Optimization of Culture Conditions and Batch Process Control for the Augmented Production of Bacteriocin by Bacillus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacterial Strains
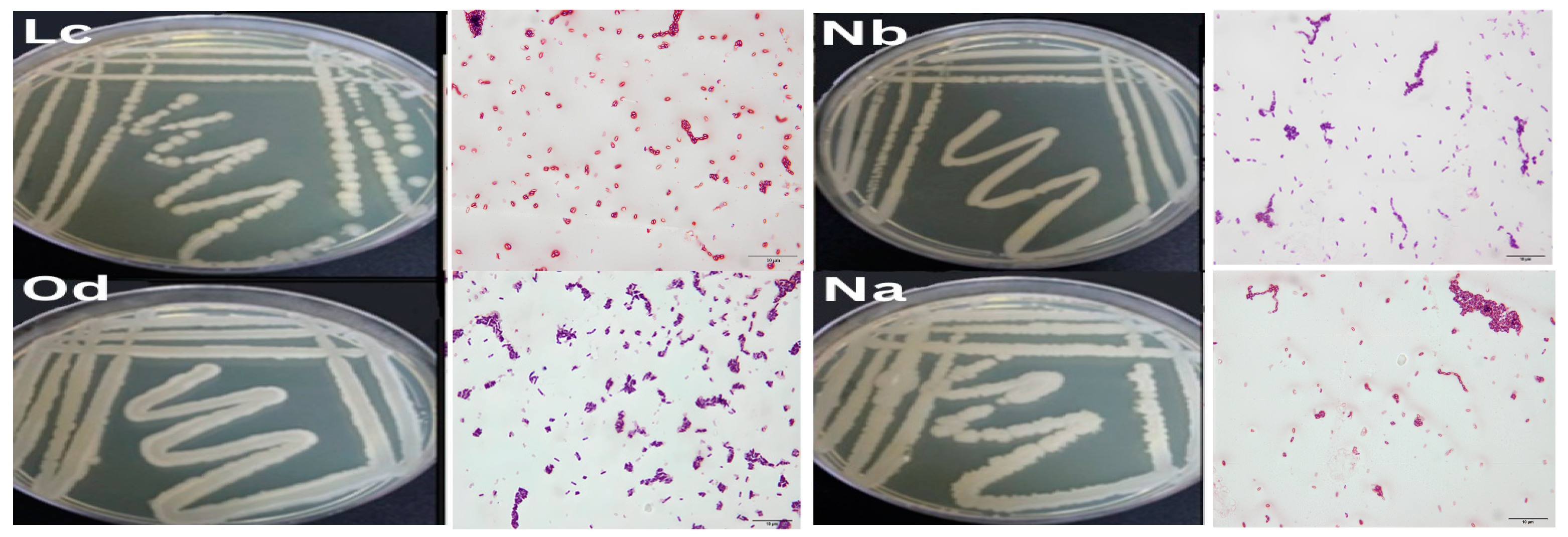
2.3. Characterization and Identification of Test Isolates
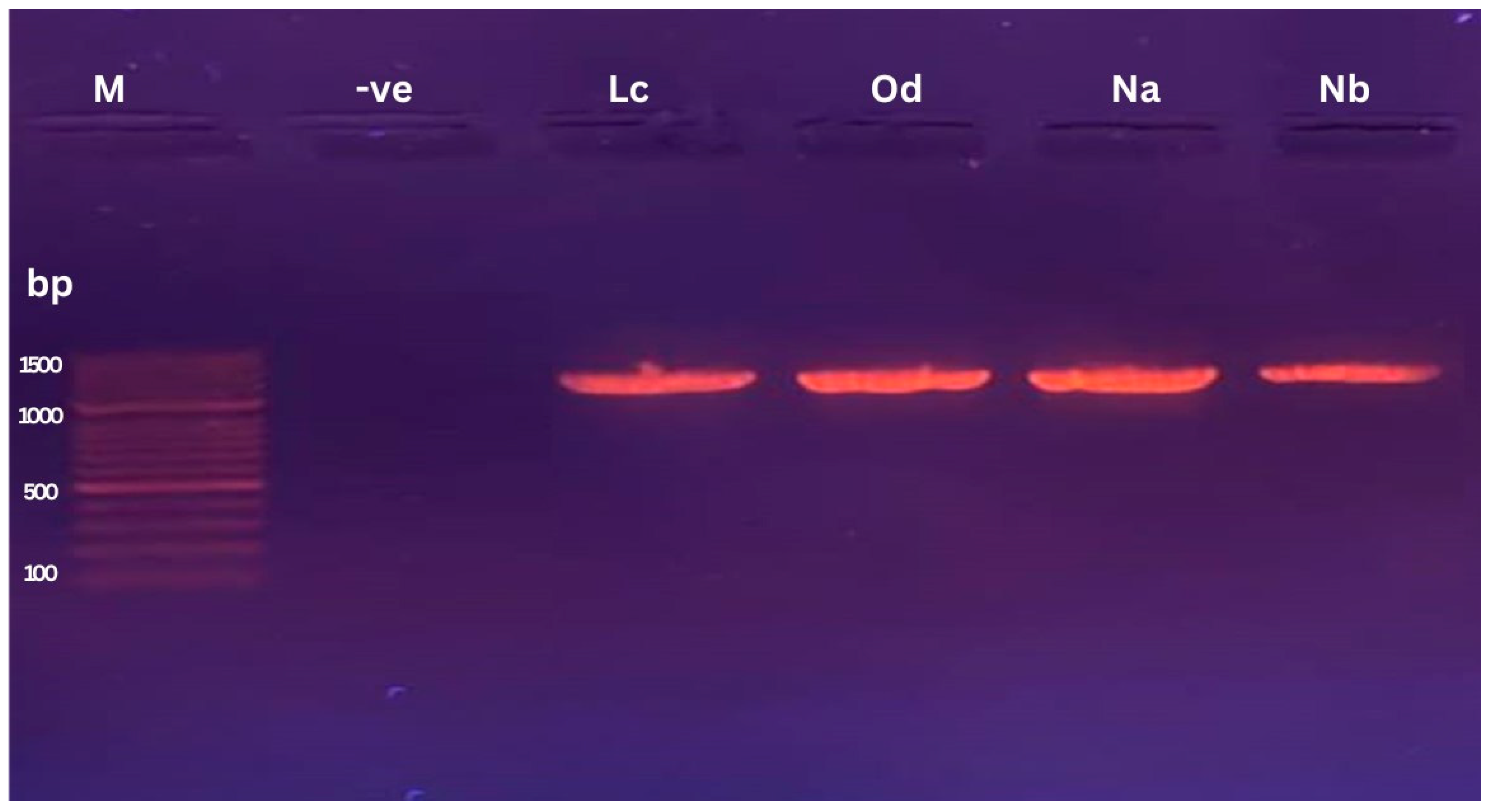
2.4. 16S rRNA Gene Amplification by PCR
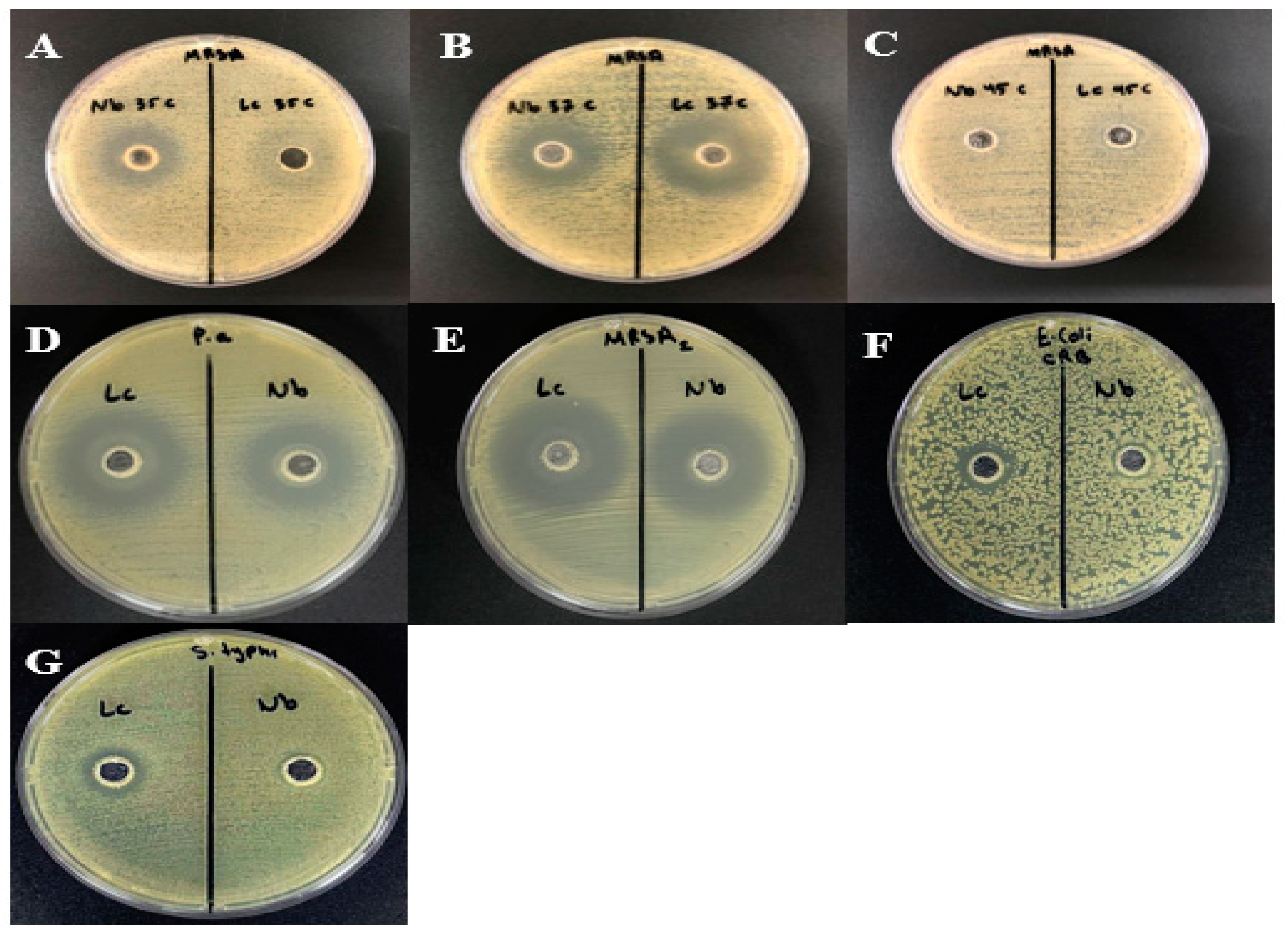
2.5. Antimicrobial Susceptibility Test
2.6. Evaluating the Influence of Incubation Duration, pH, and Temperature on Bacteriocin Synthesis
2.7. Assessing the Impact of Varied Culture Media on Bacteriocin Efficacy
2.8. Bioreactor Cultivation Procedures
2.8.1. Dissolved Oxygen (DO) Control
2.8.2. Temperature Control
2.8.3. pH Control
2.8.4. Agitation Speed and Impeller Type
2.8.5. Foam Control
2.8.6. Analytical Measurements
Quantification of Total Carbohydrates
Evaluation of Total Nitrogen
Measurement of Cell Dry Weight
3. Results
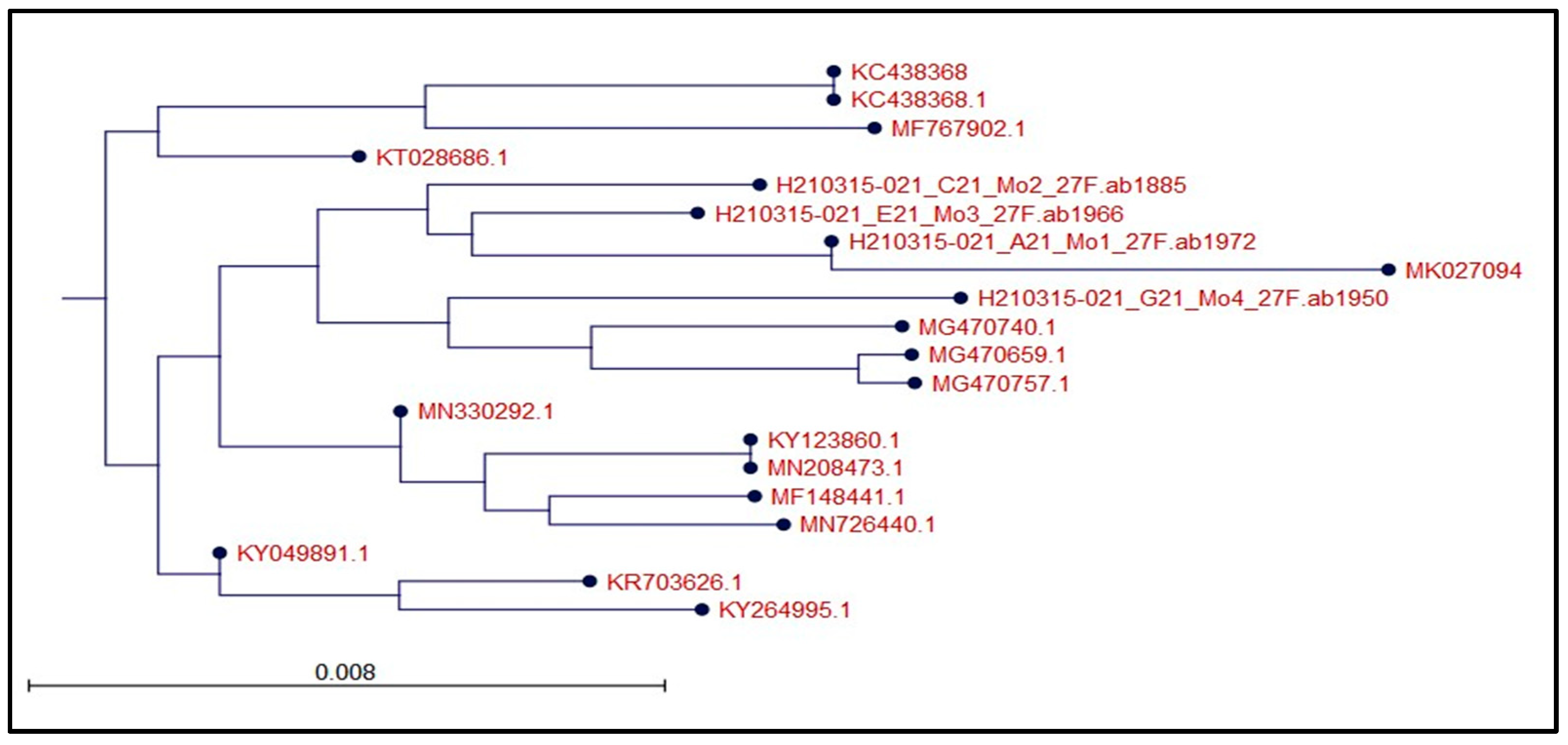
3.1. Bacterial Isolation and Identification
3.2. Optimization of Bacteriocin Production
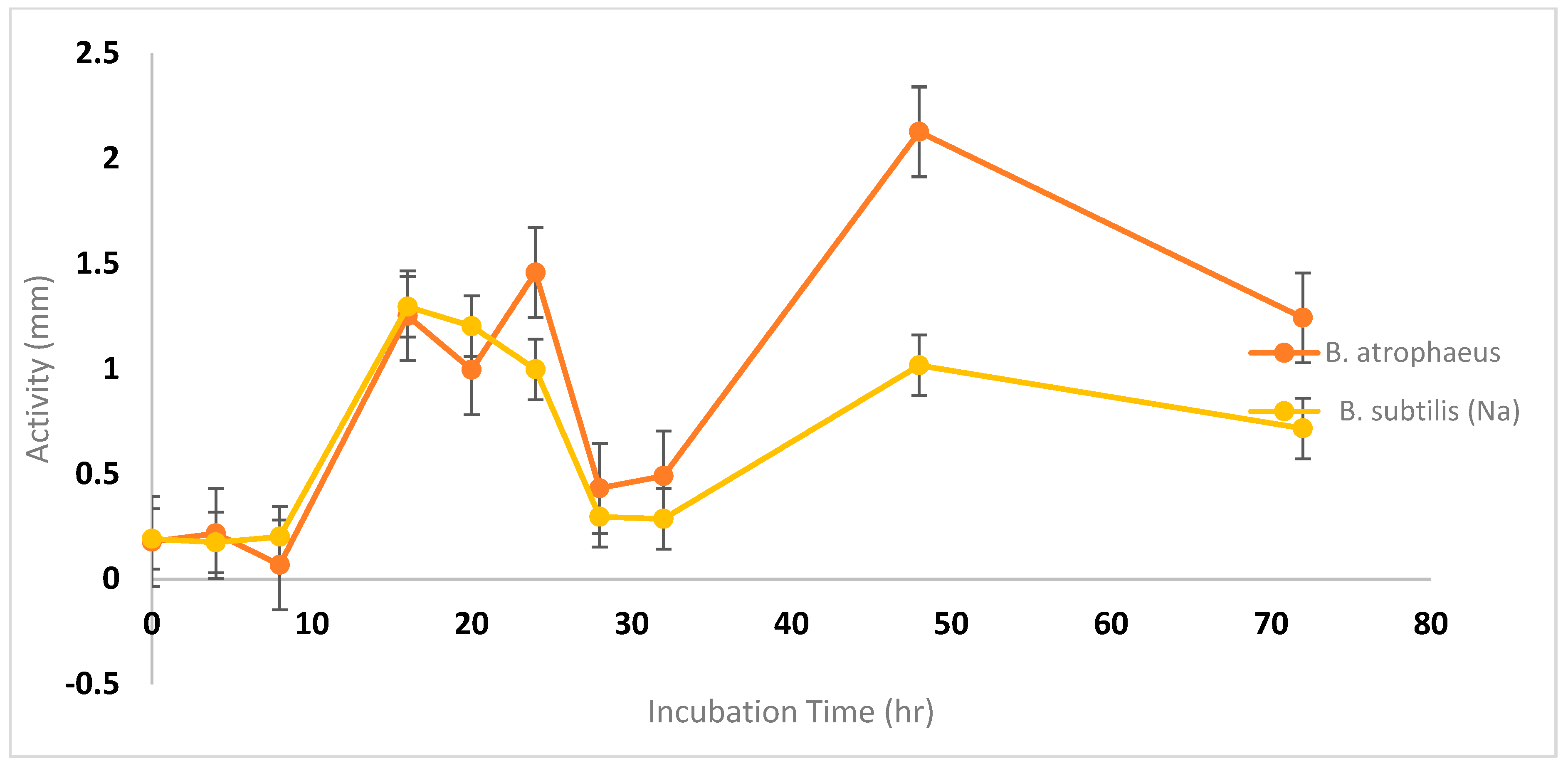
3.2.1. Impact of Various Incubation Conditions on Bacteriocin Activity
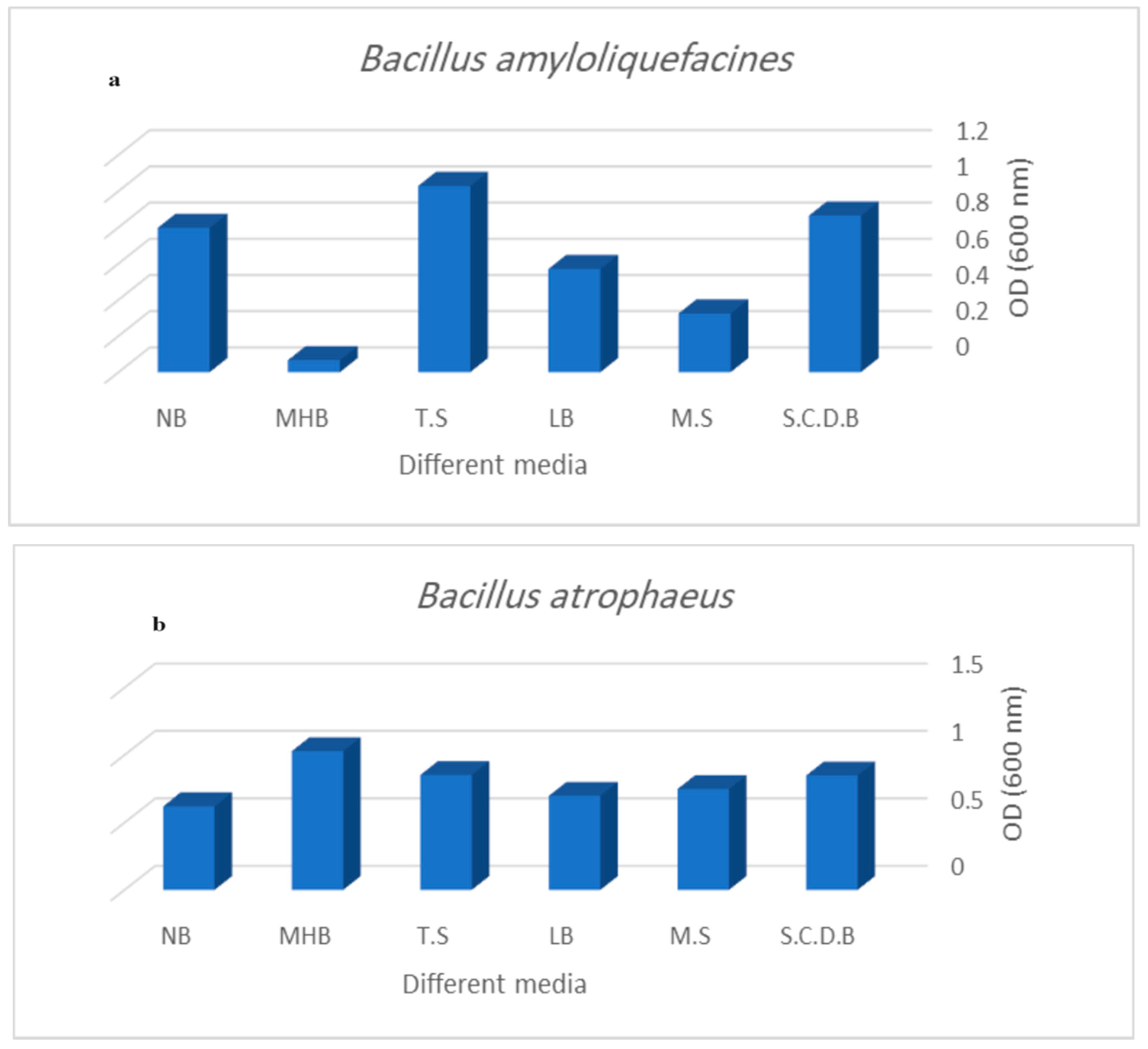
