Repeated Occurrence of Mobile Colistin Resistance Gene-Carrying Plasmids in Pathogenic Escherichia coli from German Pig Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Inclusion Criteria for Farms
2.2. Whole Genome Sequencing
2.3. Phenotypic Resistance Testing, Antimicrobial Resistance Genes, Virulence-Associated Genes
2.4. Determination of Genoserotypes, Clonotypes, Multilocus Sequence Types, Core Genome MLS Types and Phylogroups
2.5. Genomic Location of Virulence-Associated Genes and mcr Genes, Plasmid Analysis
3. Results
3.1. Farm Selection
3.2. E. coli Pathotypes
3.3. Virulence Associated Genes and Virulence Plasmids
3.4. Resistance Phenotypes and Genotypes
3.5. Multilocus Sequence Types, Clonotypes, Phylogenetic Groups
3.6. Genoserotypes
3.7. Genomic Location of mcr Genes and Plasmid Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Mora, A.; Díaz-Jiménez, D.; Flament-Simon, S.C.; Alonso, M.P.; Blanco, J.E.; Blanco, M.; Blanco, J. Swine Enteric Colibacillosis in Spain: Pathogenic Potential of mcr-1 ST10 and ST131 E. coli Isolates. Front. Microbiol. 2018, 9, 2659. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Gyles, C.L. Colibacillosis. In Disease of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 723–747. [Google Scholar]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.; van Hout, J.; Wiegel, J.; Iatridou, D.; Chantziaras, I.; de Briyne, N. Colistin Use in European Livestock: Veterinary Field Data on Trends and Perspectives for Further Reduction. Vet. Sci. 2022, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health; EMA: London, UK, 2016. [Google Scholar]
- Gunn, J.S.; Lim, K.B.; Krueger, J.; Kim, K.; Guo, L.; Hackett, M.; Miller, S.I. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 1998, 27, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Diene, S.M.; Kempf, M.; Berrazeg, M.; Bakour, S.; Gupta, S.K.; Thongmalayvong, B.; Akkhavong, K.; Somphavong, S.; Paboriboune, P.; et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: An epidemiological and molecular study. Int. J. Antimicrob. Agents 2014, 44, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016, 21, 30280. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli. mBio 2017, 8, e0054317. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017, 22, 30589. [Google Scholar] [CrossRef]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef]
- AbuOun, M.; Stubberfield, E.J.; Duggett, N.A.; Kirchner, M.; Dormer, L.; Nunez-Garcia, J.; Randall, L.P.; Lemma, F.; Crook, D.W.; Teale, C.; et al. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 2018, 73, 2904. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Q.; Li, Y.-X.; Lei, C.-W.; Zhang, A.-Y.; Wang, H.-N. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1791–1795. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhou, Y.; Li, J.; Yin, W.; Wang, S.; Zhang, S.; Shen, J.; Shen, Z.; Wang, Y. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microbes Infect. 2018, 7, 122. [Google Scholar] [CrossRef]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of Novel Mobilized Colistin Resistance Gene mcr-9 in a Multidrug-Resistant, Colistin-Susceptible Salmonella enterica Serotype Typhimurium Isolate. mBio 2019, 10, e0085319. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Caldes, C.; de Waard, J.H.; Salgado, M.S.; Villacís, M.J.; Coral-Almeida, M.; Yamamoto, Y.; Calvopiña, M. Worldwide Prevalence of mcr-mediated Colistin-Resistance Escherichia coli in Isolates of Clinical Samples, Healthy Humans, and Livestock-A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Hamame, A.; Davoust, B.; Cherak, Z.; Rolain, J.-M.; Diene, S.M. Mobile Colistin Resistance (mcr) Genes in Cats and Dogs and Their Zoonotic Transmission Risks. Pathogens 2022, 11, 698. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.-B.; Zeng, Z.-L.; Yang, X.-W.; Huang, Y.; Liu, J.-H. The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool. Res. 2017, 38, 55–80. [Google Scholar] [CrossRef]
- Dantas Palmeira, J.; V Cunha, M.; Ferreira, H.; Fonseca, C.; Tinoco Torres, R. Worldwide Disseminated IncX4 Plasmid Carrying mcr-1 Arrives to Wild Mammal in Portugal. Microbiol. Spectr. 2022, 10, e0124522. [Google Scholar] [CrossRef] [PubMed]
- Mmatli, M.; Mbelle, N.M.; Osei Sekyere, J. Global epidemiology, genetic environment, risk factors and therapeutic prospects of mcr genes: A current and emerging update. Front. Cell. Infect. Microbiol. 2022, 12, 941358. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Loureiro, D.; Henriques, A.; Ramos, F.; Pomba, C.; Domingues, S.; Da Silva, G.J. Occurrence and Biological Cost of mcr-1-Carrying Plasmids Co-harbouring Beta-Lactamase Resistance Genes in Zoonotic Pathogens from Intensive Animal Production. Antibiotics 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.; Snesrud, E.; Maybank, R.; Corey, B.; Ong, A.C.; Clifford, R.; Hinkle, M.; Whitman, T.; Lesho, E.; Schaecher, K.E. Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First Report of mcr-1 in the United States. Antimicrob. Agents Chemother. 2016, 60, 4420–4421. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.-Y.; Jiang, Y.; Ma, Q.-C.; Lu, M.-J.; Wu, H.; Wang, Z.-Y.; Jiao, X.; Wang, J. Chromosomally and Plasmid-Located mcr in Salmonella from Animals and Food Products in China. Microbiol. Spectr. 2022, 10, e0277322. [Google Scholar] [CrossRef]
