Effects of Cellulase and Xylanase on Fermentation Characteristics, Chemical Composition and Bacterial Community of the Mixed Silage of King Grass and Rice Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Chemical Composition, Fermentation Parameter and Enzyme Activity Analysis
2.3. Bacterial Community Analysis
2.4. Statistical Analysis
3. Results
3.1. Chemical Composition of the Raw Materials for Silage Production
3.2. Fermentation Quality of Mixed Silage of King Grass and Rice Straw
3.3. Chemical Composition of Mixed Silage of King Grass and Rice Straw
3.4. Enzyme Activity of Mixed Silage of King Grass and Rice Straw
3.5. Bacterial Community of Mixed Silage of King Grass and Rice Straw
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Xia, B.; Meng, Y.; Yang, Z.; Pan, L.; Zhou, M.; Zhang, X. Transcriptome Analysis to Shed Light on the Molecular Mechanisms of Early Responses to Cadmium in Roots and Leaves of King Grass (Pennisetum americanum × P. purpureum). Int. J. Mol. Sci. 2019, 20, 2532. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zi, X.; Zhou, H.; Hou, G.; Cai, Y. Effects of sucrose, glucose, molasses and cellulase on fermentation quality and in vitro gas production of king grass silage. Anim. Feed. Sci. Technol. 2014, 197, 206–212. [Google Scholar] [CrossRef]
- Mcdonald, P.; Henderson, A.R.; Heron, S. The Biochemistry of Silage; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Zi, X.; Li, M.; Chen, Y.; Lv, R.; Zhou, H.; Tang, J. Effects of Citric Acid and Lactobacillus plantarum on Silage Quality and Bacterial Diversity of King Grass Silage. Front. Microbiol. 2021, 12, 631096. [Google Scholar] [CrossRef] [PubMed]
- Kamphayae, S.; Kumagai, H.; Bureenok, S.; Narmseelee, R.; Butcha, P. Effects of graded levels of liquid brewer’s yeast on chemical composition and fermentation quality in cassava pulp and rice straw-based total mixed ration silage. Anim. Sci. J. 2017, 88, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Wang, Q.; Cao, X.; Zhang, Z. Effects of fatty acid salts on fermentation characteristics, bacterial diversity and aerobic stability of mixed silage prepared with alfalfa, rice straw and wheat bran. J. Sci. Food Agric. 2022, 102, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Lactobacillus plantarum and molasses alter dynamic chemical composition, microbial community, and aerobic stability of mixed (amaranth and rice straw) silage. J. Sci. Food Agric. 2021, 101, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, J.; Liu, Z.; Zhang, G.; Zhang, Y. Fermentation Quality and Microbial Community of Corn Stover or Rice Straw Silage Mixed with Soybean Curd Residue. Animals 2022, 12, 919. [Google Scholar] [CrossRef]
