Abstract
Studies investigating the taxonomic diversity and structure of soil bacteria in areas with enhanced radioactive backgrounds have been ongoing for three decades. An analysis of data published from 1996 to 2024 reveals changes in the taxonomic structure of radioactively contaminated soils compared to the reference, showing that these changes are not exclusively dependent on contamination rates or pollutant compositions. High levels of radioactive exposure from external irradiation and a high radionuclide content lead to a decrease in the alpha diversity of soil bacterial communities, both in laboratory settings and environmental conditions. The effects of low or moderate exposure are not consistently pronounced or unidirectional. Functional differences among taxonomic groups that dominate in contaminated soil indicate a variety of adaptation strategies. Bacteria identified as multiple-stress tolerant; exhibiting tolerance to metals and antibiotics; producing antioxidant enzymes, low-molecular antioxidants, and radioprotectors; participating in redox reactions; and possessing thermophilic characteristics play a significant role. Changes in the taxonomic and functional structure, resulting from increased soil radionuclide content, are influenced by the combined effects of ionizing radiation, the chemical toxicity of radionuclides and co-contaminants, as well as the physical and chemical properties of the soil and the initial bacterial community composition. Currently, the quantification of the differential contributions of these factors based on the existing published studies presents a challenge.
1. Introduction
The soil is rich in microorganism diversity [1,2], as evidenced by the presence of 106–1010 prokaryotic cells per gram, including bacteria and archaea [3,4]. The activity of these microorganisms, often referred to as soil engineers, plays a crucial role in maintaining the stability and health of the soil environment by significantly contributing to soil biochemical processes [5,6]. Additionally, these microorganisms play a vital role in maintaining the material and energy cycles in the biosphere [7].
Soil contamination has adverse effects on all organisms inhabiting the soil, particularly bacterial communities. Prokaryotic organisms, characterized by features such as a high rate of growth and reproduction, and horizontal gene exchange, exhibit a superior and faster adaptation to changing environments compared to other organisms. A specific group of bacteria known as extremophiles are exceptionally well-adapted to survive in extreme conditions, including environments with elevated [8] concentrations of heavy metals (HMs), extreme temperatures, and high levels of radiation exposure [9,10,11].
Soils in various regions exhibit elevated concentrations of radionuclides (RNs) due to both the natural conditions and anthropogenic activities, posing a potential hazard to living organisms. The causes of radioactive contamination can be attributed to several factors: areas with increased natural radioactivity [12,13,14,15,16], technologically enhanced natural radioactivity [17,18,19,20,21,22,23,24,25], radiation accidents [26,27,28,29], nuclear tests [30,31,32,33], and oil pollution [34,35,36,37,38].
In turn, the activity of microorganisms plays a significant role in influencing the chemical form, mobility, and toxicity of radionuclides in soils, thereby either impeding or promoting their incorporation into biological migration pathways [39,40,41,42,43,44,45]. This is why bacteria, together with microscopic fungi, are used as a key component in complex biostimulants for remediating contaminated soils, particularly in cases of polymetallic and radionuclide pollution [46,47,48].
In a global context, the radioactive contamination of the soil poses a long-term radiation hazard to non-human biota, public health, and agriculture [49,50,51,52,53]. Therefore, understanding the mechanisms of the soil bacterial communities’ response to ionizing radiation is crucial for identifying issues in soil functioning and making informed decisions regarding the reclamation of radioactively contaminated territories.
The culture-dependent approach traditionally employed in microbiological research is notably time-consuming, involving the isolation and cultivation of bacteria on specific culture media. While this method enables the comprehensive exploration of the taxonomic and functional characteristics of isolated organisms, it exhibits a limited efficiency in community analysis, typically identifying only around 5% of the dominant representatives within a given community [54,55,56]. In contrast, the utilization of modern next-generation sequencing (NGS) techniques facilitates the acquisition of detailed insights into the taxonomic structure and functional profile of entire bacterial communities. By combining a metagenomic strategy that entails the extraction of total DNA from the soil with a subsequent NGS analysis, researchers gain access to a profound examination of the vast nonculturable diversity that was previously inaccessible [57]. Metagenomic approaches have emerged as primary methodologies for investigating the diversity of soil communities, supplanting the limitations of culture-dependent methods [56,57,58,59]. These advanced techniques offer a more comprehensive and efficient means to study microbial communities in soil environments.
