Physicochemical Properties and Microbiome of Vineyard Soils from DOP Ribeiro (NW Spain) Are Influenced by Agricultural Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Physicochemical Characterization of Soils
2.3. Determination of Pesticide Residues in Soil Samples
2.3.1. Sample Preparation
2.3.2. Determination Conditions
2.3.3. Quality Control and Quality Assurance
2.4. Soil Microbiome Analysis
2.4.1. DNA Extraction and Library Preparation
2.4.2. Bioinformatic Processing
2.4.3. Computation of Microbiome Indexes and Network Properties
2.5. Statistical Analyses
3. Results and Discussion
3.1. Influence of Vineyard Management on Soil Physicochemical Properties
3.2. Presence of Pesticide Residues in Vineyard Soil from DOP Ribeiro
3.3. Soil Microbiome
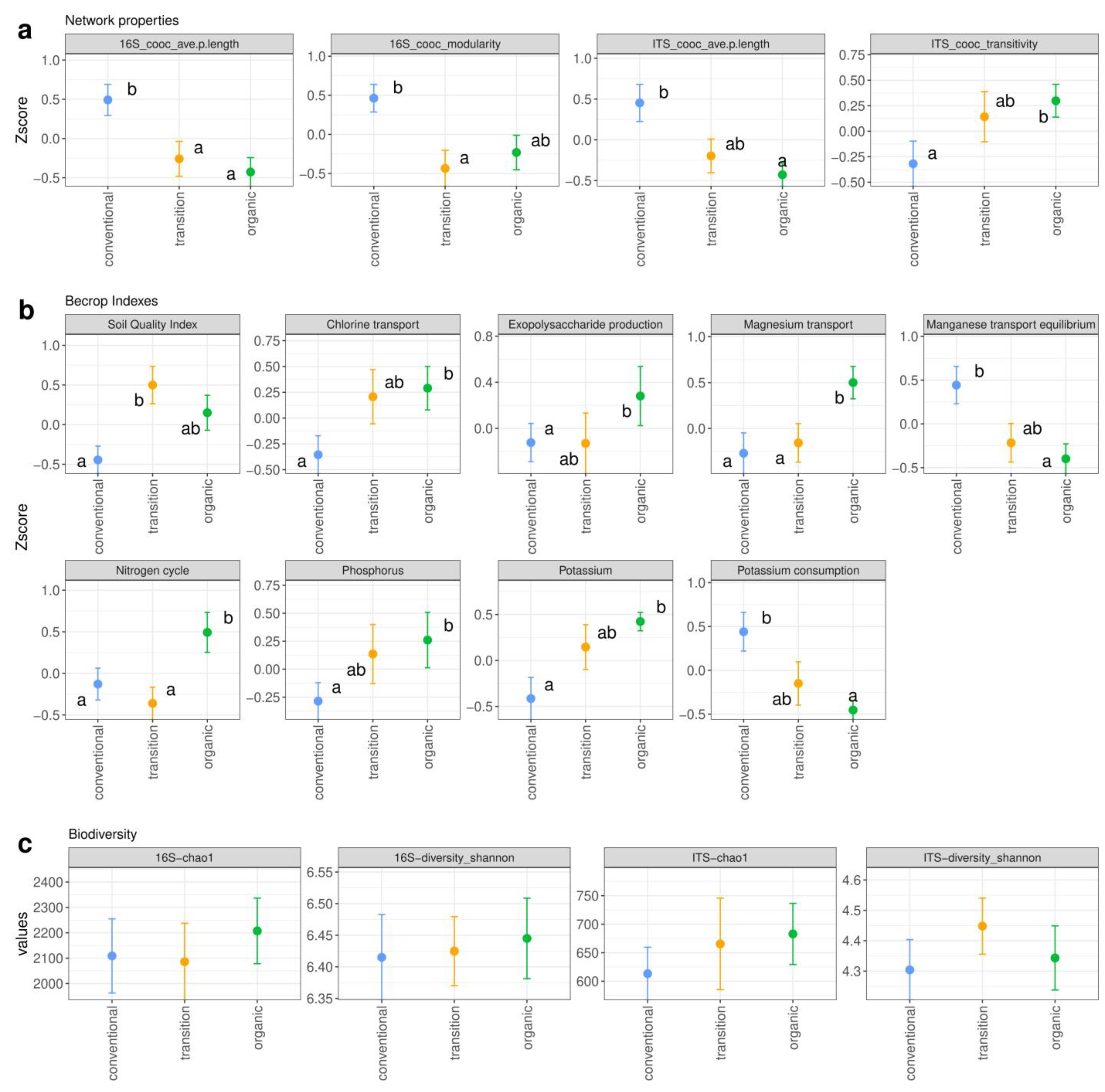
3.3.1. Transition from Conventional to Organic Management Improves Soil Microbiome Indicators
3.3.2. Factors Affecting Microbial Diversity
3.3.3. Preserved and Variable Taxonomic Fractions across DOP Ribeiro with Different Management Practices
4. Final Remarks and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- OIV Definition of Vitivinicultural “Terroir”. Available online: https://www.oiv.int/public/medias/379/viti-2010-1-en.pdf (accessed on 20 December 2023).