- Lynch, J.P.; Baah, J.; Beauchemin, K.A. Conservation, fiber digestibility, and nutritive value of corn harvested at 2 cutting heights and ensiled with fibrolytic enzymes, either alone or with a ferulic acid esterase-producing inoculant. J. Dairy Sci. 2015, 98, 1214–1224. [Google Scholar] [CrossRef]
- Xian, Z.; Wu, J.; Deng, M.; Wang, M.; Tian, H.; Liu, D.; Li, Y.; Liu, G.; Sun, B.; Guo, Y. Effects of Cellulase and Lactiplantibacillus plantarum on the Fermentation Parameters, Nutrients, and Bacterial Community in Cassia alata Silage. Front. Microbiol. 2022, 13, 926065. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Liu, J.; Yang, F.; Zhu, W.; Yuan, X.; Hu, Y.; Cui, Z.; Wang, X. Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresour. Technol. 2017, 241, 349–359. [Google Scholar] [CrossRef]
- Zhou, G.; Liang, X.; He, X.; Li, J.; Tian, G.; Liu, Y.; Wang, X.; Chen, Y.; Yang, Y. Compound enzyme preparation supplementation improves the production performance of goats by regulating rumen microbiota. Appl. Microbiol. Biot. 2023, 107, 7287–7299. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Rockville, MD, USA, 1990; pp. 152–169. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.P. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Ke, W.C.; Ding, W.R.; Xu, D.M.; Ding, L.M.; Zhang, P.; Li, F.D.; Guo, X.S. Effects of addition of malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J. Dairy Sci. 2017, 100, 8958–8966. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Pian, R.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of citric acid on fermentation characteristics and bacterial diversity of Amomum villosum silage. Bioresour. Technol. 2020, 307, 123290. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.V.; Reat, V.; Finney, J.; Ferrand, M.; Smith, J.C.; Daniel, R.M. Enzyme activity and dynamics: Xylanase activity in the absence of fast anharmonic dynamics. Biochem. J. 2000, 346 Pt 2, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Nordmark, T.S.; Bakalinsky, A.; Penner, M.H. Measuring cellulase activity: Application of the filter paper assay to low-activity enzyme preparations. Appl. Biochem. Biotechnol. 2007, 137–140, 131–139. [Google Scholar] [CrossRef]
