Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense
Abstract
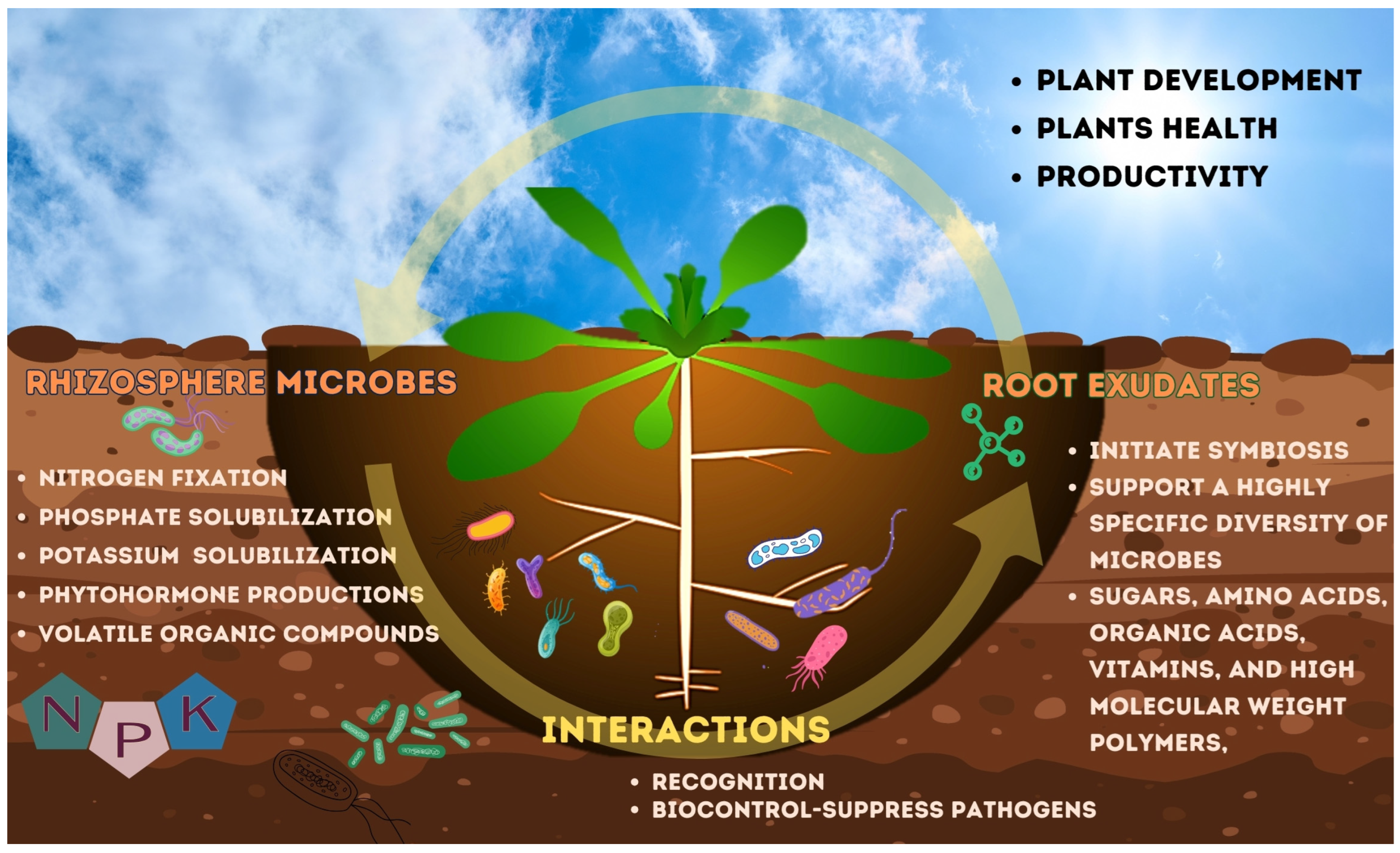
1. Introduction
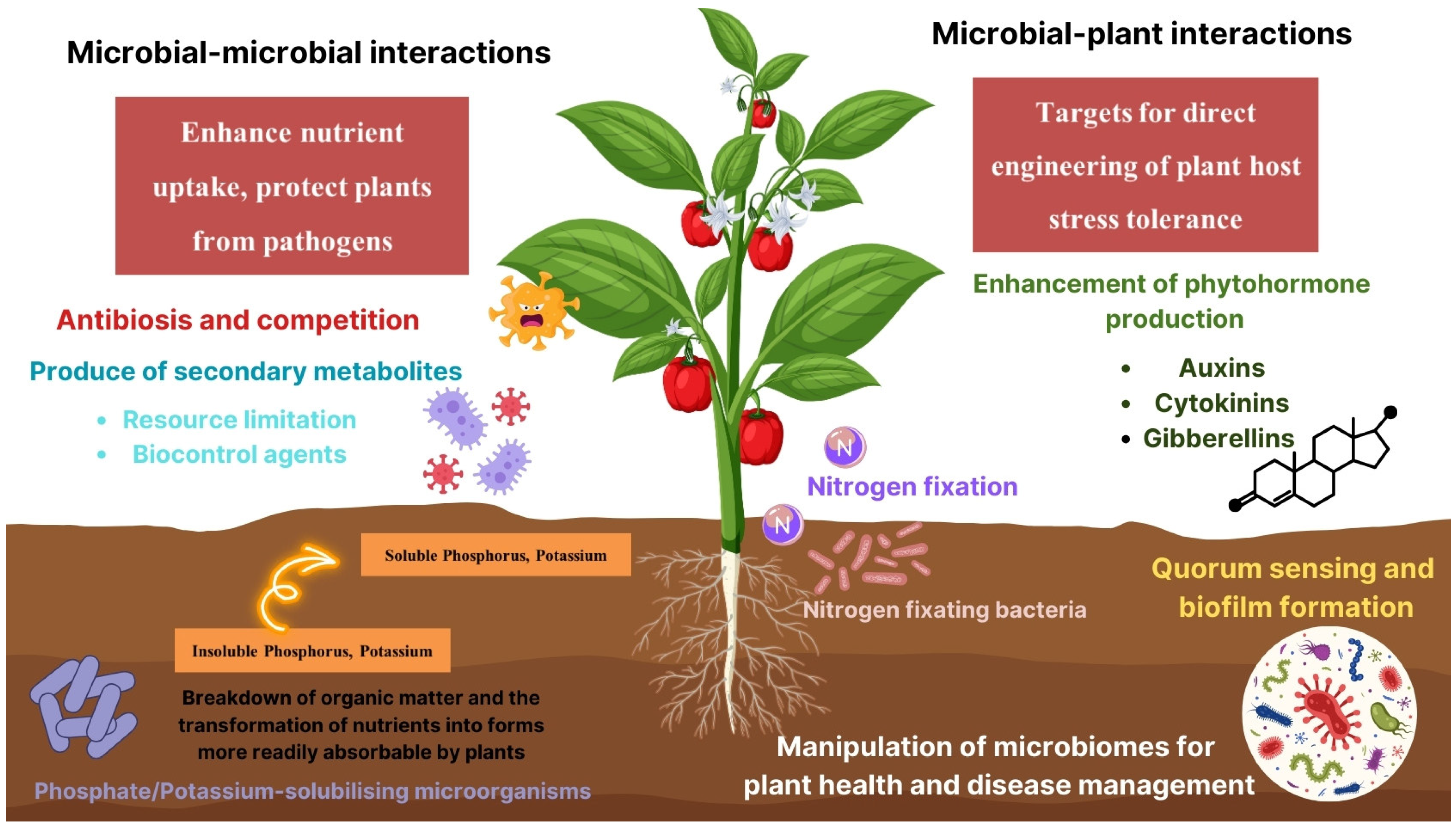
2. Soil Macronutrients
| Microorganisms | Plant | Mechanisms | Beneficial Effects | References |
|---|---|---|---|---|
| Flaisolibacter gensengsoil Williams 82 | Soybean | Nitrogen fixation, reduced the stress of molybdenum nanoparticles | Improved growth; roots, stems, leaves, nodules | [88] |
| Bacillus velezensis S141 | Soybean | Nitrogen fixation, auxin, cytokinin biosynthesis | Improved growth; nodule numbers, nodule dry weight, root-shoot dry weight, plant dry weigh | [89] |
| Bacillus pumilus | Tomato | Nitrogen fixation, Improved N uptake | Improved growth; shoot fresh weight, shoot dry weight, root fresh weight, root dry weight | [90] |
| Pseudomonas stutzeri A1501 | Maize | Nitrogen fixation, ammonia oxidizers | Improved growth; shoot, root, yields | [91] |
| Pseudomonas stutzeri Spirulina platensis extract | Onion | Nitrogen fixation, Hydrogen cyanide, indole acetic acid, ammonia, pectinase activity | Improved growth; marketable yield, total bulb yield, bulb weight, bulb diameter | [92] |
| Pseudomonas aeruginosa CQ-40 | Tomato | Nitrogen fixation, Phosphates solubilization, cellulase, protease, ferrophilin, antibacterial metabolites, | Promote Growth; plant height, stem thickness, dry and fresh weight, main root length and Control Botrytis cinerea | [93] |
| Bradyrhizobium sp. A2-10, Pseudomonas sp. A6-05 | Calopo | Nitrogen fixation, Phosphates-solubilization, siderophore production, indole acetic acid | Dry mass of shoots (SDM) and roots (RDM) Nodules | [94] |
| Lysinibacillus varians KUBM17 | Radish | Nitrogen fixation, Phosphates solubilization, siderophore production, indole acetic acid | Plant growth under cadmium stress condition. | [95] |
| Bradyrhizobium japonicum SAY3-7, Bradyrhizobium elkanii BLY3-8, Streptomyces griseoflavus | Mung Bean, Cowpea, Soybean | Nitrogen fixation, nitrogen, phosphorus, and potassium uptake | Plant growth, nodulation, yield components | [96] |
| Arthrobacter sp. V54, Bacillus sp. V62 | Tomato | Phosphates solubilization | Promote Growth; plant height, shoot, and root dry weights, | [97] |
| Aspergillus niger, Purpureocillium lilacinum, Bacillusamyloliquefaciens, Paenibacillus polymyxa | Apple | Increased uptake N, P, K, Mg, and Ca | Increase of N, P, K, Mg content in apple orchard soil | [98] |
| Klebsiella variicola | Jerusalem artichoke | Phosphates solubilization | Improved growth/Tuber | [99] |
| Pseudomonas sp. | Wheat | Phosphates solubilization | Improved root and shoot dry matter, grain yield, | [100] |
| Staphylococcus sp. | Banana | Phosphates solubilization | Promote plants growth | [101] |
| Citrobacter amalonaticus, Bacillus safensis | Maize | Phosphates solubilization | Promote plants growth | [102] |
| Bacillus licheniformis | Rice | Phosphates solubilization | Promote plants growth | [103] |
| Paenibacillus mucilaginosus | Apple | Potassium solubilization | Promote Growth; seedlings | [104] |
| Pantoea agglomerans, Rahnella aquatilis, Pseudomonas orientalis | Rice | potassium solubilization | Improved grain yield | [105] |
| Bacillus sp. MN54 | Pearl millet | enhance dry matter remobilization and translocation to the grains | Improved grain yield; Grains Per Ear, grain weight, Improved protein, iron and zinc concentration in pearl millet seeds | [106] |
| Bacillus megaterium, Bacillus mucilaginous | Chili pepper | Potassium solubilization, soil organic carbon, total nitrogen, available Phosphorus | Promote Growth; Fruits, Shoots, Roots | [107] |
| Halomonas aquamarina EU-B2RNL2, Pseudomonas extremorientalis | Chili | Potassium solubilization, phosphates solubilization | Shoot/root biomass and length, number of leaves, branches and fruits per plant | [108] |
3. Induced Plant Defense
| Microorganisms/Agents | Plant | Target Pathogens | Indirect Mechanisms | References |
|---|---|---|---|---|
| Aspergillus fumigatus Rhizopus oryzae | Tomato | Fusarium oxysporum | Increased POD, PPO | [108] |
| Trichoderma harzianum, Trichoderma asperellum Paenibacillus dendritiformis | Chilli Pepper | Colletotrichum truncatum | Increased SOD, POX, PPO, CAT, APX, GPX, PAL | [136] |
| Trichoderma hamatum Strain Th23 | Tomato | Tobacco Mosaic Virus | Increase PPO, CAT, SOD PR-1 and PR-7 | [137] |
| Bacillus amyloliquefaciens TBorg1 | Tomato | Tobacco Mosaic Virus | PPO, POX, C4H, HCT, and CHI PR-1 and PR-5 | [138] |
| Trichoderma harzianum | Cucumber | Fusarium oxysporum | Regulating ROS and RNS Metabolism | [139] |
| Trichoderma strains viz., T-42, MV-41, DFL, and RO, | Chickpea | Fusarium oxysporum f. sp. ciceris | Lignifications | [140] |