- White, R.E. The Value of Soil Knowledge in Understanding Wine Terroir. Front. Environ. Sci. 2020, 8, 12. [Google Scholar] [CrossRef]
- Setati, M.E.; Jacobson, D.; Andong, U.C.; Bauer, F. The Vineyard Yeast Microbiome, a Mixed Model Microbial Map. PLoS ONE 2012, 7, e52609. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.N.; Kluepfel, D.A.; Strauss, S.L.; Bokulich, N.A.; Cantu, D.; Steenwerth, K.L. Vineyard Soil Bacterial Diversity and Composition Revealed by 16S RRNA Genes: Differentiation by Geographic Features. Soil. Biol. Biochem. 2015, 91, 232–247. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial Biogeography of Wine Grapes Is Conditioned by Cultivar, Vintage, and Climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Morrison-Whittle, P.; Goddard, M.R. Quantifying the Relative Roles of Selective and Neutral Processes in Defining Eukaryotic Microbial Communities. ISME J. 2015, 9, 2003–2011. [Google Scholar] [CrossRef]
- Knight, S.J.; Karon, O.; Goddard, M.R. Small Scale Fungal Community Differentiation in a Vineyard System. Food Microbiol. 2020, 87, 103358. [Google Scholar] [CrossRef]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A Global Microbiome Survey of Vineyard Soils Highlights the Microbial Dimension of Viticultural Terroirs. Commun. Biol. 2022, 5, 241. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Millsa, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional Microbial Signatures Positively Correlate with Differential Wine Phenotypes: Evidence for a Microbial Aspect to Terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “Terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Q.; Zhang, P.; Chen, D.; Howell, K.S. The Fungal Microbiome Is an Important Component of Vineyard Ecosystems and Correlates with Regional Distinctiveness of Wine. mSphere 2020, 5, e0053420. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and Assembly of Microbial Communities in Vineyards as a Functional Component of Winegrowing. Front. Microbiol. 2021, 12, 673810. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Wagner, P.; Müller, H.; Matzer, R.; Unterfrauner, H.; Cernava, T.; Berg, G. Unraveling the Complexity of Soil Microbiomes in a Large-Scale Study Subjected to Different Agricultural Management in Styria. Front. Microbiol. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Burns, K.N.; Bokulich, N.A.; Cantu, D.; Greenhut, R.F.; Kluepfel, D.A.; O’Geen, A.T.; Strauss, S.L.; Steenwerth, K.L. Vineyard Soil Bacterial Diversity and Composition Revealed by 16S RRNA Genes: Differentiation by Vineyard Management. Soil. Biol. Biochem. 2016, 103, 337–348. [Google Scholar] [CrossRef]
- Chou, M.Y.; Vanden Heuvel, J.; Bell, T.H.; Panke-Buisse, K.; Kao-Kniffin, J. Vineyard Under-Vine Floor Management Alters Soil Microbial Composition, While the Fruit Microbiome Shows No Corresponding Shifts. Sci. Rep. 2018, 8, 11039. [Google Scholar] [CrossRef] [PubMed]
- Coll, P.; Le Cadre, E.; Blanchart, E.; Hinsinger, P.; Villenave, C. Organic Viticulture and Soil Quality: A Long-Term Study in Southern France. Appl. Soil Ecol. 2011, 50, 37–44. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Pérez-Rodríguez, P.; Insolia, E.; Soto-Gómez, D.; Fernández-Calviño, D.; Zornoza, R. The Impact of Crop Diversification, Tillage and Fertilization Type on Soil Total Microbial, Fungal and Bacterial Abundance: A Worldwide Meta-Analysis of Agricultural Sites. Agric. Ecosyst. Environ. 2022, 329, 107867. [Google Scholar] [CrossRef]
- Meissner, G.; Athmann, M.; Fritz, J.; Kauer, R.; Stoll, M.; Schultz, H.R. Conversion to Organic and Biodynamin Viticultural Practices: Impact on Soil, Grapevine Development and Grape Quality. Oeno One 2019, 4, 639–659. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Lee, S.A.; Goddard, M.R. Fungal Communities Are Differentially Affected by Conventional and Biodynamic Agricultural Management Approaches in Vineyard Ecosystems. Agric. Ecosyst. Environ. 2017, 246, 306–313. [Google Scholar] [CrossRef]
- Sumby, K.M.; Caliani, N.S.; Jiranek, V. Yeast Diversity in the Vineyard: How It Is Defined, Measured and Influenced by Fungicides. Aust. J. Grape Wine Res. 2021, 27, 169–193. [Google Scholar] [CrossRef]
- EU 2018/1981; European Union Commission Implementing Regulation (EU) 2018/1981. European Union: Luxembourg, 2018; p. L 317/16.
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in Plant Protection: Current Situation and Prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; de Palma, M.; Donko, A.; Experton, C.; et al. Use of Copper-Based Fungicides in Organic Agriculture in Twelve European Countries. Agronomy 2022, 12, 673. [Google Scholar] [CrossRef]
- MAPA 2023. Spanish Statistics of Land Uses. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 20 December 2023).
- CRAEGA. Consello Regulador da Agricultura Ecolóxica de Galicia. Available online: https://www.craega.es (accessed on 20 December 2023).