- Kung, L.J.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Eun, J.S.; Beauchemin, K.A. Enhancing in vitro degradation of alfalfa hay and corn silage using feed enzymes. J. Dairy Sci. 2007, 90, 2839–2851. [Google Scholar] [CrossRef]
- Liao, C.; Tang, X.; Li, M.; Lu, G.; Huang, X.; Li, L.; Zhang, M.; Xie, Y.; Chen, C.; Li, P. Effect of lactic acid bacteria, yeast, and their mixture on the chemical composition, fermentation quality, and bacterial community of cellulase-treated Pennisetum sinese silage. Front. Microbiol. 2022, 13, 1047072. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Ma, G.; Jiang, X.; Yang, J.; Lv, J.; Zhang, Y. Cellulase Interacts with Lactic Acid Bacteria to Affect Fermentation Quality, Microbial Community, and Ruminal Degradability in Mixed Silage of Soybean Residue and Corn Stover. Animals 2021, 11, 334. [Google Scholar] [CrossRef]
- Blajman, J.E.; Paez, R.B.; Vinderola, C.G.; Lingua, M.S.; Signorini, M.L. A meta-analysis on the effectiveness of homofermentative and heterofermentative lactic acid bacteria for corn silage. J. Appl. Microbiol. 2018, 125, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, F.; Wang, Y.; Fan, X.; Feng, C.; Wang, Y. Dynamics of Fermentation Parameters and Bacterial Community in High-Moisture Alfalfa Silage with or without Lactic Acid Bacteria. Microorganisms 2021, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Yuan, X.; Li, L.; Wen, A.; Shao, T. Effects of fibrolytic enzymes, molasses and lactic acid bacteria on fermentation quality of mixed silage of corn and hulless–barely straw in the Tibetan Plateau. Grassl. Sci. 2014, 60, 240–246. [Google Scholar] [CrossRef]
- Hashemzadeh-Cigari, F.; Khorvash, M.; Ghorbani, G.R.; Ghasemi, E.; Taghizadeh, A.; Kargar, S.; Yang, W.Z. Interactive effects of molasses by homofermentative and heterofermentative inoculants on fermentation quality, nitrogen fractionation, nutritive value and aerobic stability of wilted alfalfa (Medicago sativa L.) silage. J. Anim. Physiol. Anim. Nutr. 2014, 98, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Wccds, A.; Wgdn, A.; Alrm, A.; Dkas, A.; Wjcss, A.; Avss, B.; Gscs, B. Nutritive value, total losses of dry matter and aerobic stability of the silage from three varieties of sugarcane treated with commercial microbial additives. Anim. Feed Sci. Technol. 2015, 204, 1–8. [Google Scholar] [CrossRef]
