Impact of Zero-Valent Iron Nanoparticles and Ampicillin on Adenosine Triphosphate and Lactate Metabolism in the Cyanobacterium Fremyella diplosiphon
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Strain, Growth Conditions, and Treatments
2.2. ATP Extraction and Measurement
2.3. Extracellular and Intracellular L-Lactate Quantification
2.4. Transmission Electron Microscopy
2.5. Statistical Analysis
3. Results and Discussion
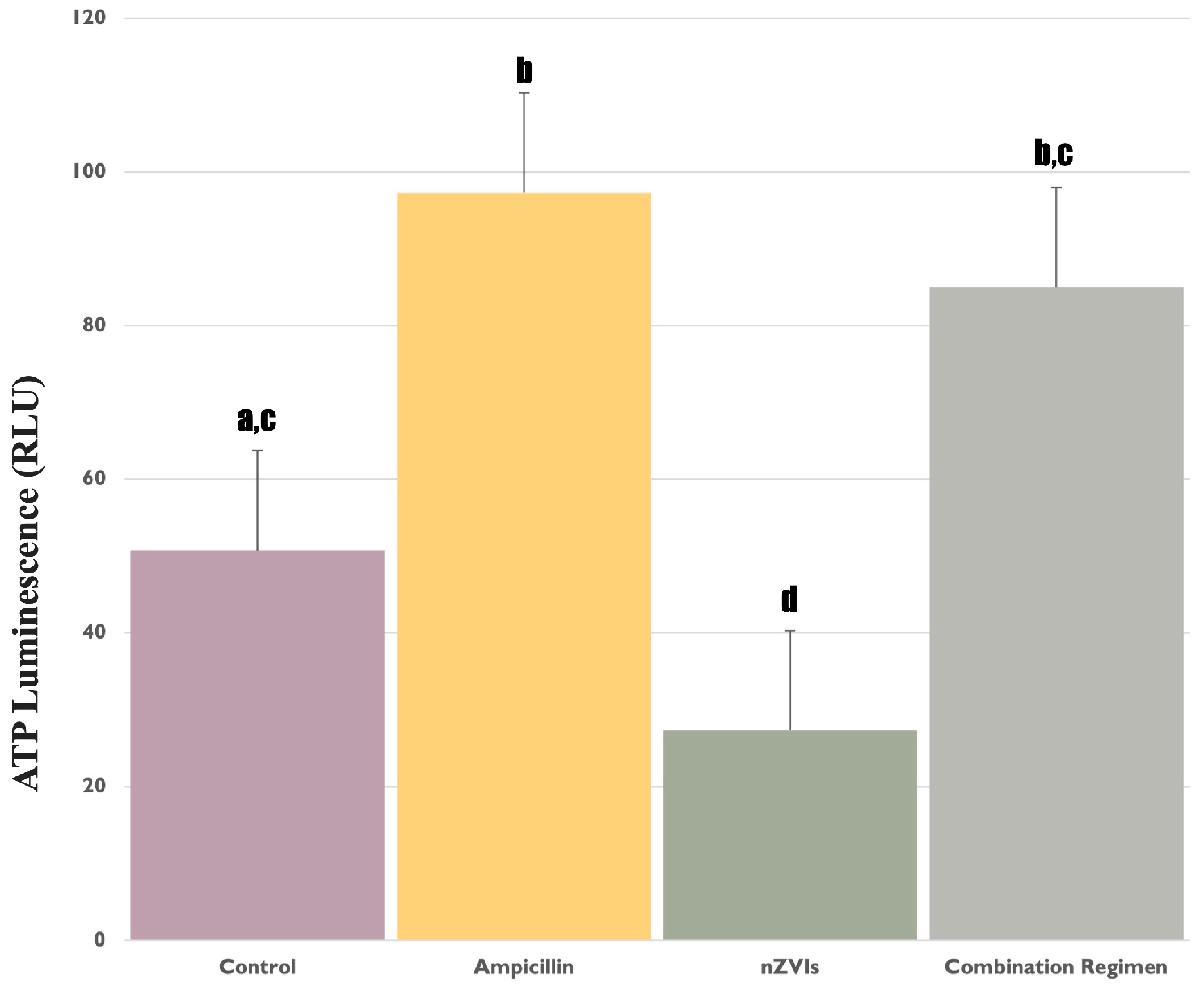
3.1. Assessment of ATP Levels in F. diplosiphon under Ampicillin and nZVIs’ Treatments
3.2. Impact of Ampicillin and nZVIs’ Treatments on Lactate Levels
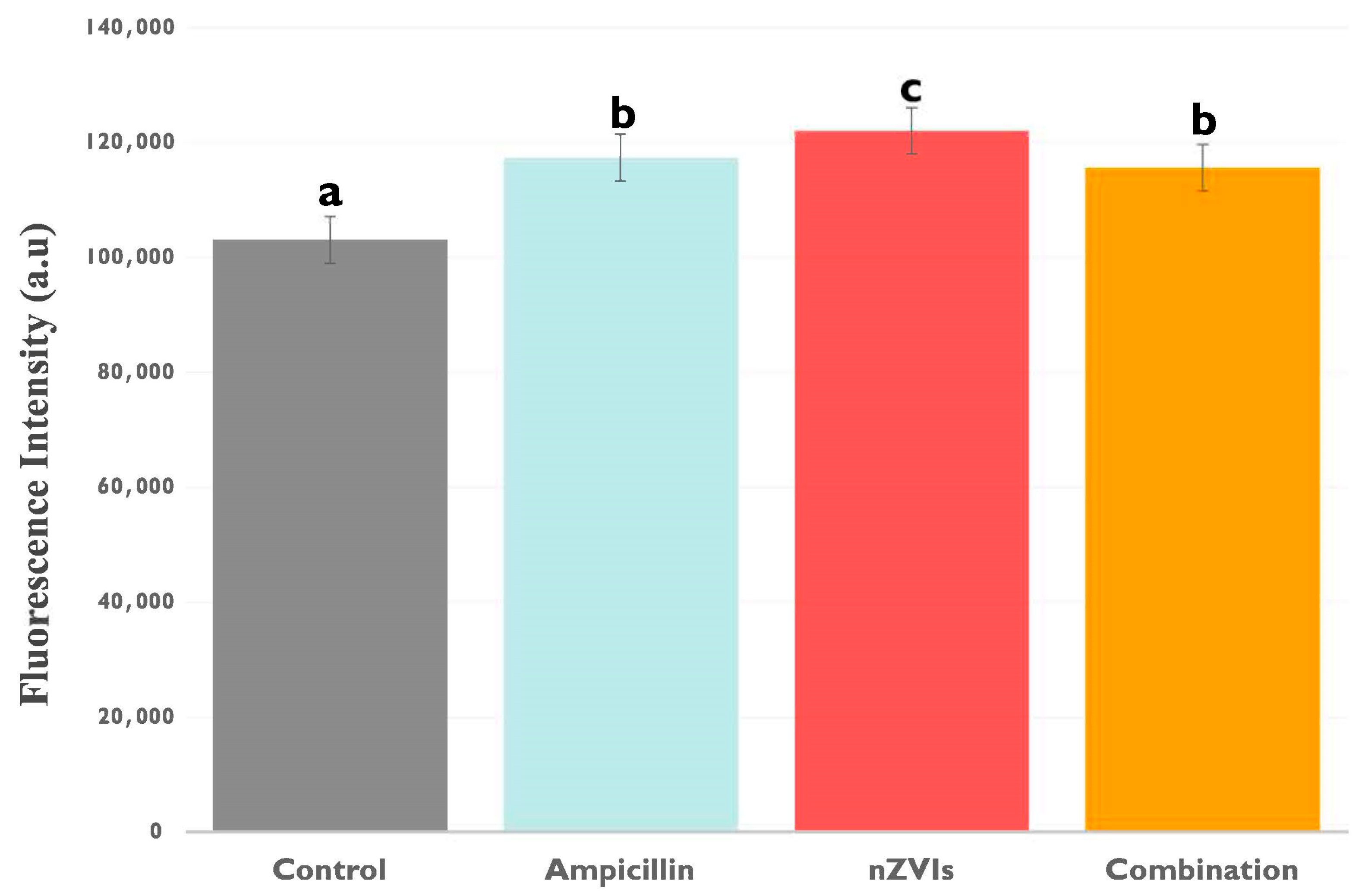
3.2.1. Extracellular Lactate Levels
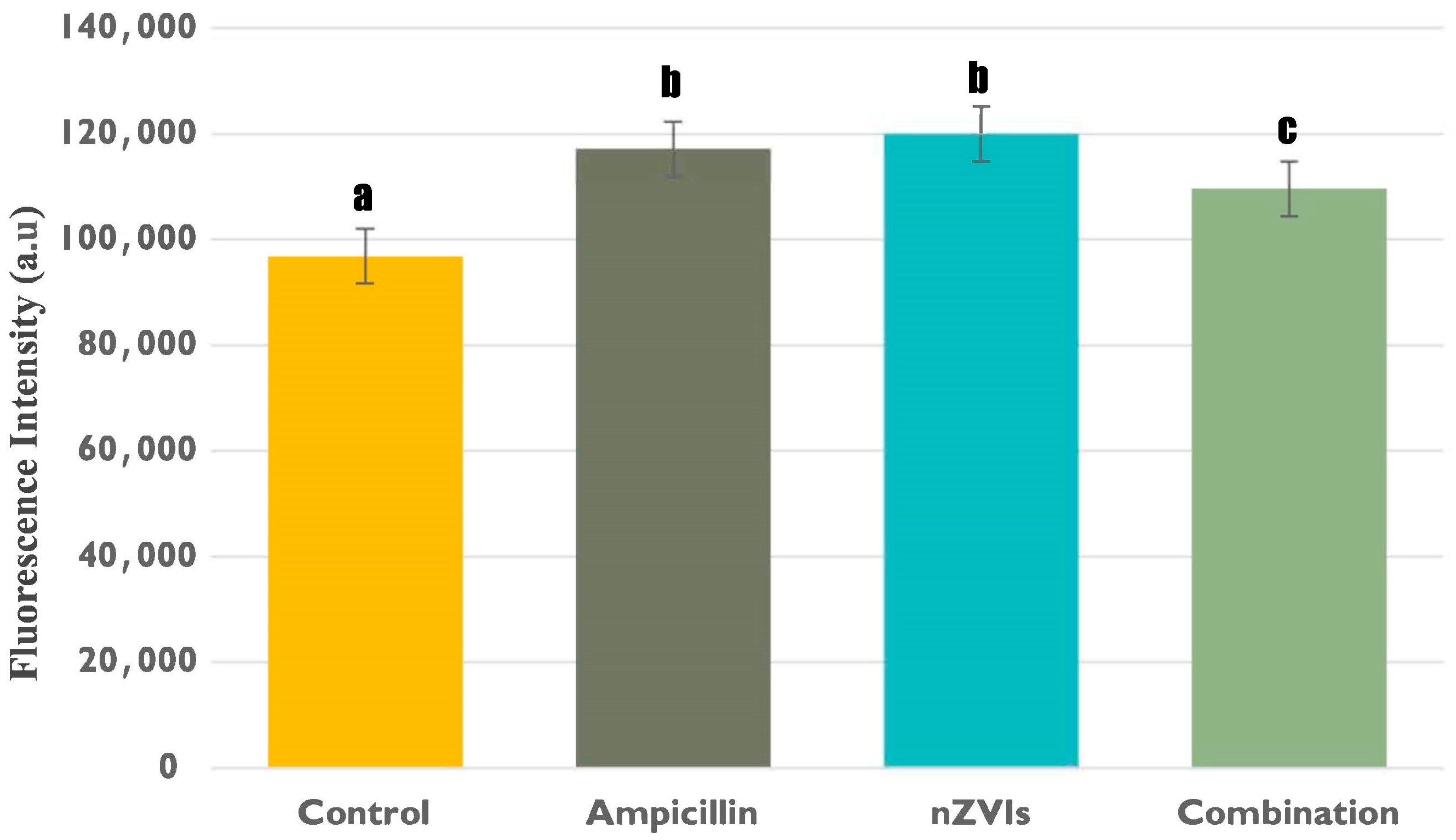
3.2.2. Intracellular Lactate Levels
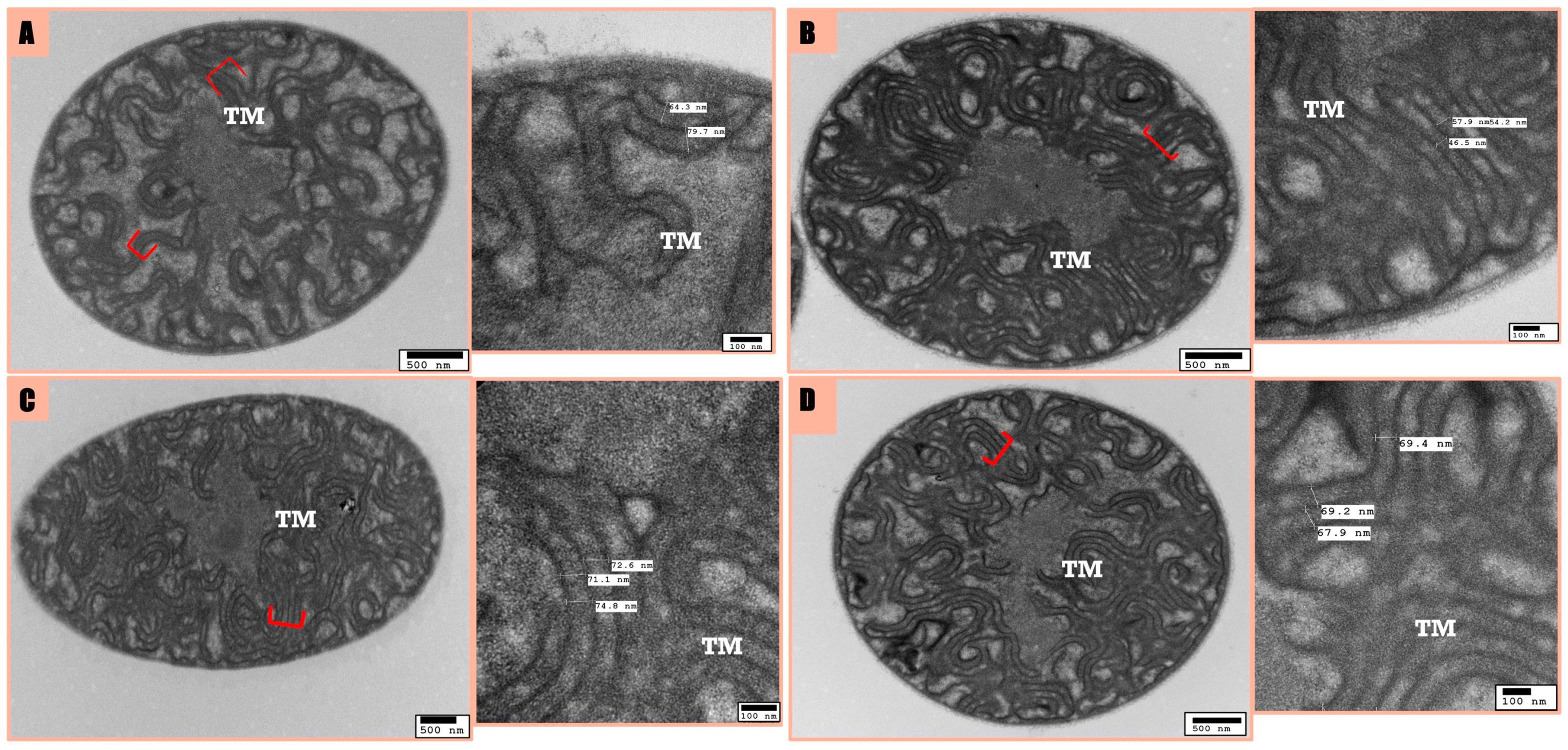
3.3. Microscopic Analysis
ATP Dynamics and Cellular Morphology of F. diplosiphon in Response to nZVI and Ampicillin Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Sahu, O. Stress response under some selected herbicides and heavy metal ions over different strains of cyanobacteria. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Ueno, Y.; Akimoto, S. Long-term light adaptation of light-harvesting and energy-transfer processes in the glaucophyte Cyanophora paradoxa under different light conditions. Photosynth. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Ho, S.H. Microalgae as a solution of third world energy crisis for biofuels production from wastewater toward carbon neutrality: An updated review. Chemosphere 2022, 291, 132863. [Google Scholar] [CrossRef] [PubMed]
