Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs
Abstract
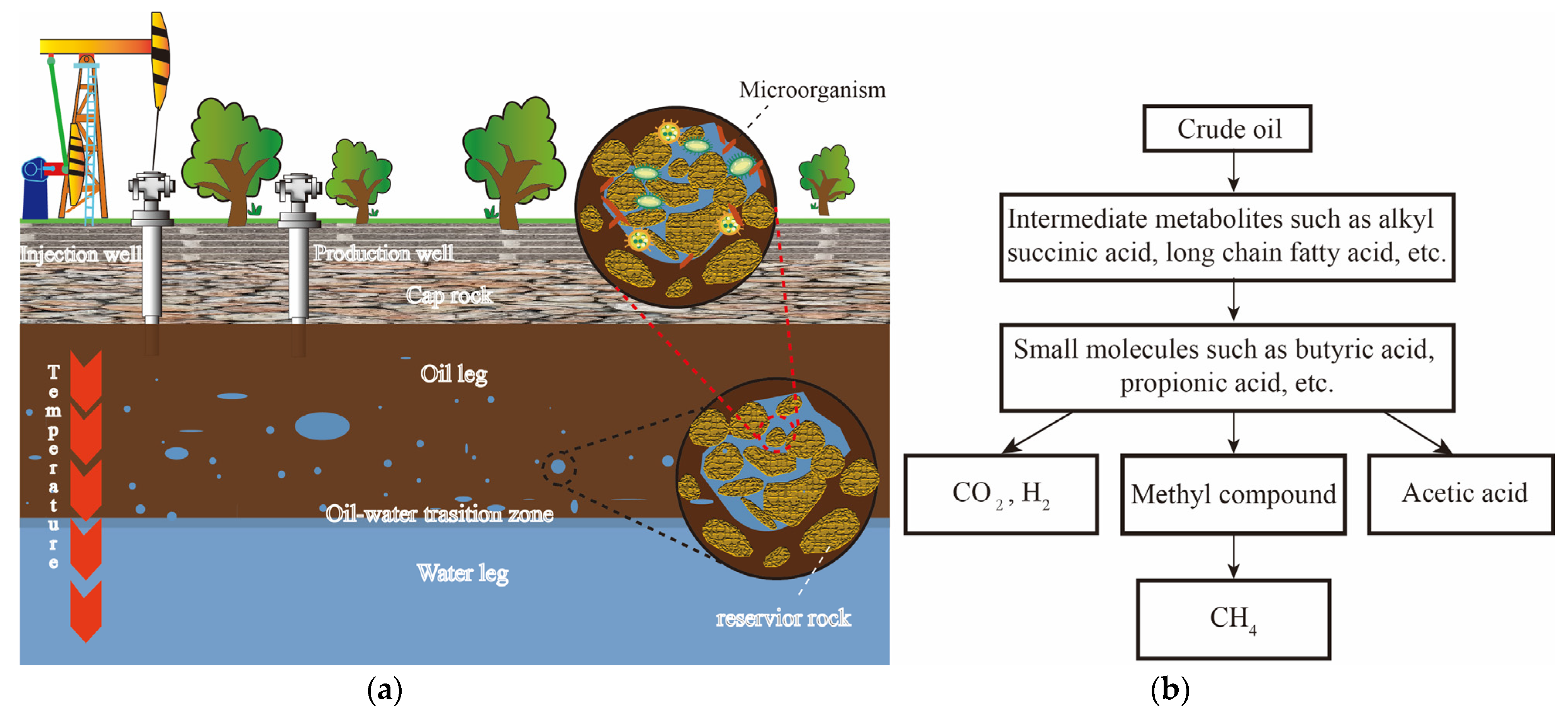
1. Introduction
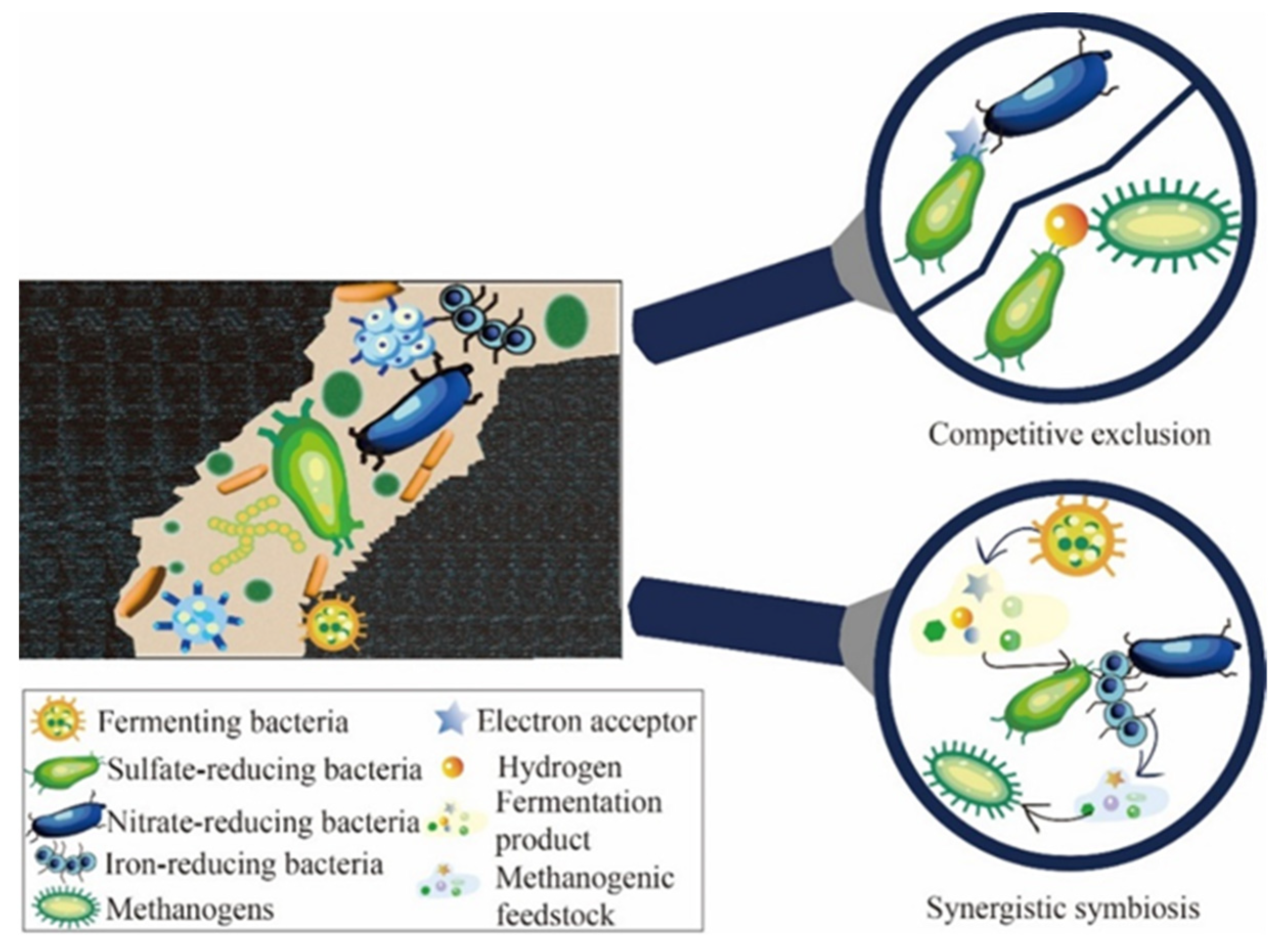
2. Microbial Flora Involved in Gasified Degradation of Crude Oil and Their Synergistic Effects
3. Metabolic Mechanisms of Reservoir Crude Oil Gasification
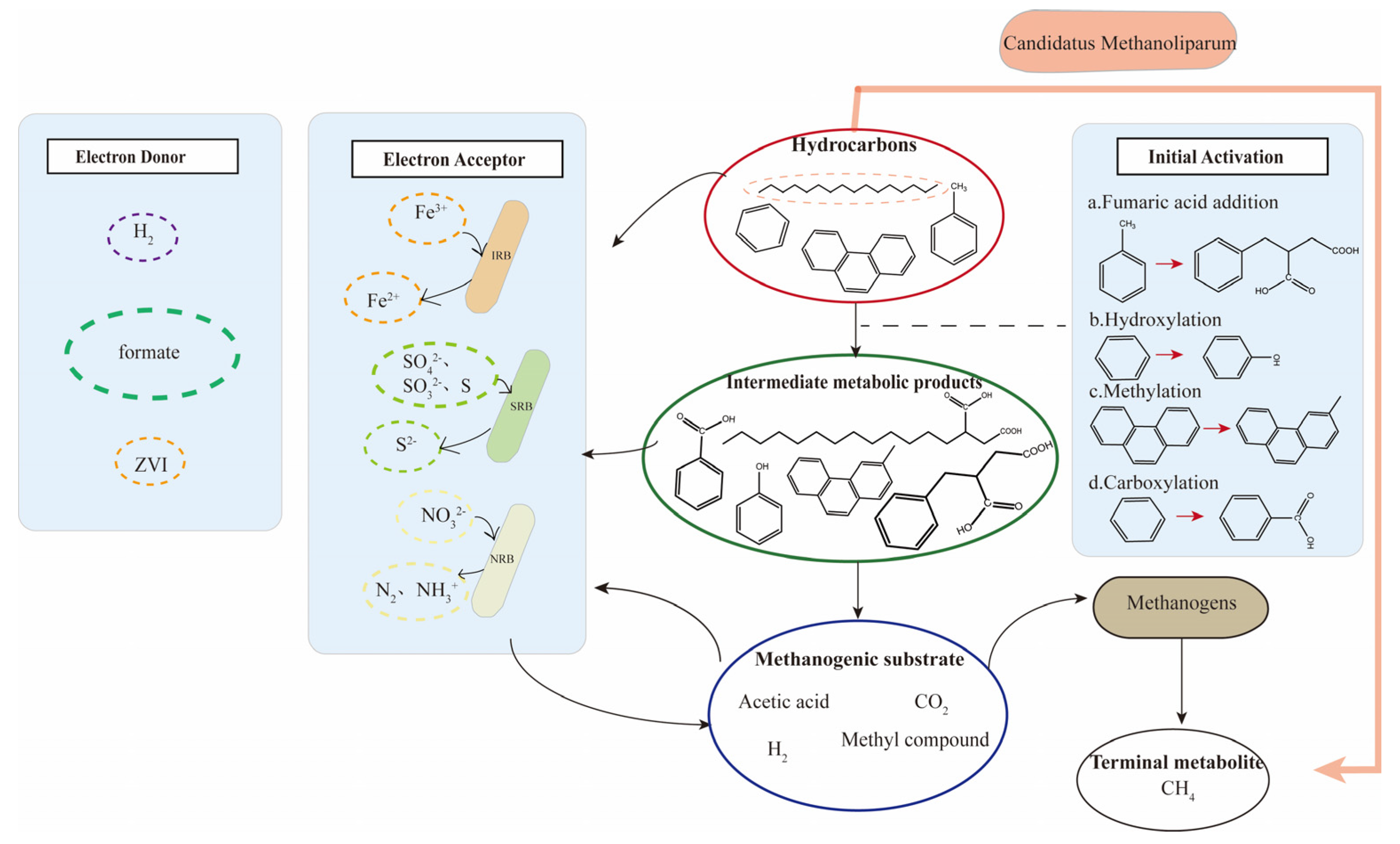
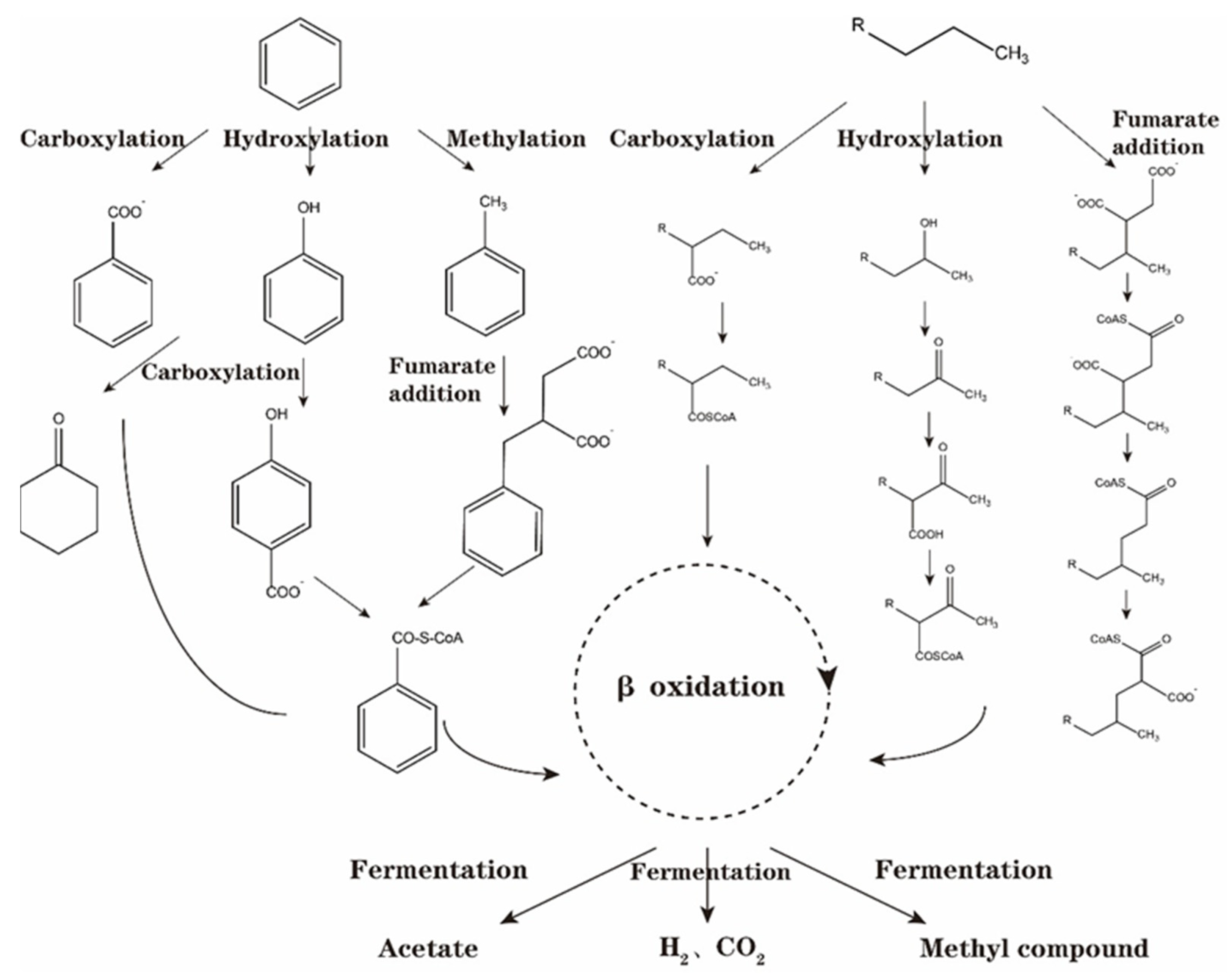
3.1. Mechanism of Anaerobic Degradation Activation in Reservoir Crude Oil
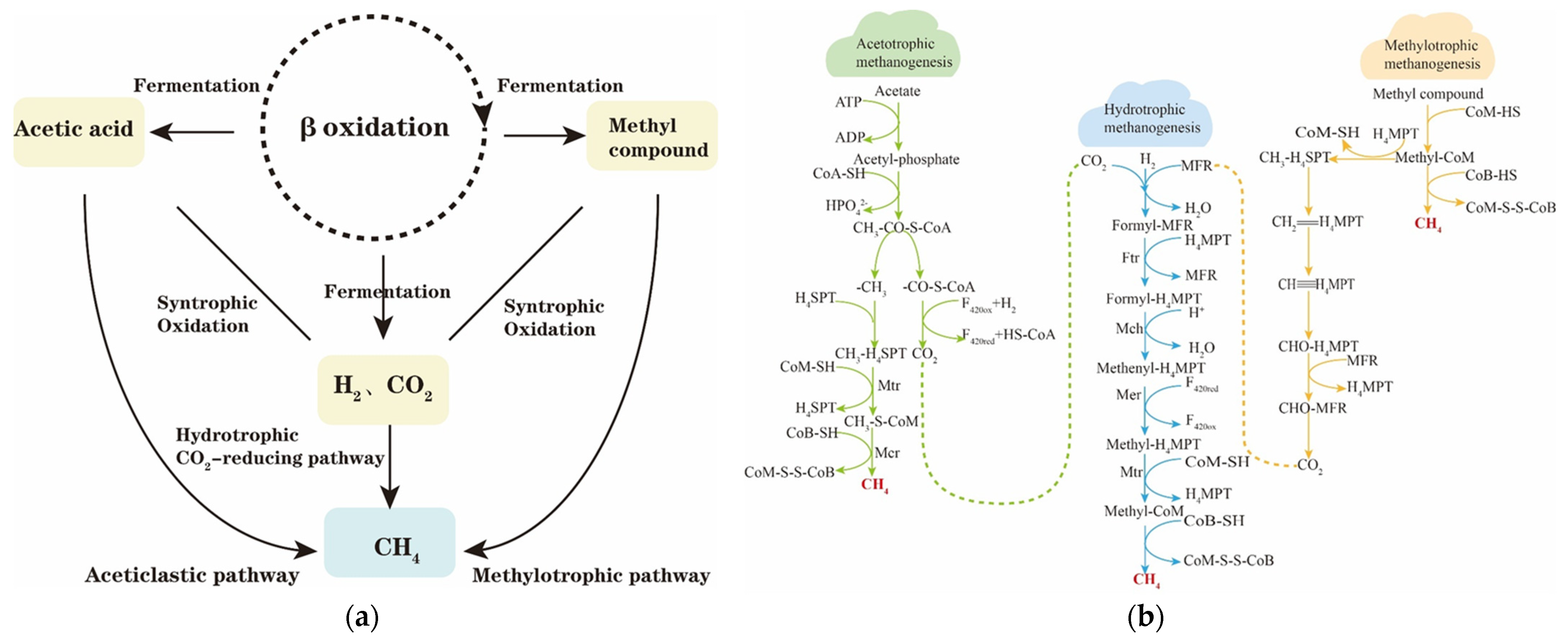
3.2. Mechanism of Methanogenesis
4. Assessment of the Significance of Various Factors Influencing the Efficiency of the Reservoir Crude Oil Gasification Process
4.1. Factors Affecting the Efficiency of Crude Oil Gasification
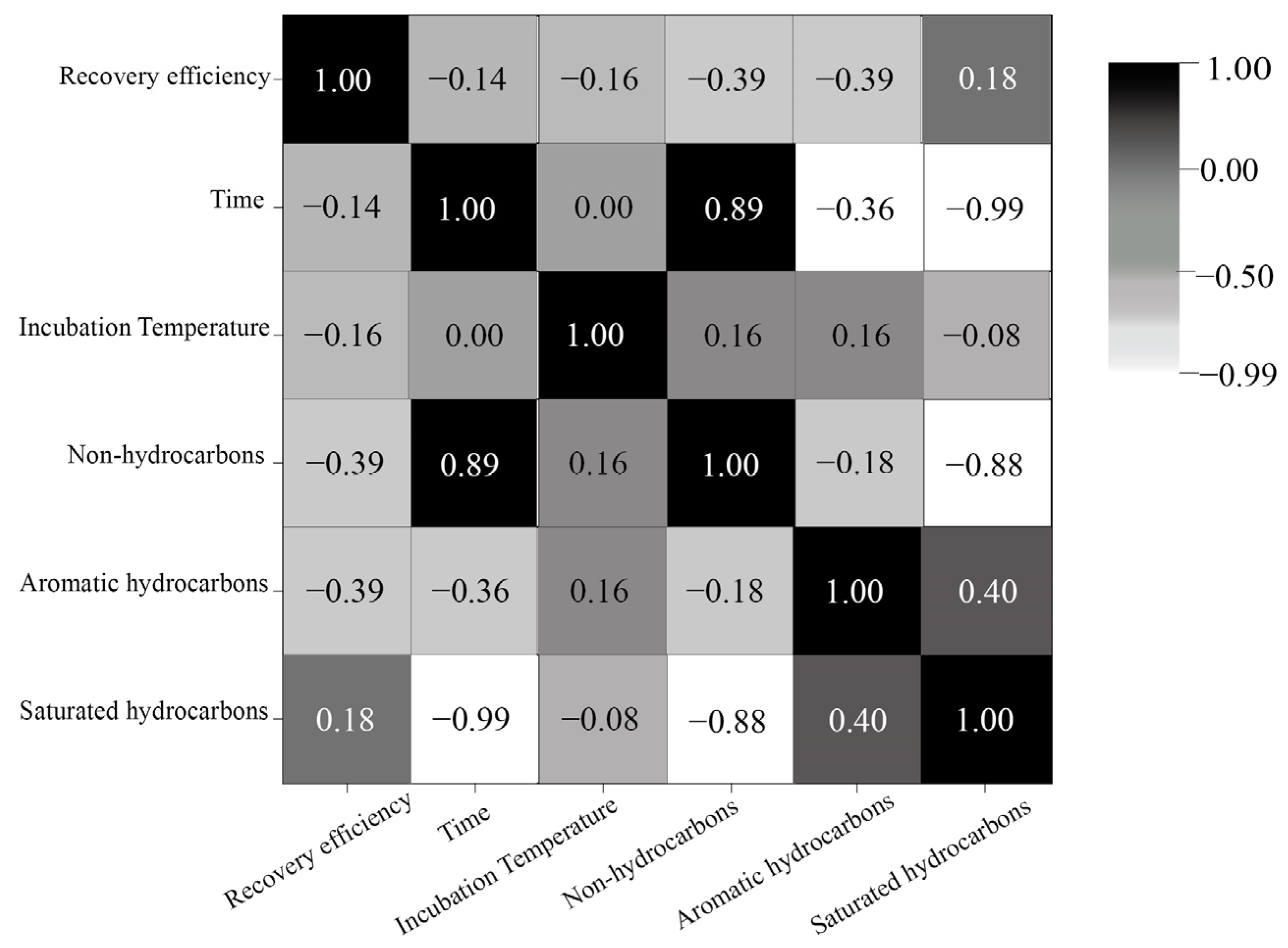
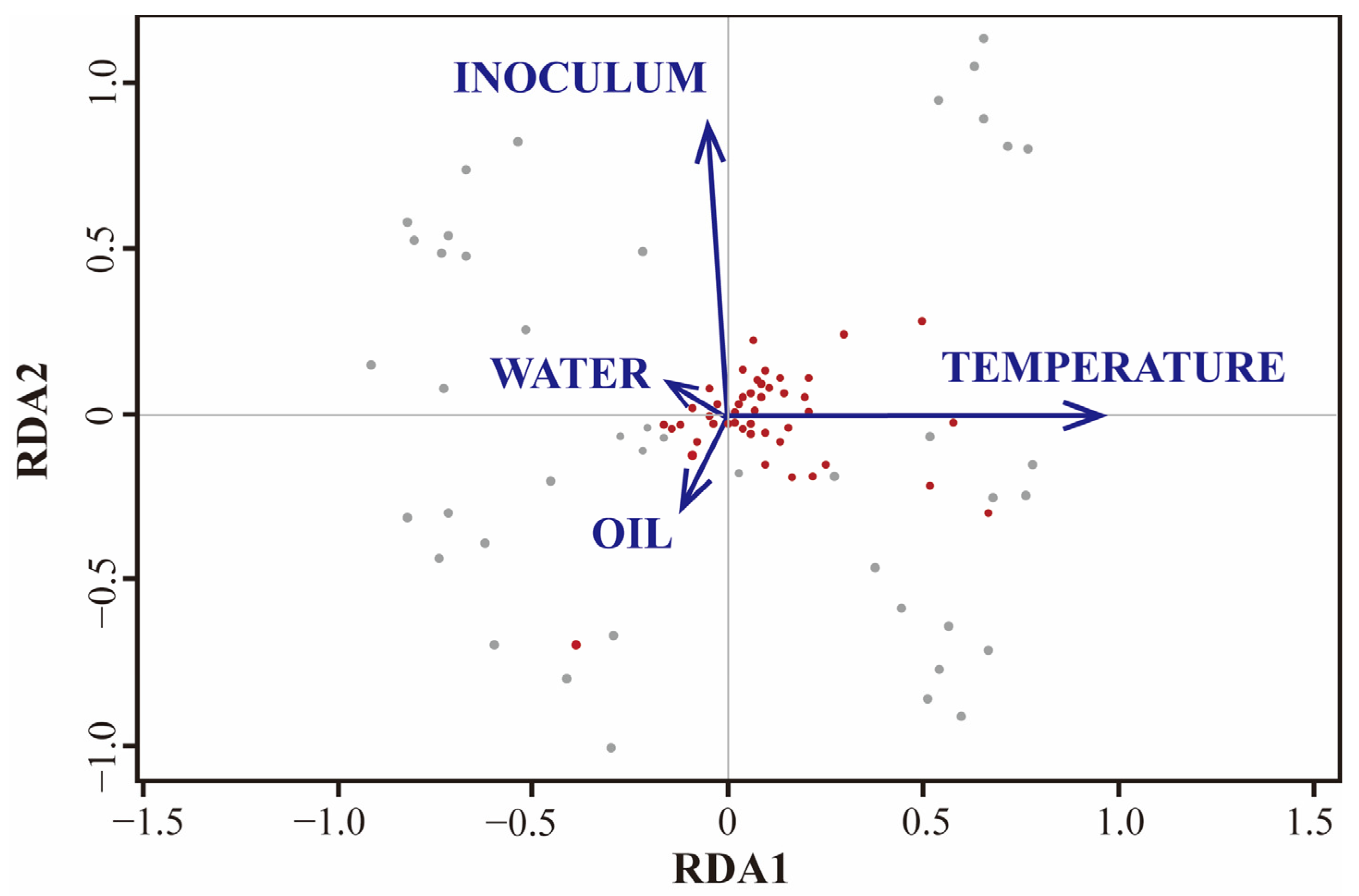
4.2. Multivariate Importance Evaluation Methods for Enhancing Oil Reservoir Gasification Efficiency
5. Opportunities and Challenges of Microbial Assisted Crude Oil Gasification in Practical Applications
6. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youssef, N.; Elshahed, M.S.; McInerney, M.J. Microbial processes in oil fields: Culprits, problems, and opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [PubMed]
- Zengler, K.; Richnow, H.H.; Rosselló-Mora, R.; Michaelis, W.; Widdel, F. Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 1999, 401, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.T.; Lovley, D.R. Naphthalene and benzene degradation under Fe (III)-reducing conditions in petroleum-contaminated aquifers. Bioremediat. J. 1999, 3, 121–135. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, S.; Li, Q.; Chen, J.; Zhang, H.; Lu, Y. Progressive degradation of crude oil n-alkanes coupled to methane production under mesophilic and thermophilic conditions. PLoS ONE 2014, 9, e113253. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Xiong, Y.; Hou, Z.; Xie, H.; Zhao, J.; Tan, X.; Luo, J. Microbial-mediated CO2 methanation and renewable natural gas storage in depleted petroleum reservoirs: A review of biogeochemical mechanism and perspective. Gondwana Res. 2022, 22, 184–198. [Google Scholar] [CrossRef]
- Bian, X.-Y.; Maurice Mbadinga, S.; Liu, Y.-F.; Yang, S.-Z.; Liu, J.-F.; Ye, R.-Q.; Gu, J.-D.; Mu, B.-Z. Insights into the anaerobic biodegradation pathway of n-alkanes in oil reservoirs by detection of signature metabolites. Sci. Rep. 2015, 5, 9801. [Google Scholar] [CrossRef]
- Xia, W.; Shen, W.; Yu, L.; Zheng, C.; Yu, W.; Tang, Y. Conversion of petroleum to methane by the indigenous methanogenic consortia for oil recovery in heavy oil reservoir. Appl. Energy 2016, 171, 646–655. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, K.; Li, B.-G.; Mbadinga, S.M.; Zhou, L.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Simulation of in situ oil reservoir conditions in a laboratory bioreactor testing for methanogenic conversion of crude oil and analysis of the microbial community. Int. Biodeterior. Biodegrad. 2019, 136, 24–33. [Google Scholar] [CrossRef]
- Bueno de Mesquita, C.P.; Wu, D.; Tringe, S.G. Methyl-based methanogenesis: An ecological and genomic review. Microbiol. Mol. Biol. Rev. 2023, 87, e00024-22. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 1987, 56, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; De Vrieze, J.; He, G.; Li, X.; Li, J. Temperature regulates methane production through the function centralization of microbial community in anaerobic digestion. Bioresour. Technol. 2016, 216, 150–158. [Google Scholar] [CrossRef]
- Murdoch, W.J.; Singh, C.; Kumbier, K.; Abbasi-Asl, R.; Yu, B. Definitions, methods, and applications in interpretable machine learning. Proc. Natl. Acad. Sci. USA 2019, 116, 22071–22080. [Google Scholar] [CrossRef] [PubMed]
- Conard, A.M.; DenAdel, A.; Crawford, L. A spectrum of explainable and interpretable machine learning approaches for genomic studies. Wiley Interdiscip. Rev.-Comput. Stat. 2023, 15, e1617. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Chen, C.; Yan, B.; Tao, J.; Chen, G. Interpretable machine learning for predicting and evaluating hydrogen production via supercritical water gasification of biomass. J. Clean. Prod. 2021, 316, 128244. [Google Scholar] [CrossRef]
- Soeder, D.J. Greenhouse gas sources and mitigation strategies from a geosciences perspective. Adv. Geo-Energy Res. 2021, 5, 274–285. [Google Scholar] [CrossRef]
- Dou, L.; Sun, L.; Lyu, W.; Wang, M.; Gao, F.; Gao, M.; Jiang, H. Trend of global carbon dioxide capture, utilization and storage industry and challenges and countermeasures in China. Pet. Explor. Dev. 2023, 50, 1246–1260. [Google Scholar] [CrossRef]
- Tyne, R.; Barry, P.; Lawson, M.; Byrne, D.; Warr, O.; Xie, H.; Hillegonds, D.; Formolo, M.; Summers, Z.; Skinner, B. Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs. Nature 2021, 600, 670–674. [Google Scholar] [CrossRef]
- Duncan, K.E.; Gieg, L.M.; Parisi, V.A.; Tanner, R.S.; Tringe, S.G.; Bristow, J.; Suflita, J.M. Biocorrosive thermophilic microbial communities in Alaskan North Slope oil facilities. Environ. Sci. Technol. 2009, 43, 7977–7984. [Google Scholar] [CrossRef]
- Orphan, V.; Goffredi, S.; Delong, E.; Boles, J. Geochemical influence on diversity and microbial processes in high temperature oil reservoirs. Geomicrobiol. J. 2003, 20, 295–311. [Google Scholar] [CrossRef]
- Stetter, K.O.; Huber, R.; Blöchl, E.; Kurr, M.; Eden, R.; Fielder, M.; Cash, H.; Vance, I. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 1993, 365, 743–745. [Google Scholar] [CrossRef]
- Nazina, T.; Sokolova, D.; Grouzdev, D.; Semenova, E.; Babich, T.; Bidzhieva, S.; Serdukov, D.; Volkov, D.; Bugaev, K.; Ershov, A.; et al. The Potential Application of Microorganisms for Sustainable Petroleum Recovery from Heavy Oil Reservoirs. Sustainability 2020, 12, 15. [Google Scholar] [CrossRef]