- Bermúdez-Couso, A.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; López-Periago, E.; Soto-González, B.; Simal-Gándara, J. Seasonal Distributions of Fungicides in Soils and Sediments of a Small River Basin Partially Devoted to Vineyards. Water Res. 2007, 41, 4515–4525. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mayán, L.; Ramil, M.; Cela, R.; Rodríguez, I. Multiresidue Procedure to Assess the Occurrence and Dissipation of Fungicides and Insecticides in Vineyard Soils from Northwest Spain. Chemosphere 2020, 261, 127696. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Yeast Diversity on Grapes from Galicia, NW Spain: Biogeographical Patterns and the Influence of the Farming System. Oeno One 2019, 53, 573–587. [Google Scholar] [CrossRef]
- Castrillo, D.; Blanco, P. Influence of Vintage, Geographical Location and Agricultural Management on Yeast Populations in Galician Grape Musts (NW Spain). Oeno One 2022, 56, 65–79. [Google Scholar] [CrossRef]
- MeteoGalicia. Available online: https://www.meteogalicia.gal/observacion/estacionshistorico/ (accessed on 20 December 2023).
- Peech, M.; Alexander, M.T.; Dean, L.; Reed, J.F. Methods of Soil Analysis for Soil-Fertility Investigations; U.S. Department of Agriculture: Washington, DC, USA, 1947. [Google Scholar]
- Lin, C.; Coleman, N.T. The Measurement of Exchangeable Aluminium in Soils and Clays. Soil Sci. Soc. Am. J. 1960, 24, 444–446. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle Size Analysis. In Methods in Soil Analysis. Part I—Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–412. [Google Scholar]
- García-Rodeja, E.; Nóvoa, J.C.; Pontevedra, X.; Martínez-Cortizas, A.; Buurman, P. Aluminium Fractionation of European Volcanic Soils by Selective Dissolution Techniques. Catena 2004, 56, 155–183. [Google Scholar] [CrossRef]
- Lakanen, E.; Ervio, R. A Comparison of Eight Extractants for the Determination of Plant Available Micronutrients in Soil. Acta Agral. Fenn. 1971, 123, 223–232. [Google Scholar]
- Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Díaz-Raviña, M.; Arias-Estévez, M. Copper Accumulation and Fractionation in Vineyard Soils from Temperate Humid Zone (NW Iberian Peninsula). Geoderma 2009, 153, 119–129. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Pérez-Novo, C.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Copper Fractionation and Release from Soils Devoted to Different Crops. J. Hazard. Mater. 2009, 167, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Becares, A.; Fernández, A. Microbiome Based Identification, Monitoring and Enhancement of Fermentation Processes and Products. World Intellectual Property. Organization Patent No. WO2017096385A1, 8 June 2017. [Google Scholar]
- Gobbi, A.; Santini, R.G.; Filippi, E.; Ellegaard-Jensen, L.; Jacobsen, C.S.; Hansen, L.H. Quantitative and Qualitative Evaluation of the Impact of the G2 Enhancer, Bead Sizes and Lysing Tubes on the Bacterial Community Composition during DNA Extraction from Recalcitrant Soil Core Samples Based on Community Sequencing and QPCR. PLoS ONE 2019, 14, e0200979. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, Q.; Xu, H.; Huang, D. Natural Farming Improves Soil Quality and Alters Microbial Diversity in a Cabbage Field in Japan. Sustainability 2019, 11, 3131. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Edgar, R.C.; Flyvbjerg, H. Error Filtering, Pair Assembly and Error Correction for next-Generation Sequencing Reads. Bioinformatics 2015, 31, 3476–3482. [Google Scholar] [CrossRef]
- Mahé, F.; Czech, L.; Stamatakis, A.; Quince, C.; De Vargas, C.; Dunthorn, M.; Rognes, T. Swarm v3: Towards Tera-Scale Amplicon Clustering. Bioinformatics 2022, 38, 267–269. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 Years of Serving the Community with Ribosomal RNA Gene Reference Databases and Tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Álvarez, R.; Ortega-Arranz, H.; Ontiveros, V.J.; de Celis, M.; Ravarani, C.; Acedo, A.; Belda, I. Network Properties of Local Fungal Communities Reveal the Anthropogenic Disturbance Consequences of Farming Practices in Vineyard Soils. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Veech, J.A. A Probabilistic Model for Analysing Species Co-Occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Acedo, A.; Ortega-Arranz, H.; Almonacid, D.; Ferrero, A. Methods and Systems for Generatting and Applying Agronomic Indices from Microbiome-Derived Parameters. US Patent US20220268756A1, 25 August 2022. [Google Scholar]
- Milke, F.; Rodas-Gaitan, H.; Meissner, G.; Masson, V.; Oltmanns, M.; Möller, M.; Wohlfahrt, Y.; Kulig, B.; Acedo, A.; Athmann, M.; et al. Enrichment of Putative Plant Growth Promoting Microorganisms in Biodynamic Compared to Organic Agriculture Soils. ISME Commun. 2024, ycae021. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e0061217. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package (2.6-2) [R Programming]. 2022. pp. 1–285. Available online: https://cran.r-project.org/ (accessed on 25 March 2023).