- United Nations Framework Convention on Climate Change (UNFCCC). In Paris Agreement; UNFCCC: New York, NY, USA, 2015.
- IPCC. 2023: Sections. In Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Rashid, D.; Khalimat, T. Climate change, greenhouse gases and the bioeconomy. BIO Web Conf. 2023, 76, 07008. [Google Scholar] [CrossRef]
- Sitther, V.; Wyatt, L.; Jones, C.; Yalcin, Y. Bioactive compounds and pigments from cyanobacteria: Applications in the pharmaceutical industry. In Expanding Horizon of Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 65–90. [Google Scholar] [CrossRef]
- Manoj, K.M.; Jaeken, L. Synthesis of theories on cellular powering, coherence, homeostasis and electro-mechanics: Murburn concept and evolutionary perspectives. J. Cell. Physiol. 2023, 238, 931–953. [Google Scholar] [CrossRef]
- Kato, Y.; Oyama, T.; Inokuma, K.; Vavricka, C.J.; Matsuda, M.; Hidese, R.; Satoh, K.; Oono, Y.; Chang, J.S.; Hasunuma, T.; et al. Enhancing carbohydrate repartitioning into lipid and carotenoid by disruption of microalgae starch debranching enzyme. Commun. Biol. 2021, 4, 450. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Zhang, J. Mechanisms for the increase in lipid production in cyanobacteria during the degradation of antibiotics. Environ. Pollut. 2023, 322, 121171. [Google Scholar] [CrossRef]
