Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 in a Rural Community Hospital during Omicron Predominance
Abstract
:1. Introduction
2. Methods
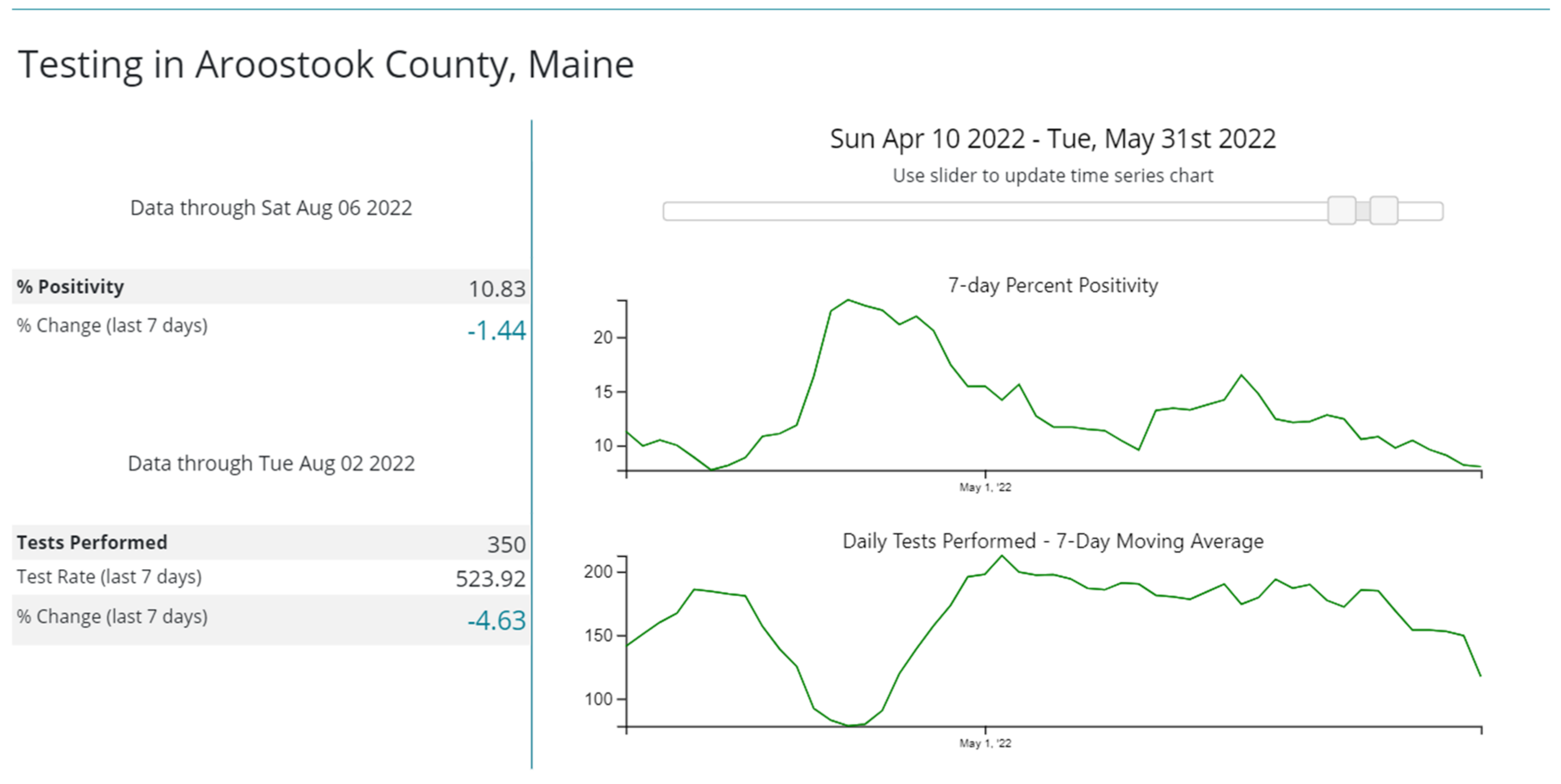