- Miguela-Villoldo, P.; Moreno, M.A.; Rodríguez-Lázaro, D.; Gallardo, A.; Hernández, M.; Serrano, T.; Sáez, J.L.; de Frutos, C.; Agüero, M.; Quesada, A.; et al. Longitudinal study of the mcr-1 gene prevalence in Spanish food-producing pigs from 1998 to 2021 and its relationship with the use of polymyxins. Porc. Health Manag. 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Roschanski, N.; Falgenhauer, L.; Grobbel, M.; Guenther, S.; Kreienbrock, L.; Imirzalioglu, C.; Roesler, U. Retrospective survey of mcr-1 and mcr-2 in German pig-fattening farms, 2011–2012. Int. J. Antimicrob. Agents 2017, 50, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Göpel, L.; Prenger-Berninghoff, E.; Wolf, S.A.; Semmler, T.; Bauerfeind, R.; Ewers, C. Occurrence of Mobile Colistin Resistance Genes mcr-1–mcr-10 including Novel mcr Gene Variants in Different Pathotypes of Porcine Escherichia coli Isolates Collected in Germany from 2000 to 2021. Appl. Microbiol. 2024, 4, 70–84. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 6th ed.; CLSI supplement VET01S; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2023. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2022. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 16 December 2023).
- Weissman, S.J.; Johnson, J.R.; Tchesnokova, V.; Billig, M.; Dykhuizen, D.; Riddell, K.; Rogers, P.; Qin, X.; Butler-Wu, S.; Cookson, B.T.; et al. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genom. 2018, 4, e000192. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Dixit, O.V.A.; Vangchhia, B.; Condamine, B.; Dion, S.; Bridier-Nahmias, A.; Denamur, E.; Gordon, D. Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environ Microbiol 2019, 21, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.-H.; Bertrand, X.; Madec, J.-Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Martiny, H.-M.; Munk, P.; Brinch, C.; Szarvas, J.; Aarestrup, F.M.; Petersen, T.N. Global Distribution of mcr Gene Variants in 214 K Metagenomic Samples. mSystems 2022, 7, e0010522. [Google Scholar] [CrossRef]
- Hamame, A.; Davoust, B.; Hasnaoui, B.; Mwenebitu, D.L.; Rolain, J.-M.; Diene, S.M. Screening of colistin-resistant bacteria in livestock animals from France. Vet. Res. 2022, 53, 96. [Google Scholar] [CrossRef]
- Tu, Z.; Shui, J.; Liu, J.; Tuo, H.; Zhang, H.; Lin, C.; Feng, J.; Feng, Y.; Su, W.; Zhang, A. Exploring the abundance and influencing factors of antimicrobial resistance genes in manure plasmidome from swine farms. J. Environ. Sci. 2023, 124, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, J.; Zheng, X.; Chang, J.; Ma, J.; Wang, J.; Ji, X.; Yang, H.; Ding, B. Antimicrobial resistance surveillance of Escherichia coli from chickens in the Qinghai Plateau of China. Front. Microbiol. 2022, 13, 885132. [Google Scholar] [CrossRef] [PubMed]
- Khine, N.O.; Lugsomya, K.; Niyomtham, W.; Pongpan, T.; Hampson, D.J.; Prapasarakul, N. Longitudinal Monitoring Reveals Persistence of Colistin-Resistant Escherichia coli on a Pig Farm Following Cessation of Colistin Use. Front. Vet. Sci. 2022, 9, 845746. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.P.; Horton, R.A.; Lemma, F.; Martelli, F.; Duggett, N.A.D.; Smith, R.P.; Kirchner, M.J.; Ellis, R.J.; Rogers, J.P.; Williamson, S.M.; et al. Longitudinal study on the occurrence in pigs of colistin-resistant Escherichia coli carrying mcr-1 following the cessation of use of colistin. J. Appl. Microbiol. 2018, 125, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Svennerholm, A.-M.; Faruque, A.S.G.; Sack, R.B. Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef]