- Bogatyrenko, E.A.; Kim, A.V.; Polonik, N.S.; Dunkai, T.I.; Ponomareva, A.L.; Dashkov, D.V. Psychrotrophic Hydrocarbon-Oxidizing Bacteria Isolated from Bottom Sediments of Peter the Great Bay, Sea of Japan. Oceanology 2022, 62, 379–389. [Google Scholar] [CrossRef]
- Grouzdev, D.S.; Sokolova, D.S.; Poltaraus, A.B.; Nazina, T.N. Draft Genome Sequence of Halomonas titanicae Strain TAT1, a Hydrocarbon-Oxidizing Halophilic Bacterium Isolated from a Petroleum Reservoir in Russia. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Semenova, E.M.; Ershov, A.P.; Sokolova, D.S.; Tourova, T.P.; Nazina, T.N. Diversity and Biotechnological Potential of Nitrate-Reducing Bacteria from Heavy-Oil Reservoirs (Russia). Microbiology 2020, 89, 685–696. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wei, F.D.; Xu, R.; Cheng, T.; Ma, Y.L. Characterization and genomic analysis of a nitrate reducing bacterium from shale oil in the Ordos Basin and the associated biosurfactant production. J. Environ. Chem. Eng. 2022, 10, 108776. [Google Scholar] [CrossRef]
- Jurelevicius, D.; Ramos, L.; Abreu, F.; Lins, U.; de Sousa, M.P.; dos Santos, V.; Penna, M.; Seldin, L. Long-term souring treatment using nitrate and biocides in high-temperature oil reservoirs. Fuel 2021, 288, 119731. [Google Scholar] [CrossRef]
- Marietou, A. Sulfate reducing microorganisms in high temperature oil reservoirs. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 116, pp. 99–131. [Google Scholar]
- Gao, P.K.; Fan, K.Y. Sulfur-oxidizing bacteria (SOB) and sulfate-reducing bacteria (SRB) in oil reservoir and biological control of SRB: A review. Arch. Microbiol. 2023, 205, 162. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, F.; Xu, T.; Liu, Y.L.; Du, Y.; Wang, C.; Liu, T.S.; Gao, J.; He, Y.L.; Wang, X.T.; et al. Culture-dependent and culture-independent methods reveal microbe-clay mineral interactions by dissimilatory iron-reducing bacteria in an integral oilfield. Sci. Total Environ. 2022, 840, 156577. [Google Scholar] [CrossRef]
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Q.Q.; Lu, Y.W.; Mbadinga, S.M.; Liu, Y.F.; Li, X.X.; Wang, B.; Lv, H.M.; Liu, J.F.; Yang, S.Z.; et al. Dominant and Active Methanogens in the Production Waters From a High-Temperature Petroleum Reservoir by DNA- and RNA-Based Analysis. Geomicrobiol. J. 2021, 38, 191–198. [Google Scholar] [CrossRef]
- Christman, G.D.; León-Zayas, R.I.; Summers, Z.M.; Biddle, J.F. Methanogens Within a High Salinity Oil Reservoir From the Gulf of Mexico. Front. Microbiol. 2020, 11, 570714. [Google Scholar] [CrossRef] [PubMed]
- Aitken, C.M.; Jones, D.M.; Maguire, M.J.; Gray, N.D.; Sherry, A.; Bowler, B.F.J.; Ditchfield, A.K.; Larter, S.R.; Head, I.M. Evidence that crude oil alkane activation proceeds by different mechanisms under sulfate-reducing and methanogenic conditions. Geochim. Cosmochim. Acta 2013, 109, 162–174. [Google Scholar] [CrossRef]
- Wentzel, A.; Ellingsen, T.E.; Kotlar, H.-K.; Zotchev, S.B.; Throne-Holst, M. Bacterial metabolism of long-chain n-alkanes. Appl. Microbiol. Biotechnol. 2007, 76, 1209–1221. [Google Scholar] [CrossRef]
- Aeckersberg, F.; Bak, F.; Widdel, F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 1991, 156, 5–14. [Google Scholar] [CrossRef]
- Liu, C.; Gu, R.; Zhu, L. Modified Biological Carbon Immobilized Polycyclic Aromatic Hydrocarbon Degradation Mixed Microbial Inoculum for Degrading Polycyclic Aromatic Hydrocarbon and Repairing Polycyclic Aromatic Hydrocarbon Contaminated Soil, Comprises Manganese Dioxide Modified Biological Carbon and Microorganisms Flora. CN115369108-A, 22 August 2022. [Google Scholar]
- Radice, R.P.; De Fabrizio, V.; Donadoni, A.; Scopa, A.; Martelli, G. Crude Oil Bioremediation: From Bacteria to Microalgae. Processes 2023, 11, 442. [Google Scholar] [CrossRef]
- Laczi, K.; Kis, A.E.; Szilagyi, A.; Bounedjoum, N.; Bodor, A.; Vincze, G.E.; Kovacs, T.; Rakhely, G.; Perei, K. New Frontiers of Anaerobic Hydrocarbon Biodegradation in the Multi-Omics Era. Front. Microbiol. 2020, 11, 590049. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 425106. [Google Scholar] [CrossRef]
- Mbadinga, S.M.; Li, K.-P.; Zhou, L.; Wang, L.-Y.; Yang, S.-Z.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Analysis of alkane-dependent methanogenic community derived from production water of a high-temperature petroleum reservoir. Appl. Microbiol. Biotechnol. 2012, 96, 531–542. [Google Scholar] [CrossRef]
- Ping, H.; Li, Z.; Zhi-Song, C.; Xiu-Chun, G.; Li, T. Isolation, identification and diversity analysis of petroleum-degrading bacteria in Shengli Oil Field wetland soil. Yingyong Shengtai Xuebao 2009, 20, 1202–1208. [Google Scholar]
- Zhou, L.; Li, K.-P.; Mbadinga, S.M.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Analyses of n-alkanes degrading community dynamics of a high-temperature methanogenic consortium enriched from production water of a petroleum reservoir by a combination of molecular techniques. Ecotoxicology 2012, 21, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, J.; Bai, Z.; Hecker, M.; Giesy, J.P. Distribution of petroleum degrading genes and factor analysis of petroleum contaminated soil from the Dagang Oilfield, China. Sci. Rep. 2015, 5, 11068. [Google Scholar] [CrossRef]
- Liu, B.; Ju, M.; Liu, J.; Wu, W.; Li, X. Isolation, identification, and crude oil degradation characteristics of a high-temperature, hydrocarbon-degrading strain. Mar. Pollut. Bull. 2016, 106, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Yu, C.; Wang, R.; Si, Y.; Masakorala, K.; Yuan, H.; Yao, J.; Zhang, J. Effects of oxygen injection on oil biodegradation and biodiversity of reservoir microorganisms in Dagang oil field, China. Int. Biodeterior. Biodegrad. 2015, 98, 59–65. [Google Scholar] [CrossRef]