3.2.2. Effect of Different Growth Media on Bacteriocin Activity
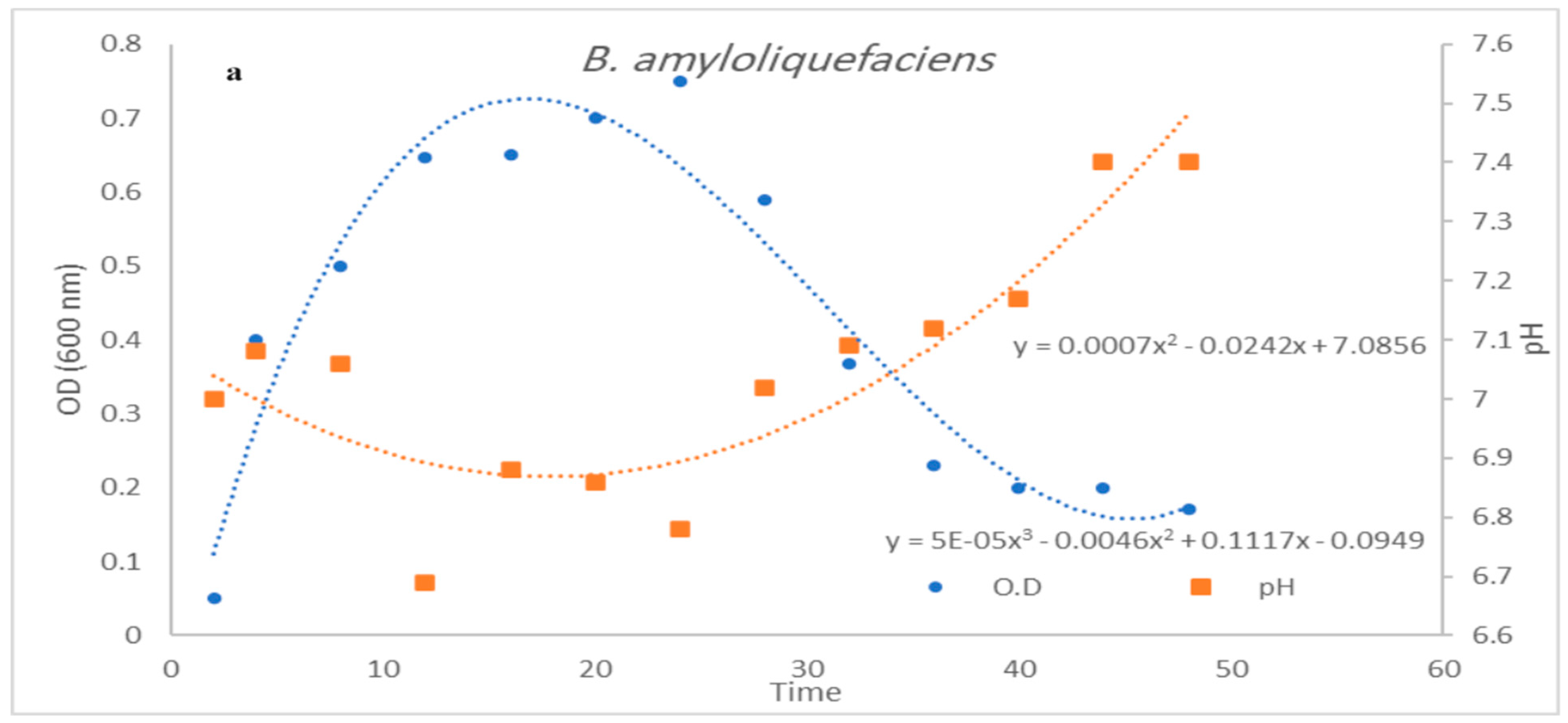
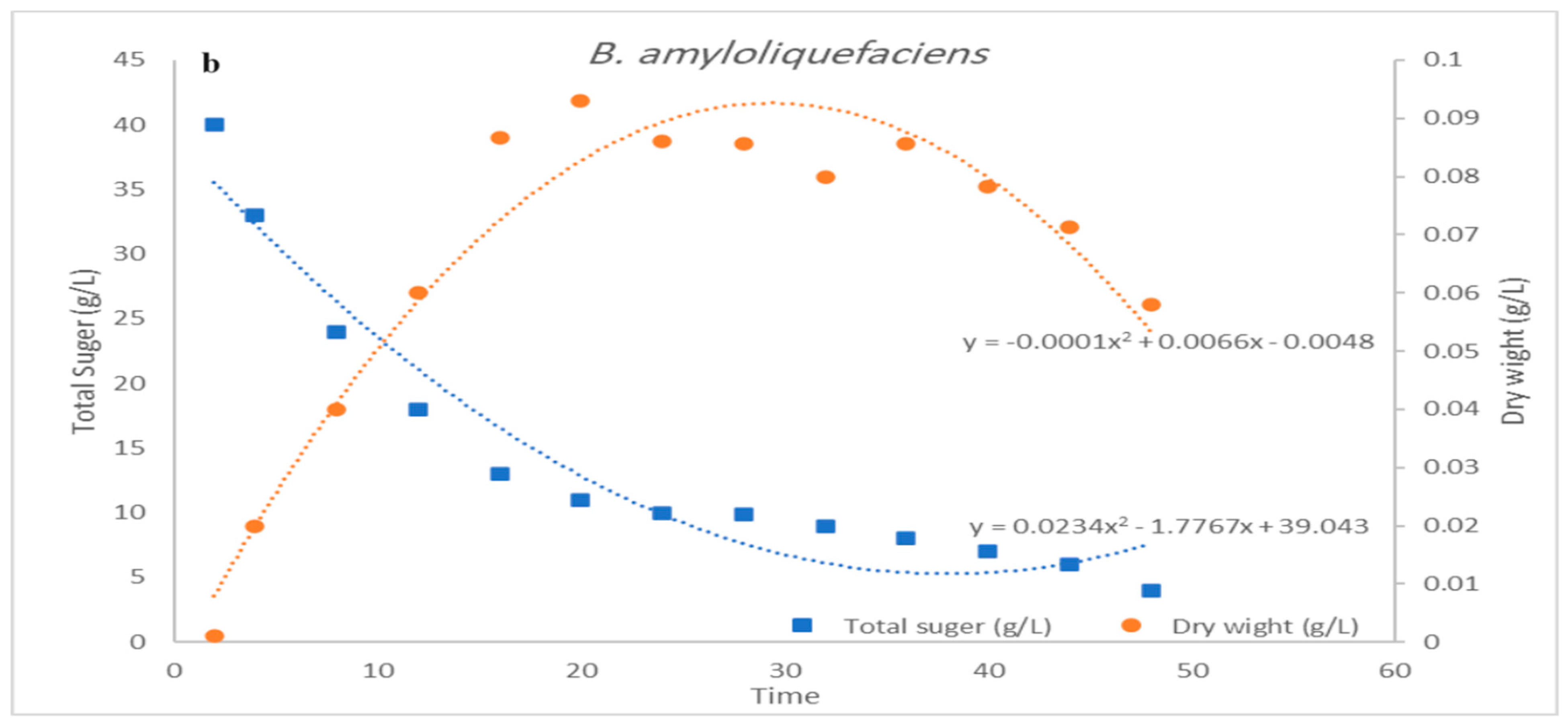
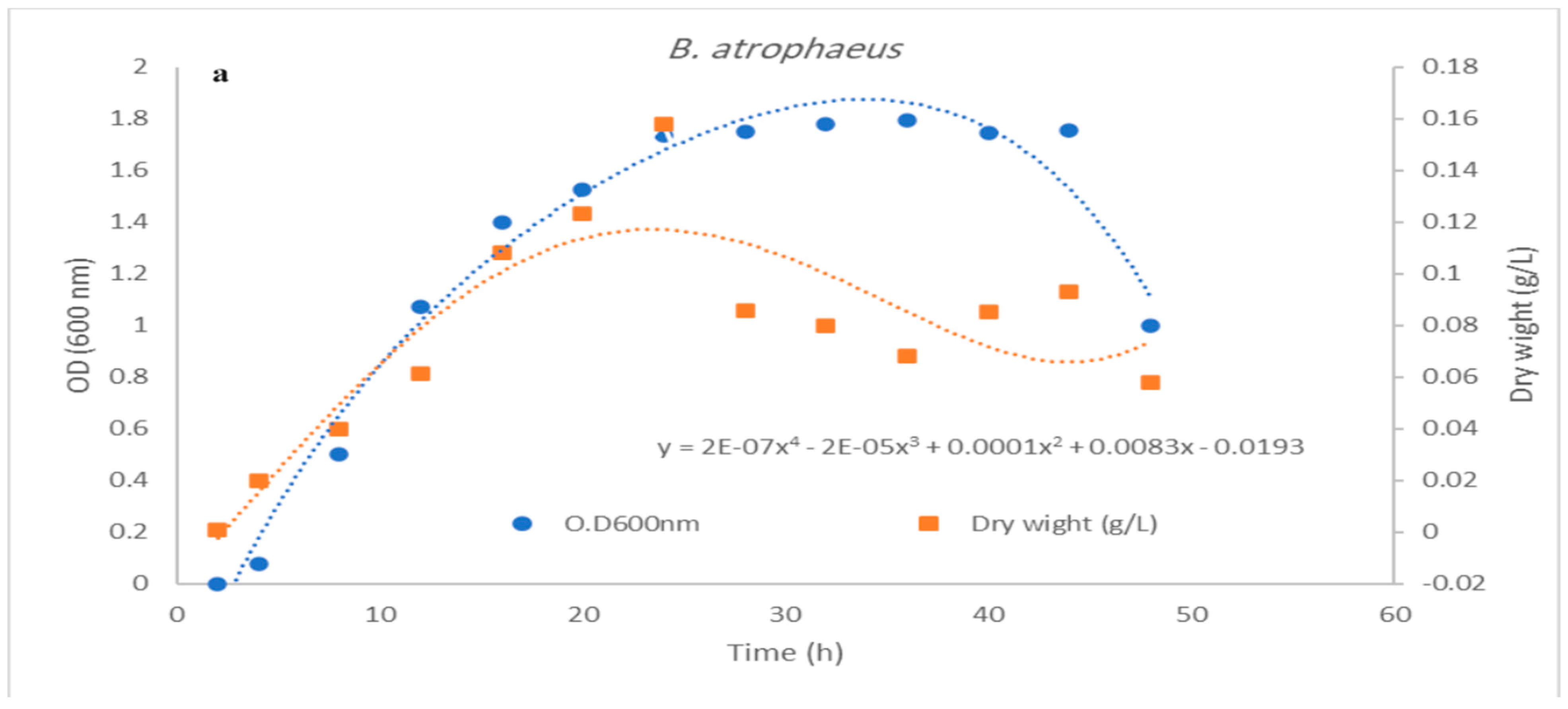
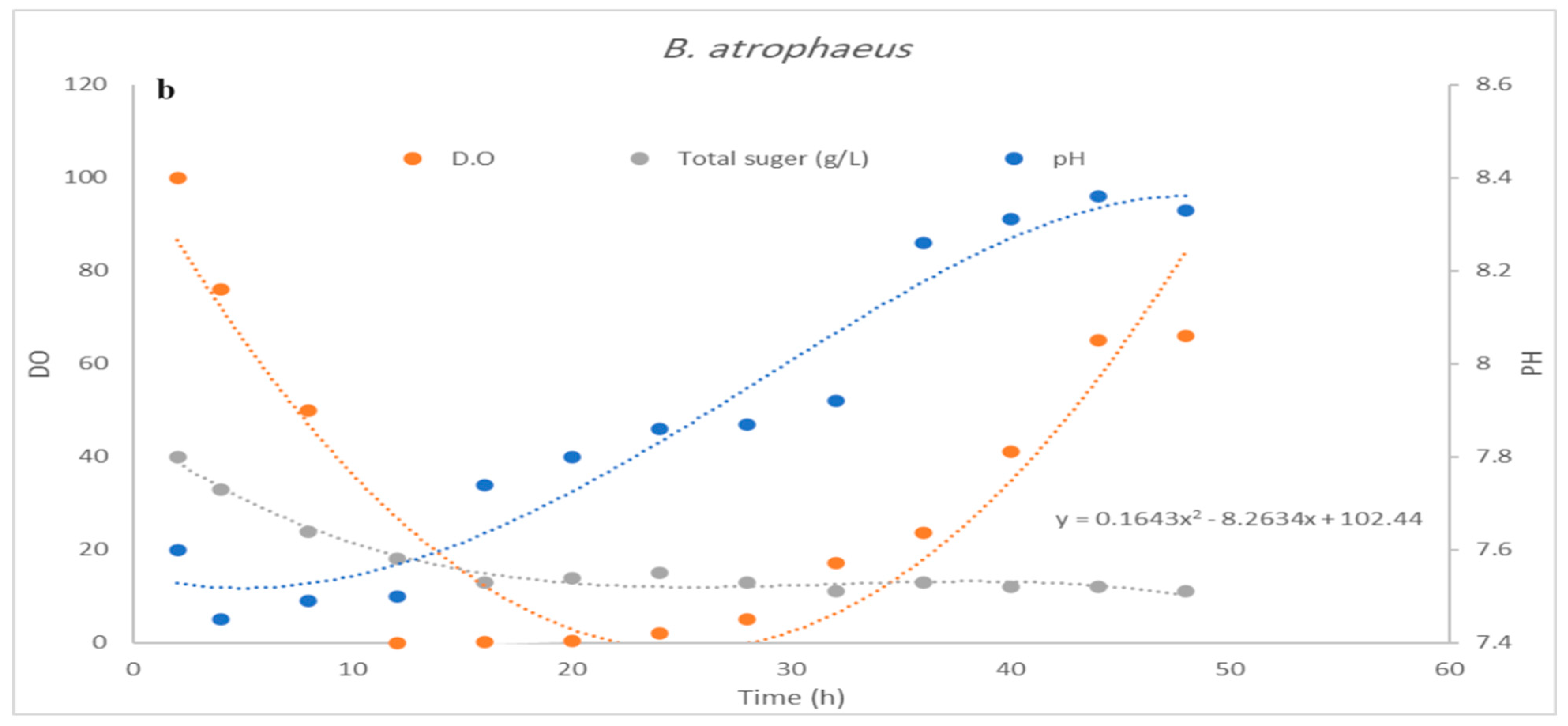
3.3. Impact of pH Control on Cell Growth and Bacteriocin Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 707330. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Predicting drug resistance evolution: Insights from antimicrobial peptides and antibiotics. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172687. [Google Scholar] [CrossRef]
- Seyfi, R.; Kahaki, F.A.; Ebrahimi, T.; Montazersaheb, S.; Eyvazi, S.; Babaeipour, V.; Tarhriz, V. Antimicrobial peptides (AMPs): Roles, functions and mechanism of action. Int. J. Pept. Res. Ther. 2020, 26, 1451–1463. [Google Scholar] [CrossRef]
- Sun, E.; Belanger, C.R.; Haney, E.F.; Hancock, R.E. 10.1 Overview of host defense peptides. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Oliveira, M.; Gomes-Alves, A.G.; Sousa, C.; Marani, M.M.; Plácido, A.; Vale, N.; Delerue-Matos, C.; Gameiro, P.; Kückelhaus, S.A.S.; Tomas, A.M.; et al. Ocellatin-PT antimicrobial peptides: High-resolution microscopy studies in antileishmania models and interactions with mimetic membrane systems. Biopolymers 2016, 105, 873–886. [Google Scholar] [CrossRef]
