Effect of Saccharina japonica Intake on Blood Pressure and Gut Microbiota Composition in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. The Experimental Diets
2.3. Blood Pressure Measurements
2.4. 16S rRNA Gene Extraction and Sequencing
2.5. Measurements of SCFAs
2.6. Statistical Analysis
3. Results
3.1. Effect of Saccharina japonica Intake on SBP and MAP in SHRs
3.2. Effects of Saccharina japonica Intake on the Microbial Taxonomic Composition in SHRs
3.3. Effects of Saccharina japonica on the SCFAs Concentration in SHRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunlay, S.M.; Weston, S.A.; Jacobsen, S.J.; Roger, V.L. Risk factors for heart failure: A population-based case-control study. Am. J. Med. 2009, 122, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, B.P.; Khelton, K.P.; Sorond, F.; Carey, M.R. Blood pressure management in stroke. Hypertension 2020, 76, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Cirigliano, M. Hypertension in renal failure. Dis. Mon. 1998, 44, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Lu, B.; Chen, M.; Zhang, Y. Evaluation of bamboo shoot peptide preparation with angiotensin converting enzyme inhibitory and antioxidant abilities from byproducts of canned bamboo shoots. J. Agric. Food Chem. 2013, 61, 5526–5533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Salih Ibrahim, R.M.; Chi, H.L.; Xiao, T.; Xia, W.J.; Li, H.B.; Kang, Y.M. Altered Gut Microbiota is Involved in the Anti–Hypertensive Effects of Vitamin C in Spontaneously Hypertensive Rat. Mol. Nutr. Food Res. 2021, 65, e2000885. [Google Scholar] [CrossRef]
- Ishida, A.; Enomoto, Y.; Sasaki, M.; Makio, A. Preventive and curative effects of powdered seaweed diets on hypertension in rat. Mem. Fac. Educ. Kumamoto Univ. Nat. Sci. 2003, 52, 57–62. (In Japanese) [Google Scholar]
- Maruyama, S.; Segawa, Y.; Hashimoto, H.; Kitamura, S.; Kimura, M.; Osera, T.; Kurihara, N. Role of Alginate in the Mechanism by Which Brown Seaweed Saccharina japonica Intake Alleviates an Increase in Blood Pressure in 2-Kidney, 1-Clip Renovascular Hypertensive Rats. Clin. Exp. Hypertens. 2022, 44, 72–82. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Su, Z.; Zou, P.; Pang, J.; Chen, J.C. ACE-inhibitory peptides from Laminaria japonica and their potential anti-hypertensive mechanism. J. Food Sci. 2021, 19, 333–340. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Han, Z.L.; Chen, M.; Fu, X.D.; Yang, M.; Hrmova, M.; Zhao, Y.H.; Mou, H.J. Potassium alginate oligosaccharides alter gut microbiota, and have potential to prevent the development of hypertension and heart failure in spontaneously hypertensive rats. Int. J. Mol. Sci. 2021, 22, 9823. [Google Scholar] [CrossRef]