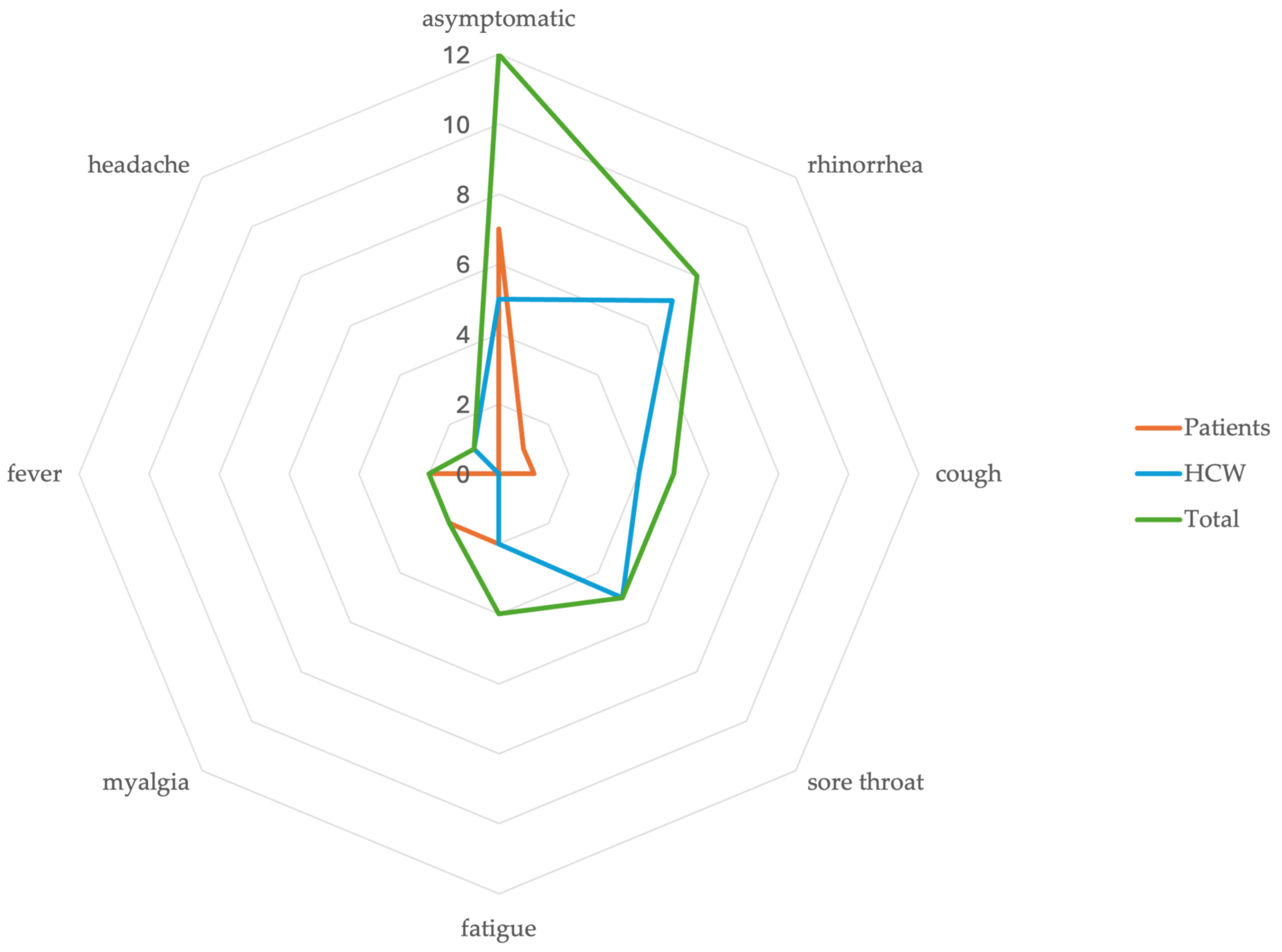
3. Results
4. Outbreak Control Measures
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 22 January 2024).
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 22 January 2024).
