Production of a Rich Fertilizer Base for Plants from Waste Organic Residues by Microbial Formulation Technology
Abstract
:1. Introduction
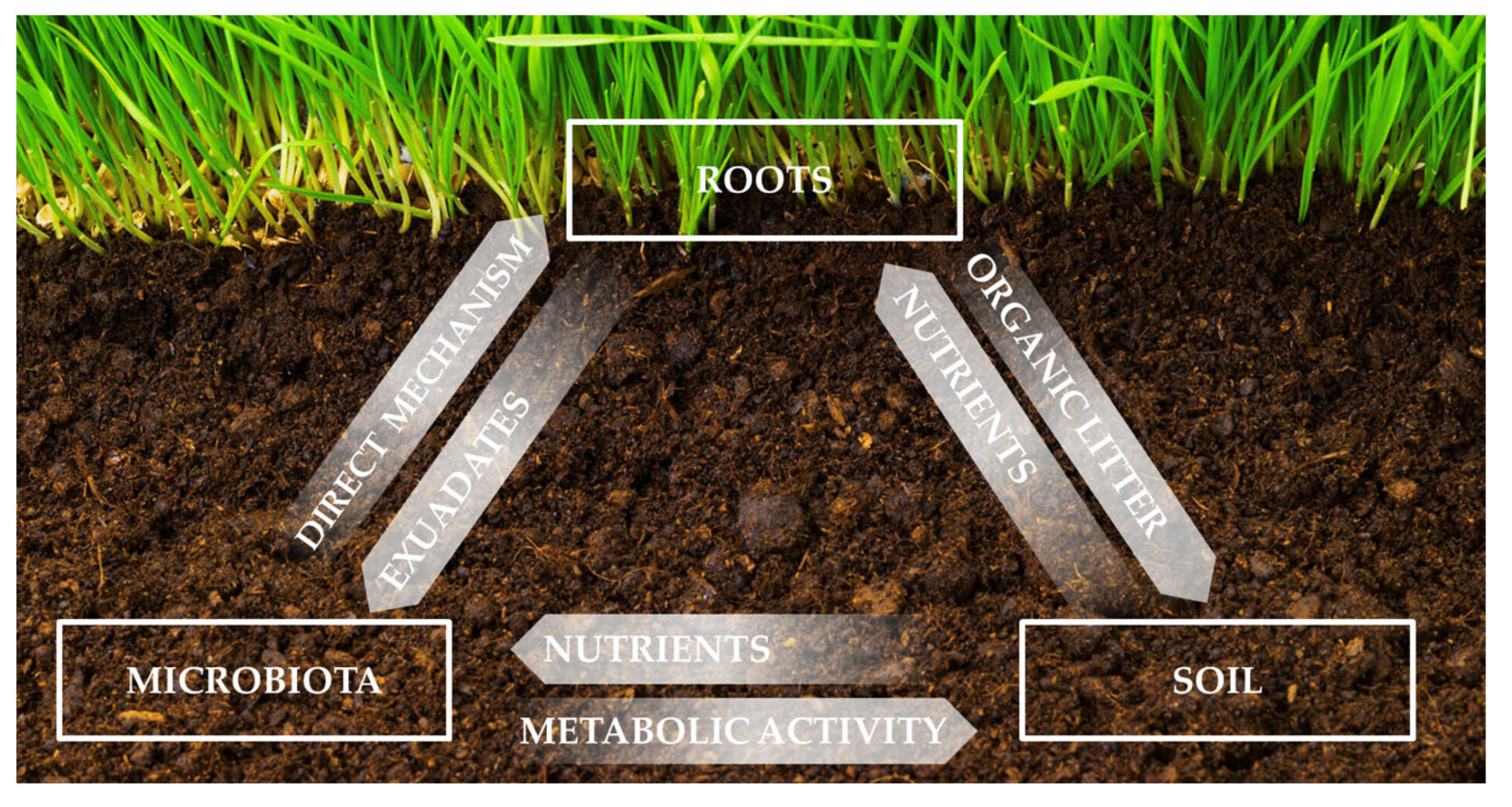
2. Complexity of the Soil System
2.1. Bio-Waste Materials as Source of Nutrients
2.2. Nutrient-Rich Formulations of Biowaste Materials
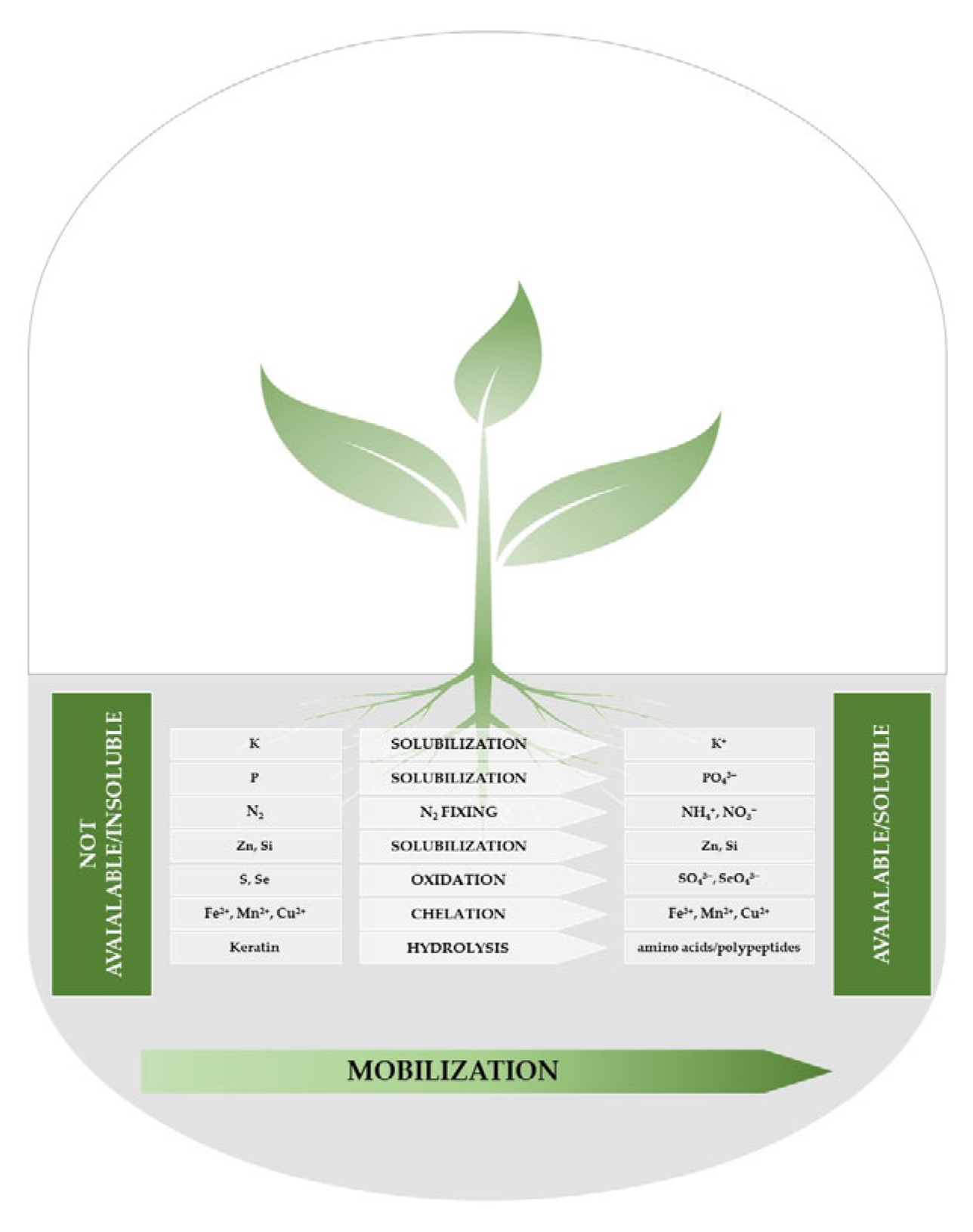
2.3. Microorganisms
3. Overview of Different Forms of Microbial Formulations
3.1. Microbial Formulation Technology
3.1.1. Single Inoculants
3.1.2. Co-Inoculants
3.1.3. Microbial Consortia
3.2. Delivery Methods of Microbial Inoculants
3.2.1. Seed Treatment
3.2.2. Biopriming of Seeds
3.2.3. Seed Encapsulation Technology
3.2.4. Soil Application
3.2.5. Foliar Spray Application
3.2.6. Root Dip Method
4. Development of Microbial Waste Compound Formulations
5. Challenges and Limitations in Microbial Formulation Technology
6. Future Aspects
- Many researchers have proven the use of bioremediation to remove poisons from actual waste. It is necessary to extensively explore bioremediation applications to assess their potential for deployment.
- Most studies focused on batch-scale bioremediation techniques for pollutant removal. The commercial potential of bioremediation as a cost-effective and fulfilling option should be investigated.
- A multidisciplinary approach is necessary to solve contemporary issues and broaden the practical applications of microbial formulation techniques.
- Microbial genome engineering can lead to the development of modified microorganisms with improved biodegradation capabilities.
- Degradation mechanisms, operational factors, and favorable environments for bacteria must all be properly assessed.