- Maruyama, S.; Segawa, Y.; Harui, A.; Yamamoto, K.; Hashimoto, H.; Osera, T.; Kurihara, N. Influence of Intestinal Barrier on Alleviating an Increase in Blood Pressure by Sodium Alginate Intake in 2-Kidney, 1-Clip Renovascular Hypertensive Rats. Mar. Drugs 2023, 21, 324. [Google Scholar] [CrossRef]
- An, C.; Kuda, T.; Yazaki, T.; Kimura, B. FLX pyrosequencing analysis of the effects of the brown-algal fermentable polysaccharides alginate and laminaran on rat cecal microbiotas. Appl. Environ. Microbiol. 2013, 79, 860–866. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T.; Matsuda, N.; Nishizawa, M. Inhibitory effects of laminaran and low molecular alginate against the putrefactive compounds produced by intestinal microflora in vitro and in rats. Food Chem. 2005, 91, 745–749. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Nelson, J.W.; Eskew, J.R.; Ganesan, A.; Ajami, N.J.; Petrosino, J.F.; Bryan, M.R., Jr.; Durgan, D.J. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 2018, 72, 1141–1150. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, G.E.; et al. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, K.J.; Kuruppu, S.; Rajapakse, W.N.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Chu, X.; Jiang, S.; Li, C.; Li, G.; He, Y.; Liu, Y.; Li, Y.; Sun, C. Vinegar decreases blood pressure by down-regulating AT1R expression via the AMPK/PGC-1α/PPARγ pathway in spontaneously hypertensive rats. Eur. J. Nutr. 2016, 55, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Magee, L.K.; Colon-Perez, M.L.; Larkin, R.; Liao, Y.S.; Balazic, E.; Cowart, R.J.; Arocha, R.; Redler, T.; Febo, M.; et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol. 2019, 226, e13256. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, E.D.; Pluznick, L.J. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Robles-Vera, I.; De La Visitación, N.; Romero, M.; Yang, T.; Sánchez, M.; Gómez-Guzmán, M.; Jiménez, R.; Raizada, K.M.; Duarte, J. Critical role of the interaction gut microbiota-sympathetic nervous system in the regulation of blood pressure. Front. Physiol. 2019, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; De La Visitación, N.; Aguilera-Sánchez, N.; Redondo, J.M.; Duarte, J. Protective Effects of Short-Chain Fatty Acids on Endothelial Dysfunction Induced by Angiotensin II. Front. Physiol. 