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Albanese, D.; Stegen, J.; Franceschi, P.; Coller, E.; Zanzotti, R.; Ioriatti, C.; Stefani, E.; Pindo, M.; Cestaro, A.; et al. Distinct and Temporally Stable Assembly Mechanisms Shape Bacterial and Fungal Communities in Vineyard Soils. Microb. Ecol. 2023, 86, 337–349. [Google Scholar] [CrossRef]
- Murphy, B.W.; Hazelton, P. What Do All the Numbers Mean? A Guide to the Interpretation of Soil Test Results, 2nd ed.; Hazelton, P., Murphy, B., Eds.; CSIRO Publishing: Melbourne, Australia, 2007. [Google Scholar]
- Pérez-Rodríguez, P.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Calviño, D. Soil Abandonment as a Trigger for Changes in Zn Fractionation in Afforested Former Vineyard Acidic Soils. Horticulturae 2023, 9, 1121. [Google Scholar] [CrossRef]
- Amorós, J.A.; Bravo, S.; Pérez-de-los-Reyes, C.; García-Navarro, F.J.; Campos, J.A.; Sánchez-Ormeño, M.; Jiménez-Ballesta, R.; Higueras, P. Iron Uptake in Vineyard Soils and Relationships with Other Elements (Zn, Mn and Ca). The Case of Castilla-La Mancha, Central Spain. Appl. Geochem. 2018, 88, 17–22. [Google Scholar] [CrossRef]
- Ramos, M.C. Metals in Vineyard Soils of the Penedès Area (NE Spain) after Compost Application. J. Environ. Manag. 2006, 78, 209–215. [Google Scholar] [CrossRef]
- Chopin, E.I.B.; Marin, B.; Mkoungafoko, R.; Rigaux, A.; Hopgood, M.J.; Delannoy, E.; Cancès, B.; Laurain, M. Factors Affecting Distribution and Mobility of Trace Elements (Cu, Pb, Zn) in a Perennial Grapevine (Vitis vinifera L.) in the Champagne Region of France. Environ. Pollut. 2008, 156, 1092–1098. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Hummes, A.P.; Bortoluzzi, E.C.; Tonini, V.; da Silva, L.P.; Petry, C. Transfer of Copper and Zinc from Soil to Grapevine-Derived Products in Young and Centenarian Vineyards. Water Air Soil. Pollut. 2019, 230, 150. [Google Scholar] [CrossRef]
- Likar, M.; Vogel-Mikuš, K.; Potisek, M.; Hančević, K.; Radić, T.; Nečemer, M.; Regvar, M. Importance of Soil and Vineyard Management in the Determination of Grapevine Mineral Composition. Sci. Total Environ. 2015, 505, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Macías-Vázquez, F.; Calvo de Anta, R. Niveles Genéricos de Referencia de Metales Pesados y Otros Elementos Traza En Suelos de Galicia; Xunta de Galicia: Santiago de Compostela, Spain, 2009. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2010. [Google Scholar]