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.; Ahmad, S.; Qadir, F.-H.; Hayat, G.; Shaheen, R.; Raza, F.A. Innovative processes and Technologies for Nutrient Recovery from Wastes: A Comprehensive Review? Sustainability 2019, 11, 4938. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C.P.R. Phosphorus recovery and Recycling-Closing the Loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on Efficiency of Various Techniques in Treatment of Waste and Sewage Water ? A Comprehensive Review. Resour. Effic. Technol. 2016, 2, 175–184. [Google Scholar]
- Yuan, Z.-S.; Liu, F.; Liu, Z.-Y.; Huang, Q.-L.; Zhang, G.-F.; Pan, H. Structural variability and Differentiation of Niches in the Rhizosphere and Endosphere Bacterial Microbiome of Moso Bamboo (Phyllostachys edulis). Sci. Rep. 2021, 11, 1574. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are Mycorrhizal Fungi Our Sustainable Saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking Crop Nutrition in Times of Modern Microbiology: Innovative Biofertilizer Technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Coulon, F.; Brassington, K.J.; Bazin, R.; Linnet, P.E.; Thomas, K.A.; Mitchell, T.R.; Lethbridge, G.; Smith, J.W.N.; Pollarda, S.J.T. Effect of Fertilizer Formulation and Bioaugmentation on Biodegradation and Leaching of Crude Oils and Refined Products in Soils. Environ. Technol. 2012, 33, 1879–1893. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Malinowski, P.; Chojnacka, K. Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus Ferrooxidans. Molecules 2017, 22, 473. [Google Scholar] [CrossRef]
- Mertens, B.; Boon, N.; Verstraete, W. Slow-Release Inoculation Allows Sustained Biodegradation of γ-Hexachlorocyclohexane. Appl. Environ. Microbiol. 2006, 72, 622–627. [Google Scholar] [CrossRef]
- Fan, T.W.-M.; Lane, A.N.; Shenker, M.; Bartley, J.P.; Crowley, D.; Higashi, R.M. Comprehensive Chemical Profiling of Gramineous Plant Root Exudates Using High-Resolution NMR and MS. Phytochemistry 2001, 57, 209–221. [Google Scholar] [CrossRef]
- Lebeau, T.; Jézéquel, K.; Braud, A. Bioaugmentation-Assisted Phytoextraction Applied to Metal-Contaminated Soils: State of the Art and Future Prospects. In Microbes and Microbial Technology; Springer: New York, NY, USA, 2011; pp. 229–266. [Google Scholar]
- Vurukonda, S.S.K.P.; Chaudhary, S.; Bodhankar, S.A.O. Interaction between Microbial Endophytes and Root Exudates. Int. J. Plant Soil Sci. 2022, 34, 25–38. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- P?triacq, P.; Williams, A.; Cotton, A.; McFarlane, A.E.; Rolfe, S.A.; Ton, J. Metabolite Profiling of non-sterile Rhizosphere Soil. Plant J. 2017, 92, 147–162. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Sojka, M.; Saeid, A. Production of Phosphorus Biofertilizer Based on the Renewable Materials in Large Laboratory Scale. Open Chem. 2019, 17, 893–901. [Google Scholar] [CrossRef]
- Smol, M.; Adam, C.; Kugler, S.A. Thermochemical Treatment of Sewage Sludge Ash (SSA)-Potential and Perspective in Poland. Energies 2020, 13, 5461. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and Its Importance on Nutrient Dynamics in Soil and Plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Stępień, A.; Wojtkowiak, K.; Kolankowska, E. Use of Meat Industry Waste in the Form of Meat-and-Bone Meal in Fertilising Maize (Zea Mays l.) for Grain. Sustainability 2021, 13, 2857. [Google Scholar] [CrossRef]
- Bomfim, A.S.C.D.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2022, 1, 2. [Google Scholar] [CrossRef]
- Szekely, I.; Jijakli, M.H. Bioponics as a Promising Approach to Sustainable Agriculture: A Review of the Main Methods for Producing Organic Nutrient Solution for Hydroponics. Water 2022, 14, 3975. [Google Scholar] [CrossRef]
- Vavrova, K.; Wimmerova, L.; Knapek, J.; Weger, J.; Keken, Z.; Kastanek, F.; Solcova, O. Waste Feathers Processing to Liquid Fertilizers for Sustainable Agriculture—LCA, Economic Evaluation, and Case Study. Processes 2022, 10, 2478. [Google Scholar] [CrossRef]
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent Substrate from Mushroom Cultivation: Exploitation Potential toward Various Applications and Value-Added Products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial Enzymes Catalyzing Keratin Degradation: Classification, Structure, Function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef]
- Zabaleta, I.; Rodic, L. Recovery of Essential Nutrients from Municipal Solid Waste-Impact of Waste Management Infrastructure and Governance Aspects. Waste Man. 2015, 44, 178–187. [Google Scholar] [CrossRef]
- De Medina-Salas, L.; Castillo-González, E.; Giraldi-Díaz, M.R.; Jamed-Boza, L.O. Valorisation of the Organic Fraction of Municipal Solid Waste. Waste Manag. Res. 2019, 37, 59–73. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An Integrated Green Biorefinery Approach towards Simultaneous Recovery of Pectin and Polyphenols Coupled with Bioethanol Production from Waste Pomegranate Peels. Bioresour. Technol. 2018, 266, 322–334. [Google Scholar] [CrossRef]
- Akao, S. Nitrogen, phosphorus, and antioxidant contents in crop residues for potential cascade utilization. Waste Biomass Valoriz. 2018, 9, 1535–1542. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Integrated Process for Sequential Extraction of Bioactive Phenolic Compounds and Proteins from Mill and Field Olive Leaves and Effects on the Lignocellulosic Profile. Foods 2019, 8, 531. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2019, 295, 122223. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of Silicon on Plant-Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Yan, G.-C.; Nikolic, M.; Ye, M.-J.; Xiao, Z.-X.; Liang, Y.-C. Silicon acquisition and Accumulation in Plant and Its Significance for Agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Brienza, C.; Snauwaert, E.; De Dobbelaere, A.; De Mey, J.; Vaneeckhaute, C.; Michels, E.; Schoumans, O.; Adani, F.; Meers, E. Production and Performance of Bio-Based Mineral Fertilizers from Agricultural Waste Using Ammonia (Stripping-) Scrubbing Technology. Waste Manag. 2019, 89, 265. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling Agricultural Wastes and By-Products in Organic Farming: Biofertilizer Production, Yield Performance and Carbon Footprint Analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- Bian, B.; Hu, X.; Zhang, S.; Lv, C.; Yang, Z.; Yang, W.; Zhang, L. Pilot-Scale Composting of Typical Multiple Agricultural Wastes: Parameter Optimization and Mechanisms. Bioresour. Technol. 2019, 287, 12148. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of Biostimulants in Tomato Plants (Solanum Lycopersicum) to Enhance Plant Growth and Salt Stress Tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Kennedy, A.C. Bacterial Diversity in Agroecosystems. Agric. Ecosys. Environ. 1999, 74, 65. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G.M.D.; Functions, S. Microbial diversity and soil functions. Eur. J. Soil. Sci. 2003, 54, 655. [Google Scholar] [CrossRef]
- Babalola, O.O.; Glick, B.R. The Use of Microbial Inoculants in African Agriculture: Current Practice and Future Prospects. J. Food Agric. Environ. 2012, 10, 540–549. [Google Scholar]
- Vitorino, L.C.; Bessa, L.A. Technological Microbiology: Development and Applications. Front. Microbiol. 2017, 8, 827. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Selvaraj, G.; Bais, H.P.F.S.M. Belowground Solutions to an Aboveground Problem. Plant Physiol. 2014, 166, 689–700. [Google Scholar] [CrossRef]
- Sarwar, M. Usage of Biorational Pesticides with Novel Modes of Action, Mechanism and Application in Crop Protection. Int. J. Mater. Chem. Phys. 2015, 1, 156–162. [Google Scholar]
- Sanford, G.B. Some Factors Affecting the Pathogenicity of Actinomyces Scabies. Can. J. Plant Pathol. S 2006, 48, S48–S70. [Google Scholar] [CrossRef]
- Weindling, R. Trichoderma Lignorum as a Parasite of Other Soil Fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Grossbard, E. Production of an Antibiotic Substance on Wheat Straw and Other Organic Materials and in Soil. Nature 1948, 161, 614. [Google Scholar] [CrossRef]