- Kryachko, Y.; Dong, X.; Sensen, C.W.; Voordouw, G. Compositions of microbial communities associated with oil and water in a mesothermic oil field. Antonie Van Leeuwenhoek 2012, 101, 493–506. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Caffrey, S.M.; Soh, J.; Agrawal, A.; Brown, D.; Budwill, K.; Dong, X.; Dunfield, P.F.; Foght, J.; Gieg, L.M. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ. Sci. Technol. 2013, 47, 10708–10717. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Clavijo, C.; Gieg, L.M. Conversion of crude oil to methane by a microbial consortium enriched from oil reservoir production waters. Front. Microbiol. 2014, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Penner, T.J.; Foght, J.M. Mature fine tailings from oil sands processing harbour diverse methanogenic communities. Can. J. Microbiol. 2010, 56, 459–470. [Google Scholar] [CrossRef]
- Siddique, T.; Penner, T.; Semple, K.; Foght, J.M. Anaerobic biodegradation of longer-chain n-alkanes coupled to methane production in oil sands tailings. Environ. Sci. Technol. 2011, 45, 5892–5899. [Google Scholar] [CrossRef]
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of short-chain n-alkanes in oil sands tailings under methanogenic conditions. Environ. Sci. Technol. 2006, 40, 5459–5464. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Mochimaru, H.; Tamaki, H.; Yamamoto, K.; Yoshioka, H.; Suzuki, Y.; Kamagata, Y.; Sakata, S. Methane production from coal by a single methanogen. Science 2016, 354, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, Y.-N.; Evans, P.N.; Guo, Q.; Zhou, F.; Nie, M.; Jin, Q.; Zhang, Y.; Zhai, X.; Zhou, M. Evidence for nontraditional mcr-containing archaea contributing to biological methanogenesis in geothermal springs. Sci. Adv. 2023, 9, eadg6004. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, Y.Y.; Yao, H.Y.; Chapman, S.J. Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci. Total Environ. 2022, 823, 153636. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.Q.H.; Enault, F.; Midoux, C.; Mariadassou, M.; Chapleur, O.; Mazéas, L.; Loux, V.; Bouchez, T.; Krupovic, M.; Bize, A. Diversity of novel archaeal viruses infecting methanogens discovered through coupling of stable isotope probing and metagenomics. Environ. Microbiol. 2022, 24, 4853–4868. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, M.; Groussin, M.; Gouy, M.; Brochier-Armanet, C. The Molecular Determinants of Thermoadaptation: Methanococcales as a Case Study. Mol. Biol. Evol. 2021, 38, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.Z.; Huang, X.Y.; Ma, R.J.; Li, J.T.; Wang, F.P.; Jiao, N.Z.; Zhang, R. Potential metabolic and genetic interaction among viruses, methanogen and methanotrophic archaea, and their syntrophic partners. Isme Commun. 2022, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.F.A.; Day, L.A.; Costa, K.C. Formate-Dependent Heterodisulfide Reduction in a Methanomicrobiales Archaeon. Appl. Environ. Microbiol. 2021, 87, e02698-20. [Google Scholar] [CrossRef] [PubMed]
- Weidenbach, K.; Wolf, S.; Kupczok, A.; Kern, T.; Fischer, M.A.; Reetz, J.; Urbanska, N.; Künzel, S.; Schmitz, R.A.; Rother, M. Characterization of Blf4, an Archaeal Lytic Virus Targeting a Member of the Methanomicrobiales. Viruses 2021, 13, 1934. [Google Scholar] [CrossRef]
- Thomas, C.M.; Taib, N.; Gribaldo, S.; Borrel, G. Comparative genomic analysis of Methanimicrococcus blatticola provides insights into host adaptation in archaea and the evolution of methanogenesis. Isme Commun. 2021, 1, 47. [Google Scholar] [CrossRef]
- García-Maldonado, J.Q.; Latisnere-Barragán, H.; Escobar-Zepeda, A.; Cadena, S.; Ramírez-Arenas, P.J.; Vázquez-Juárez, R.; Rojas-Contreras, M.; López-Cortés, A. Revisiting Microbial Diversity in Hypersaline Microbial Mats from Guerrero Negro for a Better Understanding of Methanogenic Archaeal Communities. Microorganisms 2023, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Dridi, B.; Raoult, D.; Drancourt, M. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of Archaea: Towards the universal identification of living organisms. Apmis 2012, 120, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Brugère, J.F.; Borrel, G.; Gaci, N.; Tottey, W.; O’Toole, P.W.; Malpuech-Brugère, C. Archaebiotics Proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 2014, 5, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [PubMed]
- Gieg, L.M.; Davidova, I.A.; Duncan, K.E.; Suflita, J.M. Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ. Microbiol. 2010, 12, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.; Hubert, C. Mechanisms and monitoring of oil reservoir souring control by nitrate or perchlorate injection. Microb. Communities Util. Hydrocarb. Lipids Memb. Metagenomics Ecophysiol. 2019, 225, 249. [Google Scholar]
- Fan, F.; Zhang, B.; Liu, J.; Cai, Q.; Lin, W.; Chen, B. Towards sulfide removal and sulfate reducing bacteria inhibition: Function of biosurfactants produced by indigenous isolated nitrate reducing bacteria. Chemosphere 2020, 238, 124655. [Google Scholar] [CrossRef]
- Stucki, G.; Hanselmann, K.W.; Hürzeler, R.A. Biological sulfuric acid transformation: Reactor design and process optimization. Biotechnol. Bioeng. 1993, 41, 303–315. [Google Scholar] [CrossRef]
- Overton, E.B.; Wade, T.L.; Radović, J.R.; Meyer, B.M.; Miles, M.S.; Larter, S.R. Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 2016, 29, 50–63. [Google Scholar] [CrossRef]
- Difan, H.; Jinchao, L.; Dajiang, Z. Maturation sequence of continental crude oils in hydrocarbon basins in China and its significance. Org. Geochem. 1990, 16, 521–529. [Google Scholar] [CrossRef]