- Kato, Y.; Inabe, K.; Haraguchi, Y.; Shimizu, T.; Kondo, A.; Hasunuma, T. L-Lactate treatment by photosynthetic cyanobacteria expressing heterogeneous l-lactate dehydrogenase. Sci. Rep. 2023, 13, 7249. [Google Scholar] [CrossRef] [PubMed]
- Toepel, J.; Karande, R.; Klähn, S.; Bühler, B. Cyanobacteria as whole-cell factories: Current status and future prospectives. Curr. Opin. Biotechnol. 2023, 80, 102892. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Li, Y.; Duan, J.; Guo, S.; Cai, X.; Zhang, X.; Long, H.; Ren, W.; Xie, Z. Metabolomic, proteomic and lactylated proteomic analyses indicate lactate plays important roles in maintaining energy and C:N homeostasis in Phaeodactylum tricornutum. Biotechnol. Biofuels Bioprod. 2022, 15, 61. [Google Scholar] [CrossRef]
- Onyeabor, M.; Martinez, R.; Kurgan, G.; Wang, X. Engineering transport systems for microbial production. Adv. Appl. Microbiol. 2022, 111, 33–87. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Shahbazi, A. Syntheses and applications of iron-based functional materials for bioenergy production: A review. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Le Page, G.; Gunnarsson, L.; Trznadel, M.; Wedgwood, K.C.A.; Baudrot, V.; Snape, J.; Tyler, C.R. Variability in cyanobacteria sensitivity to antibiotics and implications for environmental risk assessment. Sci. Total. Environ. 2019, 695, 133804. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; Cordoba-Diaz, M.; García-Balboa, C.; Costas, E.; López-Rodas, V.; Cordoba-Diaz, D. Assessing the effect of high doses of ampicillin on five marine and freshwater phytoplankton species: A biodegradation perspective. J. Appl. Phycol. 2019, 31, 2999–3010. [Google Scholar] [CrossRef]