- Villa, L.; García-Fernández, A.; Fortini, D.; Carattoli, A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 2010, 65, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, M.; Perreten, V.; Donà, V. Multidrug resistance and multivirulence plasmids in enterotoxigenic and hybrid Shiga toxin-producing/enterotoxigenic Escherichia coli isolated from diarrheic pigs in Switzerland. Vet. J. 2019, 244, 60–68. [Google Scholar] [CrossRef]
- Shepard, S.M.; Danzeisen, J.L.; Isaacson, R.E.; Seemann, T.; Achtman, M.; Johnson, T.J. Genome sequences and phylogenetic analysis of K88- and F18-positive porcine enterotoxigenic Escherichia coli. J. Bacteriol. 2012, 194, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Effelsberg, N.; Kobusch, I.; Linnemann, S.; Hofmann, F.; Schollenbruch, H.; Mellmann, A.; Boelhauve, M.; Köck, R.; Cuny, C. Prevalence and zoonotic transmission of colistin-resistant and carbapenemase-producing Enterobacterales on German pig farms. One Health 2021, 13, 100354. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Falgenhauer, L.; Semmler, T.; Imirzalioglu, C.; Chakraborty, T.; Roesler, U.; Roschanski, N. Environmental emission of multiresistant Escherichia coli carrying the colistin resistance gene mcr-1 from German swine farms. J. Antimicrob. Chemother. 2017, 72, 1289–1292. [Google Scholar] [CrossRef]
- Matamoros, S.; van Hattem, J.M.; Arcilla, M.S.; Willemse, N.; Melles, D.C.; Penders, J.; Vinh, T.N.; Thi Hoa, N.; Bootsma, M.C.J.; van Genderen, P.J.; et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci. Rep. 2017, 7, 15364. [Google Scholar] [CrossRef] [PubMed]
- Treilles, M.; Châtre, P.; Drapeau, A.; Madec, J.-Y.; Haenni, M. Spread of the mcr-1 colistin-resistance gene in Escherichia coli through plasmid transmission and chromosomal transposition in French goats. Front. Microbiol. 2022, 13, 1023403. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Hikoda, Y.; Fujii, Y.; Murata, M.; Miyoshi, H.; Ogura, Y.; Gotoh, Y.; Iwata, T.; Hayashi, T.; Akiba, M. Emergence of a Multidrug-Resistant Shiga Toxin-Producing Enterotoxigenic Escherichia coli Lineage in Diseased Swine in Japan. J. Clin. Microbiol. 2016, 54, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal Pathogenic Escherichia coli: Virulence Factors and Antibiotic Resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Bok, E.; Kożańska, A.; Mazurek-Popczyk, J.; Wojciech, M.; Baldy-Chudzik, K. Extended Phylogeny and Extraintestinal Virulence Potential of Commensal Escherichia coli from Piglets and Sows. Int. J. Environ. Res. Public Health 2020, 17, 366. [Google Scholar] [CrossRef] [PubMed]