- Fang, D.; Dong, Z.; Wang, D.; Li, B.; Shi, P.; Yan, J.; Zhuang, D.; Shao, T.; Wang, W.; Gu, M. Evaluating the fermentation quality and bacterial community of high-moisture whole-plant quinoa silage ensiled with different additives. J. Appl. Microbiol. 2022, 132, 3578–3589. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Ensiling as pretreatment of rice straw: The effect of hemicellulase and Lactobacillus plantarum on hemicellulose degradation and cellulose conversion. Bioresour. Technol. 2018, 266, 158–165. [Google Scholar] [CrossRef]
- Guo, J.; Xie, Y.; Yu, Z.; Meng, G.; Wu, Z. Effect of Lactobacillus plantarum expressing multifunctional glycoside hydrolases on the characteristics of alfalfa silage. Appl. Microbiol. Biotechnol. 2019, 103, 7983–7995. [Google Scholar] [CrossRef]
- Segato, F.; Damasio, A.R.; de Lucas, R.C.; Squina, F.M.; Prade, R.A. Genomics review of holocellulose deconstruction by aspergilli. Microbiol. Mol. Biol. Rev. 2014, 78, 588–613. [Google Scholar] [CrossRef]
- Decker, S.R.; Adney, W.S.; Jennings, E.; Vinzant, T.B.; Himmel, M.E. Automated filter paper assay for determination of cellulase activity. Appl. Biochem. Biotechnol. 2003, 105–108, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Park, J.Y.; Jeong, H.R.; Heo, H.J.; Han, N.S.; Kim, J.H. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 2012, 18, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from An inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.E.; Borneman, W.S. Role of rumen fungi in fiber degradation. J. Dairy Sci. 1990, 73, 3023–3032. [Google Scholar] [CrossRef]
- Joblin, K.N.; Campbell, G.P.; Richardson, A.J.; Stewart, C.S. Fermentation of barley straw by anaerobic rumen bacteria and fungi in axenic culture and in co-culture with methanogens. Lett. Appl. Microbiol. 2010, 9, 195–197. [Google Scholar] [CrossRef]
- Williams, A.G.; Orpin, C.G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can. J. Microbiol. 1987, 33, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, C.; Wang, Y.; Zhang, W.; He, L. Effect of tea polyphenols on the fermentation quality, protein preservation, antioxidant capacity and bacterial community of stylo silage. Front. Microbiol. 2022, 13, 993750. [Google Scholar] [CrossRef]