- Ferreira, G.W.; Lourenzi, C.R.; Comin, J.J.; Loss, A.; Girotto, E.; Ludwig, M.P.; Freiberg, J.A.; de Oliveira Camera, D.; Marchezan, C.; Palermo, N.M.; et al. Effect of Organic and Mineral Fertilizers Applications in Pasture and No-Tillage System on Crop Yield, Fractions and Contaminant Potential of Cu and Zn. Soil. Tillage Res. 2023, 225, 105523. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. El Cobre En Suelos de Viñedo Del Noroeste de La Península Ibérica; Servicio de Publicacións Universidade de Vigo: Vigo, Spain, 2018. [Google Scholar]
- Brataševec, K.; Sivilotti, P.; Vodopivec, B.M. Soil and Foliar Fertilization Affects Mineral Contents in Vitis vinifera L. Cv. “rebula” Leaves. J. Soil Sci. Plant Nutr. 2013, 13, 650–663. [Google Scholar]
- Colautti, A.; Civilini, M.; Contin, M.; Celotti, E.; Iacumin, L. Organic vs. Conventional: Impact of Cultivation Treatments on the Soil Microbiota in the Vineyard. Front. Microbiol. 2023, 14, 1242267. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wagg, C.; Veresoglou, S.D.; Hempel, S.; Rillig, M.C. How Soil Biota Drive Ecosystem Stability. Trends Plant Sci. 2018, 23, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Imam, N.; Belda, I.; García-Jiménez, B.; Duehl, A.J.; Doroghazi, J.R.; Almonacid, D.E.; Thomas, V.P.; Acedo, A. Local Network Properties of Soil and Rhizosphere Microbial Communities in Potato Plantations Treated with a Biological Product Are Important Predictors of Crop Yield. mSphere 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Beaumelle, L.; Giffard, B.; Tolle, P.; Winter, S.; Entling, M.H.; Benítez, E.; Zaller, J.G.; Auriol, A.; Bonnard, O.; Charbonnier, Y.; et al. Biodiversity Conservation, Ecosystem Services and Organic Viticulture: A Glass Half-Full. Agric. Ecosyst. Environ. 2023, 351, 108474. [Google Scholar] [CrossRef]
- Alonso, A.; De Celis, M.; Ruiz, J.; Vicente, J.; Navascués, E.; Acedo, A.; Ortiz-Álvarez, R.; Belda, I.; Santos, A.; Gómez-Flechoso, M.Á.; et al. Looking at the Origin: Some Insights into the General and Fermentative Microbiota of Vineyard Soils. Fermentation 2019, 5, 78. [Google Scholar] [CrossRef]
- Martínez-Diz, M.d.P.; Andrés-Sodupe, M.; Bujanda, R.; Díaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-Plant Compartments Affect Fungal Microbiome Diversity and Composition in Grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]