| Bacillus subtilis MBI600 | Tomato | Rhizoctonia solani, Pythium ultimum, and Fusarium oxysporum f. sp. | SiPin6 and SiLax4, PR-1A, GLUA, CHI3, LOXD, PAL | [141] |
| Bacillus amyloliquefaciens FZB42 | Tomato | Sclerotinia sclerotiorum | GST, SOD, PAL, HMGR, and MPK3 | [142] |
| Bacillus amyloliquefaciens Aspergillus spinulosporus | Rice | Xanthomonas oryzae pv. oryzae | SOD, PAL, PO, PPO TPC | [143] |
| Bacillus vallismortis TU-Oraga21 | Rice | Magnaporthe oryzae | Increased salicylic acid, phenolic compounds, and hydrogen peroxide (H2O2) | [117] |
| Pseudomonas putida BP25 | Rice | Magnaporthe oryzae | OsPR1.1, peroxidase and total phenol compound | [144] |
| Pseudomonas fluorescens SP007s | Kale Soybean | Pectobacterium carotovorum Xanthomonas axonopodis pv. glycines | Increased salicylic acid and phenolic compounds | [118,119] |
| Trichoderma spp. | Grapevine | Plasmopara viticola | PR-2, OSM2, HSR hypersensitive response | [145] |
| Trichoderma asperellum | Onion | Sclerotium cepivorum | AcPR1, AcPAL1 | [146] |
| Trichoderma spp. | Soybean | Fusarium virguliforme | PR-3, PR-2, PR-12, CHS | [147] |
| Trichoderma asperelloides | Muskmelon | Stagonosporopsis cucurbitacearum | POD, PPO, chitinase, β-1,3-glucanase | [148] |
| Azospirillum brasilense REC3 | Strawberry | Macrophomina phaseolina | Accumulation of ROS, salicylic acid, callose, lignin, the increase of biofilm formation on leaves, and stomatal closure | [149] |
4. Rhizosphere Communication and Signaling for Soil Health
5. Advances in Microbiome Research and Omics Technologies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van, D.M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Beckers, V.; Poelmans, L.; Van Rompaey, A.; Dendoncker, N. The impact of urbanization on agricultural dynamics: A case study in Belgium. J. Land Use Sci. 2020, 15, 626–643. [Google Scholar] [CrossRef]
- Ruzzante, S.; Labarta, R.; Bilton, A. Adoption of agricultural technology in the developing world: A meta-analysis of the empirical literature. World Dev. 2021, 146, 105599. [Google Scholar] [CrossRef]
- Hu, W.; Ran, J.; Dong, L.; Du, Q.; Ji, M.; Yao, S.; Sun, Y.; Gong, C.; Hou, Q.; Gong, H.; et al. Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, X.; Dang, K.; Li, J.; Yang, P.; Gao, X.; Deng, X.; Feng, B. Linkages between nutrient ratio and the microbial community in rhizosphere soil following fertilizer management. Environ. Res. 2020, 184, 109261. [Google Scholar] [CrossRef]
- Dlamini, S.P.; Akanmu, A.O.; Babalola, O.O. Rhizospheric microorganisms: The gateway to a sustainable plant health. Front. Sustain. Food Syst. 2022, 6, 925802. [Google Scholar] [CrossRef]
- Checcucci, A.; Marchetti, M. The Rhizosphere Talk Show: The Rhizobia on Stage. Front. Agron. 2020, 2, 591494. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, A.; Srivastava, P.; Choudhary, K.K.; Dikshit, A. 1—Plant growth-promoting microorganisms in sustainable agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–19. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Solomon, R.; Wein, T.; Levy, B.; Eshed, S.; Dror, R.; Reiss, V.; Zehavi, T.; Furman, O.; Mizrahi, I.; Jami, E. Protozoa populations are ecosystem engineers that shape prokaryotic community structure and function of the rumen microbial ecosystem. ISME J. 2022, 16, 1187–1197. [Google Scholar] [CrossRef]
- Wright, C.L.; Lehtovirta-Morley, L.E. Nitrification and beyond: Metabolic versatility of ammonia oxidising archaea. ISME J. 2023, 17, 1358–1368. [Google Scholar] [CrossRef]
- Braga, L.P.P.; Spor, A.; Kot, W.; Breuil, M.-C.; Hansen, L.H.; Setubal, J.C.; Philippot, L. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome 2020, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, H.; Chen, L.; Yuan, Y.; Fang, H.; Luan, L.; Chen, Y.; Wang, X.; Liu, M.; Li, H.; et al. Nematodes and Microorganisms Interactively Stimulate Soil Organic Carbon Turnover in the Macroaggregates. Front. Microbiol. 2018, 9, 2803. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, T.; Gen, S.; Zhang, X.-Q.; Wang, X.; Jiang, D.; Li, C.; Li, C.; Wang, J.; Zhang, W.; et al. Soil properties and agricultural practices shape microbial communities in flooded and rainfed croplands. Appl. Soil Ecol. 2020, 147, 103449. [Google Scholar] [CrossRef]
- Leff, J.W.; Bardgett, R.D.; Wilkinson, A.; Jackson, B.G.; Pritchard, W.J.; De Long, J.R.; Oakley, S.; Mason, K.E.; Ostle, N.J.; Johnson, D.; et al. Predicting the structure of soil communities from plant community taxonomy, phylogeny, and traits. ISME J. 2018, 12, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef]