2020, 11, 277. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-linked pathophysiological alterations in the gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molinaet, F. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, R.V.; Petrosino, F.J.; Bryan, M.R., Jr.; Durgan, J.D. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Luo, H.; Wang, J.; Tang, W.; Lu, J.; Wu, S.; Xiong, Z.; Yang, G.; Chen, Z.; Lan, T.; et al. Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Exp. Mol. Med. 2017, 49, e370. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Jin, H.; Wu, Z.; Yan, C.; Liu, Z.; Xu, X.; Liu, S.; Zhu, F. Zhengganxifeng Decoction affects gut microbiota and reduces blood pressure via renin–angiotensin system. Biol. Pharm. Bull. 2019, 42, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.J.; Xu, M.L.; Yu, X.J.; Du, M.M.; Li, X.H.; Yang, T.; Lu, L.; Li, Y.; Kang, B.K.; Su, Q.; et al. Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat: Short Title: Exercise, hypertension and gut-brain axis. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Galla, S.; Chakraborty, S.; Cheng, X.; Yeo, J.; Mell, B.; Zhang, H.; Mathew, A.V.; Vijay-Kumar, M.; Joe, B. Disparate effects of antibiotics on hypertension. Physiol. Genom. 2018, 50, 837–845. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Toral, M.; Romero, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Zarzuelo, M.J.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 2326–2336. [Google Scholar] [CrossRef]
- Luo, X.; Han, Z.; Kong, Q.; Wang, Y.; Mou, H.; Duan, X. Clostridium butyricum Prevents Dysbiosis and the Rise in Blood Pressure in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2023, 24, 4955. [Google Scholar] [CrossRef]
- Yang, X.P.; Liu, Y.H.; Rhaleb, N.E.; Kurihara, N.; Kim, H.E.; Carretero, O.A. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am. J. Physiol. 1999, 277, 1967–1974. [Google Scholar] [CrossRef]
- Segawa, Y.; Hashimoto, H.; Maruyama, S.; Shintani, M.; Ohno, H.; Nakai, Y.; Osera, T.; Kurihara, N. Dietary capsaicin-mediated attenuation of hypertension in a rat model of renovascular hypertension. Clin. Exp. Hypertens. 2020, 42, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Kunihiro, T.; Takahashi, S.; Hisada, T.; Nagashima, K.; Mochizuki, J.; Mizushima, K.; Naito, Y. A newly developed solution for the preservation of short-chain fatty acids, bile acids, and microbiota in fecal specimens. J. Clin. Biochem. Nutr. 2023, 72, 263–269. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, A.; Ai, C. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by noemalizing the gut microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Fang, Z.; Pei, Z.; Wang, H.; Zhu, J.; Lee, K.Y.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. A low molecular weight brown algae Laminaria japonica glycan modulation of gut microbiota and body weight in mice. Food Funct. 2021, 12, 12606–12620. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, Y.M.; Kim, I.S.; Kim, J.A.; Yu, D.Y.; Adhikari, B.; Lee, S.S.; Choi, I.S.; Cho, K.K. Effects of the Brown Seaweed Laminaria japonica Supplementation on Serum Concentrations of IgG, Triglycerides, and Cholesterol, and Intestinal Microbiota Composition in Rats. Front. Nutr. 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, X.Y.; Guo, W.L.; Cao, Y.J.; Lin, Y.C.; Cheng, W.J.; Chen, L.J.; Rao, P.F.; Ni, L.; Lv, X.C. The protective mechanisms of macroalgae Laminaria japonica consumption against lipid metabolism disorders in high-fat diet-induced hyperlipidemic rats. Food Funct. 2020, 11, 3256–3270. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.N.; Kuda, T.; Taniguchi, M.; Nakamura, S.; Hajime, T.; Kimura, B. Detection and isolaion of molecular weight alginate- and laminaran- susceptible gut indigenous bacteria from ICR mice. Carbohydr. Polym. 2020, 238, 116205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal. Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 Receptor Activation and Epac2 Link Atrial Natriuretic Peptide Secretion to Control of Blood Pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef]
- Yu, M.; Moreno, C.; Hoagland, K.M.; Dahly, A.; Ditter, K.; Mistry, M.; Roman, R.J. Antihypertensive Effect of Glucagon-Like Peptide 1 in Dahl Salt-Sensitive Rats. J. Hypertens. 2003, 21, 1125–1135. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Muñoz, R.; Algieri, F.; Vezza, T.; Jiménez, R.; Gálvez, J.; et al. Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br. J. Pharmacol. 2020, 177, 2006–2023. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Yang, H.T.; Jiang, Z.H.; Yang, Y.; Wu, T.T.; Zheng, Y.Y.; Ma, Y.T.; Xie, X. Faecalibacterium prausnitzii as a potential Antiatherosclerotic microbe. Cell Commun. Signal 2024, 19, 54. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, K.; Quan, D.; Kang, L.; Yang, D.; Wu, H.; Yan, M.; Wu, S.; Lv, L.; Zhang, G. The Combination of Scutellaria baicalensis Georgi and Sophora japonica L. ameliorate Renal Function by Regulating Gut Microbiota in Spontaneously Hypertensive Rats. Front. Pharmacol. 2021, 12, 575294. [Google Scholar] [CrossRef]