- Romero, J.J.; Zhao, Y.; Balseca-Paredes, M.A.; Tiezzi, F.; Gutierrez-Rodriguez, E.; Castillo, M.S. Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. J. Dairy Sci. 2017, 100, 1812–1828. [Google Scholar] [CrossRef]
- Yuan, X.J.; Dong, Z.H.; Li, J.F.; Shao, T. Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 2020, 75, 37–44. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Kampfer, P.; Glaeser, S.P. Prokaryotic taxonomy in the sequencing era--the polyphasic approach revisited. Environ. Microbiol. 2012, 14, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Fuhs, G.W.; Chen, M. Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microb. Ecol. 1975, 2, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, M.; Yang, J.; Chen, L.; Zi, X.; Zhou, H.; Tang, J. Exploring the effect of wilting on fermentation profiles and microbial community structure during ensiling and air exposure of king grass silage. Front. Microbiol. 2022, 13, 971426. [Google Scholar] [CrossRef] [PubMed]
- Puntillo, M.; Peralta, G.H.; Burgi, M.; Huber, P.; Gaggiotti, M.; Binetti, A.G.; Vinderola, G. Metaprofiling of the bacterial community in sorghum silages inoculated with lactic acid bacteria. J. Appl. Microbiol. 2022, 133, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, S.; Awasthi, M.K.; Wang, Y.; Xu, P. Exploring the bacterial community and fermentation characteristics during silage fermentation of abandoned fresh tea leaves. Chemosphere 2021, 283, 131234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zou, X.; Wu, S.; Wu, N.; Chen, X.; Zhou, W. Effects of Pyroligneous Acid on Diversity and Dynamics of Antibiotic Resistance Genes in Alfalfa Silage. Microbiol. Spectr. 2022, 10, e155422. [Google Scholar] [CrossRef] [PubMed]
- Junges, D.; Morais, G.; Spoto, M.; Santos, P.S.; Adesogan, A.T.; Nussio, L.G.; Daniel, J. Short communication: Influence of various proteolytic sources during fermentation of reconstituted corn grain silages. J. Dairy Sci. 2017, 100, 9048–9051. [Google Scholar] [CrossRef]
- Du, G.; Zhang, G.; Shi, J.; Zhang, J.; Ma, Z.; Liu, X.; Yuan, C.; Li, X.; Zhang, B. Keystone Taxa Lactiplantibacillus and Lacticaseibacillus Directly Improve the Ensiling Performance and Microflora Profile in Co-Ensiling Cabbage Byproduct and Rice Straw. Microorganisms 2021, 9, 1099. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wang, Z.; Bao, J.; Zhao, M.; Ge, G.; Jia, Y.; Du, S. Effects of Isolated LAB on Chemical Composition, Fermentation Quality and Bacterial Community of Stipa grandis Silage. Microorganisms 2022, 10, 2463. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, K.; Lin, Y.; Li, M.; Wang, X.; Yu, Q.; Sun, H.; Cheng, Q.; Xie, Y.; Wang, C.; et al. Effect of cellulase and lactic acid bacteria on the fermentation quality, carbohydrate conversion, and microbial community of ensiling oat with different moisture contents. Front. Microbiol. 2022, 13, 1013258. [Google Scholar] [CrossRef]
- Refat, B.; Christensen, D.A.; McKinnon, J.J.; Yang, W.; Beattie, A.D.; McAllister, T.A.; Eun, J.S.; Abdel-Rahman, G.A.; Yu, P. Effect of fibrolytic enzymes on lactational performance, feeding behavior, and digestibility in high-producing dairy cows fed a barley silage-based diet. J. Dairy Sci. 2018, 101, 7971–7979. [Google Scholar] [CrossRef]
- Alsersy, H.; Salem, A.Z.; Borhami, B.E.; Olivares, J.; Gado, H.M.; Mariezcurrena, M.D.; Yacuot, M.H.; Kholif, A.E.; El-Adawy, M.; Hernandez, S.R. Effect of Mediterranean saltbush (Atriplex halimus) ensilaging with two developed enzyme cocktails on feed intake, nutrient digestibility and ruminal fermentation in sheep. Anim. Sci. J. 2015, 86, 51–58. [Google Scholar] [CrossRef]
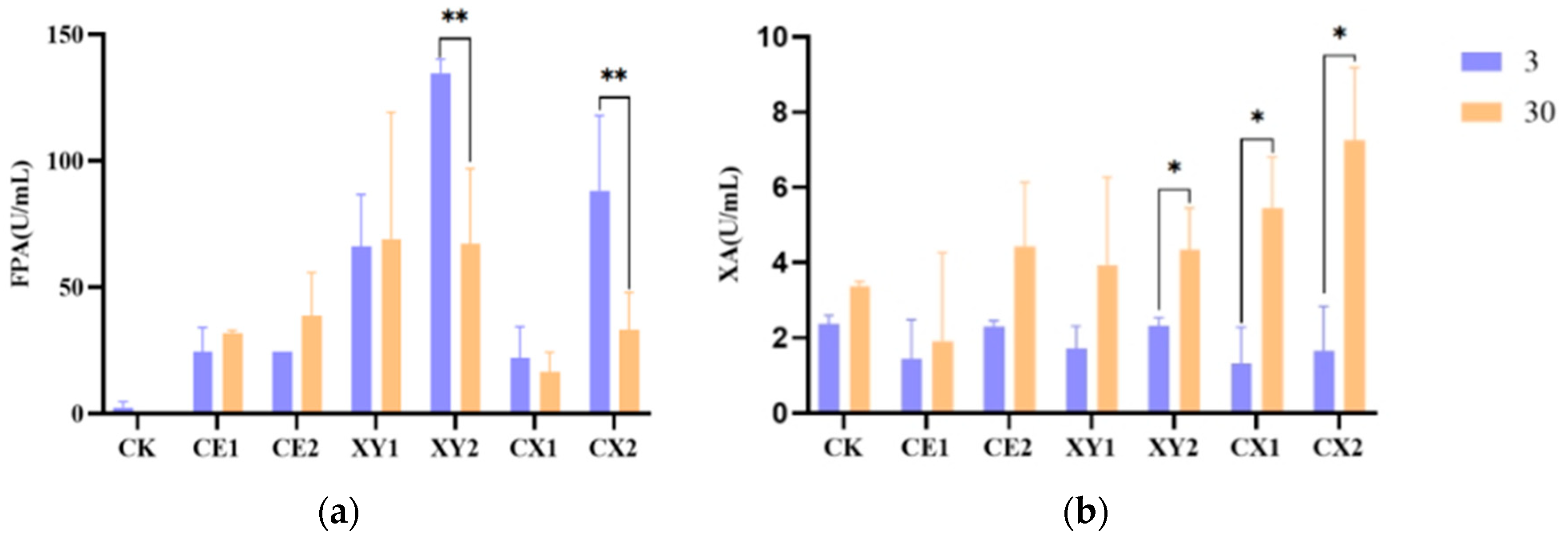
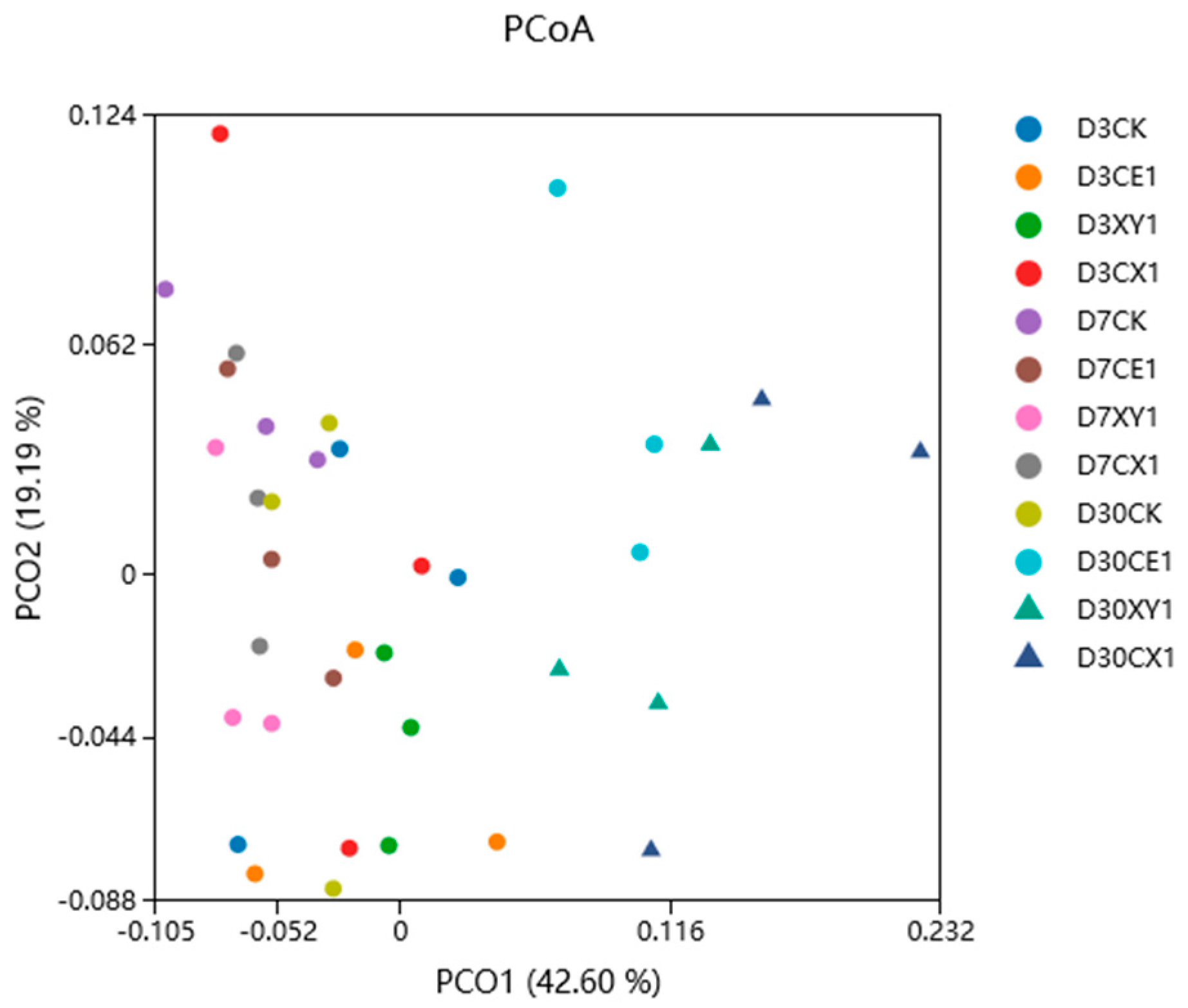
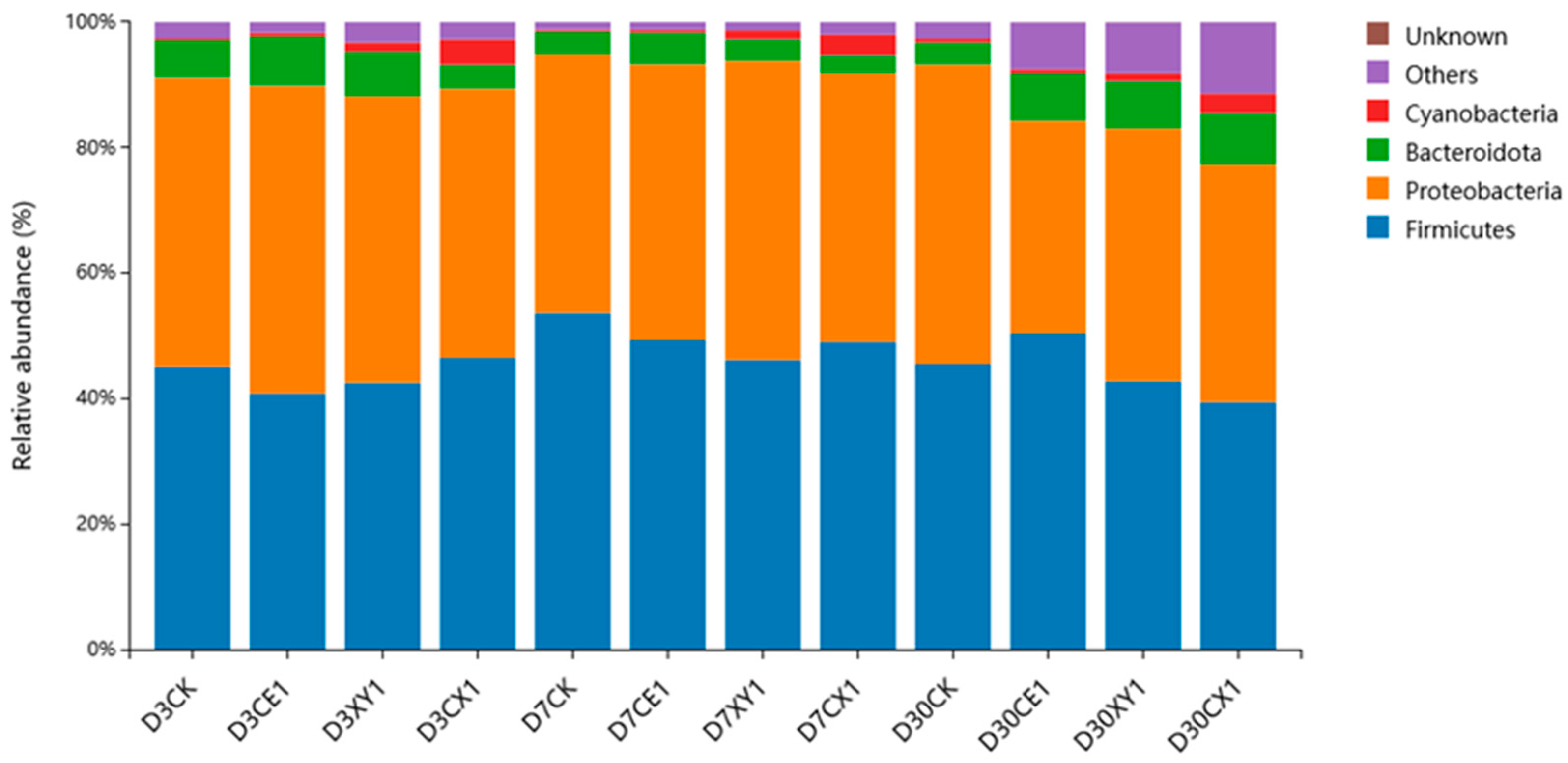
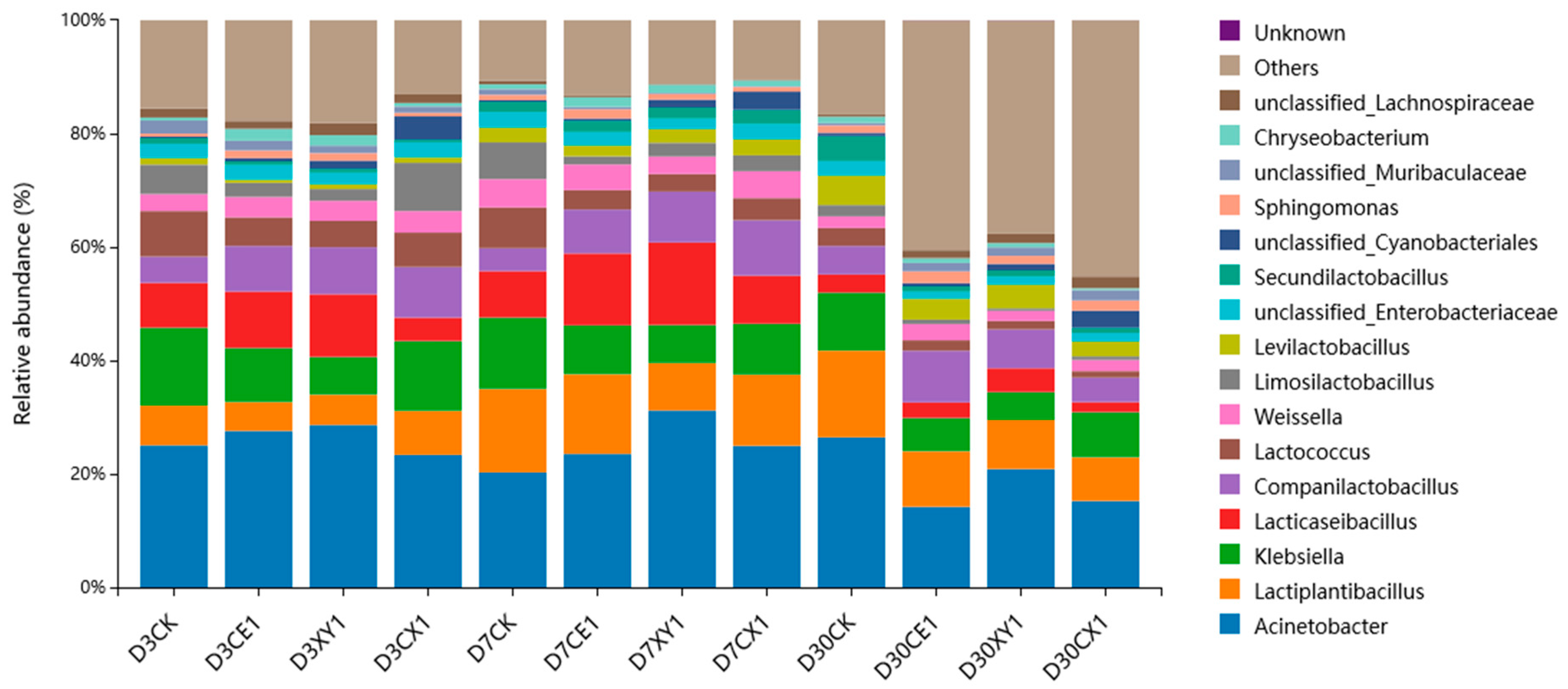
| Item | King Grass | Rice Straw |
|---|---|---|
| Dry matter (% Fresh matter) | 17.60 | 88.26 |
| Crude protein (% Dry matter) | 8.33 | 3.43 |
| Neutral detergent fiber (% Dry matter) | 70.69 | 72.30 |
| Acid detergent fiber (% Dry matter) | 35.99 | 39.33 |
| Acid detergent lignin (% Dry matter) | 4.62 | 5.08 |
| Item | Treatment | Ensiling Days | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 30 | D | T | D*T | |||
| pH | CK | 4.06 a | 4.04 a | 4.06 a | 0.026 | 0.14 | <0.01 | 0.16 |
| CE1 | 3.76 b | 3.74 b | 3.75 b | |||||
| CE2 | 3.65 Bcd | 3.72 Abc | 3.68 Bbc | |||||
| XY1 | 3.64 d | 3.65 cd | 3.65 bc | |||||
| XY2 | 3.69 bcd | 3.67 bcd | 3.62 d | |||||
| CX1 | 3.75 Abc | 3.65 Bcd | 3.70 Abc | |||||
| 3.72 Abcd | 3.63 Bd | 3.64 Bbc | ||||||
| LA (% DM) | CK | 3.88 b | 4.58 b | 5.08 | 1.208 | 0.30 | <0.01 | 0.97 |
| CE1 | 7.22 ab | 6.76 ab | 6.57 | |||||
| CE2 | 7.46 a | 6.28 ab | 8.00 | |||||
| XY1 | 7.33 ab | 8.82 a | 7.62 | |||||
| XY2 | 8.41 a | 8.36 ab | 9.59 | |||||
| CX1 | 5.35 ab | 6.96 ab | 7.80 | |||||
| CX2 | 5.86 Bab | 6.95 Abab | 7.98 A | |||||