- Wright, S.S.; Purcell, E.M.; Wilcox, C.; Broderick, M.K.; Finland, M. Antibiotic combinations And Resistance: Response of E. Coli to Antibiotics, Singly and in Pairs. Proc. Soc. Exp. Biol. Med. 1954, 85, 128–133. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Pseudomonas siderophores: A mechanism explaining disease suppression in soils. Curr. Microbiol. 1980, 4, 317–320. [Google Scholar] [CrossRef]
- Howell, C.R.; Beier, R.C.; Stipanovi, R.D. Production of Ammonia by Enterobacter Caloacae and Its Possible Role in the Biological Control of Pythium Pre- Emergence Damping off by the Bacterium. Phytopathology 1998, 78, 1075–1078. [Google Scholar] [CrossRef]
- Junaid, J.M.; Dar, N.A.; Bhat, T.A.; Bhat, A.H.; Bhat, M.A. Commercial Biocontrol Agents and Their Mechanism of Action in the Management of Plant Pathogens. Int. J. Mod. Plant Anim. Sci. 2013, 1, 39–57. [Google Scholar]
- Mcrae, C.F.C.; Approaches, I. To Biological Weed Control Compared. Plant Prot. Quart. 1988, 3, 124. [Google Scholar]
- Mohiddin, F.A.; Khan, M.R.; Khan, S.M. Why Trichoderma Is Considered Superhero (Super Fungus) against the Evil Parasites? Plant Pathol. J. 2010, 9, 92–102. [Google Scholar] [CrossRef]
- Peighami-Ashnaei, S.; Sharifi-Tehrani, A.; Ahmadzadeh, M.; Behboudi, K. Interaction of Different Media on Production and Biocontrol Efficacy of Pseudomonas Fluorescens P-35 and Bacillus Subtilis B-3 against Grey Mould of Apple. J. Plant Pathol. 2009, 91, 65–70. [Google Scholar]
- Dawar, S.; Wahab, S.; Tariq, M.; Zaki, M.J. Application of Bacillus species in the control of root rot diseases of crop plants. Arch. Phytopathol. Plant Prot. 2010, 43, 412–418. [Google Scholar] [CrossRef]
- Ngo, B.H.; Vu, D.N.; Tran, D.Q. Analyze Antagonist Effects of Trichoderma Spp. for Controlling Southern Stem Rot Caused by Sclerotium Rolfsii on Peanut. Plant Prot. 2006, 1, 12–14. [Google Scholar]
- Manczinger, L.; Antal, Z.; Kredics, L.E. Ecophysiology and Breeding of Mycoparasitic Trichoderma Strains (a Review). Acta Microbiol. Immunol. Hung. 2002, 49, 1–14. [Google Scholar] [CrossRef]
- Podile, A.R.; Prakash, A.P.L.; Control, B. Of Aspergillus Niger by Bacillus Subtilis AF 1. Can. J. Microbiol. 1996, 42, 533–538. [Google Scholar] [CrossRef]
- Takayanagi, T.; Ajisaka, K.; Takiguchi, Y.; Shimahara, K. Isolation and Characterization of Thermostable Chitinases from Bacillus Licheniformis X-7u. Biochimica. et Biophysica. Biochimica. et Biophysica. Acta (BBA) Protein Struct. Mol. Enzymol. 1991, 1078, 404. [Google Scholar] [CrossRef]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus Subtilis. Curr. Opin. Microbiol. 2004, 7, 579. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus Subtilis against Infection of Arabidopsis Roots by Pseudomonas Syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef]
- Lemessa, F.; Zeller, W. Screening Rhizobacteria for Biological Control of Ralstonia Solanacearum in Ethiopia. Biol. Control 2007, 42, 336–344. [Google Scholar] [CrossRef]
- Aliye, N.; Fininsa, C.; Hiskias, Y. Evaluation of Rhizosphere Bacterial Antagonists for Their Potential to Bioprotect Potato (Solanum tuberosum) against Bacterial Wilt (Ralstonia solanacearum). Biol. Control 2008, 47, 282–288. [Google Scholar] [CrossRef]
- Ji, X.; Lu, G.; Gai, Y.; Zheng, C.; Mu, Z. Biological Control against Bacterial Wilt and Colonization of Mulberry by an Endophytic Bacillus Subtilis Strain: Biological Control against Bacterial Wilt. FEMS Microbiol. Ecol. 2008, 65, 565–573. [Google Scholar] [CrossRef]
- Glick, B.R.M. load And Heterologous Gene-Expression. Biotechnol. Adv. 1995, 13, 247–261. [Google Scholar] [CrossRef]
- Kuiper, I.; Bloemberg, G.V.; Lugtenberg, B.J. Selection of a Plant-Bacterium Pair as a Novel Tool for Rhizostimulation of Polycyclic Aromatic Hydrocarbon-Degrading Bacteria. Mol. Plant 2001, 14, 1197–1205. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Khan, M.R. Plant Nematodes: Methodology, Morphology, Systematics, Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Pal, K.K.; Tilak, K.V.; Saxena, A.K.; Dey, R.; Singh, C.S. Antifungal Characteristics of a Fluorescent Pseudomonas Strain Involved in the Biological Control of Rhizoctonia Solani. Microbiol. Res. 2000, 155, 233–242. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mhamdi, R.M.I.; Impact, T. On Soil Microbial Communities: A Review. Biomed. Res. 2013, 2013, 86324. [Google Scholar]
- Wilson, M.J.; Trevor, A.J. Progress in the Commercialization of Bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
- Mendoza, A.R.; Kiewnick, S.; Sikora, R.A. In Vitro Activity of Bacillus Firmus against the Burrowing Nematode Radopholus Similis, the Root-Knot Nematode Meloidogyne Incognita and the Stem Nematode Ditylenchus Dipsaci. Biocontrol Sci. Technol. 2008, 18, 377–389. [Google Scholar] [CrossRef]
- Marimuthu, S.; Subbian, P.; Ramamoorthy, V.; Samiyappan, R. Synergistic effect of combined application of Azospirillum and Pseudomonas fluorescens with inorganic fertilizers on root rot incidence and yield of cotton/Synergistische Wirkung der kombinierten Anwendung von Azospirillum und Pseudomonas fluorescens mit anorganischen Düngern auf die Wurzelfäule und den Ertrag von Baumwolle. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2002, 109, 569–577. [Google Scholar]
- Janisiewicz, W.E.D.; Overlap, N. Ecological diversity, niche overlap, and Coexistence of Antagonists Used in Developing Mixtures for Biocontrol of Postharvest Diseases of Apples. Phytopathology 1996, 86, 473–479. [Google Scholar] [CrossRef]
- Duffy, B.K.; Weller, D.M. Use of Gaeunianno; Nycesgrarninis Var. Granhinis Alone and in Combination with Fluorescent Pseudomonas Spp. to Suppress Take-All of Wheat. Plant Dis. 1995, 79, 907–911. [Google Scholar] [CrossRef]
- Schisler, D.A.; Slininger, P.J.; Bothast, R.J. Effects of Antagonist Cell Concentration and Two-Strain Mixtures on Biological Control of Fusarium Dry Rot of Potatoes. Phytopathology 1997, 87, 177–183. [Google Scholar] [CrossRef]
- Khaosaad, T.; Garcia-Garrido, J.M.; Steinkellner, S.; Vierheilig, H. Take-All Disease Is Systemically Reduced in Roots of Mycorrhizal Barley Plants. Soil. Biol. 2007, 39, 727–734. [Google Scholar] [CrossRef]
- Thygesen, K.; Larsen, J.; Bødker, L. Arbuscular Mycorrhizal Fungi Reduce Development of Pea Root-Rot Caused by Aphanomyces Euteiches Using Oospores as Pathogen Inoculum. Eur. J. Plant Pathol. 2004, 110, 411–419. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; El-Haddad, S.A.; Hafez, E.E.; Rashad, Y.M. Induction of Defense Responses in Common Bean Plants by Arbuscular Mycorrhizal Fungi. Microbiol. Res. 2011, 166, 268–281. [Google Scholar] [CrossRef]
- De Oliveira, M.A.L.; De Canuto, E.L.; Urquiaga, S.; Reis, V.M.; Baldani, J.I. Yield of Micro Propagated Sugarcane Varieties in Different Soil Types Following Inoculation with Diazotrophic Bacteria. Plant Soil. 2006, 284, 23–32. [Google Scholar] [CrossRef]
- Sundaramoorthy, S.; Raguchander, T.; Ragupathi, N.; Samiyappan, R. Combinatorial Effect of Endophytic and Plant Growth Promoting Rhizobacteria against Wilt Disease of Capsicum Annum L. Caused By Fusarium Solani. Biol. Control. 2012, 60, 59–67. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next Generation of Microbial Inoculants for Agriculture and Bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H. Microbial Consortia Are Needed to Degrade Soil Pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef]
- Bhatt, P.; Gangola, S.; Bhandari, G.; Zhang, W.; Maithani, D.; Mishra, S.; Chen, S. New Insights into the Degradation of Synthetic Pollutants in Contaminated Environments. Chemosphere 2021, 268, 128827. [Google Scholar] [CrossRef]
- Varjani, S.; Pandey, A.; Upasani, V.N. Petroleum Sludge Polluted Soil Remediation: Integrated Approach Involving Novel Bacterial Consortium and Nutrient Application. Sci. Total Environ. 2021, 763, 142934. [Google Scholar] [CrossRef]
- Kang, D.; Huang, Y.; Nesme, J.; Herschend, J.; Jacquiod, S.; Kot, W.; Hansen, L.H.; Lange, L.; Sørensen, S.J. Metagenomic Analysis of a Keratin-Degrading Bacterial Consortium Provides Insight into the Keratinolytic Mechanisms. Sci. Total Environ. 2021, 761, 143281. [Google Scholar] [CrossRef]