- Xiong, W.; Song, Y.; Yang, K.; Gu, Y.; Wei, Z.; Kowalchuk, G.A.; Xu, Y.; Jousset, A.; Shen, Q.; Geisen, S. Rhizosphere protists are key determinants of plant health. Microbiome 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xing, P.; Niu, M.; Wei, G.; Shi, P. Taxonomic Compositions and Co-occurrence Relationships of Protists in Bulk Soil and Rhizosphere of Soybean Fields in Different Regions of China. Front. Microbiol. 2021, 12, 738129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Delmont, T.O.; Vogel, T.M.; Bromberg, Y. Functional Basis of Microorganism Classification. PLoS Comput. Biol. 2015, 11, e1004472. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial adaptation to different environmental conditions: Molecular perspective of evolved genetic and cellular systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef] [PubMed]
- Counts, J.A.; Zeldes, B.M.; Lee, L.L.; Straub, C.T.; Adams, M.W.W.; Kelly, R.M. Physiological, metabolic and biotechnological features of extremely thermophilic microorganisms. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1377. [Google Scholar] [CrossRef] [PubMed]
- Kiri, I. Mechanisms of Nutrient Uptake and Assimilation Processes in Some Plants: A Review. Dutse J. Pure Appl. Sci. 2023, 9, 223–237. [Google Scholar] [CrossRef]
- Kochhar, N.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the microorganism of extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Aroney, S.T.N.; Poole, P.S.; Sánchez-Cañizares, C. Rhizobial Chemotaxis and Motility Systems at Work in the Soil. Front. Plant Sci. 2021, 12, 725338. [Google Scholar] [CrossRef] [PubMed]
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.V.; Willett, D.S. The Effects of Biostimulants on Induced Plant Defense. Front. Agron. 2021, 3, 630596. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Ziyu, Z.; Shujun, Y.; Hao, L.; Noman, A.; Zhang, S.-H. Cellulose Degradation Microorganisms and Environmental-Friendly Solution to the Agricultural Waste Management. In Beneficial Microorganisms in Agriculture; Prasad, R., Zhang, S.-H., Eds.; Springer Nature: Singapore, 2022; pp. 307–328. [Google Scholar]
- Olanrewaju, O.S.; Babalola, O.O. The rhizosphere microbial complex in plant health: A review of interaction dynamics. J. Integr. Agric. 2022, 21, 2168–2182. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Sinha, D.; Tandon, P. An Overview of Nitrogen, Phosphorus and Potassium: Key Players of Nutrition Process in Plants. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Springer: Singapore, 2020; pp. 85–117. [Google Scholar]
- López-Arredondo, D.L.; Sánchez-Calderón, L.; Yong-Villalobos, L. Chapter 1—Molecular and genetic basis of plant macronutrient use efficiency: Concepts, opportunities, and challenges. In Plant Macronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Tran, L.-S.P., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–29. [Google Scholar]
- Singh, S.K.; Wu, X.; Shao, C.; Zhang, H. Microbial enhancement of plant nutrient acquisition. Stress Biol. 2022, 2, 3. [Google Scholar] [CrossRef]
- Cao, T.; Luo, Y.; Shi, M.; Tian, X.; Kuzyakov, Y. Microbial interactions for nutrient acquisition in soil: Miners, scavengers, and carriers. Soil Biol. Biochem. 2024, 188, 109215. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 147–203. [Google Scholar]
- Hua, F.; Wang, H.Q. Uptake and trans-membrane transport of petroleum hydrocarbons by microorganisms. Biotechnol. Biotechnol. Equip. 2014, 28, 165–175. [Google Scholar] [CrossRef]
- Singh, D.; Ghosh, P.; Kumar, J.; Kumar, A. Plant Growth-Promoting Rhizobacteria (PGPRs): Functions and Benefits. In Microbial Interventions in Agriculture and Environment: Volume 2: Rhizosphere, Microbiome and Agro-Ecology; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 205–227. [Google Scholar]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and Signaling Aspects Underpinning the Regulation of Plant Carbon Nitrogen Interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Gojon, A. Nitrogen nutrition in plants: Rapid progress and new challenges. J. Exp. Bot. 2017, 68, 2457–2462. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Dybowski, D.; Dzierzbicka-Glowacka, L.A.; Pietrzak, S.; Juszkowska, D.; Puszkarczuk, T. Estimation of nitrogen leaching load from agricultural fields in the Puck Commune with an interactive calculator. PeerJ 2020, 8, e8899. [Google Scholar] [CrossRef]
- Aeron, A.; Khare, E.; Jha, D.C.; Meena, V.; Abdel-Aziz, S.; Islam, T.; Kim, K.; Meena, S.; Pattanayak, A.; Hosahatti, R.; et al. Revisiting the plant growth-promoting rhizobacteria: Lessons from the past and objectives for the future. Arch. Microbiol. 2020, 202, 665–676. [Google Scholar] [CrossRef]
- Muratore, C.; Espen, L.; Prinsi, B. Nitrogen Uptake in Plants: The Plasma Membrane Root Transport Systems from a Physiological and Proteomic Perspective. Plants 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and Molecular Mechanisms Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 2018, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T. Symbiosis Between Rhizobia and Legumes. In Ecology and Evolution of Rhizobia: Principles and Applications; Wang, E.T., Tian, C.F., Chen, W.F., Young, J.P.W., Chen, W.X., Eds.; Springer: Singapore, 2019; pp. 3–19. [Google Scholar]