- Toma, I.; Kang, J.J.; Sipos, A.; Vargas, S.; Bansal, E.; Hanner, F.; Meer, E.; Peti-Peterdi, J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Investig. 2008, 118, 2526–2534. [Google Scholar] [CrossRef]
- He, W.; Miao, F.J.; Lin, D.C.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004, 429, 188–193. [Google Scholar] [CrossRef]
- Sadagopan, N.; Li, W.; Roberds, S.L.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar]
- Huć, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Nakagawa, Y.; Ichikawa, T. Effects of dietary potassium alginate on blood pressuure, mineral balance and serum cholesterol levels in spontaneously hypertensive rats. J. Home Econ. Jpn. 1993, 44, 3–9. (In Japanese) [Google Scholar]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef] [PubMed]


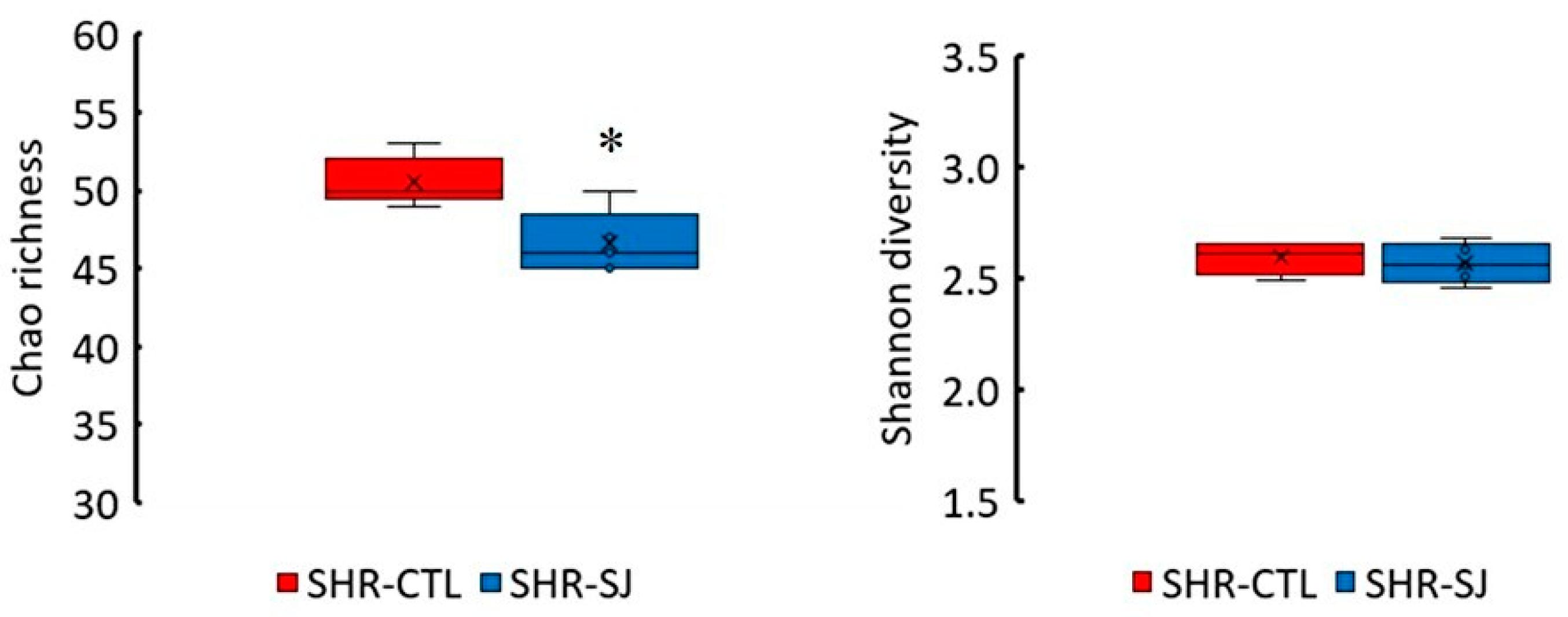


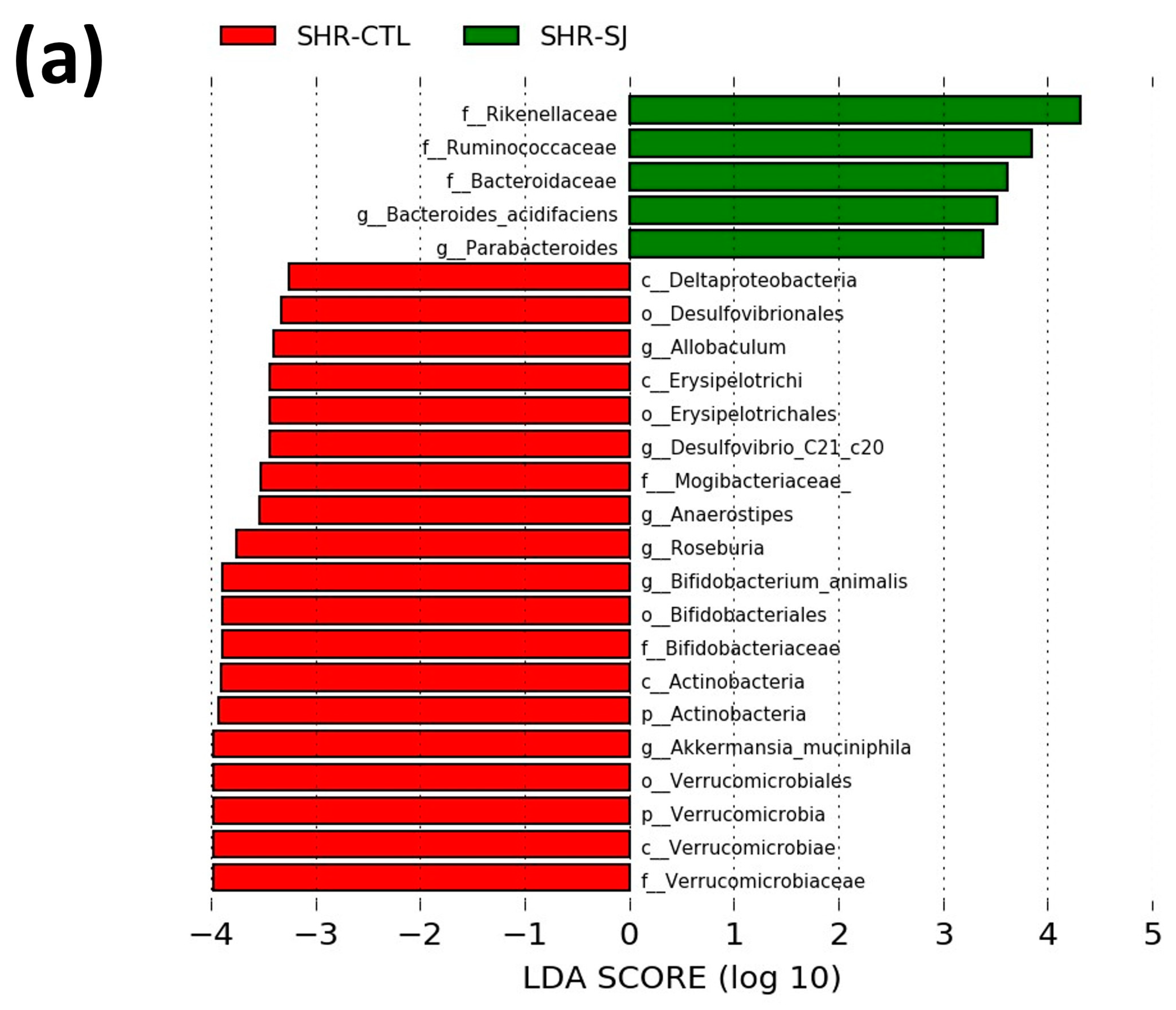

| Phylum | Relative Abundance (%) | p Value | |
|---|---|---|---|
| CTL | SJ | ||
| Firmicutes | 65.0 (58.2–70.6) | 60.9 (60.2–66.2) | 0.548 |
| Bacteroidetes | 30.1 (23.2–37.0) | 34.5 (30.8–36.9) | 0.548 |
| Verrucomicrobia | 1.8 (1.3–2.8) | 0.6 (0.3–1.2) | 0.016 * |
| Protepbacteria | 1.4 (1.2–2.4) | 2.0 (1.7–2.4) | 0.222 |
| Actinobacteria | 0.9 (0.6–2.6) | 0.2 (0.1–0.5) | 0.032 * |
| SCFAs | Mean Concentration (mg/g) | p Value | |
|---|---|---|---|
| CTL | SJ | ||
| Succinate | 0.05 ± 0.01 | N/D | - |
| Lactate | 0.22 ± 0.07 | 0.06 ± 0.02 | 0.059 |
| Acetate | 2.76 ± 0.17 | 2.55 ± 0.13 | 0.356 |
| Propionate | 0.73 ± 0.09 | 0.69 ± 0.04 | 0.617 |
| Butyrate | 2.21 ± 0.30 | 2.43 ± 0.23 | 0.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harui, A.; Maruyama, S.; Segawa, Y.; Kurihara, N. Effect of Saccharina japonica Intake on Blood Pressure and Gut Microbiota Composition in Spontaneously Hypertensive Rats. Microorganisms 2024, 12, 556. https://doi.org/10.3390/microorganisms12030556
Harui A, Maruyama S, Segawa Y, Kurihara N. Effect of Saccharina japonica Intake on Blood Pressure and Gut Microbiota Composition in Spontaneously Hypertensive Rats. Microorganisms. 2024; 12(3):556. https://doi.org/10.3390/microorganisms12030556
Chicago/Turabian StyleHarui, Ayaka, Saki Maruyama, Yukiko Segawa, and Nobutaka Kurihara. 2024. "Effect of Saccharina japonica Intake on Blood Pressure and Gut Microbiota Composition in Spontaneously Hypertensive Rats" Microorganisms 12, no. 3: 556. https://doi.org/10.3390/microorganisms12030556