- Olajire, A.; Essien, J. Aerobic degradation of petroleum components by microbial consortia. J. Pet. Environ. Biotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Wang, B.; Kuang, S.; Shao, H.; Wang, L.; Wang, H. Anaerobic-petroleum degrading bacteria: Diversity and biotechnological applications for improving coastal soil. Ecotoxicol. Environ. Saf. 2021, 224, 112646. [Google Scholar] [CrossRef]
- Rajbongshi, A.; Gogoi, S.B. A review on anaerobic microorganisms isolated from oil reservoirs. World J. Microbiol. Biotechnol. 2021, 37, 111. [Google Scholar] [CrossRef]
- Wartell, B.; Boufadel, M.; Rodriguez-Freire, L. An effort to understand and improve the anaerobic biodegradation of petroleum hydrocarbons: A literature review. Int. Biodeterior. Biodegrad. 2021, 157, 105156. [Google Scholar] [CrossRef]
- Biegert, T.; Fuchs, G.; Heider, J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 1996, 238, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R.; Widdel, F. Utilization of alkylbenzenes during anaerobic growth of pure cultures of denitrifying bacteria on crude oil. Appl. Environ. Microbiol. 1996, 62, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Kniemeyer, O.; Fischer, T.; Wilkes, H.; Glöckner, F.O.; Widdel, F. Anaerobic degradation of ethylbenzene by a new type of marine sulfate-reducing bacterium. Appl. Environ. Microbiol. 2003, 69, 760–768. [Google Scholar] [CrossRef]
- Mbadinga, S.M.; Wang, L.-Y.; Zhou, L.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Microbial communities involved in anaerobic degradation of alkanes. Int. Biodeterior. Biodegrad. 2011, 65, 1–13. [Google Scholar] [CrossRef]
- Agrawal, A.; Gieg, L.M. In situ detection of anaerobic alkane metabolites in subsurface environments. Front. Microbiol. 2013, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Herath, A.; Wawrik, B.; Qin, Y.; Zhou, J.; Callaghan, A.V. Transcriptional response of Desulfatibacillum alkenivorans AK-01 to growth on alkanes: Insights from RT-qPCR and microarray analyses. FEMS Microbiol. Ecol. 2016, 92, fiw062. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of volatile organic compounds and their effects on biodegradability under co-existing conditions. Microbes Environ. 2017, 32, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic aromatic hydrocarbons: A critical review of environmental occurrence and bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Boll, M.; Estelmann, S.; Heider, J. Anaerobic degradation of hydrocarbons: Mechanisms of hydrocarbon activation in the absence of oxygen. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2020; pp. 3–29. [Google Scholar]
- Abu Laban, N.; Selesi, D.; Rattei, T.; Tischler, P.; Meckenstock, R.U. Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ. Microbiol. 2010, 12, 2783–2796. [Google Scholar] [CrossRef]
- Ji, J.-H.; Liu, Y.-F.; Zhou, L.; Irfan, M.; Mbadinga, S.M.; Pan, P.; Chen, J.; Liu, J.-F.; Yang, S.-Z.; Sand, W. Methanogenic biodegradation of C13 and C14 n-alkanes activated by addition to fumarate. Int. Biodeterior. Biodegrad. 2020, 153, 104994. [Google Scholar] [CrossRef]
- Gieg, L.M.; Toth, C.R. Signature metabolite analysis to determine in situ anaerobic hydrocarbon biodegradation. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2020; pp. 361–390. [Google Scholar]
- Tremblay, P.-L.; Zhang, T. Functional genomics of metal-reducing microbes degrading hydrocarbons. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2020; pp. 233–253. [Google Scholar]
- Bozinovski, D.; Herrmann, S.; Richnow, H.-H.; von Bergen, M.; Seifert, J.; Vogt, C. Functional analysis of an anaerobic m-xylene-degrading enrichment culture using protein-based stable isotope probing. FEMS Microbiol. Ecol. 2012, 81, 134–144. [Google Scholar] [CrossRef]
- Godin, S.; Kubica, P.; Ranchou-Peyruse, A.; Le Hecho, I.; Patriarche, D.; Caumette, G.; Szpunar, J.; Lobinski, R. An LC-MS/MS method for a comprehensive determination of metabolites of BTEX anaerobic degradation in bacterial cultures and groundwater. Water 2020, 12, 1869. [Google Scholar] [CrossRef]
- Marozava, S.; Mouttaki, H.; Müller, H.; Laban, N.A.; Probst, A.J.; Meckenstock, R.U. Anaerobic degradation of 1-methylnaphthalene by a member of the Thermoanaerobacteraceae contained in an iron-reducing enrichment culture. Biodegradation 2018, 29, 23–39. [Google Scholar] [CrossRef]
- Heider, J.; Szaleniec, M.; Suenwoldt, K.; Boll, M. Ethylbenzene dehydrogenase and related molybdenum enzymes involved in oxygen-independent alkyl chain hydroxylation. J. Mol. Microbiol. Biotechnol. 2016, 26, 45–62. [Google Scholar] [CrossRef]
- Aeckersberg, F.; Rainey, F.A.; Widdel, F. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 1998, 170, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, A.V.; Tierney, M.; Phelps, C.D.; Young, L. Anaerobic biodegradation of n-hexadecane by a nitrate-reducing consortium. Appl. Environ. Microbiol. 2009, 75, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, A.-K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R.; Boll, M.; Heider, J.; Meckenstock, R.U.; Buckel, W.; Einsle, O.; Ermler, U.; Golding, B.T.; Gunsalus, R.P.; Kroneck, P.M. Anaerobic microbial degradation of hydrocarbons: From enzymatic reactions to the environment. Microb. Physiol. 2016, 26, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Basha, K.M.; Rajendran, A.; Thangavelu, V. Recent advances in the biodegradation of phenol: A review. Asian J. Exp. Biol. Sci. 2010, 1, 219–234. [Google Scholar]