- Hawkins, J.P.; Oresnik, I.J. The Rhizobium-Legume Symbiosis: Co-opting Successful Stress Management. Front. Plant Sci. 2021, 12, 796045. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- LILLO, C.; LEA, U.S.; RUOFF, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Liese, R.; Schulze, J.; Cabeza, R.A. Nitrate application or P deficiency induce a decline in Medicago truncatula N2-fixation by similar changes in the nodule transcriptome. Sci. Rep. 2017, 7, 46264. [Google Scholar] [CrossRef]
- Cui, S.; Inaba, S.; Suzaki, T.; Yoshida, S. Developing for nutrient uptake: Induced organogenesis in parasitic plants and root nodule symbiosis. Curr. Opin. Plant Biol. 2023, 76, 102473. [Google Scholar] [CrossRef]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular Dialog Between Parasitic Plants and Their Hosts. Annu. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Scheerer, U.; Trube, N.; Netzer, F.; Rennenberg, H.; Herschbach, C. ATP as Phosphorus and Nitrogen Source for Nutrient Uptake by Fagus sylvatica and Populus x canescens Roots. Front. Plant Sci. 2019, 10, 378. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Luyckx, L.; Correia, D.; Caneghem, J. Linking Phosphorus Extraction from Different Types of Biomass Incineration Ash to Ash Mineralogy, Ash Composition and Chemical Characteristics of Various Types of Extraction Liquids. Waste Biomass Valorization 2021, 12, 5235–5248. [Google Scholar] [CrossRef]
- Li, J.-t.; Lu, J.-L.; Wang, H.-Y.; Fang, Z.; Wang, X.-J.; Feng, S.-W.; Wang, Z.; Yuan, T.; Cheung, S.-C.; Ou, S.-N.; et al. A comprehensive synthesis unveils the mysteries of phosphate-solubilizing microbes. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2771–2793. [Google Scholar] [CrossRef] [PubMed]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Olivares, F.L.; Canellas, L.P.; Smith, D.S.; Paul Voroney, R. Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chem. Biol. Technol. Agric. 2023, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, K.; Hetrick, B.A.D.; Schwab, A.P. Mycorrhizal Mediation of Phosphorus Availability: Synthetic Iron Chelate Effects on Phosphorus Solubilization. Soil Sci. Soc. Am. J. 1989, 53, 1701–1706. [Google Scholar] [CrossRef]
- Djuuna, I.A.F.; Prabawardani, S.; Massora, M. Population distribution of phosphate-solubilizing microorganisms in agricultural soil. Microbes Environ. 2022, 37, ME21041. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Saheewala, H.; Sanadhaya, S.; Joshi, A.; Bhojiya, A.A.; Verma, A.K.; Mohanty, S.R. Chapter 11—Potassium solubilizing microorganisms as soil health engineers: An insight into molecular mechanism. In Rhizosphere Engineering; Dubey, R.C., Kumar, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 199–214. [Google Scholar]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.; Bano, A. Mechanism of Potassium Solubilization in the Agricultural Soils by the Help of Soil Microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 137–147. [Google Scholar] [CrossRef]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The use of soil microbial potassium solubilizers in potassium nutrient availability in soil and its dynamics. Ann. Microbiol. 2022, 72, 45. [Google Scholar] [CrossRef]
- Askegaard, M.; Hansen, H.; Schjoerring, J. A cation exchange resin method for measuring long-term potassium release rates from soil. Plant Soil 2005, 271, 63–74. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of potassium solubilizing bacteria in improved potassium assimilation and cytosolic K+/Na+ ratio in rice (Oryza sativa L.) under saline-sodic conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Bahadur, D.; Maurya, R.; Roy, P.; Kumar, A. Potassium-Solubilizing Bacteria (KSB): A Microbial Tool for K-Solubility, Cycling, and Availability to Plants. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability; Springer: Singapore, 2019; pp. 257–265. [Google Scholar]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F.; et al. A critical review of mineral-microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, nwac128. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Singh, R.L. Microbes and Environment. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, B.N.; Dwivedi, P.; Rajawat, M.V.S. Interference of Climate Change on Plant-Microbe Interaction: Present and Future Prospects. Front. Agron. 2022, 3. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Araneda, N.; Valdebenito, E.; Hansen, F.; Nuti, M. Microbial Community in the Composting Process and Its Positive Impact on the Soil Biota in Sustainable Agriculture. Agronomy 2023, 13, 542. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, X.; Li, J.; Chu, L.; Wang, F.; Shan, H.; Hu, F. Microbial communities and nitrogen cycling in Litopenaeus vannamei and Mercenaria mercenaria polyculture ponds. Aquac. Rep. 2023, 33, 101769. [Google Scholar] [CrossRef]