- Ali, S.S.; Kornaros, M.; Manni, A.; Sun, J.; El-Shanshoury, A.E.R.R.; Kenawy, E.R.; Khalil, M.A. Enhanced Anaerobic Digestion Performance by Two Artificially Constructed Microbial Consortia Capable of Woody Biomass Degradation and Chlorophenols Detoxification. J. Hazard. Mater. 2020, 389, 122076. [Google Scholar] [CrossRef]
- Vieira, G.A.L.; Cabral, L.; Otero, I.V.R.; Ferro, M.; Faria, A.U.D.; de Oliveira, V.M.; Bacci, M.; Sette, L.D. Marine Associated Microbial Consortium Applied to RBBR Textile Dye Detoxification and Decolorization: Combined Approach and Metatranscriptomic Analysis. Chemosphere 2021, 267, 129190. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, B.; Lan, Y.; Ma, T. Enhanced Degradation of Different Crude Oils by Defined Engineered Consortia of Acinetobacter Venetianus RAG-1 Mutants Based on Their Alkane Metabolism. Bioresour. Technol. 2021, 327, 124787. [Google Scholar] [CrossRef]
- Abou Khalil, C.; Prince, V.L.; Prince, R.C.; Greer, C.W.; Lee, K.; Zhang, B.; Boufadel, M.C. Occurrence and Biodegradation of Hydrocarbons at High Salinities. Sci. Total Environ. 2021, 762, 143165. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, A.; Wang, S.; Cheng, S.; Yin, X.; Yue, X. Quorum Sensing Shaped Microbial Consortia and Enhanced Hydrogen Recovery from Waste Activated Sludge Electro-Fermentation on Basis of Free Nitrous Acid Treatment. Sci. Total Environ. 2021, 766, 144348. [Google Scholar] [CrossRef]
- Basak, B.; Patil, S.M.; Saha, S.; Kurade, M.B.; Ha, G.S.; Govindwar, S.P.; Lee, S.S.; Chang, S.W.; Chung, W.J.; Jeon, B.H. Rapid Recovery of Methane Yield in Organic Overloaded-Failed Anaerobic Digesters through Bioaugmentation with Acclimatized Microbial Consortium. Sci. Total Environ. 2021, 764, 144219. [Google Scholar] [CrossRef]
- Jamir, L.; Singhal, P.; Goyal, S.; Khajuria, R.; Rakhra, G.; Aochen, C.; Gupta, M. Development of Microbial Consortium for Degradation of Organic Kitchen Waste. Nova Biotechnol. Chim. 2022, 21, 20220282138. [Google Scholar] [CrossRef]
- Hua, B.; Cai, Y.; Cui, Z.; Wang, X. Bioaugmentation with Methanogens Cultured in a Micro-Aerobic Microbial Community for Overloaded Anaerobic Digestion Recovery. Anaerobe 2022, 76, 102603. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, M.; Song, L.; Wang, C.; Yang, S.; Yan, Z.; Wang, Y. Study on Nitrogen-Retaining Microbial Agent to Reduce Nitrogen Loss during Chicken Manure Composting and Nitrogen Transformation Mechanism. J. Clean. Prod. 2021, 285, 124813. [Google Scholar] [CrossRef]
- Xu, M.; Sun, H.; Yang, M.; Xie, D.; Sun, X.; Meng, J.; Wang, Q.; Wu, C. Biodrying of Biogas Residue through a Thermophilic Bacterial Agent Inoculation: Insights into Dewatering Contribution and Microbial Mechanism. Bioresour. Technol. 2022, 355, 127256. [Google Scholar] [CrossRef]
- Zhou, S.P.; Zhou, H.Y.; Xia, S.N.; Ying, J.M.; Ke, X.; Zou, S.P.; Xue, Y.P.; Zheng, Y.G. Efficient Bio-Degradation of Food Waste through Improving the Microbial Community Compositions by Newly Isolated Bacillus Strains. Bioresour. Technol. 2021, 321, 124451. [Google Scholar] [CrossRef]
- Krainara, S.; Mistry, A.N.; Malee, C.; Chavananikul, C.; Pinyakong, O.; Assavalapsakul, W.; Jitpraphai, S.M.; Kachenchart, B.; Luepromchai, E. Development of a Plastic Waste Treatment Process by Combining Deep Eutectic Solvent (DES) Pretreatment and Bioaugmentation with a Plastic-Degrading Bacterial Consortium. J. Hazard. Mater. 2023, 460, 132507. [Google Scholar] [CrossRef]
- Ritika; Pant, S.; Komesu, A.; Penteado, E.D.; Diniz, A.A.R.; Rahman, M.A.; Bhunia, R.K.; Kuila, A. Simultaneous Fermentation of Glucose and Xylose by Using Co-Culture of S. Cerevisiae and a Potential Robust Pentose Fermenting Fungi (Fusarium Incarnatum). Biomass Convers. Biorefin 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Matusiak, K.; Oleksy, M.; Borowski, S.; Nowak, A.; Korczyński, M.; Dobrzański, Z.; Gutarowska, B. The Use of Yucca Schidigera and Microbial Preparation for Poultry Manure Deodorization and Hygienization. J. Environ. Manag. 2016, 170, 50–59. [Google Scholar] [CrossRef]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; American Phytopathological Society: St. Paul, MN, USA, 1983. [Google Scholar]
- Chandra, K.; Greep, S. Liquid Based Bio-Fertilizers. J. Eco-Friend Agric. 2010, 5, 1–7. [Google Scholar]
- Woods, T.S.P.F. AGR 185 in Encyclopedia of Agrochemicals; Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Callan, N.W.; Mathre, D.E.; Miller, J.B. Bio-Priming Seed Treatment for Control of Pythium Ultimum Pre-Emergence Damping-off in Sh-2 Sweet Corn. Plant Dis. 1990, 74, 368–372. [Google Scholar] [CrossRef]
- Cliquet, S.; Scheffer, R.J. Influence of Culture Conditions on Growth and Survival of Conidia of Trichoderma spp. Coated on Seeds. Biocontrol Sci. Technol. 1997, 7, 171–182. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-Sowing Seed Treatment-A Shotgun Approach to Improve Germination, Plant Growth, and Crop Yield Under Saline and Non-Saline Conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- McQuilken, M.P.; Halmer, P.; Rhodes, D.J. Application of Microorganisms to Seeds. In Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments; Burges, H., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 255–285. [Google Scholar]
- Abuamsha, R.; Salman, M.; Ehlers, R.-U. Effect of Seed Priming with Serratia Plymuthica and Pseudomonas Chlororaphis to Control Leptosphaeria Maculans in Different Oilseed Rape Cultivars. Eur. J. Plant Pathol. 2011, 130, 287–295. [Google Scholar] [CrossRef]
- Reddy, P.P. Recent Advances in Crop Protection; Springer India: New Delhi, India, 2012; Volume 83. [Google Scholar]
- Anitha, D.; Vijaya, T.; Reddy, N.V.M. endophytes And Their Potential for Improved Bioremediation and Biotransformation: A Review. Indo Am. 2013, 3, 6408-17. [Google Scholar]
- Taylor, A.G.; Harman, G.E. Concepts and Technologies of Selected Seed Treatments. Annu. Rev. Phytopathol. 1990, 28, 321–339. [Google Scholar] [CrossRef]
- Moeinzadeh, A.; Sharif-Zadeh, F.; Ahmadzadeh, M.; Tajabadi, F. Biopriming of Sunflower (Helianthus Annuus L.) Seed with Pseudomonas Fluorescens for Improvement of Seed Invigoration and Seedling Growth. Aust. J. Crop Sci. 2010, 4, 564–570. [Google Scholar]
- Jensen, B.; Povlsen, F.V.; Knudsen, I.M.B.; Jensen, S.D.F. Combining Microbial Seed Treatment with Priming of Carrot Seeds for Control of Seed Borne Alternaria spp.; Bulletin, I.W., Elad, Y., Freeman, S., Monte, E., Eds.; University of Copenhagen: Copenhagen, Denmark, 2001; pp. 197–201. [Google Scholar]
- Callan, N.W.; Mathre, D.E.; Miller, J.B. Field Performance of Sweet Corn Seed Bio-Primed and Coated with Pseudomonas Fluorescens AB254. HortScience 1991, 26, 1163–1165. [Google Scholar] [CrossRef]
- Legro, B.; Satter, H. Biological Control of Pythium through Seed Coating and Seed Priming with Trichoderma. In Monterey Proceedings of the 4th National Symposium on Stand Establishment of Horticultural Crops; Bradford, K., Hartz, T., Eds.; Monterey: California, CA, USA, 1995; pp. 235–237. [Google Scholar]
- Warren, J.E.; Bennett, M.A. Bio-Osmopriming Tomato (Lycopersicon esculentum Mill.) Seeds for Improved Stand Establishment. Seed Sci. Technol. 1999, 27, 489. [Google Scholar]
- Harman, G.E. Combining Effective Strains of Trichoderma Harzianum and Solid Matrix Priming to Improve Biological Seed Treatments. Plant Dis. 1989, 73, 631–637. [Google Scholar] [CrossRef]
- Bennett, A.J.; Mead, A.; Whipps, J.M. Performance of Carrot and Onion Seed Primed with Beneficial Microorganisms in Glasshouse and Field Trials. Biol. Control. 2009, 51, 417–426. [Google Scholar] [CrossRef]
- Chakraborty, U.; Roy, S.; Chakraborty, A.P.; Dey, P.; Chakraborty, B. Plant growth promotion and amelioration of salinity stress in crop plants by a salt-tolerant bacterium. Recent Res. Sci. Technol. 2011, 3, 61. [Google Scholar]
- Sharifi, R.S. Study of Nitrogen Rates Effects and Seed Biopriming with PGPR on Quantitative and Qualitative Yield of Safflower (Carthamus Tinctorius L.). Tech. J. Eng. Appl. Sci. 2012, 2, 162–166. [Google Scholar]