- Yalcin, Y.S.; Aydin, B.N.; Sayadujjhara, M.; Sitther, V. Antibiotic-induced changes in pigment accumulation, photosystem II, and membrane permeability in a model cyanobacterium. Front. Microbiol. 2022, 13, 930357. [Google Scholar] [CrossRef] [PubMed]
- Fathabad, S.G.; Arumanayagam, A.C.S.; Tabatabai, B.; Chen, H.; Lu, J.; Sitther, V. Augmenting Fremyella diplosiphon cellular lipid content and unsaturated fatty acid methyl esters via sterol desaturase gene overexpression. Appl. Biochem. Biotechnol. 2019, 189, 1127–1140. [Google Scholar] [CrossRef]
- Nikkanen, L.; Solymosi, D.; Jokel, M.; Allahverdiyeva, Y. Regulatory electron transport pathways of photosynthesis in cyanobacteria and microalgae: Recent advances and biotechnological prospects. Physiol. Plant. 2021, 173, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, H.; Roussou, S.; Lindblad, P. Current advances in engineering cyanobacteria and their applications for photosynthetic butanol production. Curr. Opin. Biotechnol. 2022, 73, 143–150. [Google Scholar] [CrossRef]
- Gichuki, S.M.; Yalcin, Y.S.; Wyatt, L.; Ghann, W.; Uddin, J.; Kang, H.; Sitther, V. Zero-valent iron nanoparticles induce reactive oxygen species in the cyanobacterium, Fremyella diplosiphon. ACS Omega 2021, 6, 32730–32738. [Google Scholar] [CrossRef]