- Hayatsu, M.; Katsuyama, C.; Tago, K. Overview of recent researches on nitrifying microorganisms in soil. Soil Sci. Plant Nutr. 2021, 67, 619–632. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.; Yang, J.; Lv, Z.; Song, Z.; Han, H. Soybean rhizosphere microorganisms alleviate Mo nanomaterials induced stress by improving soil microbial community structure. Chemosphere 2022, 310, 136784. [Google Scholar] [CrossRef] [PubMed]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-i.; Teaumroong, N. Co-Inoculation of Bacillus velezensis Strain S141 and Bradyrhizobium Strains Promotes Nodule Growth and Nitrogen Fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen-fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Cai, Z.; Guo, L.; Chen, X.; Chen, X.; Liu, J.; Feng, M.; Qiu, Y.; Zhang, Y.; et al. A Biocontrol Strain of Pseudomonas aeruginosa CQ-40 Promote Growth and Control Botrytis cinerea in Tomato. Pathogens 2021, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- González, A.H.; Morales Londoño, D.; Pille da Silva, E.; Nascimento, F.X.I.; de Souza, L.F.; da Silva, B.G.; Canei, A.D.; de Armas, R.D.; Giachini, A.J.; Soares, C.R.F.S. Bradyrhizobium and Pseudomonas strains obtained from coal-mining areas nodulate and promote the growth of Calopogonium muconoides plants used in the reclamation of degraded areas. J. Appl. Microbiol. 2019, 126, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Sengupta, C. Isolation of Cadmium and Lead Tolerant Plant Growth Promoting Rhizobacteria: Lysinibacillus varians and Pseudomonas putida from Indian Agricultural Soil. Soil Sediment Contam. Int. J. 2019, 28, 601–629. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Soe, K.M.; Moe, K.; Yamakawa, T. Effects of Biofertilizer Produced from Bradyrhizobium and Streptomyces griseoflavus on Plant Growth, Nodulation, Nitrogen Fixation, Nutrient Uptake, and Seed Yield of Mung Bean, Cowpea, and Soybean. Agronomy 2019, 9, 77. [Google Scholar] [CrossRef]
- Tchakounté, G.V.T.; Berger, B.; Patz, S.; Becker, M.; Fankem, H.; Taffouo, V.D.; Ruppel, S. Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses. Microorganisms 2020, 8, 1844. [Google Scholar] [CrossRef] [PubMed]
- Lisek, J.; Sas-Paszt, L.; Mika, A.; Lisek, A. The Response of Weeds and Apple Trees to Beneficial Soil Microorganisms and Mineral Fertilizers Applied in Orchards. Agronomy 2022, 12, 2882. [Google Scholar] [CrossRef]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Kuyper, T.W.; Boonlue, S. Interaction between Phosphate Solubilizing Bacteria and Arbuscular Mycorrhizal Fungi on Growth Promotion and Tuber Inulin Content of Helianthus tuberosus L. Sci. Rep. 2020, 10, 4916. [Google Scholar] [CrossRef]
- Emami, S.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Motasharezadeh, B.; Sarmadian, F. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere 2020, 14, 100196. [Google Scholar] [CrossRef]
- Shah, C.; Mali, H.; Mesara, S.; Dhameliya, H.; Subramanian, R.B. Combined inoculation of phosphate solubilizing bacteria with mycorrhizae to alleviate the phosphate deficiency in Banana. Biologia 2022, 77, 2657–2666. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Dong, Y.; Liu, H.; Peng, J.; Hu, Y.; Sun, Y. Profiling of Plant Growth-Promoting Metabolites by Phosphate-Solubilizing Bacteria in Maize Rhizosphere. Plants 2021, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Shankhdhar, D.; Shankhdhar, S.C. Synergistic Impact of Phosphate Solubilizing Bacteria and Phosphorus Rates on Growth, Antioxidative Defense System, and Yield Characteristics of Upland Rice (Oryza sativa L.). J. Plant Growth Regul. 2022, 41, 2449–2461. [Google Scholar] [CrossRef]
- Chen, Y.-h.; Yang, X.-z.; Li, Z.; An, X.-h.; Ma, R.-p.; Li, Y.-q.; Cheng, C.-g. Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. J. Integr. Agric. 2020, 19, 2458–2469. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Pirdashti, H.; Rahimian, H.; Nematzadeh, G.; Ghajar Sepanlou, M. The role of potassium solubilizing bacteria (KSB) inoculations on grain yield, dry matter remobilization and translocation in rice (Oryza sativa L.). J. Plant Nutr. 2019, 42, 1165–1179. [Google Scholar] [CrossRef]