- Sharifi, R.S.; Khavazi, K. Effects of Seed Priming with Plant Growth Promotion Rhizobacteria (PGRP) on Yield and Yield Attribute of Maize (Zea Mays L.) Hybrids. J. Food Agric. Environ. 2011, 9, 496–500. [Google Scholar]
- Mirshekari, B.; Hokmalipour, S.; Sharifi, R.S.; Farahvash, F.; Gadim, A.E.K. Effect of Seed Biopriming with Plant Growth Promoting Rhizobacteria (PGPR) on Yield and Dry Matter Accumulation of Spring Barley (Hordeum vulgare L.) at Various Levels of Nitrogen and Phosphorus Fertilizers. J. Food Agric. Environ. 2012, 10, 314–320. [Google Scholar]
- Green, B.K. Pressure Sensitive Record Material. U.S. Patent No. 2,712,507, 5 July 1955. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Hunt, N.C.; Grover, L.M. Cell Encapsulation Using Biopolymer Gels for Regenerative Medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Orive, G.; Hernandez, R.M.; Gascon, A.R.; Calafiore, R.; Chang, T.M.S.; De Vos, P.; Hortelano, G.; Hunkeler, D.; Lacık, I.; Pedraz, J.L. History, Challenges and Perspectives of Cell Microencapsulation. Trends Biotechnol. 2004, 22, 87–92. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Bhakyaraj, R.; Subhasree, R.S. Encapsulation “The Future of Probiotics”—A Review. Adv. Biol. Res. 2009, 3, 96–103. [Google Scholar]
- Onwulata, C.I. Encapsulation of New Active Ingredients. Annu. Rev. Food Sci. Technol. 2012, 3, 183–202. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.Q. Encapsulation of Food Ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef]
- Poncelet, D.; Boh, B. Microcapsules deliver. Chem. Ind. 2008, 2, 23–25. [Google Scholar]
- McLoughlin, A.J. Controlled Release of Immobilized Cells as a Strategy to Regulate Ecological Competence of Inocula. In Biotechnics/Wastewater; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–45. [Google Scholar]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental Applications of Immobilized Microbial Cells: A Review. J. Ind. Microbiol. Biotechnol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohravandi, S. Principles and Methods of Microencapsulation of Probiotic Microorganisms. Iranian J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Holcberg, I.B.; Margalith, P. Alcoholic Fermentation by Immobilized Yeast at High Sugar Concentrations. Eur. J. Appl. Microbiol. Biotechnol. 1981, 13, 133–140. [Google Scholar] [CrossRef]
- Groboillot, A.; Boadi, D.K.; Poncelet, D.; Neufeld, R.J. Immobilization of Cells for Application in the Food Industry. Crit. Rev. Biotechnol. 1994, 14, 75–107. [Google Scholar] [CrossRef]
- Diefenbach, R.; Keweloh, H.; Rehm, H.J. Fatty Acid Impurities in Alginate Influence the Phenol Tolerance of Immobilized Escherichia Coli. Appl. Microbiol. Biotechnol. 1992, 36, 530–534. [Google Scholar] [CrossRef]
- Keweloh, H.; Heipieper, H.J.; Rehm, H.J. Phenol Tolerance of Immobilized Bacteria. In Physiology of Immobilized Cells; Bont, D., Visser, J.A.M., Mattiasson, J., Tramper, B., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990; pp. 545–550. [Google Scholar]
- Brannon-Peppas, L. Polymers in Controlled Drug Delivery. Biomaterials 1997, 11. [Google Scholar]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-Encapsulation of Microbial Cells for Targeted Agricultural Delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Warrior, P.; Konduru, K.; Vasudevan, P. Formulation of Biological Control Agents for Pest and Disease Management. In Biological Control of Crop Diseases; Gnanamanickam, S.S., Ed.; Markel Dekker Inc.: New York, NY, USA, 2002; pp. 421–442. [Google Scholar]
- Lumsden, R.D.; Lewis, J.A.; Fravel, D.R. Formulation and Delivery of Biocontrol Agents for Use against Soil Borne Plant Pathogens. ACS Symp. Ser. 1995, 595, 166–182. [Google Scholar]
- Sesan, T.; Csep, N. Prevention of White Rot (Sclerotinia sclerotiorum) of Sunflower and Soybean by the Biological Control Agent Coniothyrium Minitans. International Organization for Biological and Integrated Control for Noxious Animals and Plants. West. Palearct. Reg. Sect. 1992, 15, 60. [Google Scholar]
- Bankole, S.A.; Adebanjo, A. Efficacy of Some Fungal and Bacterial Isolates in Controlling Wet Rot Disease of Cowpea Caused by Pythium Aphanidermatum. J. Plant Prot. Trop. 1998, 11, 37–43. [Google Scholar]
- Kulkarni, S.A.; Anahosur, K.H. Effect of Age of Groundnut Plant to Infection of Sclerotium Rolfsii Sacc. - A Causal Agent of Stem Rot Disease. Karnataka J. Agric. Sci. 1994, 7, 367–368. [Google Scholar]
- Weststeijn, W.A. Fluorescent Pseudomonads Isolate E11-2 as Biological Agent for Pythium Root Rot in Tulips. Neth. J. Plant Pathol. 1990, 96, 262. [Google Scholar] [CrossRef]
- Hebbar, P.; Berge, O.; Heulin, T.; Singh, S.P. Bacterial Antagonists of Sunflower (Helianthus Annuus L.) Fungal Pathogens. Plant Soil. 1991, 133, 131–140. [Google Scholar] [CrossRef]
- Herzfeld, D.; Sargent, K. Private Pesticide Applicator Safety Education Manual. In Pesticide Safety & Environ Educ Program University of Minnesota Extension, 19th ed.; University of Minnesota: St. Paul, MN, USA, 2011. [Google Scholar]
- Matthews, G.A. Developments in Application Technology. Environmentalist 2008, 28, 19–24. [Google Scholar] [CrossRef]
- Van de Zande, J.C.; Huijsmans, J.F.M.; Porskamp, H.A.J.; Michielsen, J.M.G.P.; Stallinga, H.; Holterman, H.J.; de Jong, A.S.T. How to Optimise Spray Deposition and Minimise Spray Drift. Environmentalist 2008, 28, 9–17. [Google Scholar] [CrossRef]
- Dorr, G.J.; Hewitt, A.J.; Adkins, S.W.; Hanan, J.; Zhang, H.; Noller, B.A. Comparison of Initial Spray Characteristics Produced by Agricultural Nozzles. Crop Prot. 2013, 53, 109–117. [Google Scholar] [CrossRef]
- Preininger, C.; Sauer, U.; Bejarano, A.; Berninger, T. Concepts and Applications of Foliar Spray for Microbial Inoculants. Appl. Microbiol. Biotechnol. 2018, 102, 7265–7282. [Google Scholar] [CrossRef]
- Puente, M.L.; Gualpa, J.L.; Lopez, G.A.; Molina, R.M.; Carletti, S.M.; Cassán, F.D. The Benefits of Foliar Inoculation with Azospirillum Brasilense in Soybean Are Explained by an Auxin Signaling Model. Symbiosis 2018, 76, 41–49. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Calonego, J.C.; Moreira, A.; Garcia, A.; Momesso, L.; Kuramae, E.E.; Hungria, M. Beneficial Microbial Species and Metabolites Alleviate Soybean Oxidative Damage and Increase Grain Yield during Short Dry Spells. Eur. J. Agron. 2021, 127, 126293. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Momesso, L.; Garcia, A.; Kuramae, E.E.; Hungria, M. Bacterial Consortium and Microbial Metabolites Increase Grain Quality and Soybean Yield. J. Soil. Sci. Plant Nutr. 2020, 20, 1923–1934. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and Induction of Plant-Stress Tolerance and Defense Genes by Seed and Foliar Inoculation with Azospirillum Brasilense Cells and Metabolites Promote Maize Growth. AMB Express 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; de la Osa, C.; Valderrama-Fernández, R.; Nogueira, M.A.; Megías, M.; Hungria, M. Antioxidant Activity and Induction of Mechanisms of Resistance to Stresses Related to the Inoculation with Azospirillum Brasilense. Arch. Microbiol. 2018, 200, 1191–1203. [Google Scholar] [CrossRef]
- Puente, M.L.; Zawoznik, M.; de Sabando, M.L.; Perez, G.; Gualpa, J.L.; Carletti, S.M.; Cassán, F.D. Improvement of Soybean Grain Nutritional Quality under Foliar Inoculation with Azospirillum Brasilense Strain Az39. Symbiosis 2019, 77, 41–47. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Gómez, I.; Franco-Andreu, L.; Benítez, C.; Parrado, J. Use of Biofertilizers Obtained from Sewage Sludges on Maize Yield. Eur. J. Agron. 2016, 78, 13–19. [Google Scholar] [CrossRef]
- Alberola, C.; Lichtfouse, E.; Navarrete, M.; Debaeke, P.; Souchère, V. Agronomy for Sustainable Development. Proc. Ital. J. Agron. 2008, 3. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of Growth, Yield, and the Nutraceutical and Antioxidative Potential of Soybean through the Use of Synthetic Biostimulants. Front. Plant Sci. 2018, 871, 1401. [Google Scholar] [CrossRef]
- Singh, U.S.; Zaidi, N.W. Current Status of Formulation and Delivery of Fungal and Bacterial Antagonists for Disease Management in India. In Microbial Biopesticide Formulations and Application; Rabindra, R.J., Hussaini, S.S., Ramanujam, B., Eds.; Project Directorate of Biological Control: Bangalore, India, 2002; p. 269. [Google Scholar]