| AA (% DM) | CK | 0.73 B | 0.71 B | 1.21 A | 0.181 | 0.04 | <0.01 | 0.98 |
| CE1 | 0.98 | 0.79 | 1.05 | |||||
| CE2 | 0.87 | 0.83 | 1.30 | |||||
| XY1 | 1.08 | 1.26 | 1.29 | |||||
| XY2 | 1.13 | 1.29 | 1.62 | |||||
| CX1 | 0.89 | 1.03 | 1.36 | |||||
| CX2 | 0.96 B | 0.96 B | 1.32 A | |||||
| NH3-N (% DM) | CK | 0.32 Ba | 0.32 Ba | 0.88 Aa | 0.036 | <0.01 | <0.01 | 0.03 |
| CE1 | 0.30 Bab | 0.30 Bab | 0.80 Aab | |||||
| CE2 | 0.22 Bc | 0.23 Bbc | 0.55 Ac | |||||
| XY1 | 0.22 Bc | 0.22 Bc | 0.70 Abc | |||||
| XY2 | 0.23 Bbc | 0.27 Babc | 0.66 Abc | |||||
| CX1 | 0.25 Babc | 0.26 Babc | 0.58 Ac | |||||
| CX2 | 0.23 Bbc | 0.20 Bc | 0.61 Ac | |||||
| Item | Treatment | Ensiling Days | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 30 | D | T | D*T | |||
| DM (% FM) | CK | 22.50 | 24.08 | 21.59 | 1.086 | 0.48 | 0.62 | 0.85 |
| CE1 | 23.59 | 22.30 | 22.94 | |||||
| CE2 | 23.36 | 23.19 | 21.75 | |||||
| XY1 | 21.35 | 23.05 | 21.11 | |||||
| XY2 | 22.18 | 20.98 | 21.36 | |||||
| CX1 | 23.02 | 21.85 | 22.93 | |||||
| CX2 | 23.15 | 22.23 | 22.65 | |||||
| WSC (% DM) | CK | 0.06 Cc | 0.28 Bc | 0.65 Ac | 0.454 | <0.01 | <0.01 | <0.01 |
| CE1 | 0.93 Bab | 0.41 Bbc | 2.35 Abc | |||||
| CE2 | 1.23 Ba | 0.48 Bab | 3.17 Aab | |||||
| XY1 | 0.38 Bbc | 0.54 Bab | 5.52 Aa | |||||
| XY2 | 0.30 Bbc | 0.61 Ba | 4.88 Aa | |||||
| CX1 | 0.46 Bbc | 0.46 Babc | 4.97 Aa | |||||
| CX2 | 0.12 Bc | 0.51 Bab | 4.65 Aab | |||||
| NDF (% DM) | CK | 68.26 Aba | 70.14 Aa | 66.89 Ba | 1.050 | <0.01 | <0.01 | 0.17 |
| CE1 | 63.07 b | 62.91 b | 58.14 b | |||||
| CE2 | 62.58 Ab | 62.15 Abc | 57.86 Bb | |||||
| XY1 | 62.91 Ab | 61.31 Abc | 56.52 Bb | |||||
| XY2 | 62.11 Ab | 58.49 Bd | 56.56 Bb | |||||
| CX1 | 61.88 Ab | 61.83 Abc | 54.09 Bb | |||||
| CX2 | 60.06 Ab | 59.76 Acd | 55.61 Bb | |||||
| ADF (% DM) | CK | 35.29 Ba | 38.21 Aa | 36.15 Ba | 0.715 | <0.01 | <0.01 | 0.04 |
| CE1 | 32.49 b | 31.95 b | 30.87 ab | |||||
| CE2 | 32.77 b | 32.19 b | 31.61 b | |||||
| XY1 | 32.48 Ab | 32.17 Ab | 28.97 Bab | |||||
| XY2 | 30.85 b | 29.22 c | 29.18 ab | |||||
| CX1 | 32.71 Ab | 31.72 Ab | 28.72 Bb | |||||
| CX2 | 31.48 b | 30.53 bc | 29.34 ab | |||||
| ADL (% DM) | CK | 4.07 B | 5.57 Aab | 4.15 B | 0.276 | <0.01 | 0.04 | 0.25 |
| CE1 | 4.71 | 4.22 d | 4.03 | |||||
| CE2 | 4.82 | 5.63 ab | 4.64 | |||||
| XY1 | 5.14 AB | 5.89 Aa | 4.45 B | |||||
| XY2 | 4.77 | 4.94 bc | 4.26 | |||||
| CX1 | 4.98 | 4.60 cd | 4.45 | |||||