- Majeed, A.; Farooq, M.; Naveed, M.; Hussain, M. Combined Application of Inorganic and Organic Phosphorous with Inoculation of Phosphorus Solubilizing Bacteria Improved Productivity, Grain Quality and Net Economic Returns of Pearl Millet (Pennisetum glaucum [L.] R. Br.). Agronomy 2022, 12, 2412. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Effects of microbial inoculants on phosphorus and potassium availability, bacterial community composition, and chili pepper growth in a calcareous soil: A greenhouse study. J. Soils Sediments 2019, 19, 3597–3607. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.N.; Suman, A. Potential applications of mineral solubilizing rhizospheric and nitrogen fixing endophytic bacteria as microbial consortium for the growth promotion of chilli (Capsicum annum L.). Biologia 2022, 77, 2933–2943. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Tiwari, M. Plant Defense Priming: A New Tool for Sustainable Global Food Security. In Agriculture and Innovations and Sustainability; Agrobios Research: Rajasthan, India, 2021; pp. 133–153. [Google Scholar]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.; Singh, R.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and Mechanism of Action Against Phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- David, L.; Harmon, A.C.; Chen, S. Plant immune responses—From guard cells and local responses to systemic defense against bacterial pathogens. Plant Signal. Behav. 2019, 14, e1588667. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, J.; Xiang, J.; Wang, J.; Yin, K.; Liu, Q. Isolation and identification of a novel protein elicitor from a Bacillus subtilis strain BU412. AMB Express 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Thepbandit, W.; Srisuwan, A.; Siriwong, S.; Nawong, S.; Athinuwat, D. Bacillus vallismortis TU-Orga21 blocks rice blast through both direct effect and stimulation of plant defense. Front. Plant Sci. 2023, 14, 1103487. [Google Scholar] [CrossRef] [PubMed]
- Nilmat, A.; Thepbandit, W.; Chuaboon, W.; Athinuwat, D. Pseudomonas fluorescens SP007S Formulations in Controlling Soft Rot Disease and Promoting Growth in Kale. Agronomy 2023, 13, 1856. [Google Scholar] [CrossRef]
- Choorin, M.; Athinuwat, D.; Kasem, S.; Thowthampitak, J.; Prathuangwong, S. CarAB in Pseudomonas fluorescens SP007s reduces symptoms on soybean caused by Xanthomonas axonopodis pv. glycines that links to the role of multiple genes. Thai J. Agric. Sci. 2017, 50, 166–197. [Google Scholar]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef]
- Vlot, C.; Sales, J.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2020, 229, 1234–1250. [Google Scholar] [CrossRef]
- Checker, V.G.; Kushwaha, H.R.; Kumari, P.; Yadav, S. Role of Phytohormones in Plant Defense: Signaling and Cross Talk. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 159–184. [Google Scholar]
- De Vleesschauwer, D.; Höfte, M. Chapter 6 Rhizobacteria-Induced Systemic Resistance. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 51, pp. 223–281. [Google Scholar]
- Métraux, J.P. Systemic Acquired Resistance. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 627–629. [Google Scholar]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- van Wees, S.C.; de Swart, E.A.; van Pelt, J.A.; van Loon, L.C.; Pieterse, C.M. Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2000, 97, 8711–8716. [Google Scholar] [CrossRef] [PubMed]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef]
- Kour, D.; Negi, R.; Khan, S.S.; Kumar, S.; Kaur, S.; Kaur, T.; Sharma, B.; Dasila, H.; Kour, H.; Ramniwas, S.; et al. Microbes mediated induced systemic response in plants: A review. Plant Stress 2024, 11, 100334. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Almeida, O.A.C.; de Araujo, N.O.; Dias, B.H.S.; de Sant’Anna Freitas, C.; Coerini, L.F.; Ryu, C.-M.; de Castro Oliveira, J.V. The power of the smallest: The inhibitory activity of microbial volatile organic compounds against phytopathogens. Front. Microbiol. 2023, 13, 951130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, S.; Yang, Y.; Zhang, J.; Zhao, D.; Pan, Y.; Fan, S.; Yang, Z.; Zhu, J. Antifungal Effects of Volatiles Produced by Bacillus subtilis Against Alternaria solani in Potato. Front. Microbiol. 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.A.C.; de Araujo, N.O.; Mulato, A.T.N.; Persinoti, G.F.; Sforça, M.L.; Calderan-Rodrigues, M.J.; Oliveira, J.V.d.C. Bacterial volatile organic compounds (VOCs) promote growth and induce metabolic changes in rice. Front. Plant Sci. 2023, 13, 1056082. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Rana, A.; Dhaka, R.K.; Singh, A.P.; Chahar, M.; Singh, S.; Nain, L.; Singh, K.P.; Minz, D. Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: Current understanding and future scope. Biotechnol. Adv. 2023, 63, 108078. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Aseel, D.G.; Király, L.; Künstler, A.; Moawad, H.; Al-Askar, A.A. Induction of Systemic Resistance to Tobacco mosaic virus in Tomato through Foliar Application of Bacillus amyloliquefaciens Strain TBorg1 Culture Filtrate. Viruses 2022, 14, 1830. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Ren, J.J.; Zhao, H.J.; Wang, X.L.; Wang, T.H.; Jin, S.D.; Wang, Z.H.; Li, C.Y.; Liu, A.R.; Lin, X.M.; et al. Trichoderma harzianum Improves Defense Against Fusarium oxysporum by Regulating ROS and RNS Metabolism, Redox Balance, and Energy Flow in Cucumber Roots. Phytopathology 2019, 109, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Meshram, S.; Patel, J.S.; Yadav, S.K.; Kumar, G.; Singh, D.P.; Singh, H.B.; Sarma, B.K. Trichoderma mediate early and enhanced lignifications in chickpea during Fusarium oxysporum f. sp. ciceris infection. J. Basic Microbiol. 2019, 59, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chatterjee, A.; Das, S. Synergistic consortium of beneficial microorganisms in rice rhizosphere promotes host defense to blight-causing Xanthomonas oryzae pv. oryzae. Planta 2020, 252, 106. [Google Scholar] [CrossRef]
- Ashajyothi, M.; Kumar, A.; Sheoran, N.; Ganesan, P.; Gogoi, R.; Subbaiyan, G.K.; Bhattacharya, R. Black pepper (Piper nigrum L.) associated endophytic Pseudomonas putida BP25 alters root phenotype and induces defense in rice (Oryza sativa L.) against blast disease incited by Magnaporthe oryzae. Biol. Control 2020, 143, 104181. [Google Scholar] [CrossRef]
- Lazazzara, V.; Vicelli, B.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense-related processes against downy mildew. Physiol. Plant. 2021, 172, 1950–1965. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma asperellum biocontrol activity and induction of systemic defenses against Sclerotium cepivorum in onion plants under tropical climate conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- Pimentel, M.F.; Arnão, E.; Warner, A.J.; Subedi, A.; Rocha, L.F.; Srour, A.; Bond, J.P.; Fakhoury, A.M. Trichoderma Isolates Inhibit Fusarium virguliforme Growth, Reduce Root Rot, and Induce Defense-Related Genes on Soybean Seedlings. Plant Dis. 2020, 104, 1949–1959. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-i.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Mol. Plant Pathol. 2021, 115, 101663. [Google Scholar] [CrossRef]
- Elías, J.M.; Ramírez-Mata, A.; Albornóz, P.L.; Baca, B.E.; Díaz-Ricci, J.C.; Pedraza, R.O. The Polar Flagellin of Azospirillum brasilense REC3 Induces a Defense Response in Strawberry Plants Against the Fungus Macrophomina phaseolina. J. Plant Growth Regul. 2022, 41, 2992–3008. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.; Kögel-Knabner, I.; Rillig, M. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Molefe, R.R.; Amoo, A.E.; Babalola, O.O. Communication between plant roots and the soil microbiome; involvement in plant growth and development. Symbiosis 2023, 90, 231–239. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere Signaling: Insights into Plant-Rhizomicrobiome Interactions for Sustainable Agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Ofaim, S.; Ofek-Lalzar, M.; Sela, N.; Jinag, J.; Kashi, Y.; Minz, D.; Freilich, S. Analysis of Microbial Functions in the Rhizosphere Using a Metabolic-Network Based Framework for Metagenomics Interpretation. Front. Microbiol. 2017, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Manresa-Grao, M.; Sanchez-Bel, P.; Gamir, J.; Pastor, V.; Flors, V. Chapter 6—The chemical dialogue between plants and beneficial arbuscular fungi in disease resistance. In The Chemical Dialogue Between Plants and Beneficial Microorganisms; Sharma, V., Salwan, R., Moliszewska, E., Ruano-Rosa, D., Jędryczka, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 99–115. [Google Scholar]
- Schikora, A.; Schenk, S.T.; Hartmann, A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of theN-acyl homoserine lactone group. Plant Mol. Biol. 2016, 90, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef] [PubMed]
- Saccá, M.L.; Barra Caracciolo, A.; Di Lenola, M.; Grenni, P. Ecosystem Services Provided By Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–24. [Google Scholar]
- Majeed, A.; Muhammad, Z.; Ullah, R.; Ullah, K.; Ali, H.; Inayat, N. Chapter 18—Plant growth-promoting rhizobacteria as bioremediators of polluted agricultural soils: Challenges and prospects. In Hazardous and Trace Materials in Soil and Plants; Naeem, M., Aftab, T., Ali Ansari, A., Gill, S.S., Macovei, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 265–275. [Google Scholar]
- Shrestha, A.; Elhady, A.; Adss, S.; Wehner, G.; Böttcher, C.; Heuer, H.; Ordon, F.; Schikora, A. Genetic Differences in Barley Govern the Responsiveness to N-Acyl Homoserine Lactone. Phytobiomes J. 2019, 3, 191–202. [Google Scholar] [CrossRef]