- Jambhulkar, P.P.; Sharma, P. Promotion of Rice Seedling Growth Characteristics by Development and Use of Bioformulation of Pseudomonas Fluorescens. Indian. J. Agric. Sci. 2013, 83, 136–142. [Google Scholar]
- Rabindran, R.; Vidhyasekaran, P. Development of a Formulation of Pseudomonas Fluorescens PfALR2 for Management of Rice Sheath Blight. Crop Prot. 1996, 15, 715–721. [Google Scholar] [CrossRef]
- Nandakumar, R.; Babu, S.; Viswanathan, R.; Sheela, J.; Samiyappan, R. Induction of Systemic Resistance in Rice against Sheath Blight Diseases by Plant Growth Promoting Rhizobacteria. Soil. Biol. 2001, 33, 603–612. [Google Scholar] [CrossRef]
- Yadav, K.S.; Mishra, M.M.; Kapoor, K.K. The Effect of Fungal Inoculation on Composting. Agric. Wastes 1982, 4, 329–333. [Google Scholar] [CrossRef]
- Faure, D.; Deschamps, A.M. The Effect of Bacterial Inoculation on the Initiation of Composting of Grape Pulps. Bioresour. Technol. 1991, 37, 235–238. [Google Scholar] [CrossRef]
- Xi, B.-D.; He, X.-S.; Wei, Z.-M.; Jiang, Y.-H.; Li, M.-X.; Li, D.; Li, Y.; Dang, Q.-L. Effect of Inoculation Methods on the Composting Efficiency of Municipal Solid Wastes. Chemosphere 2012, 88, 744–750. [Google Scholar] [CrossRef]
- Xi, B.; He, X.; Dang, Q.; Yang, T.; Li, M.; Wang, X.; Li, D.; Tang, J. Effect of Multi-Stage Inoculation on the Bacterial and Fungal Community Structure during Organic Municipal Solid Wastes Composting. Bioresour. Technol. 2015, 196, 399–405. [Google Scholar] [CrossRef]
- Zeng, G.M.; Huang, H.L.; Huang, D.L.; Yuan, X.Z.; Jiang, R.Q.; Yu, M.; Yu, H.Y.; Zhang, J.C.; Wang, R.Y.; Liu, X.L. Effect of Inoculating White-Rot Fungus during Different Phases on the Compost Maturity of Agricultural Wastes. Process Biochem. 2009, 44, 396–400. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Huang, Z.-L.; Dong, M.; Yu, X.-L.; Ning, P.A. New Strategy for Co-Composting Dairy Manure with Rice Straw: Addition of Different Inocula at Three Stages of Composting. Waste Manag. 2015, 40, 38–43. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Q.; Wei, Y.; Cui, H.; Zhang, X.; Wang, X.; Shan, S.; Wei, Z. Effect of Actinobacteria Agent Inoculation Methods on Cellulose Degradation during Composting Based on Redundancy Analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Xia, J.; Chen, Y. Effect of Microbial Inoculation on Physicochemical Properties and Bacterial Community Structure of Citrus Peel Composting. Bioresour. Technol. 2019, 291, 12184. [Google Scholar] [CrossRef]
- Fan, Y.V.; Lee, C.T.; Ho, C.S.; Klemeš, J.J.; Wahab, R.A.; Chua, L.S.; Sarmidi, M.R. Evaluation of Microbial Inoculation Technology for Composting. Chem. Eng. Trans. 2017, 56, 433–438. [Google Scholar]
- Jusoh, M.L.C.; Manaf, L.A.; Latiff, P.A. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. Iran. J. Environ. Health Sci. Eng. 2013, 10, 1–19. [Google Scholar] [CrossRef]
- Lim, L.Y.; Chua, L.S.; Lee, C.T. Effects of Microbial Additive on the Physiochemical and Biological Properties of Oil Palm Empty Fruit Bunches Compost. J. Eng. Sci. Technol. 2015, 10, 10–18. [Google Scholar]
- Abdel-Rahman, M.A.; El-Din, N.; Refaat, M.; Abdel-Shakour, B.M.; Ewais, E.H.; Alrefaey, E.E.D.; Biotechnological, H.M.A. Application of Thermotolerant Cellulose-Decomposing Bacteria in Composting of Rice Straw. Ann. Agric. Sci. 2016, 61, 135–143. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Qu, F.; Mohamed, T.A.; Zhu, L.; Zhao, Y.; Jia, L.; Zhao, R.; Liu, L.; Li, P. Effect of Fenton Pretreatment Combined with Bacteria Inoculation on Humic Substances Formation during Lignocellulosic Biomass Composting Derived from Rice Straw. Bioresour. Technol. 2020, 303, 122849. [Google Scholar] [CrossRef]
- Yang, L.; Jie, G.; She-Qi, Z.; Long-Xiang, S.; Wei, S.; Xun, Q.; Man-Li, D.; Ya-Nan, Y.; Xiao-Juan, W. Effects of Adding Compound Microbial Inoculum on Microbial Community Diversity and Enzymatic Activity during Co-Composting. Environ. Eng. Sci. 2018, 35, 270–278. [Google Scholar] [CrossRef]
- Henry, B.A.; Maung, C.E.H.; Kim, K.Y. Metagenomic Analysis Reveals Enhanced Biodiversity and Composting Efficiency of Lignocellulosic Waste by Thermoacidophilic Effective Microorganism (TEM). J. Environ. Manage 2020, 276, 111252. [Google Scholar] [CrossRef]
- Estrada-Bonilla, G.A.; Lopes, C.M.; Durrer, A.; Alves, P.R.L.; Passaglia, N.; Cardoso, E.J.B.N. Effect of Phosphate-Solubilizing Bacteria on Phosphorus Dynamics and the Bacterial Community during Composting of Sugarcane Industry Waste. Syst. Appl. Microbiol. 2017, 40, 308–313. [Google Scholar] [CrossRef]
- Zainudin, M.H.M.; Zulkarnain, A.; Azmi, A.S.; Muniandy, S.; Sakai, K.; Shirai, Y.; Hassan, M.A. Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review. Agronomy 2022, 12, 198. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuge, C.; Weng, Q.; Hu, B. Additional Strains Acting as Key Microbes Promoted Composting Process. Chemosphere 2022, 287, 132304. [Google Scholar] [CrossRef]
- Jia, X.; Qin, X.; Tian, X.; Zhao, Y.; Yang, T.; Huang, J. Inoculating with the Microbial Agents to Start up the Aerobic Composting of Mushroom Residue and Wood Chips at Low Temperature. J. Environ. Chem. Eng. 2021, 9, 10529. [Google Scholar] [CrossRef]
- Wan, L.; Wang, X.; Cong, C.; Li, J.; Xu, Y.; Li, X.; Hou, F.; Wu, Y.; Wang, L. Effect of Inoculating Microorganisms in Chicken Manure Composting with Maize Straw. Bioresour. Technol. 2020, 301, 12273. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Duan, W.; Qiao, C.; Shen, Q.; Li, R. Microbial Community Composition Turnover and Function in the Mesophilic Phase Predetermine Chicken Manure Composting Efficiency. Bioresour. Technol. 2020, 313, 123658. [Google Scholar] [CrossRef]
- Cao, R.; Ben, W.; Qiang, Z.; Zhang, J. Removal of Antibiotic Resistance Genes in Pig Manure Composting Influenced by Inoculation of Compound Microbial Agents. Bioresour. Technol. 2020, 317, 12396. [Google Scholar] [CrossRef]
- Du, X.; Li, B.; Chen, K.; Zhao, C.; Xu, L.; Yang, Z.; Sun, Q.; Chandio, F.A.; Wu, G. Rice Straw Addition and Biological Inoculation Promote the Maturation of Aerobic Compost of Rice Straw Biogas Residue. Biomass Convers. Biorefinery 2021, 11, 1885–1896. [Google Scholar] [CrossRef]
- Hu, T.; Wang, X.; Zhen, L.; Gu, J.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H. Effects of Inoculation with Lignocellulose-Degrading Microorganisms on Antibiotic Resistance Genes and the Bacterial Community during Co-Composting of Swine Manure with Spent Mushroom Substrate. Environ. Pollut. 2019, 252, 110–118. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Li, Y.; Wu, Y.; Zeng, Z.; Xu, R.; Wang, S.; Li, H.; Zhang, J. Changes of Heavy Metal Fractions during Co-Composting of Agricultural Waste and River Sediment with Inoculation of Phanerochaete Chrysosporium. J. Hazard. Mater. 2019, 378, 12075. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Z.; Li, M.; Li, Q.A. Compost-Derived Thermophilic Microbial Consortium Enhances the Humification Process and Alters the Microbial Diversity during Composting. J. Environ. Manag. 2019, 243, 240–249. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J.; Lan, X.; Zhang, Y.; Yang, X.; et al. Microbial Inoculation Influences Bacterial Community Succession and Physicochemical Characteristics during Pig Manure Composting with Corn Straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhou, B.; Wang, Q.; Gu, J.; Liu, G.; Qin, Z.; Li, Z. Changes in Antibiotic Resistance Genes and Mobile Genetic Elements during Cattle Manure Composting after Inoculation with Bacillus Subtilis. Bioresour. Technol. 2019, 292, 122011. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved Lignocellulose-Degrading Performance during Straw Composting from Diverse Sources with Actinomycetes Inoculation by Regulating the Key Enzyme Activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Zhang, Z.; Wei, Y.; Wang, H.; Lu, Q.; Li, Y.; Wei, Z. Effect of Thermo-Tolerant Actinomycetes Inoculation on Cellulose Degradation and the Formation of Humic Substances during Composting. Waste Manag. 2017, 68, 64–73. [Google Scholar] [CrossRef]