- Khanawapee, A.; Kerdsin, A.; Chopjitt, P.; Boueroy, P.; Hatrongjit, R.; Akeda, Y.; Tomono, K.; Nuanualsuwan, S.; Hamada, S. Distribution and Molecular Characterization of Escherichia coli Harboring mcr Genes Isolated from Slaughtered Pigs in Thailand. Microb. Drug Resist. 2021, 27, 971–979. [Google Scholar] [CrossRef]
- Pazos-Rojas, L.A.; Cuellar-Sánchez, A.; Romero-Cerón, A.L.; Rivera-Urbalejo, A.; van Dillewijn, P.; Luna-Vital, D.A.; Muñoz-Rojas, J.; Morales-García, Y.E.; Del Bustillos-Cristales, M.R. The Viable but Non-Culturable (VBNC) State, a Poorly Explored Aspect of Beneficial Bacteria. Microorganisms 2023, 12, 39. [Google Scholar] [CrossRef]
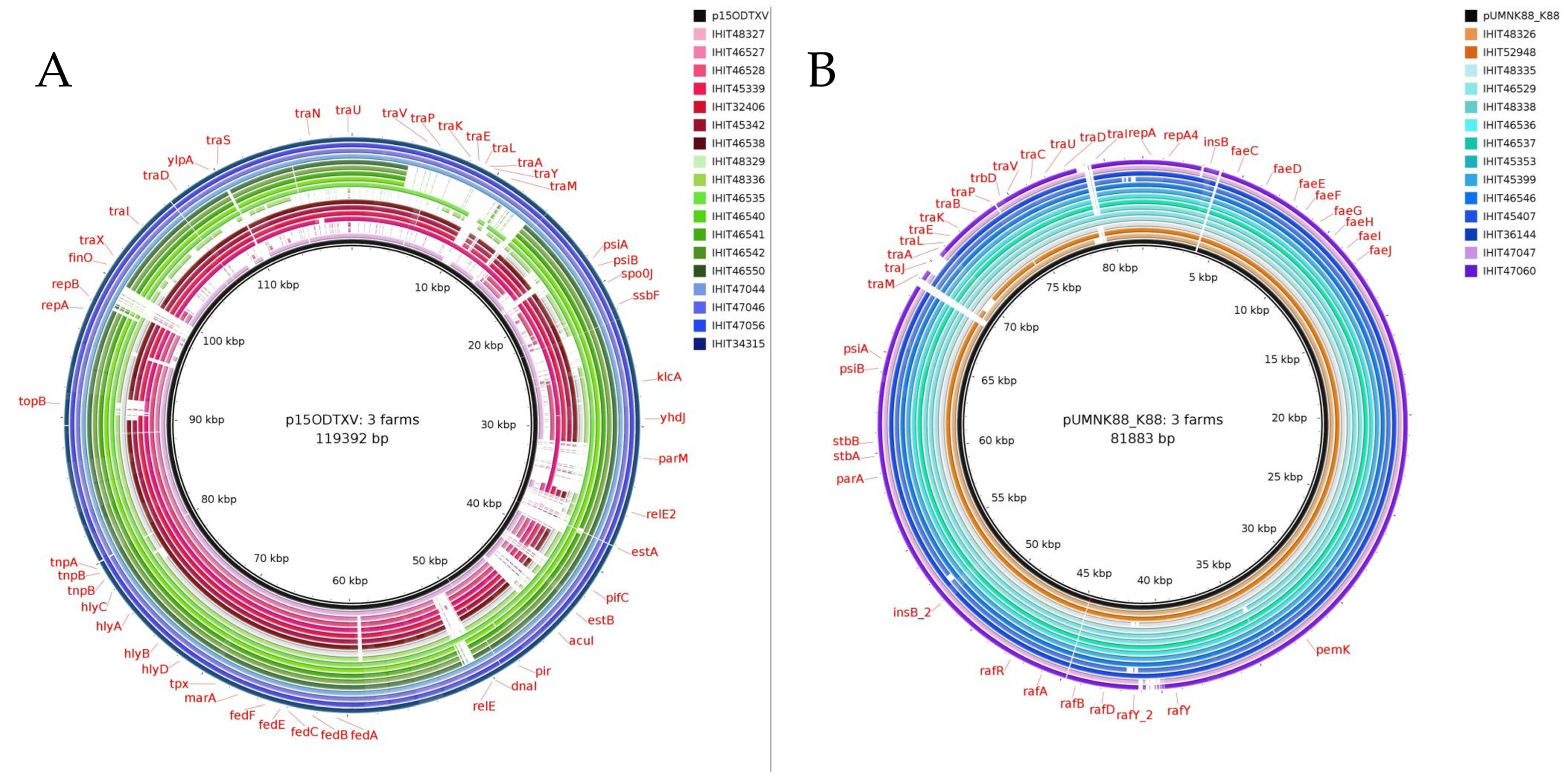
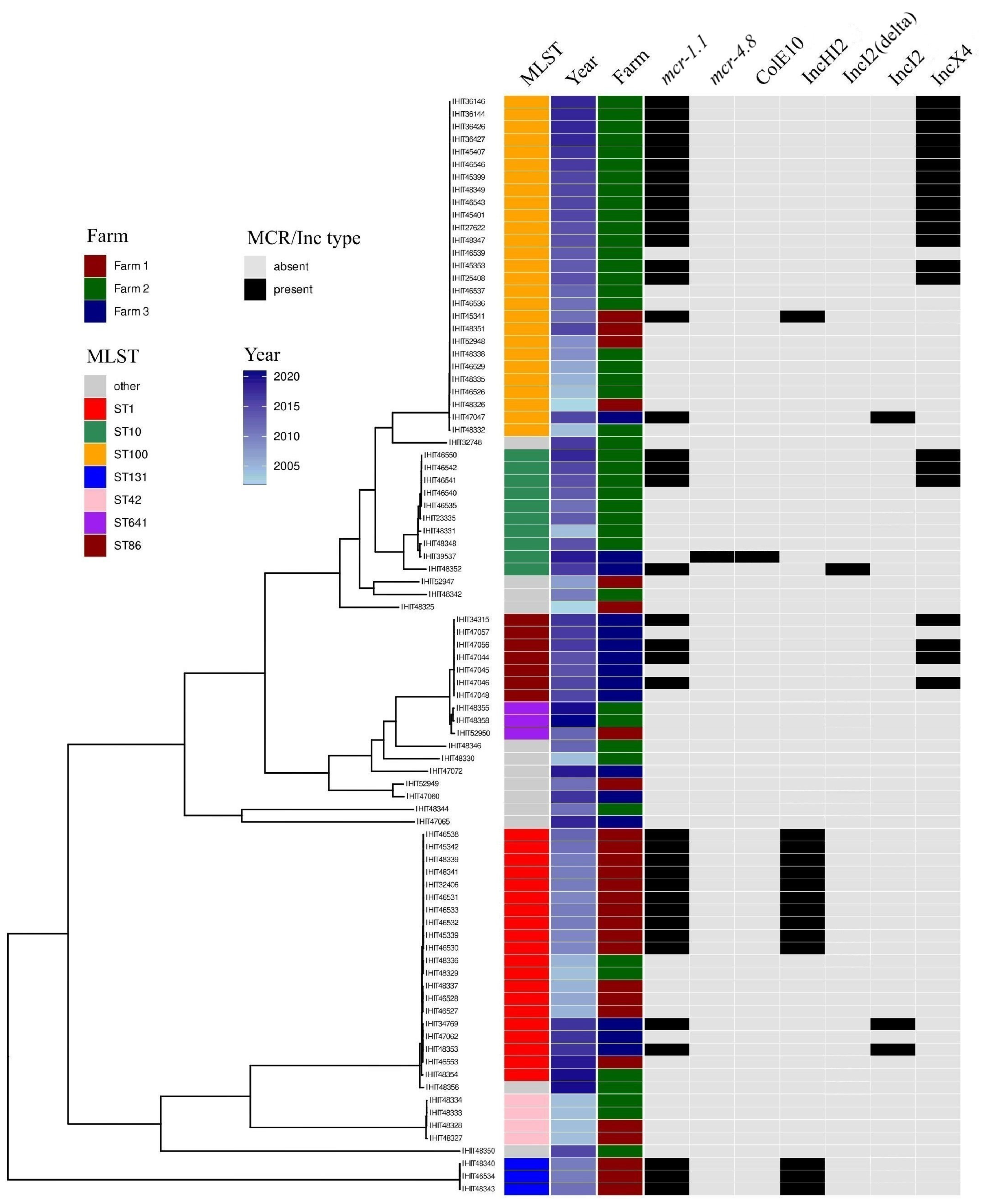
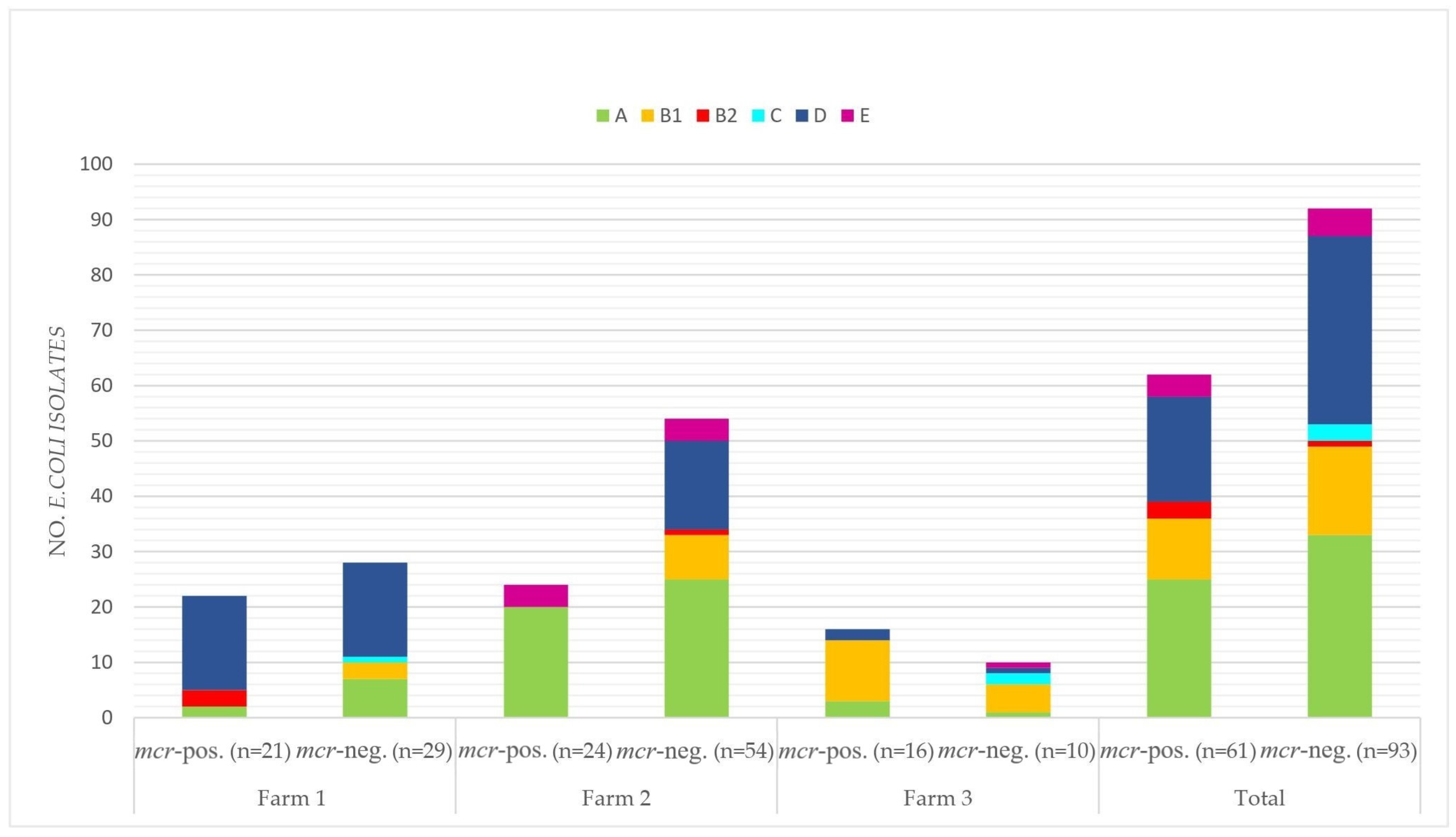

| E. coli Isolates Possessing InPEC-Related VAGs * | mcr-Positive E. coli Isolates Possessing InPEC-Related VAGs | |||||||
|---|---|---|---|---|---|---|---|---|
| Farm No. | No. of Sampled Pigs | Sample Type (No.) | No. | Predicted Pathotypes ** | Sample Collection Period | No. | Predicted Pathotypes | Isolate Collection Period |
| Farm 1 | 43 | feces (41), intestine + feces (1), isolate (1) | 50 | EDEC (23), ETEC (21), STEC (3), ETEC-like (2), AdhF-Ec (1) | 05/2002–10/2019 | 22 | EDEC (17), ETEC (5) | 07/2009–09/2012 |
| Farm 2 | 69 | feces (43), isolate (22), intestine + feces (4) | 78 | ETEC (53), ETEC-like (7), EDEC (6), AEEC (6), AdhF-Ec (3), ETEC/STEC (2), STEC (1) | 06/2004–02/2021 | 24 | ETEC (24) | 07/2013–02/2018 |
| Farm 3 | 25 | isolate (23), feces (2) | 26 | ETEC/STEC (14), ETEC (4), EDEC (3), ETEC-like (3), STEC (2) | 10/2014–04/2019 | 16 | ETEC/STEC (11), EDEC (2), ETEC (1), ETEC-like (1), STEC (1) | 10/2014–04/2019 |
| Strain ID | Source * | Date of Isolation | VAGs ** | Pathotype | MLST | Clonotype | Genoserotype *** | Phylogroup | mcr Gene/pmrB Mutation | Colistin MIC (µg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Farm 1 (n = 27) | ||||||||||
| IHIT46527 | Feces | 06/2005 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | - | 1 |
| IHIT48337 | Feces | 12/2005 | fedAab, stx2e | EDEC | 1 | 2-54 | Ont:H1 | D | - | 8 |
| IHIT46528 | Feces | 01/2006 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | - | 4 |
| IHIT46530 | Feces | 07/2009 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT45339 | Feces | 08/2009 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT46531 | Feces | 12/2009 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 4 |
| IHIT46532 | Feces | 01/2010 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT46533 | Feces | 03/2010 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT48339 | Feces | 04/2010 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT32406 | Feces | 06/2010 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT48341 | Feces | 06/2010 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT45342 | Feces | 07/2011 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT46538 | Feces | 09/2012 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 8 |