- Kumar, M.; Sabu, S.; Sangela, V.; Meena, M.; Rajput, V.D.; Minkina, T.; Vinayak, V. The mechanism of nanoparticle toxicity to cyanobacteria. Arch. Microbiol. 2023, 205, 30. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Zhang, J. Antibiotic contaminants reduced the treatment efficiency of UV-C on Microcystis aeruginosa through hormesis. Environ. Pollut. 2020, 261, 114193. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; López-Rosales, L.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. Rapid method for the assessment of cell lysis in microalgae cultures. J. Appl. Phycol. 2016, 28, 105–112. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Al-Zaban, M.I. Lethal mechanisms of Nostoc-synthesized silver nanoparticles against different pathogenic bacteria. Int. J. Nanomed. 2020, 15, 10499–10517. [Google Scholar] [CrossRef] [PubMed]
- Pádrová, K.; Lukavský, J.; Nedbalová, L.; Čejková, A.; Cajthaml, T.; Sigler, K.; Vítová, M.; Řezanka, T. Trace concentrations of iron nanoparticles cause overproduction of biomass and lipids during cultivation of cyanobacteria and microalgae. J. Appl. Phycol. 2015, 27, 1443–1451. [Google Scholar] [CrossRef]
- Yalcin, Y.S.; Aydin, B.; Chen, H.; Gichuki, S.; Sitther, V. Lipid production and cellular changes in Fremyella diplosiphon exposed to nanoscale zerovalent iron nanoparticles and ampicillin. Microb. Cell Factories 2023, 22, 108. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.S.; Konhauser, K.O.; Robbins, L.J.; Sánchez-Baracaldo, P. Timing the evolution of antioxidant enzymes in cyanobacteria. Nat. Commun. 2021, 12, 4742. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Baumgartner, D.; Hagemann, M.; Muro-Pastor, A.M.; Maaß, S.; Becher, D.; Hess, W.R. AtpΘ is an inhibitor of F0F1 ATP synthase to arrest ATP hydrolysis during low-energy conditions in cyanobacteria. Curr. Biol. 2022, 32, 136–148.e5. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.F.; Lima, S.; Tamagnini, P.; Oliveira, P. Cyanobacterial secretion systems: Understanding fundamental mechanisms toward technological applications. In Cyanobacteria: From Basic Science to Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 359–381. [Google Scholar] [CrossRef]
- Fal, S.; Aasfar, A.; Rabie, R.; Smouni, A.; Arroussi, H.E. Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon 2022, 8, e08811. [Google Scholar] [CrossRef] [PubMed]
- Shukla, E.; Singh, S.S.; Singh, P.; Mishra, A.K. Chemotaxonomy of heterocystous cyanobacteria using FAME profiling as species markers. Protoplasma 2012, 249, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, D.H.; Woo, H.M. Microbial bioprocess for extracellular squalene production and formulation of nanoemulsions. ACS Sustain. Chem. Eng. 2021, 9, 14263–14276. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, K.; Choudhary, S.; Kumawat, G.; Nigam, S.; Joshi, G.; Saharan, V.; Meena, M.; Gupta, A.K. Toxicity evaluation of iron oxide nanoparticles to freshwater cyanobacteria Nostoc ellipsosporum. Environ. Sci. Pollut. Res. 2023, 30, 55742–55755. [Google Scholar] [CrossRef]