| CX2 | 4.66 | 4.85 cd | 3.78 | |||||
| Item | Treatment | Ensiling Days | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 30 | D | T | D*T | |||
| Shannon | CK | 5.18 | 4.93 | 5.22 b | 0.25 | <0.01 | <0.01 | <0.01 |
| CE1 | 5.46 AB | 5.07 C | 6.81 Aa | |||||
| XY1 | 5.54 B | 4.86 C | 7.14 Aa | |||||
| CX1 | 4.97 B | 4.83 B | 7.52 Aa | |||||
| Simpson | CK | 0.93 | 0.92 | 0.91 b | 0.01 | <0.01 | <0.01 | 0.01 |
| CE1 | 0.94 | 0.93 | 0.96 a | |||||
| XY1 | 0.95 B | 0.93 C | 0.97 aA | |||||
| CX1 | 0.92 B | 0.93 B | 0.97 aA | |||||
| Chao1 | CK | 1144.47 C | 1361.63 B | 1753.86 A | 75.49 | 0.12 | <0.01 | 0.28 |
| CE1 | 1190.27 B | 1406.58 B | 1740.20 A | |||||
| XY1 | 1366.88 B | 1356.11 B | 1776.59 A | |||||
| CX1 | 1225.17 B | 1459.28 B | 2008.01 A | |||||
| Ace | CK | 1396.75 B | 1771.80 A | 1738.45 A | 105.01 | <0.01 | 0.10 | 0.51 |
| CE1 | 1410.83 | 1748.33 | 1601.87 | |||||
| XY1 | 1637.24 | 1871.92 | 1602.43 | |||||
| CX1 | 1510.46 | 1966.78 | 1925.63 | |||||
| Coverage | CK | 0.99 | 0.99 | 0.99 | - | - | - | - |
| CE1 | 0.99 | 0.99 | 0.99 | |||||
| XY1 | 0.99 | 0.99 | 0.99 | |||||
| CX1 | 0.99 | 0.99 | 0.99 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Liu, N.; Diao, X.; He, L.; Zhou, H.; Zhang, W. Effects of Cellulase and Xylanase on Fermentation Characteristics, Chemical Composition and Bacterial Community of the Mixed Silage of King Grass and Rice Straw. Microorganisms 2024, 12, 561. https://doi.org/10.3390/microorganisms12030561
Qiu C, Liu N, Diao X, He L, Zhou H, Zhang W. Effects of Cellulase and Xylanase on Fermentation Characteristics, Chemical Composition and Bacterial Community of the Mixed Silage of King Grass and Rice Straw. Microorganisms. 2024; 12(3):561. https://doi.org/10.3390/microorganisms12030561
Chicago/Turabian StyleQiu, Chenchen, Nanbing Liu, Xiaogao Diao, Liwen He, Hanlin Zhou, and Wei Zhang. 2024. "Effects of Cellulase and Xylanase on Fermentation Characteristics, Chemical Composition and Bacterial Community of the Mixed Silage of King Grass and Rice Straw" Microorganisms 12, no. 3: 561. https://doi.org/10.3390/microorganisms12030561
APA StyleQiu, C., Liu, N., Diao, X., He, L., Zhou, H., & Zhang, W. (2024). Effects of Cellulase and Xylanase on Fermentation Characteristics, Chemical Composition and Bacterial Community of the Mixed Silage of King Grass and Rice Straw. Microorganisms, 12(3), 561. https://doi.org/10.3390/microorganisms12030561