- Elnagdy, S.; AlKhazindar, M. The Potential of Antimicrobial Peptides as an Antiviral Therapy against COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Wei, Z.; Guan, Z.; Cai, Y.; Liao, X. Antifungal activity of isolated Bacillus amyloliquefaciens SYBC H47 for the biocontrol of peach gummosis. PLoS ONE 2016, 11, e0162125. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Mangmee, S.; Reamtong, O.; Kalambaheti, T.; Roytrakul, S.; Sonthayanon, P. Antimicrobial Peptide Modifications against Clinically Isolated Antibiotic-Resistant Salmonella. Molecules 2021, 26, 4654. [Google Scholar] [CrossRef]
- Dramé, O.; Leclair, D.; Parmley, E.J.; Deckert, A.; Ouattara, B.; Daignault, D.; Ravel, A. Antimicrobial resistance of Campylobacter in broiler chicken along the food chain in Canada. Foodborne Pathog. Dis. 2020, 17, 512–520. [Google Scholar] [CrossRef]
- Lisoba, M.P.; Bonatto, D.; Bizani, J.A.; Henriques; Brandelli, A. Characterization of a bacteriocin like substance produced by Bacillus amyloliquefaciens isolated from Brazilian atlantic forest. Int. Microbiol. 2006, 9, 111–118. [Google Scholar]