| IHIT46553 | Isolate | 10/2019 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | - | 0.5 |
| IHIT52949 | Feces | 06/2011 | estb, estap, fedAac | ETEC | 23 | 4-54 | O8:H17 | C | - | 0.25 |
| IHIT48327 | Feces | 04/2004 | estb, eltB-Ip, fedAac | ETEC | 42 | 28-65 | O147:H14 | D | pmrB V161G | 2 |
| IHIT48328 | Feces | 07/2004 | estb, estap #, fedAac | ETEC | 42 | 28-65 | Ont:H14 | D | pmrB V161G | 0.5 |
| IHIT48326 | Feces | 05/2002 | estb, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 1 |
| IHIT52948 | Feces | 02/2008 | estb, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 0.25 |
| IHIT45341 | Feces | 07/2011 | estb, eltB-Ip, faeGac | ETEC | 100 | 27-65 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT48351 | Feces | 12/2015 | estb #, eltB-Ip, faeGac | ETEC | 100 | 27-0 | Ont:H10 | A | - | 0.5 |
| IHIT46534 | Feces | 06/2010 | estb, estap, fedAac | ETEC | 131 | 40-683 | O25:H4 | B2 | mcr-1.1 | 4 |
| IHIT48340 | Feces | 06/2010 | estb, estap, fedAac | ETEC | 131 | 40-683 | O25:H4 | B2 | mcr-1.1 | 4 |
| IHIT48343 | Feces | 06/2011 | estb, estap #, fedAac | ETEC | 131 | 40-683 | O25:H4 | B2 | mcr-1.1 | 8 |
| IHIT52950 | Feces | 07/2012 | fedA | AdhF-Ec | 641 | 6-289 | O121:H10 | B1 | - | 0.5 |
| IHIT48325 | Feces | 05/2002 | stx2e | STEC | 710 | 153-1582 | Ont:H30 | A | - | 0.5 |
| IHIT52947 | Feces | 12/2007 | stx2e | STEC | 12009 | 11-23 | O142:H27 | A | - | 0.5 |
| Farm 2 (n = 44) | ||||||||||
| IHIT48329 | Feces | 07/2004 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | - | 0.5 |
| IHIT48336 | Feces | 08/2005 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | - | 8 |
| IHIT48354 | Int + Fec | 11/2020 | fedAab | AdhF-Ec | 1 | 2-54 | O139:H1 | D | - | 0.5 |
| IHIT48331 | Feces | 10/2004 | estb # | ETEC-like | 10 | 11-54 | O163:H10 | A | - | 0.5 |
| IHIT46535 | Feces | 02/2011 | estb, estap, fedAac | ETEC | 10 | 11-24 | O141:H4 | A | - | 0.5 |
| IHIT46540 | Isolate | 07/2013 | estb, estap, fedAac | ETEC | 10 | 11-24 | O141:H4 | A | - | 0.25 |
| IHIT23335 | Feces | 07/2013 | estb, estap, fedAac, stx2e | ETEC/STEC | 10 | 11-24 | O141:H4 | A | - | 0.25 |
| IHIT48348 | Feces | 11/2014 | estb | ETEC-like | 10 | 11-45 | Ont:H6 | A | - | 8 |
| IHIT46541 | Feces | 11/2014 | estb, estap #, fedAac | ETEC | 10 | 11-24 | O141:H4 | A | mcr-1.1 | 4 |
| IHIT46542 | Isolate | 01/2015 | estb, estap, fedAac | ETEC | 10 | 11-24 | O141:H4 | A | mcr-1.1 | 4 |
| IHIT46550 | Isolate | 02/2018 | estb, estap, fedAac | ETEC | 10 | 11-24 | O141:H4 | A | mcr-1.1 | 4 |
| IHIT48346 | Feces | 12/2012 | eae | AEEC | 20 | 4-25 | Ont:H49 | B1 | - | 0.5 |
| IHIT48330 | Feces | 07/2004 | eae | AEEC | 29 | 4-24 | O123:H11 | B1 | - | 0.5 |