- Ng, C.Y.H.; Lim, N.A.; Bao, L.X.Y.; Quek, A.M.L.; Seet, R.C.S. Mitigating SARS-CoV-2 Transmission in Hospitals: A Systematic Literature Review. Public Health Rev. 2022, 43, 1604572. [Google Scholar] [CrossRef]
- Read, J.M.; Green, C.A.; Harrison, E.M.; Docherty, A.B.; Funk, S.; Harrison, J.; Girvan, M.; Hardwick, H.E.; Turtle, L.; Dunning, J.; et al. Hospital-acquired SARS-CoV-2 infection in the UK’s first COVID-19 pandemic wave. Lancet 2021, 398, 1037–1038. [Google Scholar] [CrossRef]
- Lindsey, B.B.; Villabona-Arenas, C.J.; Campbell, F.; Keeley, A.J.; Parker, M.D.; Shah, D.R.; Parsons, H.; Zhang, P.; Kakkar, N.; Gallis, M.; et al. Characterising within-hospitalSARS-CoV-2 transmission events using epidemiological and viral genomic data across two pandemic waves. Nat. Commun. 2022, 13, 671. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Constantinides, B.; Sanderson, N.; Rodger, G.; Street, T.L.; Swann, J.; Chau, K.K.; O’Donnell, D.; Warren, F.; Hoosdally, S.; et al. Epidemiological data and genome sequencing reveals that nosocomial transmission of SARS-CoV-2 is underestimated and mostly mediated by a small number of highly infectious individuals. J. Infect. 2021, 83, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Liang En, W.; Ko, K.K.; Conceicao, E.P.; Aung, M.K.; Oo, A.M.; Yong, Y.; Arora, S.; Venkatachalam, I. Enhanced infection-prevention measures including universal N95 usage and daily testing: The impact on SARS-CoV-2 transmission in cohorted hospital cubicles through successive Delta and Omicron waves. Clin. Infect. Dis. 2022, 75, 917–919. [Google Scholar]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target. Ther. 2022, 7, 141. [Google Scholar] [CrossRef]
- Rhee, C.; Baker, M.A.; Klompas, M. Survey of coronavirus disease 2019 (COVID-19) infection control policies at leading US academic hospitals in the context of the initial pandemic surge of the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) omicron variant. Infect. Control Hosp. Epidemiol. 2023, 44, 597–603. [Google Scholar] [CrossRef]
- Jinadatha, C.; Jones, L.D.; Choi, H.; Chatterjee, P.; Hwang, M.; Redmond, S.N.; Navas, M.E.; Zabarsky, T.F.; Bhullar, D.; Cadnum, J.L.; et al. Transmission of SARS-CoV-2 in Inpatient and Outpatient Settings in a Veterans Affairs Health Care System. Open Forum Infect. Dis. 2021, 8, ofab328. [Google Scholar] [CrossRef]
- Karan, A.; Klompas, M.; Tucker, R.; Baker, M.; Vaidya, V.; Rhee, C. The Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission from Patients With Undiagnosed Coronavirus Disease 2019 (COVID-19) to Roommates in a Large Academic Medical Center. Clin. Infect. Dis. 2022, 74, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.M.; Moreno, G.K.; Buys, A.; Somsen, E.D.; Bobholz, M.; Accola, M.A.; Anderson, L.; Rehrauer, W.M.; Baker, D.A.; Safdar, N.; et al. Viral Sequencing to Investigate Sources of SARS-CoV-2 Infection in US Healthcare Personnel. Clin. Infect. Dis. 2021, 73, e1329–e1336. [Google Scholar] [CrossRef]
- Roche Molecular Systems, Inc. Cobas® SARS-CoV-2 & Influenza A/B. 2020. Available online: https://www.fda.gov/media/142193/download?attachment (accessed on 22 January 2024).
- Zhen, W.; Manji, R.; Smith, E.; Berry, G.J. Comparison of Four Molecular In Vitro Diagnostic Assays for the Detection of SARS-CoV-2 in Nasopharyngeal Specimens. J. Clin. Microbiol. 2020, 58, e00743-20. [Google Scholar] [CrossRef]
- Division of Disease Surveillance, Maine Center for Disease Control & Prevention. Available online: https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/airborne/documents/SARS-CoV-2-Sequencing-Summary-07-29-2022.pdf (accessed on 26 August 2022).
- Zhang, L.; Kang, X.; Wang, L.; Yan, R.; Pan, Y.; Wang, J.; Chen, Z. Clinical and virological features of asymptomatic and mild symptomatic patients with SARS-CoV-2 Omicron infection at Shanghai Fangcang shelter hospital. Immun. Inflamm. Dis. 2023, 11, e1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Weng, Z.; Zheng, Y.; Zheng, M.; Chen, W.; He, H.; Ye, X.; Zheng, Y.; Xie, J.; Zheng, K.; et al. Epidemiological and clinical features of SARS-CoV-2 Omicron variant infection in Quanzhou, Fujian province: A retrospective study. Sci. Rep. 2023, 13, 22152. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, B.; Choi, Y.Y.; Um, J.; Lee, K.S.; Sung, H.K.; Kim, Y.; Park, J.S.; Lee, M.; Jang, H.C.; et al. Clinical Characteristics of 40 Patients Infected With the SARS-CoV-2 Omicron Variant in Korea. J. Korean Med. Sci. 2022, 37, e31. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.; Elliott, J.; Bodinier, B.; Barclay, W.; Ward, H.; Cooke, G.; Donnelly, C.A.; Chadeau-Hyam, M.; Elliott, P. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat. Commun. 2022, 13, 6856. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, W.; Ye, X.; Zhou, Y.; Zheng, Y.; Weng, Z.; Xie, J.; Zheng, K.; Su, Z.; Zhuang, X.; et al. Clinical characteristics of patients infected with novel coronavirus wild strain, Delta variant strain and Omicron variant strain in Quanzhou: A real-world study. Exp. Ther. Med. 2023, 25, 62. [Google Scholar] [CrossRef]
- Wang, R.C.; Gottlieb, M.; Montoy, J.C.C.; Rodriguez, R.M.; Yu, H.; Spatz, E.S.; Chandler, C.W.; Elmore, J.G.; Hannikainen, P.A.; Chang, A.M.; et al. Association Between SARS-CoV-2 Variants and Frequency of Acute Symptoms: Analysis of a Multi-institutional Prospective Cohort Study-December 20, 2020-June 20, 2022. Open Forum Infect. Dis. 2023, 10, ofad275. [Google Scholar] [CrossRef]
- Linsenmeyer, K.; Charness, M.E.; O’Brien, W.J.; Strymish, J.; Doshi, S.J.; Ljaamo, S.K.; Gupta, K. Vaccination Status and the Detection of SARS-CoV-2 Infection in Health Care Personnel Under Surveillance in Long-term Residential Facilities. JAMA Netw. Open 2021, 4, e2134229. [Google Scholar] [CrossRef]
- Smith, L.; Pau, S.; Fallon, S.; Cosgrove, S.E.; Curless, M.S.; Fabre, V.; Karaba, S.M.; Maragakis, L.L.; Milstone, A.M.; Sick-Samuels, A.C.; et al. Impact of weekly asymptomatic testing for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) in inpatients at an academic hospital. Infect. Control Hosp. Epidemiol. 2021, 44, 99–101. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html (accessed on 22 January 2024).