- Xie, J.; Zhang, R.; Shang, C.; Guo, Y. Isolation and characterization of bacteriocin produced by an isolated Bacillus subtilis LEB112 that exhibits antimicrobial activity against domestic animal pathogens. Afr. J. Biotechnol. 2009, 8, 5611–5619. [Google Scholar]
- Novotny, J.F.; Perry, J.J. Characterization of bacteriocin from two strains of Bacillus thermoleovorans, A thermophilic hydrocarbon utilizing species. Appl. Environ. Microbiol. 1992, 58, 2393–2396. [Google Scholar] [CrossRef]
- Hyronimus, B.; Le Marrec, C.; Urdaci, M.C. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 1998, 85, 42–50. [Google Scholar] [CrossRef]
- Naclerio, G.; Ricca, E.; Sacco, M.; De Felice, M. Antimicrobial activity of a newly identified bacteriocin of Bacillus cereus. Appl. Environ. Microbiol. 1993, 59, 4313–4316. [Google Scholar] [CrossRef] [PubMed]
- De Borjac, H.; Lajudie, J. Mise en evidence de facteurs antagonists du type des bacterioeoeines chez Bacillus thuringiensis. Ann. J. Microbiol. Ser. B 1974, 125, 529–537. [Google Scholar]
- Bradley, D.E. Ultra-structure of bacteriophages and bacteriocins. Bacreriol. Rev. 1967, 31, 230–314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yu, M.; Huang, J.; Zhou, K. Bacteriocins and Their Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 