- Xie, X.-Y.; Zhao, Y.; Sun, Q.-H.; Wang, X.-Q.; Cui, H.-Y.; Zhang, X.; Li, Y.-J.; Wei, Z.-M.A. Novel Method for Contributing to Composting Start-up at Low Temperature by Inoculating Cold-Adapted Microbial Consortium. Bioresour. Technol. 2017, 238, 39–47. [Google Scholar] [CrossRef]
- Gou, C.; Wang, Y.; Zhang, X.; Lou, Y.; Gao, Y. Inoculation with a Psychrotrophic-Thermophilic Complex Microbial Agent Accelerates Onset and Promotes Maturity of Dairy Manure-Rice Straw Composting under Cold Climate Conditions. Bioresour. Technol. 2017, 243, 339–346. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete Chrysosporium Inoculation on Bacterial Community and Metal Stabilization in Lead-Contaminated Agricultural Waste Composting. Bioresour. Technol. 2017, 243, 294–303. [Google Scholar] [CrossRef]
- Varma, V.S.; Ramu, K.; Kalamdhad, A.S. Carbon Decomposition by Inoculating Phanerochaete Chrysosporium during Drum Composting of Agricultural Waste. Environ. Sci. Pollut. Res. 2015, 22, 7851–7858. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Wu, S.; He, Q.; Ling, Z.; Liu, T.; Wang, Q.; Zhang, Z.; Quan, F. Cattle Manure Compost Humification Process by Inoculation Ammonia-Oxidizing Bacteria. Bioresour. Technol. 2022, 344, 126314. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, F.B.; Zhang, L.L.; Li, J. Effects of Indigenous and Exogenous Microbial Inocula on Dynamic Changes of Enzyme Activities during Composting in a Bioreactor. Adv. Mater. Res. 2011, 390, 4017–4023. [Google Scholar] [CrossRef]
- Xu, P.; Li, J. Effects of Microbial Inoculant on Physical and Chemical Properties in Pig Manure Composting. Compost. Sci. Util. 2017, 25, S37–S42. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Kan, Z.; Li, B.; Cao, Y.; Jin, H. Effects of Two-Stage Microbial Inoculation on Organic Carbon Turnover and Fungal Community Succession during Co-Composting of Cattle Manure and Rice Straw. Bioresour. Technol. 2021, 341, 12584. [Google Scholar] [CrossRef]
- Wang, W.-K.; Liang, C.-M. Enhancing the Compost Maturation of Swine Manure and Rice Straw by Applying Bioaugmentation. Sci. Rep. 2021, 11, 6103. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D. Challenges of Formulation and Quality of Biofertilizers for Successful Inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef]

| Waste Biomass | Possible Source of Nutrients | References |
|---|---|---|
| Ash | Phosphorous, Potassium, and Zinc | [16] |
| Biochar | Nitrogen, Phosphorous, Potassium, and Zinc | [17] |
| Bones meat meal | Phosphorous, Potassium, Zinc, Iron, and Selenium | [18] |
| Spent coffee grounds | Potassium, Magnesium, Selenium, Phosphorus, and Iron | [19] |
| Blood meal | Nitrogen, Phosphorous, Potassium, Zinc, Iron, and Selenium | [20] |
| Feathers meal | Amino acids and Nitrogen | [21] |
| Spent mushroom substrate | Nitrogen, Phosphorous, and Humic acid | [22] |
| Wastes Compound | Composition of Consortia | Performance | Reference |
|---|---|---|---|
| Cattle manure | Methanosarcina acetivorans and Methanosaeta thermophila | Biogas production increased by 45% | [96] |
| Chicken manure | Nitrogen-converting bacteria | Reduced ammonia loss by 59% | [97] |
| Biogas residue | Bacteria, fungi, actinomycetes, and yeasts | Drying contribution accounted for 79% | [98] |
| Food waste | Bacillus amyloliquefaciens B59, Bacillus licheniformis B58, Bacillus haynesii A31, and Bacillus amyloliquefaciens B11 | The volatile solids removal improved by 10% | [99] |
| Municipal waste | Six plastic-degrading bacterial | Improved degradation of different plastics | [100] |
| Mustard biomass | Saccharomyces cerevisiae and Fusarium incarnatum | Bioethanol production increased 33 mg/mL | [101] |
| Poultry manure | Six strains including Bacillus subtilis and Streptomyces rutgersensis | The concentration of odorants reduced 58–73% | [102] |
| Waste Compounds | Microbial Treatment | Culture Concentration | Composting Conditions | Impact on the Entire Composting Process | References |
|---|---|---|---|---|---|
| Mushroom residue | Paenibacillus GX 5 Paenibacillus GX 7 Paenibacillus GX 13 Brevibacillus, GX 5 Brevibacillus, GX 7 Brevibacillus, GX 13 | 2 mL 100 g−1 | C/N-12, T–(57 °C), MC–(60–24%), pH-(8) | Increased microbial contact, extended thermophilic period, and improved rate of lignocellulose and organic matter decomposition. | [185] |
| Mushroom residue and wood chips | Aspergillus, Penicillium Bacillus, Streptomyces | 0.2% (w/w−1) | C/N–(22), T- (58.4 °C), MC-(50%), pH-(7.8) | extended thermophilic stage, improved cellulose and hemicellulose breakdown efficiency, and optimal microbial community structure. | [186] |
| Chicken manure and maize straw | B. licheniformis, B. amyloliquefaciens, Ureibacillus thermosphaericus, B. megaterium, Geobacillus pallidus, B. pumilus, Geobacillus sp. Paracoccus denitrificans | 200 mL of 1 × 108 CFU mL−1 | C/N-(21), T-(68.4 °C), MC-(55.6–42%), pH-(8.7) | Increased germination index, NO3 content, prolonged thermophilic stage, reduced volatile solids contents, improved humification and compost maturity level. | [187] |
| Chicken manure and rice husk | Ureibacillus terrenus BE8 and B. tequilensis BG7 | 5% (v/w−1) | Total C (263 g kg−1), and Total N (34 g kg−1), T-(65 °C), MC-(78.1%) | Improved germination index values, faster compost maturity through early stimulation of many important microorganisms, and superior phytotoxicity-free compost compared to the control treatment. | [188] |
| Pig manure and wheat straw | Microbial agent solution consisting of photosynthetic bacteria, actinomycetes, yeasts, and lactic acid bacteria | 40 mL 10 kg−1 | Total C (41.2 ± 0.5%), Total N (1.79 ± 0.03%), T-(68.4 °C), MC-(55%) | The possible hosts of ARGs have changed because of changes in ARG profiles and bacterial populations, which has increased the removal of ARGs in their entirety. | [189] |
| Rice straw | Compound bacterial agent screened from rice straw composts: Aeromonas caviae sp. SD3 (KR868995.1), Shinella sp. XM2 (CP015736.1), Rhizobium sp. S8 (KF261556.1), Corynebacterium pseudotuberculosis sp. SD1 (CP020356.1) and S. clavuligerus sp. XM (CP032052.1) | 1% (w/w−1) of 1 × 109 CFU mL−1 cell concentration | C/N-(30), MC-(65%) | Improved the degradation of organic matter and coarse fiber content by 7.58% and, 8.82% due to the enhancement of core microbial metabolism. | [180] |
| Chicken manure, rice bran and pine waste | Bacteria: Bacillus spp., Alicyclobacillus spp., Pseudomonas spp., Lactobacillus spp., Pediococcuss spp., and Actinomycetes. Fungi: Rhizomucor pusillus, Aspergillus spp. | 0.2% (w/w−1) | C/N-(28.4), T-(65 °C), MC-(60 to 40%), pH-(8.5) | Enhanced mineralization, composting rate, and microbial population and variety. | [182] |
| Rice straw biogas residue and rice straw | A. niger CICIMF0410 and P. chrysosporium AF 96007 | 1% (v/w−1) of 1 × 108 CFU mL−1 cell concentration | C/N-(32), T-(68.3 °C), MC-(60%) | Reduced the time required for decomposition of organic matter, removed the toxicity risk for crops and promoted the stability of the compost. | [190] |
| Swine manure and spent mushroom substrate | Microbial suspension of lignocellulose-degrading microorganism’s consortium consisting of Bacillus, Brevibacillus, Paenibacillus and Lysinibacillus genera | 10% (v/w−1) | Mixture ratio (1:1), T-(68 °C), MC-(60%), pH-(7.6) | Promoted the changes of the bacterial community in the mesophilic phase and reduced the risk of ARGs in the final compost. | [191] |
| Maize straw and canola residue | Phanerochaete chrysosporium | 1 × 108 CFU mL−1 | C/N-(25), T-(60 °C), MC-(52%), pH-(8.17) | Improved lignin degradation during the cooling stage, enhanced compost humification. | [192] |
| River sediment, rice straw, vegetables, and bran | Phanerochaete chrysosporium | 0.5% (v/w−1) | C/N-(30), T-(69 °C), MC-(60%), pH-(8.6) | Enhanced the passivation of copper and reduced the effect of pH on the bioavailability of heavy metals. | [192] |