- Toth, C.R.; Berdugo-Clavijo, C.; O’Farrell, C.M.; Jones, G.M.; Sheremet, A.; Dunfield, P.F.; Gieg, L.M. Stable isotope and metagenomic profiling of a methanogenic naphthalene-degrading enrichment culture. Microorganisms 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Li, L.; Lin, C.; Dong, W.; Shen, W.; Yong, X.; Jia, H.; Wu, X.; Zhou, J. Anaerobic biodegradation of pyrene by Klebsiella sp. LZ6 and its proposed metabolic pathway. Environ. Technol. 2018, 41, 2130–2139. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, Y.; Fan, F.; Wang, Y.; Liu, X.; Ding, A.; Dou, J. Biodegradation of benzo (a) pyrene by Microbacterium sp. strain under denitrification: Degradation pathway and effects of limiting electron acceptors or carbon source. Biochem. Eng. J. 2017, 121, 131–138. [Google Scholar] [CrossRef]
- Ye, Q.; Liang, C.; Chen, X.; Fang, T.; Wang, Y.; Wang, H. Molecular characterization of methanogenic microbial communities for degrading various types of polycyclic aromatic hydrocarbon. J. Environ. Sci. 2019, 86, 97–106. [Google Scholar] [CrossRef]
- Bergmann, F.D.; Selesi, D.; Meckenstock, R.U. Identification of new enzymes potentially involved in anaerobic naphthalene degradation by the sulfate-reducing enrichment culture N47. Arch. Microbiol. 2011, 193, 241–250. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Cunha Tarouco, P.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. Microb. Physiol. 2016, 26, 92–118. [Google Scholar] [CrossRef]
- Safinowski, M.; Meckenstock, R.U. Methylation is the initial reaction in anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Environ. Microbiol. 2006, 8, 347–352. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Kumar, M.; Lin, J.-G. Anaerobic biotransformation of fluorene and phenanthrene by sulfate-reducing bacteria and identification of biotransformation pathway. J. Hazard. Mater. 2009, 164, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Risso, C.; Smith, J.A.; Lovley, D.R. Genome-scale analysis of anaerobic benzoate and phenol metabolism in the hyperthermophilic archaeon Ferroglobus placidus. ISME J. 2012, 6, 146–157. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Mouttaki, H. Anaerobic degradation of non-substituted aromatic hydrocarbons. Curr. Opin. Biotechnol. 2011, 22, 406–414. [Google Scholar] [CrossRef]
- Selesi, D.; Meckenstock, R.U. Anaerobic degradation of the aromatic hydrocarbon biphenyl by a sulfate-reducing enrichment culture. FEMS Microbiol. Ecol. 2009, 68, 86–93. [Google Scholar] [CrossRef][Green Version]
- Kraiselburd, I.; Brüls, T.; Heilmann, G.; Kaschani, F.; Kaiser, M.; Meckenstock, R.U. Metabolic reconstruction of the genome of candidate Desulfatiglans TRIP_1 and identification of key candidate enzymes for anaerobic phenanthrene degradation. Environ. Microbiol. 2019, 21, 1267–1286. [Google Scholar] [CrossRef] [PubMed]
- Kümmel, S.; Herbst, F.-A.; Bahr, A.; Duarte, M.; Pieper, D.H.; Jehmlich, N.; Seifert, J.; von Bergen, M.; Bombach, P.; Richnow, H.H. Anaerobic naphthalene degradation by sulfate-reducing Desulfobacteraceae from various anoxic aquifers. FEMS Microbiol. Ecol. 2015, 91, fiv006. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Dolfing, J.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Tamaki, H.; Takeuchi, M.; Nakatsu, C.H.; Kamagata, Y. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 2013, 4, 1998. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Rui, J.; Li, X. Research progress in biochemical metabolic pathways of methanogenesis. J. Appl. Environ. Biol. 2015, 21, 1–9. [Google Scholar]
- Herrmann, G.; Jayamani, E.; Mai, G.; Buckel, W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 2008, 190, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Lie, T.J.; Costa, K.C.; Lupa, B.; Korpole, S.; Whitman, W.B.; Leigh, J.A. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 15473–15478. [Google Scholar] [CrossRef] [PubMed]
- Dolfing, J.; Larter, S.R.; Head, I.M. Thermodynamic constraints on methanogenic crude oil biodegradation. ISME J. 2008, 2, 442–452. [Google Scholar] [CrossRef]
- Duszenko, N.; Buan, N.R. Physiological evidence for isopotential tunneling in the electron transport chain of methane-producing archaea. Appl. Environ. Microbiol. 2017, 83, e00950-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-C.; Zhou, L.; Mbadinga, S.M.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Formate-dependent microbial conversion of CO2 and the dominant pathways of methanogenesis in production water of high-temperature oil reservoirs amended with bicarbonate. Front. Microbiol. 2016, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, C.-j.; Liu, P.-f.; Fu, L.; Laso-Pérez, R.; Yang, L.; Bai, L.-p.; Li, J.; Yang, M.; Lin, J.-z. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 2022, 601, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, S.; Xie, L. Effect of pH on anaerobic hydrogen consumption, methane production and microbial community at high temperature. Chem. Ind. Eng. Progress 2019, 38, 3816–3822. [Google Scholar]
- Waldron, P.J.; Petsch, S.T.; Martini, A.M.; Nüsslein, K. Salinity constraints on subsurface archaeal diversity and methanogenesis in sedimentary rock rich in organic matter. Appl. Environ. Microbiol. 2007, 73, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liang, B.; Wang, L.-Y.; Zhou, L.; Mbadinga, S.M.; Gu, J.-D.; Mu, B.-Z. Microbial reduction of CO2 from injected NaH13CO3 with degradation of n-hexadecane in the enrichment culture derived from a petroleum reservoir. Int. Biodeterior. Biodegrad. 2018, 127, 192–200. [Google Scholar] [CrossRef]
- Jiménez, N.; Richnow, H.H.; Vogt, C.; Treude, T.; Krüger, M. Methanogenic hydrocarbon degradation: Evidence from field and laboratory studies. Microb. Physiol. 2016, 26, 227–242. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef] [PubMed]