- Paolinelli, M.; Martinez, L.E.; García-Lampasona, S.; Diaz-Quirós, C.; Belmonte, M.; Ahumada, G.; Pirrone, M.A.; Farber, M.; Escoriaza, G.; Longone, V.; et al. Microbiome in Soils of Mendoza: Microbial Resources for the Development of Agroecological Management in Viticulture. Oeno One 2023, 57, 191–205. [Google Scholar] [CrossRef]
- Hendgen, M.; Hoppe, B.; Döring, J.; Friedel, M.; Kauer, R.; Frisch, M.; Dahl, A.; Kellner, H. Effects of Different Management Regimes on Microbial Biodiversity in Vineyard Soils. Sci. Rep. 2018, 8, 9393. [Google Scholar] [CrossRef]
- Wright, A.H.; Ali, S.; Migicovsky, Z.; Douglas, G.M.; Yurgel, S.; Bunbury-Blanchette, A.; Franklin, J.; Adams, S.J.; Walker, A.K. A Characterization of a Cool-Climate Organic Vineyard’s Microbiome. Phytobiomes J. 2022, 6, 69–82. [Google Scholar] [CrossRef]
- Song, G.C.; Im, H.; Jung, J.; Lee, S.; Jung, M.Y.; Rhee, S.K.; Ryu, C.M. Plant Growth-Promoting Archaea Trigger Induced Systemic Resistance in Arabidopsis thaliana against Pectobacterium carotovorum and Pseudomonas syringae. Environ. Microbiol. 2019, 21, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, L.E.; Barbosa, O. Metagenomic Analysis Exploring Taxonomic and Functional Diversity of Soil Microbial Communities in Chilean Vineyards and Surrounding Native Forests. PeerJ 2017, 2017, e3098. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and Interactions in Plant Growth Promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.A.; El-Nagdi, W.M.A.; Lotfy, D.E.M. Evaluation of the Fungal Activity of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus as Biocontrol Agents against Root-Knot Nematode, Meloidogyne Incognita on Cowpea. Bull. Natl. Res. Cent. 2020, 44, 112. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]

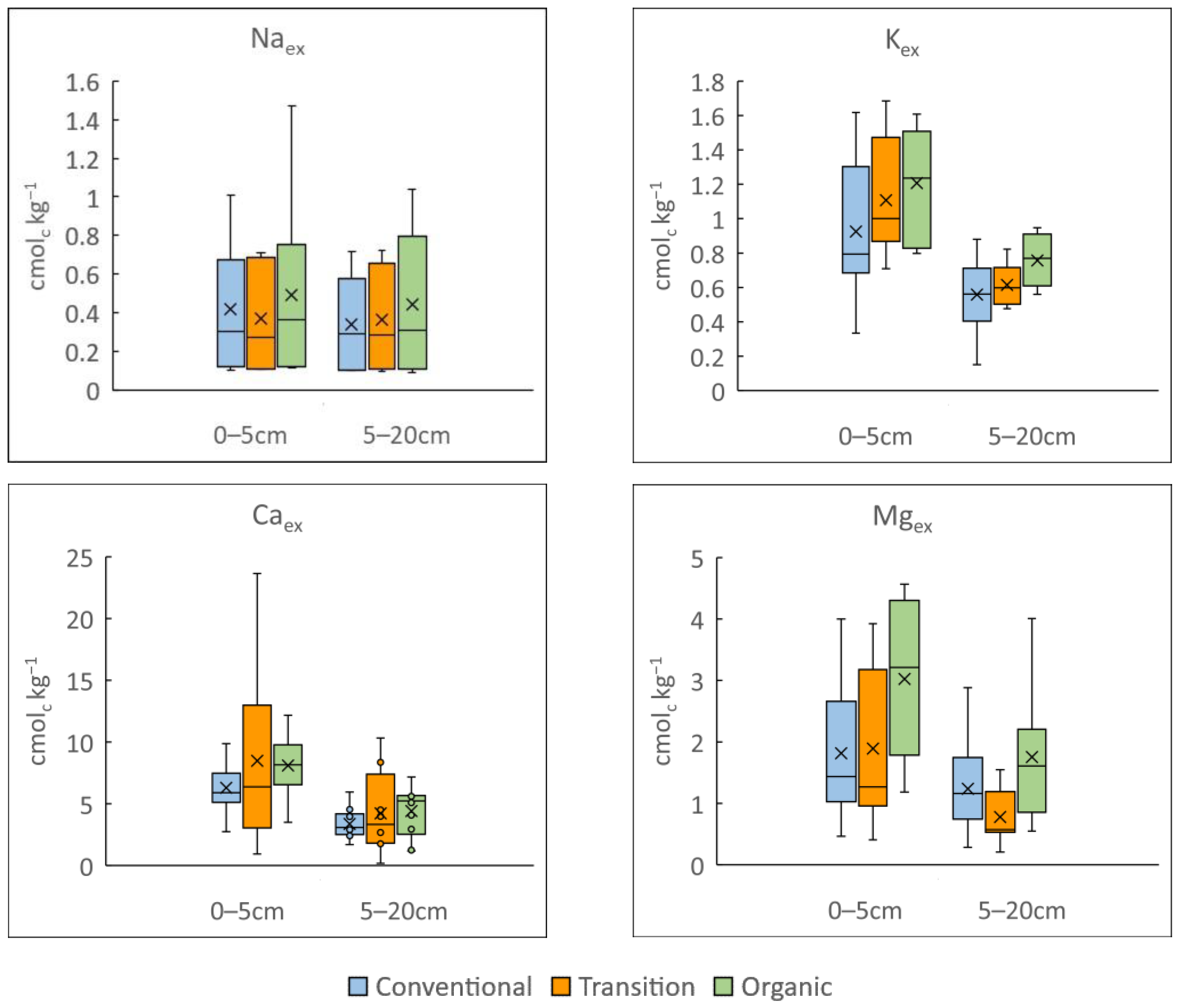
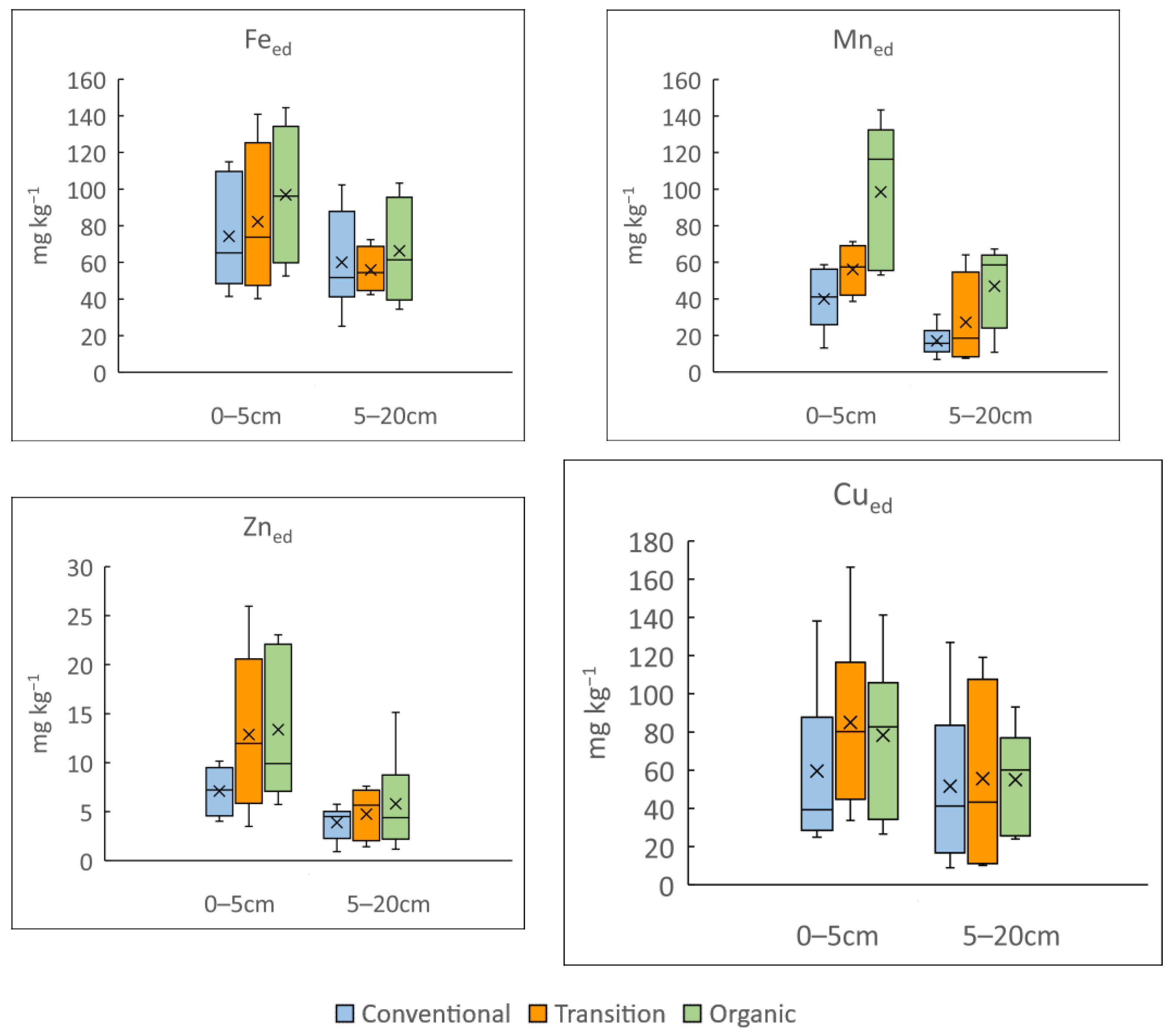
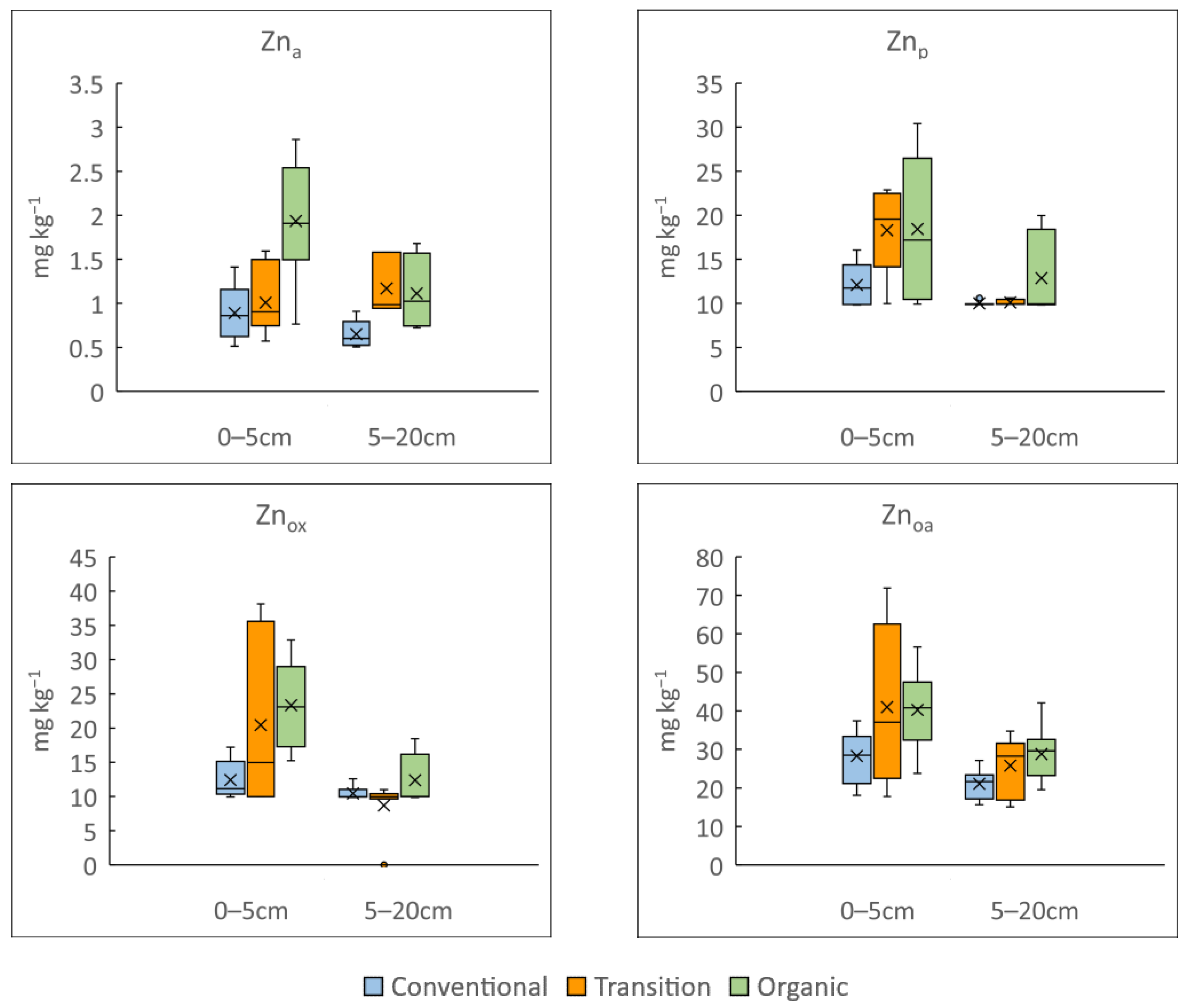



| Soil Depth | 0–5 cm | 5–20 cm | |||||
|---|---|---|---|---|---|---|---|
| Management | Conventional | Transitional | Organic | Conventional | Transitional | Organic | |
| pHw | avg ± sd | 5.6 ± 0.4 b | 5.8 ± 0.6 ab | 6.0 ± 0.5 a | 5.4 ± 0.4 b | 5.8 ± 0.5 ab | 6.0 ± 0.4 a |
| max − min | 6.7 − 5.0 | 6.6 − 4.9 | 6.9 − 5.2 | 6.0 − 4.8 | 6.5 − 5.0 | 6.6 − 5.3 | |
| pHk | avg ± sd | 4.9 ± 0.3 a | 5.2 ± 0.4 a | 5.1 ± 0.4 a | 4.7 ± 0.3 b | 5.1 ± 0.5 a | 5.1 ± 0.3 a |