- Bano, A.; Qadri, T.A.; Mahnoor; Khan, N. Bioactive metabolites of plants and microbes and their role in agricultural sustainability and mitigation of plant stress. S. Afr. J. Bot. 2023, 159, 98–109. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Сhеbоtаr, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Pham, T.-M.; Bui, X.D.; Trang, L.V.K.; Le, T.-M.; Nguyen, M.L.; Trinh, D.-M.; Phuong, N.T.D.; Khoo, K.S.; Chew, K.W.; Show, P.L. Isolation of indole-3-acetic acid-producing Azospirillum brasilense from Vietnamese wet rice: Co-immobilization of isolate and microalgae as a sustainable biorefinery. J. Biotechnol. 2022, 349, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kodera, S.M.; Das, P.; Gilbert, J.A.; Lutz, H.L. Conceptual strategies for characterizing interactions in microbial communities. iScience 2022, 25, 103775. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N. Friends or Foes-Microbial Interactions in Nature. Biology 2021, 10, 496. [Google Scholar] [CrossRef]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a promising biological control agents for plant pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef]
- Le, K.D.; Yu, N.H.; Park, A.R.; Park, D.J.; Kim, C.J.; Kim, J.C. Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases. Microorganisms 2022, 10, 791. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.E.L.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Bacteria of Soil: Designing of Consortia Beneficial for Crop Production. Microorganisms 2023, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Themaat, E.V.L.v.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Sikder, M.M.; Vestergård, M.; Kyndt, T.; Topalović, O.; Kudjordjie, E.N.; Nicolaisen, M. Genetic disruption of Arabidopsis secondary metabolite synthesis leads to microbiome-mediated modulation of nematode invasion. ISME J. 2022, 16, 2230–2241. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Rio, T.G.d.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- Almario, J.; Mahmoudi, M.; Kroll, S.; Agler, M.; Placzek, A.; Mari, A.; Kemen, E. The Leaf Microbiome of Arabidopsis Displays Reproducible Dynamics and Patterns throughout the Growing Season. mBio 2022, 13, e02825-02821. [Google Scholar] [CrossRef] [PubMed]
- Kudjordjie, E.N.; Sapkota, R.; Nicolaisen, M. Arabidopsis assemble distinct root-associated microbiomes through the synthesis of an array of defense metabolites. PLoS ONE 2021, 16, e0259171. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Frachon, L.; Bartoli, C. The Genetic Architecture of Adaptation to Leaf and Root Bacterial Microbiota in Arabidopsis thaliana. Mol. Biol. Evol. 2023, 40, msad093. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; He, D.; Shi, T.; Zhang, Y.; Liu, S.; Yan, X.; Huang, L. Responses of plant immune system and rhizosphere soil microbiome to the elicitor BAR11 in Arabidopsis thaliana. Sci. Total Environ. 2024, 914, 169920. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, J.; Zhang, Y.; Naz, M.; Afzal, M.R.; Du, D.; Dai, Z. Omics Approaches in Invasion Biology: Understanding Mechanisms and Impacts on Ecological Health. Plants 2023, 12, 1860. [Google Scholar] [CrossRef]
- Swarupa, V.; Pavitra, K.; Shivashankara, K.S.; Ravishankar, K.V. Omics-Driven Approaches in Plant–Microbe Interaction. In Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 1: Research Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 61–84. [Google Scholar]
- Tian, B.; Cao, Y.; Zhang, K.-Q. Metagenomic insights into communities, functions of endophytes, and their associates with infection by root-knot nematode, Meloidogyne incognita, in tomato roots. Sci. Rep. 2015, 5, 17087. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef]
- Köberl, M.; Wagner, P.; Müller, H.; Matzer, R.; Unterfrauner, H.; Cernava, T.; Berg, G. Unraveling the Complexity of Soil Microbiomes in a Large-Scale Study Subjected to Different Agricultural Management in Styria. Front. Microbiol. 2020, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci. Rep. 2017, 7, 40093. [Google Scholar] [CrossRef]
- Dlamini, S.; Akanmu, A.; Fadiji, A.; Babalola, O. Maize rhizosphere modulates the microbiome diversity and community structure to enhance plant health. Saudi J. Biol. Sci. 2022, 30, 103499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thepbandit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. https://doi.org/10.3390/microorganisms12030558
Thepbandit W, Athinuwat D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms. 2024; 12(3):558. https://doi.org/10.3390/microorganisms12030558
Chicago/Turabian StyleThepbandit, Wannaporn, and Dusit Athinuwat. 2024. "Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense" Microorganisms 12, no. 3: 558. https://doi.org/10.3390/microorganisms12030558
APA StyleThepbandit, W., & Athinuwat, D. (2024). Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms, 12(3), 558. https://doi.org/10.3390/microorganisms12030558