- De Rezende, J.R.; Oldenburg, T.B.; Korin, T.; Richardson, W.D.; Fustic, M.; Aitken, C.M.; Bowler, B.F.; Sherry, A.; Grigoryan, A.; Voordouw, G. Anaerobic microbial communities and their potential for bioenergy production in heavily biodegraded petroleum reservoirs. Environ. Microbiol. 2020, 22, 3049–3065. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhou, L.; Mbadinga, S.M.; Gu, J.-D.; Mu, B.-Z. Accelerated CO2 reduction to methane for energy by zero valent iron in oil reservoir production waters. Energy 2018, 147, 663–671. [Google Scholar] [CrossRef]
- Hendrickson, E.L.; Haydock, A.K.; Moore, B.C.; Whitman, W.B.; Leigh, J.A. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc. Natl. Acad. Sci. USA 2007, 104, 8930–8934. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, E.L.; Leigh, J.A. Roles of coenzyme F420-reducing hydrogenases and hydrogen-and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J. Bacteriol. 2008, 190, 4818–4821. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Hendrickson, E.L.; Zhang, Y.; Wang, T.; Taub, F.; Moore, B.C.; Porat, I.; Whitman, W.B.; Hackett, M.; Leigh, J.A. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real time PCR. Mol. Cell. Proteom. 2006, 5, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, T.; Hendrickson, E.L.; Lie, T.J.; Hackett, M.; Leigh, J.A. Quantitative proteomics of nutrient limitation in the hydrogenotrophic methanogen Methanococcus maripaludis. BMC Microbiol. 2009, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, A.V.; Wawrik, B.; Chadhain, S.M.N.; Young, L.Y.; Zylstra, G.J. Anaerobic alkane-degrading strain AK-01 contains two alkylsuccinate synthase genes. Biochem. Biophys. Res. Commun. 2008, 366, 142–148. [Google Scholar] [CrossRef]
- Wilkes, H.; Buckel, W.; Golding, B.T.; Rabus, R. Metabolism of hydrocarbons in n-alkane-utilizing anaerobic bacteria. J. Mol. Microbiol. Biotechnol. 2016, 26, 138–151. [Google Scholar] [CrossRef]
- Zhu, J.; Shimizu, K. The effect of pfl gene knockout on the metabolism for optically pure D-lactate production by Escherichia coli. Appl. Microbiol. Biotechnol. 2004, 64, 367–375. [Google Scholar] [CrossRef]
- Grabarse, W.; Mahlert, F.; Shima, S.; Thauer, R.K.; Ermler, U. Comparison of three methyl-coenzyme M reductases from phylogenetically distant organisms: Unusual amino acid modification, conservation and adaptation. J. Mol. Biol. 2000, 303, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Lueders, T.; Chin, K.J.; Conrad, R.; Friedrich, M. Molecular analyses of methyl-coenzyme M reductase α-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 2001, 3, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Schauer-Gimenez, A.; Bhattad, U.; Kearney, C.; Struble, C.A.; Zitomer, D.; Maki, J.S. Methyl coenzyme M reductase (mcrA) gene abundance correlates with activity measurements of methanogenic H2/CO2-enriched anaerobic biomass. Microb. Biotechnol. 2014, 7, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hochheimer, A.; Linder, D.; Thauer, R.K.; Hedderich, R. The molybdenum formylmethanofuran dehydrogenase operon and the tungsten formylmethanofuran dehydrogenase operon from Methanobacterium thermoautotrophicum: Structures and transcriptional regulation. Eur. J. Biochem. 1996, 242, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G.; Thauer, R.K. The Na+-translocating methyltransferase complex from methanogenic archaea. Biochim. Biophys. Acta (BBA)-Bioenerg. 2001, 1505, 28–36. [Google Scholar] [CrossRef]
- Hedderich, R.; Whitman, W.B. Physiology and biochemistry of the methane-producing Archaea. Prokaryotes 2006, 2, 1050–1079. [Google Scholar]
- Darlington, R.B. Multiple regression in psychological research and practice. Psychol. Bull. 1968, 69, 161. [Google Scholar] [CrossRef]
- Johnson, J.W. A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivar. Behav. Res. 2000, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Munandar, T.A.; Sumiati, S.; Rosalina, V. Pattern of symptom correlation on type of heart disease using approach of pearson correlation coefficient. IOP Conf. Ser. Mater. Sci. Eng. 2020, 830, 022086. [Google Scholar] [CrossRef]
- Kumar, A.; Abirami, S. Aspect-based opinion ranking framework for product reviews using a Spearman’s rank correlation coefficient method. Inf. Sci. 2018, 460, 23–41. [Google Scholar]
- Yang, S.; Li, X.; Gong, Z.; Li, D.; Cheng, X.; Cai, W. New Principle of Pilot Protection Based on Kendall’s Rank Correlation Coefficient. IOP Conf. Ser. Mater. Sci. Eng. 2021, 827, 012016. [Google Scholar]
- Zhao, B.; Zhang, H.; Wang, H.; Wang, Z. Factors influencing gas well productivity in fractured tight sandstone reservoir. J. Pet. Explor. Prod. Technol. 2022, 12, 183–190. [Google Scholar] [CrossRef]
- Narayanan, C.; Narayan, V. Biological wastewater treatment and bioreactor design: A review. Sustain. Environ. Res. 2019, 29, 33. [Google Scholar] [CrossRef]
- Liu, J.; Bacosa, H.P.; Liu, Z. Potential Environmental Factors Affecting Oil-Degrading Bacterial Populations in Deep and Surface Waters of the Northern Gulf of Mexico. Front. Microbiol. 2017, 7, 2131. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Ali, S.A.; Calka, B.; Bielecka, E.; Linh, N.T.T.; Pham, Q.B. Urban flood vulnerability assessment in a densely urbanized city using multi-factor analysis and machine learning algorithms. Theor. Appl. Climatol. 2022, 149, 639–659. [Google Scholar] [CrossRef]
- Thiagarajan, N.; Kitchen, N.; Xie, H.; Ponton, C.; Lawson, M.; Formolo, M.; Eiler, J. Identifying thermogenic and microbial methane in deep water Gulf of Mexico Reservoirs. Geochim. Cosmochim. Acta 2020, 275, 188–208. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Z.; Jiang, Q.; Wang, J.; Zheng, J.; Zhang, T. Analysis of factors of productivity of tight conglomerate reservoirs based on random forest algorithm. ACS Omega 2022, 7, 20390–20404. [Google Scholar] [CrossRef]
- Qi, Y.; Cai, C.-F.; Sun, P.; Wang, D.-W.; Zhu, H.-J. Crude oil cracking in deep reservoirs: A review of the controlling factors and estimation methods. Pet. Sci. 2023, 20, 1978–1997. [Google Scholar] [CrossRef]