| IHIT48333 | Feces | 12/2004 | estb, eltB-Ip, fedAac | ETEC | 42 | 28-65 | O147:H14 | D | pmrB V161G | 4 |
| IHIT48334 | Feces | 12/2004 | fedAac | AdhF-Ec | 42 | 28-65 | O147:H14 | D | pmrB V161G | 4 |
| IHIT48342 | Feces | 06/2010 | eae # | AEEC | 93 | 11-0 | O5:H4 | A | - | 0.25 |
| IHIT46526 | Feces | 06/2004 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 1 |
| IHIT48332 | Feces | 10/2004 | estb, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 0.5 |
| IHIT48335 | Feces | 03/2005 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 0.25 |
| IHIT46529 | Feces | 02/2006 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 8 |
| IHIT48338 | Feces | 08/2008 | estb, eltB-Ip, faeGac | ETEC | 100 | 27-0 | Ont:H10 | A | - | 4 |
| IHIT46536 | Feces | 11/2011 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 0.5 |
| IHIT46537 | Feces | 01/2012 | estb, estap #, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 0.5 |
| IHIT45353 | Isolate | 07/2013 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT46539 | Isolate | 07/2013 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | - | 0.25 |
| IHIT25408 | Isolate | 03/2014 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | Ont:H10 | A | mcr-1.1 | 0.5 |
| IHIT48347 | Isolate | 05/2014 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT27622 | Isolate | 10/2014 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT45399 | Isolate | 01/2015 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT48349 | Isolate | 07/2015 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | Ont:H10 | A | mcr-1.1 | 8 |
| IHIT45401 | Isolate | 09/2015 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT46543 | Isolate | 09/2015 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 4 |
| IHIT46546 | Isolate | 09/2016 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT45407 | Isolate | 08/2017 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT36144 | Isolate | 01/2018 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT36146 | Isolate | 01/2018 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT36426 | Isolate | 02/2018 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT36427 | Isolate | 02/2018 | estb, estap, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT48355 | Int + Fec | 11/2020 | estb # | ETEC-like | 641 | 6-832 | O45:H10 | B1 | - | 2 |
| IHIT48358 | Feces | 02/2021 | estb | ETEC-like | 641 | 6-289 | O115:H10 | B1 | - | 0.25 |
| IHIT32748 | Isolate | 09/2016 | eae | AEEC | 793 | 168-555 | O49:H10 | A | - | 0.5 |
| IHIT48344 | Feces | 12/2011 | eae | AEEC | 799 | 84-305 | O108:H9 | E | - | 0.25 |
| IHIT48356 | Int + Fec | 11/2020 | stx2e | STEC | 955 | 2-65 | O139:H1 | D | - | 0.5 |
| IHIT48350 | Feces | 09/2015 | estb | ETEC-like | 2944 | 224-1082 | O17/O77:H28 | D | - | 1 |
| Farm 3 (n = 16) | ||||||||||