655–679. [Google Scholar]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing Innate Immunity for Food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Nissen-Meyer, J.; Rogne, P.; Oppegard, C.; Haugen, H.S.; Kristiansen, P.E. Structure-Function Relationships of the Non-Lanthionine-Containing Peptide (Class II) Bacteriocins Produced by Gram-Positive Bacteria. Curr. Pharm. Biotechnol. 2011, 12, 1184–1196. [Google Scholar] [CrossRef]
- Chittpurna, N.; Naidu, S.S.; Kumar, G.N. Antimicrobial Peptides from Bacillus spp.: A New Hope for Novel Antibiotics. In Microbial Biotechnology; Rajasekaran, P., Ed.; Springer: Cham, Switzerland, 2018; pp. 205–225. [Google Scholar]
- Li, J.; Huo, L.; Huang, Q.; Dai, W.; Zheng, J.; Zhao, Z.; Zhang, J.; Yang, J.; Zhang, Y.; Ma, J. Amylocyclicin, a novel cyclic bacteriocin from Bacillus amyloliquefaciens strain 7A001, displays potent antibacterial activity against Listeria monocytogenes. Food Control 2021, 121, 107630. [Google Scholar]
- Baindara, P.; Mandal, S.M.; Chawla, N.; Singh, P.K.; Pinnaka, A.K.; Korpole, S. Characterization of two antimicrobial peptides produced by a halotolerant Bacillus subtilis strain SK.DU.4 isolated from a rhizosphere soil sample. AMB Express 2013, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.H.; Holt, J.G. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Hensyl, W.R., Ed.; Williams & Wilkins: Baltimore, MD, USA, 1994; ISBN 978-0-683-00603-2. [Google Scholar]
- Azcárate-Peril, M.A.; Raya, R.R. Methods for Plasmid and Genomic DNA Isolation from Lactobacilli. In Food Microbiology Protocols. Methods in Biotechnology; Spencer, J.F.T., de Ragout Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 14. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria-A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Ebrahimipour, G.H.; Khosravibabadi, Z.; Sadeghi, H.; Aliahmadi, A. Isolation, Partial Purification and Characterization of an Antimicrobial Compound, Produced by Bacillus atrophaeus. Jundishapur J. Microbiol. 2014, 7, e11802. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ma, W.; Peng, C.; Yin, Y.; Xu, B.; Zhang, F.; Guo, Y.; Li, Z. Bacillamide C production by the optimized cultivation of the Bacillus atrophaeus strain C89 associated with the South China Sea sponge Dysidea avara. Process Biochem. 2011, 46, 1153–1159. [Google Scholar] [CrossRef]
- Ansari, A.; Aman, A.; Siddiqui, N.N.; Iqbal, S.; Ali ul Qader, S. Bacteriocin (BAC-IB17): Screening, isolation and production from Bacillus subtilis KIBGE IB-17. Pak. J. Pharm. Sci. 2012, 25, 195–201. [Google Scholar] [PubMed]
- Abada, E.A.E. Isolation and characterization of a antimicrobial compound from Bacillus coagulans. Anim. Cells Syst. 2008, 12, 41–46. [Google Scholar] [CrossRef]
- Dischinger, J.; Josten, M.; Szekat, C.; Sahl, H.G.; Bierbaum, G. Production of the Novel Two-Peptide Lantibiotic Lichenicidin by Bacillus licheniformis DSM 13. PLoS ONE 2009, 4, e6788. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Bayles, D.O. The Contribution of Lactic Acid Bacteria to Human Health. In Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 282–288. [Google Scholar]
- Hamedi, J.; Mohammadipanah, F. Biotechnological applications and potential of marine-derived microorganisms. AIMS Microbiol. 2019, 5, 233–244. [Google Scholar]
- Axelsson, L. Lactic Acid Bacteria: Classification and Physiology. In Lactic Acid Bacteria: Microbiological and Functional Aspects, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–66. [Google Scholar]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Das, S.K.; Thatoi, H. Isolation, Culture, and Biochemical Characterization of Microbes. In A Practical Guide to Environmental