- Stingaciu, L.R.; O’Neill, H.; Liberton, M.; Urban, V.S.; Pakrasi, H.B.; Ohl, M. Revealing the dynamics of thylakoid membranes in living cyanobacterial cells. Sci. Rep. 2016, 6, 19627. [Google Scholar] [CrossRef]
- Liberton, M.; Collins, A.M.; Page, L.E.; O’dell, W.B.; O’neill, H.; Urban, V.S.; Timlin, J.A.; Pakrasi, H.B. Probing the consequences of antenna modification in cyanobacteria. Photosynth. Res. 2013, 118, 17–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin, Y.S.; Aydin, B.N.; Sitther, V. Impact of Zero-Valent Iron Nanoparticles and Ampicillin on Adenosine Triphosphate and Lactate Metabolism in the Cyanobacterium Fremyella diplosiphon. Microorganisms 2024, 12, 612. https://doi.org/10.3390/microorganisms12030612
Yalcin YS, Aydin BN, Sitther V. Impact of Zero-Valent Iron Nanoparticles and Ampicillin on Adenosine Triphosphate and Lactate Metabolism in the Cyanobacterium Fremyella diplosiphon. Microorganisms. 2024; 12(3):612. https://doi.org/10.3390/microorganisms12030612
Chicago/Turabian StyleYalcin, Yavuz S., Busra N. Aydin, and Viji Sitther. 2024. "Impact of Zero-Valent Iron Nanoparticles and Ampicillin on Adenosine Triphosphate and Lactate Metabolism in the Cyanobacterium Fremyella diplosiphon" Microorganisms 12, no. 3: 612. https://doi.org/10.3390/microorganisms12030612
APA StyleYalcin, Y. S., Aydin, B. N., & Sitther, V. (2024). Impact of Zero-Valent Iron Nanoparticles and Ampicillin on Adenosine Triphosphate and Lactate Metabolism in the Cyanobacterium Fremyella diplosiphon. Microorganisms, 12(3), 612. https://doi.org/10.3390/microorganisms12030612




