problem arises when the RFI is used due to differences in feed intake, which can greatly influence the composition of the microbiota [
63]. To avoid changes associated with feed intake, metabolomics has been used to assess the effect of ruminal metabolites on ADG. Artgegoitia et al. [
63] indicated that 33 individual metabolites were associated with differences in ADG in beef cattle. The results suggested that the balance between microbial community activity and the ruminal absorption of VFA (and other acids) impacted the ADG of crossbred beef steers. More recently, de Almeida et al. [
64] utilized untargeted metabolomics to provide a snapshot of the rumen fluid metabolome and observed 1882 molecular features with 67 molecular features, including the following: amino acids, dicarboxylic acids, carboxylic acids, lactones, fatty acids derivatives and indole compounds. The production traits of beef cattle have been improved through animal selection using traditional quantitative genetics approaches [
65]; however, there is still a need to understand the physiological mechanisms that contribute to variations in feed efficiency, including the impact of changes in the composition of the ruminal microbial community. While the complexity of the rumen remains vast and not well understood, these findings elucidate the potential of the rumen as a reservoir of novel metabolites and enzymatic activities that can be used to improve sustainability and reduce the global footprint of animal agriculture.