| IHIT34769 | Isolate | 06/2017 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | mcr-1.1 | 4 |
| IHIT48353 | Isolate | 06/2017 | faeGac, stx2e | EDEC | 1 | 2-54 | Ont:H1 | D | mcr-1.1 | 8 |
| IHIT47062 | Isolate | 10/2017 | fedAab, stx2e | EDEC | 1 | 2-54 | O139:H1 | D | - | 1 |
| IHIT48352 | Feces | 12/2016 | stx2e | STEC | 10 | 11-23 | Ont.:H32 | A | mcr-1.1 | 8 |
| IHIT39537 | Isolate | 04/2019 | estb | ETEC-like | 10 | 11-2594 | O35:H6 | A | mcr-4.8 | 4 |
| IHIT47044 | Isolate | 10/2014 | estb, estap, fedAac, stx2e | ETEC/STEC | 86 | 6-32 | Ont:H10 | B1 | mcr-1.1 | 0.5 |
| IHIT47045 | Isolate | 10/2014 | estb, estap, fedAac, stx2 # | ETEC/STEC | 86 | 6-32 | O86:H10 | B1 | - | 1 |
| IHIT47046 | Isolate | 06/2015 | estb, estap, fedAac, stx2e | ETEC/STEC | 86 | 6-32 | O86:H10 | B1 | mcr-1.1 | 4 |
| IHIT47048 | Isolate | 06/2015 | estb, estap, fedAac, stx2e | ETEC/STEC | 86 | 6-32 | O86:H10 | B1 | - | 0.25 |
| IHIT47056 | Isolate | 06/2016 | estb, estap, fedAac, stx2e | ETEC/STEC | 86 | 6-32 | Ont:H10 | B1 | mcr-1.1 | 4 |
| IHIT47057 | Isolate | 11/2016 | estb, estap, fedAac | ETEC | 86 | 6-32 | O86:H10 | B1 | - | 0.5 |
| IHIT34315 | Isolate | 04/2017 | estb, estap, fedAac, stx2e | ETEC/STEC | 86 | 6-32 | Ont:H10 | B1 | mcr-1.1 | 4 |
| IHIT47060 | Isolate | 02/2017 | estb, eltB-Ip, faeGac | ETEC | 90 | 4-54 | O8:H19 | C | - | 0.5 |
| IHIT47047 | Isolate | 06/2015 | estb, eltB-Ip, faeGac | ETEC | 100 | 27-0 | O149:H10 | A | mcr-1.1 | 8 |
| IHIT47065 | Isolate | 07/2018 | estb | ETEC-like | 118 | 4-331 | O15:H45 | E | - | 0.5 |
| IHIT47072 | Isolate | 03/2019 | estb | ETEC-like | 162 | 65-32 | O8:H19 | B1 | - | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göpel, L.; Prenger-Berninghoff, E.; Wolf, S.A.; Semmler, T.; Bauerfeind, R.; Ewers, C. Repeated Occurrence of Mobile Colistin Resistance Gene-Carrying Plasmids in Pathogenic Escherichia coli from German Pig Farms. Microorganisms 2024, 12, 729. https://doi.org/10.3390/microorganisms12040729
Göpel L, Prenger-Berninghoff E, Wolf SA, Semmler T, Bauerfeind R, Ewers C. Repeated Occurrence of Mobile Colistin Resistance Gene-Carrying Plasmids in Pathogenic Escherichia coli from German Pig Farms. Microorganisms. 2024; 12(4):729. https://doi.org/10.3390/microorganisms12040729
Chicago/Turabian StyleGöpel, Lisa, Ellen Prenger-Berninghoff, Silver A. Wolf, Torsten Semmler, Rolf Bauerfeind, and Christa Ewers. 2024. "Repeated Occurrence of Mobile Colistin Resistance Gene-Carrying Plasmids in Pathogenic Escherichia coli from German Pig Farms" Microorganisms 12, no. 4: 729. https://doi.org/10.3390/microorganisms12040729
APA StyleGöpel, L., Prenger-Berninghoff, E., Wolf, S. A., Semmler, T., Bauerfeind, R., & Ewers, C. (2024). Repeated Occurrence of Mobile Colistin Resistance Gene-Carrying Plasmids in Pathogenic Escherichia coli from German Pig Farms. Microorganisms, 12(4), 729. https://doi.org/10.3390/microorganisms12040729