| max − min | 5.5 − 4.4 | 5.7 − 4.4 | 5.7 − 4.5 | 5.3 − 4.2 | 5.6 − 4.0 | 5.6 − 4.6 | |
| C (g kg−1) | avg ± sd | 21.1 ± 6.8 a | 26.7 ± 17.3 a | 24.6 ± 5.7 a | 14.1 ± 5.7 a | 13.4 ± 5.8 a | 12.7 ± 1.6 a |
| max − min | 32.5 − 11.9 | 58.6 − 11.5 | 38.7 − 17.9 | 23.3 − 5.4 | 22.1 − 7.0 | 15.3 − 10.5 | |
| N (g kg−1) | avg ± sd | 1.6 ± 0.6 a | 2.2 ± 1.5 a | 2.0 ± 0.5 a | 1.1 ± 0.5 a | 1.0 ± 0.4 a | 1.0 ± 0.2 a |
| max − min | 2.4 − 0.8 | 5.0 − 0.8 | 3.0 − 1.4 | 1.8 − 0.4 | 1.7 − 0.5 | 1.3 − 0.8 | |
| C/N | avg ± sd | 13.4 ± 1.2 a | 12.5 ± 1.0 a | 12.6 ± 1.0 a | 13.5 ± 1.6 a | 13.0 ± 1.0 a | 13.4 ± 1.3 a |
| max − min | 16.0 − 11.7 | 14.0 − 11.5 | 14.2 − 10.9 | 16.1 − 11.6 | 14.8 − 12.1 | 16.3 − 11.6 | |
| P (mg kg−1) | avg ± sd | 46.3 ± 35.1 a | 44.0 ± 25.6 a | 39.1 ± 16.1 a | 45.3 ± 22.5 a | 51.8 ± 44.3 a | 34.3 ± 15.7 a |
| max − min | 144.6 − 15.5 | 101.8 − 18.4 | 75.7 − 21.5 | 81.2 − 23.1 | 149.1 − 14.2 | 61.1 − 18.0 | |
| Soil Depth | 0–5 cm | 5–20 cm | |||||
|---|---|---|---|---|---|---|---|
| Management | Conventional | Transitional | Organic | Conventional | Transitional | Organic | |
| FeT (g kg−1) | avg ± sd | 28.7 ± 10.6 a | 31.8 ± 8.2 a | 27.6 ± 7.0 a | 30.5 ± 11.54 a | 31.5 ± 9.5 a | 29.60 ± 6.0 a |
| max − min | 49.5 − 19.7 | 43.4 − 24.6 | 35.9 − 19.1 | 52.9 − 19.7 | 45.1 − 23.3 | 37.6 − 23.7 | |
| MnT (mg kg−1) | avg ± sd | 233.5 ± 132.1 a | 253.0 ± 50.4 a | 426.1 ± 146.4 a | 218.3 ± 130 b | 233.8 ± 89.5 ab | 483.1 ± 119.8 a |
| max − min | 458.9 − 83.7 | 322.5 − 213.0 | 595.6 − 247.2 | 431.2 − 69.1 | 361.5 − 166.8 | 556.8 − 274.0 | |
| CuT (mg kg−1) | avg ± sd | 176.0 ± 96.9 a | 217.0 ± 163.3 a | 167.2 ± 58.3 a | 172.1 ± 113.7 a | 165.1 ± 154.1 a | 132.7 ± 57.4 a |
| max − min | 395.3 − 69.1 | 571.4 − 54.1 | 240.5 − 91.2 | 397.7 − 58.0 | 473.2 − 24.5 | 206.7 − 50.3 | |
| ZnT (mg kg−1) | avg ± sd | 71.1 ± 18.9 b | 127.8 ± 77.1 a | 93.4 ± 15.4 a | 63.4 ± 18.5 a | 76.3 ± 11.4 a | 79.4 ± 12.0 a |
| max − min | 105.4 − 43.8 | 287.5 − 73.3 | 127.5 − 79.4 | 89.0 − 37.0 | 91.7 − 60.5 | 106.3 − 66.8 | |
| Marker | Factor | p-Value | R2 (%) |
|---|---|---|---|
| 16S | Ribeiro zone | 0.01 | 10.71 |
| management | 0.01 | 7.54 | |
| time | 0.01 | 8.96 | |
| depth | 0.01 | 5.84 | |
| ITS | Ribeiro zone | 0.01 | 8.78 |
| management | 0.01 | 8.41 | |
| time | 0.01 | 10.25 | |
| depth | 0.02 | 2.60 |
| Mortierella spp. | Management Comparison | Fold Change (log) | adj-p-Value |