- Wu, Y.-c.; Feng, J.-w. Development and application of artificial neural network. Wirel. Pers. Commun. 2018, 102, 1645–1656. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Kumar, L.; Chugh, M.; Kumar, S.; Kumar, K.; Sharma, J.; Bharadvaja, N. Remediation of petrorefinery wastewater contaminants: A review on physicochemical and bioremediation strategies. Process Saf. Environ. Prot. 2022, 159, 362–375. [Google Scholar] [CrossRef]
- Haghsheno, H.; Arabani, M. Geotechnical properties of oil-polluted soil: A review. Environ. Sci. Pollut. Res. 2022, 29, 32670–32701. [Google Scholar] [CrossRef] [PubMed]
- De Bertoldi, M.d.; Vallini, G.; Pera, A. The biology of composting: A review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Bai, M.; Shen, A.; Meng, L.; Zhu, J.; Song, K. Well completion issues for underground gas storage in oil and gas reservoirs in China. J. Pet. Sci. Eng. 2018, 171, 584–591. [Google Scholar] [CrossRef]
- Zou, C.; Wu, S.; Yang, Z.; Pan, S.; Wang, G.; Jiang, X.; Guan, M.; Yu, C.; Yu, Z.; Shen, Y. Progress, challenge and significance of building a carbon industry system in the context of carbon neutrality strategy. Pet. Explor. Dev. 2023, 50, 210–228. [Google Scholar] [CrossRef]




| Metabolic Process | Classes of Bacteria | Representative Strain | Refs. |
|---|---|---|---|
| Hydrocarbon degradation | Fermenting bacteria | Thermococcus, Thermoanueroubacter, Haloanaerobium | [20,21,22] |
| Hydrocarbon degradation | Hydrocarbon oxidizing bacteria | Pseudomonas, Bacillus, Acinetobacter | [23,24,25] |
| Hydrocarbon degradation | Nitrate-reducing bacteria | Deferribacteres, Firmicutes, β-proteobacteria | [26,27] |
| Hydrocarbon degradation | Sulfate-reducing bacteria | Firmicutes, δ-proteobacteria, Thermodesulfbacterium | [28,29,30] |
| Hydrocarbon degradation | Iron-reducing bacteria | Deferribacteres, Firmicutes Metallireducens, | [31,32] |
| Methanogenic | Methanogens | Methanococcales, Methanomicrobiales, Methanobacteriales | [33,34,35] |
| Origin of the Inoculum | Substrate, Temperature/°C | Bacterial Flora | Refs. |
|---|---|---|---|
| Shengli oil field, PR China | C15–C20 alkanes, 55 | Firmicutes, Thermodesulfobiaceae, Thermotogaceae, Nitrospiraceae, Dictyoglomaceae, Archaeoglobales | [42,43,44] |
| Dagang oil field, PR China | Oil, 30 | Pseudomonas, Smithella, Syntrophorhabdus, Desulfobulbus, Methanosaeta, Thermoplasma | [45,46,47] |
| Medicine Hat, Canada | Oil, 33 | Smithella, Pseudomonas, Methanosaeta, Methanoculleus, Methanobacterium | [48,49,50] |
| Mildred Lake Settling Basin, Canada | C14–C18 alkanes, 20 | Syntrophus, Desulfuromonas, Desulfobacter, Methanosaeta, Methanoculleus | [51,52,53] |
| Orders | Representatives | Degradation Substrate | Refs. |
|---|---|---|---|
| Methanobacteriales | Methanobacterium Methanobrevibacter Methanothermobacter Methanothermus | Hydrogen, carbon dioxide, formates, methanol | [56,57] |
| Methanococcales | Methanococcus Methanothermococcus Methanocaldococcus | Hydrogen, carbon dioxide, formates | [58,59] |
| Methanomicrobiales | Methanomicrobium Methanoculleus Methanogenium Methanocalculus Methanospirillum Methanofollis Methanoplanus | Hydrogen, carbon dioxide, 2-propanol, 2-butanol, acetate, 2-butanone | [60,61] |
| Methanosarcinales | Methanosarcina Methanococcoides Methanohalobium Methanohalopholus Methanolobus Metahanosaeta | Hydrogen, carbon dioxide, formates, acetate, methylamine | [62] |
| Methanopyrales | Methanopyrus | Hydrogen, carbon dioxide | [62] |
| Methanocellales | Methanocellapaludicola Methanocellaarvoryzae Methanocellaconradii | Hydrogen, carbon dioxide, formates | [63] |
| Methanomassiliicccales | Methanomassiliicoccus luminyensis | Hydrogen, methylamines, methanol | [64,65] |
| Substrate | Conditions | Process | Refs. |
|---|---|---|---|
| Alkanes | Sulfate reduction Nitrate reduction Methanogenesis | Fumarate addition→alkylsuccinate | [83,89] |
| Cycloalkanes | Sulfate reduction | Fumarate addition | [90] |
| Toluene | Sulfate reduction Nitrate reduction Iron reduction Methanogenesis | Fumarate addition→benzylsuccinate→benzoyl-CoA | [91] |
| Ethylbenzene | Sulfate reduction Nitrate reduction Iron reduction | Fumarate addition→benzylsuccinate→benzoyl-CoA | [85] |
| m-Xylene | Sulfate reduction | Fumarate addition→3-methyl benzylsuccinate→m-toluic acid | [92] |
| o-Xylene | Sulfate reduction | Fumarate addition→2-methyl benzylsuccinate→o-toluic acid | [85] |
| p-Xylene | Sulfate reduction | Fumarate addition→4-methyl benzylsuccinate→p-toluic acid | [93] |
| 2-Methylnaphthalene | Sulfate reduction Iron reduction | Fumarate addition→2-Naphthylmethyl succinate→2-naphthyl-CoA | [94] |
| Substrate | Conditions | Process | Refs. |
|---|---|---|---|
| Alkanes | Sulfate reduction Nitrate reduction | Hydroxylation | [37,87,96,97] |
| Benzene | Methanogenesis | Hydroxylation to phenol | [98] |
| Ethylbenzene | Nitrate reduction | Hydroxylation→(s)-1-phenyl ethanol | [99] |
| Phenol | Sulfate reduction Nitrate reduction Iron reduction | Hydroxylation→catechol | [100] |
| Methylated phenols | Sulfate reduction | Hydroxylation→catechols | [100] |
| Naphthalene | Methanogenesis | Hydroxylation and ring cleavage | [101] |
| Phenanthrene | Sulfate reduction Nitrate reduction | Hydroxylation→2′-Hydroxypropiophenone→Phthalic acid | [102] |
| Phenanthrene | Sulfate reduction Nitrate reduction | Hydroxylation→4-Hydroxycinnamate→p-cresol→phenol | [102] |
| Benzo(a)pyrene | Nitrate reduction | Hydroxylation→4,5-dihydro benzo(a)pyrene→chrysene | [103] |
| Substrate | Conditions | Process | Refs. |
|---|---|---|---|
| Benzene | Sulfate reduction Nitrate reduction Methanogenesis | Carboxylation→benzoate | [91] |