- Baker, M.A.; Rhee, C.; Tucker, R.; Badwaik, A.; Coughlin, C.; Holtzman, M.A.; Hsieh, C.; Maguire, A.; Mermel Blaeser, E.; Seetharaman, S.; et al. Rapid Control of Hospital-Based Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Clusters Through Daily Testing and Universal Use of N95 Respirators. Clin. Infect. Dis. 2022, 75, e296–e299. [Google Scholar] [CrossRef] [PubMed]
- Rosser, J.I.; Tayyar, R.; Giardina, R.; Kolonoski, P.; Kenski, D.; Shen, P.; Steinmetz, L.M.; Hung, L.Y.; Xiao, W.; Bains, K.; et al. Case-control study evaluating risk factors for SARS-CoV-2 outbreak amongst healthcare personnel at a tertiary care center. Am. J. Infect. Control 2021, 49, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.E.; Carrion, R.; Alfson, K.J.; Staples, H.M.; Jinadatha, C.; Jarvis, W.R.; Sampathkumar, P.; Chemaly, R.F.; Khawaja, F.; Povroznik, M.; et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: Implications for environmental COVID-19 control. Infect. Control Hosp. Epidemiol. 2021, 42, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Rathnasinghe, R.; Karlicek, R.F., Jr.; Schotsaert, M.; Koffas, M.; Arduini, B.L.; Jangra, S.; Wang, B.; Davis, J.L.; Alnaggar, M.; Costa, A.; et al. Scalable, effective, and rapid decontamination of SARS-CoV-2 contaminated N95 respirators using germicidal ultraviolet C (UVC) irradiation device. Sci. Rep. 2021, 11, 19970. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Chen, L.F.; Weber, D.J.; Moehring, R.W.; Lewis, S.S.; Triplett, P.F.; Blocker, M.; Becherer, P.; Schwab, J.C.; Knelson, L.P.; et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): A cluster-randomised, multicentre, crossover study. Lancet 2017, 389, 805–814. [Google Scholar] [CrossRef]
- Myhrman, S.; Olausson, J.; Ringlander, J.; Gustavsson, L.; Jakobsson, H.E.; Sansone, M.; Westin, J. Unexpected details regarding nosocomial transmission revealed by whole-genome sequencing of severe acute respiratory coronavirus virus 2 (SARS-CoV-2). Infect. Control Hosp. Epidemiol. 2022, 43, 1403–1407. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishna, A.; Tutt, J.; Grewal, M.; Bragdon, S.; Moreshead, S. Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 in a Rural Community Hospital during Omicron Predominance. Microorganisms 2024, 12, 686. https://doi.org/10.3390/microorganisms12040686
Krishna A, Tutt J, Grewal M, Bragdon S, Moreshead S. Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 in a Rural Community Hospital during Omicron Predominance. Microorganisms. 2024; 12(4):686. https://doi.org/10.3390/microorganisms12040686
Chicago/Turabian StyleKrishna, Amar, Julie Tutt, Mehr Grewal, Sheila Bragdon, and Suzanne Moreshead. 2024. "Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 in a Rural Community Hospital during Omicron Predominance" Microorganisms 12, no. 4: 686. https://doi.org/10.3390/microorganisms12040686
APA StyleKrishna, A., Tutt, J., Grewal, M., Bragdon, S., & Moreshead, S. (2024). Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 in a Rural Community Hospital during Omicron Predominance. Microorganisms, 12(4), 686. https://doi.org/10.3390/microorganisms12040686