Biotechnology; Learning Materials in Biosciences; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Wilson-Simpson, F.; Gelinas, R.; Laviña, W.A. Antimicrobial resistance patterns of urifapathogens isolated from older adults in long-term care facilities: A scoping review. J. Gerontol. Nurs. 2021, 47, 13–20. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elazzazy, A.M.; Mobarki, M.O.; Baghdadi, A.M.; Bataweel, N.M.; Al-Hejin, A.M. Optimization of Culture Conditions and Batch Process Control for the Augmented Production of Bacteriocin by Bacillus Species. Microorganisms 2024, 12, 651. https://doi.org/10.3390/microorganisms12040651
Elazzazy AM, Mobarki MO, Baghdadi AM, Bataweel NM, Al-Hejin AM. Optimization of Culture Conditions and Batch Process Control for the Augmented Production of Bacteriocin by Bacillus Species. Microorganisms. 2024; 12(4):651. https://doi.org/10.3390/microorganisms12040651
Chicago/Turabian StyleElazzazy, Ahmed M., Mona O. Mobarki, Afra M. Baghdadi, Noor M. Bataweel, and Ahmed M. Al-Hejin. 2024. "Optimization of Culture Conditions and Batch Process Control for the Augmented Production of Bacteriocin by Bacillus Species" Microorganisms 12, no. 4: 651. https://doi.org/10.3390/microorganisms12040651