|---|---|---|---|
| Mortierella amoeboidea | organic_vs._conventional | 2.17 | 1.02 × 10−2 |
| Mortierella globulifera | organic_vs._conventional | 2.12 | 3.15 × 10−4 |
| Mortierella sclerotiella | transition_vs._conventional | 2.98 | 3.79 × 10−7 |
| Mortierella stylospora | transition_vs._conventional | 4.02 | 1.60 × 10−9 |
| Mortierella amoeboidea | organic_vs._transition | 3.10 | 8.80 × 10−4 |
| Mortierella fatshederae | organic_vs._transition | −2.01 | 1.58 × 10−2 |
| Mortierella gamsii | organic _vs._transition | −2.12 | 1.58 × 10−2 |
| Mortierella sclerotiella | organic _vs._transition | −2.10 | 9.68 × 10−4 |
| Mortierella stylospora | organic _vs._transition | −4.20 | 4.79 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, P.; Rodríguez, I.; Fernández-Fernández, V.; Ramil, M.; Castrillo, D.; Acín-Albiac, M.; Adamo, I.; Fernández-Trujillo, C.; García-Jiménez, B.; Acedo, A.; et al. Physicochemical Properties and Microbiome of Vineyard Soils from DOP Ribeiro (NW Spain) Are Influenced by Agricultural Management. Microorganisms 2024, 12, 595. https://doi.org/10.3390/microorganisms12030595
Blanco P, Rodríguez I, Fernández-Fernández V, Ramil M, Castrillo D, Acín-Albiac M, Adamo I, Fernández-Trujillo C, García-Jiménez B, Acedo A, et al. Physicochemical Properties and Microbiome of Vineyard Soils from DOP Ribeiro (NW Spain) Are Influenced by Agricultural Management. Microorganisms. 2024; 12(3):595. https://doi.org/10.3390/microorganisms12030595
Chicago/Turabian StyleBlanco, Pilar, Isaac Rodríguez, Victoria Fernández-Fernández, María Ramil, David Castrillo, Marta Acín-Albiac, Irene Adamo, Clara Fernández-Trujillo, Beatriz García-Jiménez, Alberto Acedo, and et al. 2024. "Physicochemical Properties and Microbiome of Vineyard Soils from DOP Ribeiro (NW Spain) Are Influenced by Agricultural Management" Microorganisms 12, no. 3: 595. https://doi.org/10.3390/microorganisms12030595
APA StyleBlanco, P., Rodríguez, I., Fernández-Fernández, V., Ramil, M., Castrillo, D., Acín-Albiac, M., Adamo, I., Fernández-Trujillo, C., García-Jiménez, B., Acedo, A., Calvo-Portela, N., Parente-Sendín, A., Acemel-Míguez, L., & Alonso-Vega, F. (2024). Physicochemical Properties and Microbiome of Vineyard Soils from DOP Ribeiro (NW Spain) Are Influenced by Agricultural Management. Microorganisms, 12(3), 595. https://doi.org/10.3390/microorganisms12030595