| Phenol | Sulfate reduction Nitrate reduction Iron reduction | Carboxylation →4-hydroxybenzoate | [109] |
| Benzoate | Sulfate reduction Nitrate reduction Iron reduction Methanogenesis | Carboxylation→benzoyl-CoA | [110] |
| 2-Methylnaphthalene | Methanogenesis | Carboxylation→2-naphthoic acid | [104] |
| Biphenyl | Sulfate reduction | Carboxylation→biphenyl-4-carboxylic acid | [111] |
| Phenanthrene | Sulfate reduction | Carboxylation→phenanthrene-2-carboxylic acid | [112] |
| Naphthalene | Sulfate reduction Iron reduction | Methylation→2-methylnaphthalene | [113] |
| Phenanthrene | Sulfate reduction | Methylation→4-methylphenol→phenol | [108] |
| Condition | Methanogenesis Reaction | ΔGθ(25 °C)/kJ mol−1CH4 | Equation | Refs. |
|---|---|---|---|---|
| Substrate: Hydrogen, formic acid Environment: Low, medium, and high temperature; pH 6.0~9.0 Bacteria: The vast majority of methanogens | Hydrotropic CO2-reducing pathway | −130.8 | [114] | |
| Substrate: Aceticlastic Environment: Low temperature; pH 6.0~8.5 Bacteria: Methanosarcina, Methanosaeta | Acetoclastic pathway | −75.8 | [114] | |
| Substrate: Methyl compound Environment: High salinity Bacteria: Methanocardiales, Methanosphaera | Methylotrophic pathway | −105.8 | [115] | |
| Substrate: Aceticlastic Environment: High temperature and low pH Bacteria: Methanothermobacter, Thermactogenium | Acetate oxidation | 55.0 | [114] |
| Condition | Variant | CH4 Production Rate | Refs. |
|---|---|---|---|
| Methane production rates in methanogenic-enrichment cultures originating from freshwater ditch sediments supplemented with different hydrocarbon substrates | Light oil | 360–420 (nmol CH4/g TOC/day) | [125] |
| n-alkanes (C12-C18) | 350–560 (nmol CH4/g TOC/day) | [125] | |
| Heavy oil | 170–250 (nmol CH4/g TOC/day) | [125] | |
| Paraffin (n-C32) | 40–70 (nmol CH4/g TOC/day) | [125] | |
| BTEX (ethylbenzene, toluene) | 30–70 (nmol CH4/g TOC/day) | [125] | |
| PAH (2-methylnapthalene) | 20–50 (nmol CH4/g TOC/day) | [125] | |
| Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions | 35 °C, pH 7.74 | 66 ± 14 (mL/L day) | [126] |
| 55 °C, pH 7.82 | 247 ± 27 (mL/L day) | [126] | |
| Microcosms were established by combining oil sands and formation water from the same oil sands reservoir as the only sources of microbial inoculum and organic carbon, amended with different electron acceptors | SO42− | 0.03 (μmol CH4/g oil sand/day) | [127] |
| CO32− | 0.15 (μmol CH4/g oil sand/day) | [127] | |
| NO3− | - | [127] | |
| Microbiological reduction of carbon dioxide into methane bioenergy through biochemical reaction by addition of zero-valent iron (ZVI) as an alternative electron donor in oil reservoir-production waters | ZVI | 61.67 (μmol CH4/L oil reservoir production water/day) | [128] |
| Without the addition of ZVI | 0.60 (μmol CH4/L oil reservoir production water/day) | [128] |
| Impact Factor Categories | Factors | Influence |
|---|---|---|
| Environmental factor | Temperature, pH, Salinity | Microbial activity and metabolic pathways |
| Trace elements, ammonia content, total nitrogen content, ammonia to alkalinity ratio, concentration of inhibitors like volatile fatty acids, ammonia, and heavy metals | Reaction rate of key enzymes | |
| Porosity, permeability, capillary force, and wettability | The contact area of the oil-water phase and microbial growth | |
| Substrate factor | Carbon source | Metabolic pathways and methane production efficiency |
| Electron acceptor | The continuation of the initial degradation and methanogenesis process | |
| Electron donor | Rate of reduction | |
| Biological factor | Degradation enzymes such as alkyl succinate synthetase, phenyl methyl succinic synthetase | Degradation of alkanes, aromatic substituents |
| Hydrogenases and dehydrogenases | Electron gain and loss | |
| Methyl-coenzyme M | Methane production efficiency |
| Common Machine Learning Algorithms | Advantage | Disadvantage | Refs. |
|---|---|---|---|
| Linear regression | High measurement rate and easy interpretation of results | Classification decision has an error rate | - |
| Logistic regression | The operation is simple and easy to understand and implement | Not suitable to deal with a large number of multi-class feature numbers or variables | - |
| Decision tree | Perform visual analysis, high running speed | Easy to overfit, easy to ignore the association within the data set | - |
| KNN | Easy to implement and high accuracy | High requirements on memory space | - |
| Artificial neural network | Strong parallel distributed processing capability and high accuracy | Black box process, hard to interpret the results | [154] |
| Random forest | Random forest can extend the importance of variables to all variables and identify these important variables, avoiding the elimination of important variables and high accuracy | Overfitting can occur in some noisy classification or regression problems | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, S.; Lv, W.; Ji, Z.; Wang, K.; Mei, Y.; Li, Y. Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs. Microorganisms 2024, 12, 702. https://doi.org/10.3390/microorganisms12040702
Ni S, Lv W, Ji Z, Wang K, Mei Y, Li Y. Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs. Microorganisms. 2024; 12(4):702. https://doi.org/10.3390/microorganisms12040702
Chicago/Turabian StyleNi, Shumin, Weifeng Lv, Zemin Ji, Kai Wang, Yuhao Mei, and Yushu Li. 2024. "Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs" Microorganisms 12, no. 4: 702. https://doi.org/10.3390/microorganisms12040702
APA StyleNi, S., Lv, W., Ji, Z., Wang, K., Mei, Y., & Li, Y. (2024). Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs. Microorganisms, 12(4), 702. https://doi.org/10.3390/microorganisms12040702