| Dairy manure and sugarcane leaves | Thermophilic lignocellulolytic microbes screened from dairy and sugarcane leaves compost samples: B. licheniformis (TA65), A. nidulans (GXU-1) and A. oryzae (GXU-11) | 2% (w/w−1) | C/N-(30), T-(55 °C), | Enhance the lignocellulose degradation process and the humification process, as well as the mineralization of organic carbon. | [193] |
| Pig manure and corn stalk | Compound bacterium agent comprised of Acinetobacter pittii, B. subtilis sub sp. Stercoris and B. altitudinis | 1% (v/w−1) of 1 × 109 CFU mL−1 cell concentration | C/N-(30), T-(67.3 °C), MC-(60%), pH-(8.8) | Increased the number of biomarkers, prolonged the thermophilic stage, reduced the amount of human disease-related functional genes, and improved fertility and longevity. | [194] |
| Citrus peel. bran and lime | The bacterial consortium which was screened from citrus peel compost samples | 3% (w/w−1) | C/N-(25), T-(65 °C), MC-(60%), pH-(8.5) | Decreased C/N, organic matter, moisture, pectin and cellulose content, and enhanced the richness and diversity of the bacterial community. | [175] |
| Cattle manure and wheat stalks | B. subtilis | 0.5% (w/w−1) | C/N-(25), MC-(60%), pH-(7.61) | Promoted changes in ARGs and removed many pathogenic bacteria. | [195] |
| Wheat straw, rice, corn and soybean | Actinomycetes: Streptomyces sp. H1 (KX641927.1), Mycobacerium sp. G1 (KY910181.1), Micromonospora sp. G7 (LC333394.1) and Saccha-romonospora sp. T9 (NR074713.2) | 3 mL kg−1 of 1 × 109 CFU mL−1 cell concentration | C/N-(30), T-(63 °C), MC-(50 to 60%), pH (9.4) | Improved 34.3% lignocellulose degradation and 8.3% enzyme activity. | [196] |
| Pig manure and apple tree branches | Microbial inoculum: Ralstoinia sp., Penicillium sp., Penicillium aurantiogriseum, and Acremonium alternatum | 2% (v/w−1) | C/N-(30), T-(77 °C), MC-(60%), pH-(8.1) | Enhanced cellulase, urease, and polyphenol oxidase activities and promoted the succession of the bacterial community structure. | [181] |
| Corn straw and dairy manure | Thermo-tolerant actinomycetes Streptomyces sp. H1, Streptomyces sp. G1, Streptomyces sp. G2 and Actinobacteria bacterium T9 | 2% (v/w−1) of 1 × 109 CFU mL−1 cell concentration | C/N-(30), T-(57 °C), MC-(60%) | Enhanced cellulase activities and increased degradation of cellulose, humic substances content. | [197] |
| Food waste and maize straw | Cold adapted microbial consortium comprised of stains P. fragi (KY283110), P. simiae (KY283111), Clostridium vincentii (KY283112), P. jessenii (KY283113) and Iodobacter fluviatilis (KY283114). | 1% (v/w−1) of 1 × 108 CFU mL−1 cell concentration | C/N-(18), T-(45 °C), MC-(66%) | Improved the breakdown of organic materials at low temperatures and encouraged a shift in the succession and composition of the bacterial population. | [198] |
| Dairy manure and rice straw | Psychrotrophic-thermophilic complex microbial agent (PTCMA): B. diminuta CB1, Flavobacterium glaciei CB23, A. niger CF5 and Penicillium commune CF8 | 10 mL kg−1 of 1 × 108 CFU mL−1 cell concentration | C/N-(32), T-(63 to 45 °C), MC-(60%), pH-(8.2 to 8.4) | In colder areas, raising the temperature of the composting pile, greatly enhancing the compost’s maturity, and proposing PTCMA injection are all useful strategies. | [199] |
| Sugarcane industry waste | Phosphate-solubilizing bacteria: P. aeruginosa, Bacillus sp., Lactobacillales, Bacillales, Pseudomonas sp., Clostridiales | 8 L mg−1 of 1 × 108 CFU mL−1 cell concentration | C/N-(30), T-(60 °C) | Elevated bacterial development, mostly of the Lactobacillales order, which results in the heaps heating up in the first stage of composting and having an increased phosphorus content at the end. | [183] |
| Rice straw, soil, vegetables, and bran | Phanerochaete chrysosporium | 2% (v/w−1) of 1 × 106 CFU mL−1 cell concentration | C/N-(30), T-(58 °C), MC-(60%), pH-(8) | reduced the lead’s toxicity and enhanced the composting bacterial community’s diversity | [200] |
| Chicken manure and rice straw | Ammonia-oxidizing bacteria | 5% (v/w−1) of 1 × 106 CFU mL−1 cell concentration | C/N-(25), T-(57 °C), MC-(60 to 70%), pH-(7.4) | Reduced nitrogen loss and ammonia emissions by the conversion of ammonium to nitrite and improved bacterial community abundance. | [174] |
| Rice straw | Cellulase producing bacteria: B. licheniformis 1-1v and B. sonorensis 7-1v | 1% (v/w−1) of 3.6 and 6.8 × 107 CFU mL−1 cell concentration | C/N-(35.8), T-(54 °C), MC-(35%), pH-(8.1) | Lowered the composting period by 40 to 43%, which improved the quality of the compost and led to a greater drop in the total organic carbon and C/N ratio. | [179] |
| Vegetable waste: cattle manure: sawdust | Phanerochaetechrysosporium (MTCC 787) | 107 to 108 spores g−1 of compost | Compost mixture ratio (5:4), T-(64 °C), MC-(65%), pH-(7.5) | improved the volatile solids reduction over the uninoculated compost treatment by 1.45 times in trial 2 (the initial phase) and 1.7 times in trial 3 (the thermophilic phase). | [201] |
| Rice straw and goat manure | EM: lactic acid bacteria, yeast and phototrophic bacteria. | 5% (v/w−1) | C/N-(32.4) | Improved the mineralization in composting process. | [177] |
| Wheat straw and cattle manure | Ammonium-oxidizing bacteria: Bacillaceae (strain T-AOB-2, M-AOB-4 and MT-AOB, 2–4) | 5% (v/w−1) of 1 × 108 CFU mL−1 cell concentration | C/N-(30), MC-(65%) | Enhance bacterial activity and encourage the production of humic compounds by lowering total and dissolved organic carbon. | [202] |
| Chicken manure, furfural residues and bagasse | Exogenous microbes (VT) and indigenous microbes (M3T) | 0.5% (v/w−1) | C/N-(30), T-(50 to 58 °C), MC-(55%) | Increased urease, protease, and cellulase activity, as well as a faster rate of temperature increase. | [203] |
| Maize straw and pig manure | B. subtilis, B. licheniformis, Phanerochaetechrysosporium, Trichoderma koningii, Saccharomyces cerevisiae | 0.1% (w/w−1) | C/N-(27.7), T-(66 °C), MC-(60%) | Improved rate of temperature increase, increased micronutrients (N, P, K), enhanced decomposition of organic carbon, improved germination index. | [204] |
| Wheat straw and dairy manure | Microbial agent: A. niger, Saccharomyces cerevisiae, Lactobacillus plantarum, Lactobacillus acidophilus, B. megaterium, S. albogriseus and B. subtilis | 0.2% (v/w−1) | C/N-(16), T-(60 °C), MC-(60%), pH-(8.0) | Raised essential bacterial network interaction, reduced possible pathogen abundance, and increased composting maturity and overall organic carbon decomposition. | [30] |
| Rice straw and cattle manure | Malbranchea cinnamonmea, Gloephyllumtrabeum | 10 mL kg−1 | C/N-(25), T-(73 °C), MC-(65%), pH-(8.5) | Strengthened nutrients and humus carbon, enhanced lignocellulosic fungal variety and relative abundance, and promoted decomposition of cellulose, hemicellulose, and lignin. | [205] |
| Rice straw and swine manure | Kitasatospora phosalacinea C1, Paenibacillus glycanilyticus X1, B. licheniformis S3, Brevibacillus agri E4 and Phanerochaete chrysosporium | Not mentioned | C/N-(27.5), T-(62 °C) | Increased degree of maturity and improved pace of temperature increase. | [206] |
| Wheat straw and swine manure | Gloephyllum trabeum | 1 × 108 spores kg−1 | C/N-(27), T-(73 °C), MC-(60%) | Shorten maturation period, increased decomposition rate of cellulose, hemicellulose and lignin, influencing fungal community by increasing relative abundance of Aspergillus, Mycothemus and melanocapus. | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vurukonda, S.S.K.P.; Fotopoulos, V.; Saeid, A. Production of a Rich Fertilizer Base for Plants from Waste Organic Residues by Microbial Formulation Technology. Microorganisms 2024, 12, 541. https://doi.org/10.3390/microorganisms12030541
Vurukonda SSKP, Fotopoulos V, Saeid A. Production of a Rich Fertilizer Base for Plants from Waste Organic Residues by Microbial Formulation Technology. Microorganisms. 2024; 12(3):541. https://doi.org/10.3390/microorganisms12030541
Chicago/Turabian StyleVurukonda, Sai Shiva Krishna Prasad, Vasileios Fotopoulos, and Agnieszka Saeid. 2024. "Production of a Rich Fertilizer Base for Plants from Waste Organic Residues by Microbial Formulation Technology" Microorganisms 12, no. 3: 541. https://doi.org/10.3390/microorganisms12030541