There are numerous publications that explore bacterial communities in radioactive contaminated soils or those irradiated under laboratory conditions using both culture-dependent and molecular–genetic approaches. These study results are interesting to discern common changes in the community’s taxonomic or functional transformation and adaptation mechanisms.
The level of radioactive contamination is typically not extreme for soil bacterial communities, primarily due to their high radiotolerance even in the presence of high radionuclide activity concentrations. However, the unique nature of environmental contamination exerts long-term effects through multifactorial exposure. Consequently, uncertainties arise that may impede the analysis of community structure and composition data under varying chemical, physical, and biological influences obtained in different studies. In addition to ionizing radiation, various factors, such as the soil moisture, pH levels, organic carbon content and the availability of macro- and micro-elements, the chemical toxicity of radionuclides, and the presence of other toxicants (like heavy metals and oil products), influence the activity of soil organisms. Identifying trends within extensive datasets can enhance the efficacy of selecting and estimating radionuclides and external ionizing radiation exposure amidst other environmental influences.
The objective of this review was to summarize the existing scientific data on alterations in the taxonomic and functional compositions as well as the adaptations of soil bacterial communities in highly radioactive environments with a focus on identifying the primary factors influencing these alterations. Furthermore, an endeavor was made to determine whether the ionizing radiation or chemical toxicity of radioactive elements plays a pivotal role in influencing the structure and diversity of soil bacterial communities. This review considered studies in which the radioactive contamination of the soil was estimated using measurements of the radioactive background, pollution density, radionuclide activity concentrations, or concentrations in the soil. Additionally, studies in which irradiation doses were calculated were included. The review covered the findings derived from the analysis of 55 articles spanning the period from 1996 to 2024, sourced from databases such as PubMed (https://www.ncbi.nlm.nih.gov/ (accessed on 28 March 2024)), Google Scholar (https://scholar.google.com/ (accessed on 28 March 2024)), and eLibrary (https://www.elibrary.ru/ (accessed on 28 March 2024)). The selection of articles was based on specific keywords, including ‘bacterial communities’, ‘radioactive contamination’, ‘ionizing radiation’, and ‘taxonomic diversity’. This methodological approach underpinned the structure and focus of our review, providing a comprehensive examination of the subject matter.
One of the main challenges in summarizing the data from these studies was the lack of standardization in their methodologies. There were significant differences in the structure of the analyzed studies, the methodological approaches used to estimate the state of bacterial communities, and the parameters chosen to characterize the radioactive situation.
2. Taxonomic Diversity and Structure of Soil Communities after Radiation Exposure
Table 1 provides a summary of studies conducted from 1996 to 2024, focusing on the impact of radioactive soil contamination and external gamma irradiation on bacterial diversity [15,19,20,25,26,27,29,30,34,35,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. Particular studies are discussed below in the correspondent sections.

Table 1.
Effects of the radioactive contamination of the soil and external gamma irradiation on the taxonomic diversity of bacterial communities.
2.1. Soils Contaminated by Artificial Radionuclides
Nuclear tests, the operations of nuclear facilities, and accidents stand out as the primary sources of artificially derived radionuclides released into the environment [49,104,105]. Notably, events such as the Chernobyl and Fukushima nuclear power plant accidents led to the extensive contamination of large areas with radionuclides like 137Cs, 90Sr, and others [106,107,108,109]. Nuclear tests conducted by countries such as the USSR, USA, and France have resulted in the pollution of vast territories with 137Cs, 90Sr, and isotopes of uranium and plutonium [110,111,112]. The persistent soil radioactive contamination by long-lived radionuclides continues to pose a significant environmental hazard to ecosystems and non-human species to this day [113].
The initial studies on bacterial communities in soils contaminated with radionuclides primarily focused on the examination of bacteria capable of growing solely on cultural media. Thus studies, as indicated in Table 1 [30,60,61,62,64,114], revealed a predominance of aerobic species within these communities. However, during the early years following the Chernobyl accident, when the radioactive background levels reached 629 kBq/kg [60,61], a decrease in the diversity of chemoorganotrophic bacteria and a reduction in the abundance of cellulose-degrading, nitrifying, and sulfate-reducing bacteria were noted in the soil. Furthermore, the authors observed a significant presence of Bacillus cereus from the Bacciliota phylum [61] and representatives of the Methylobacterium genus from the Pseudomonadota phylum [60,61]. Additionally, other studies employing a culture-dependent approach reported no differences in the diversity and ability of bacteria in contaminated soils [62,64]. In fact, an increase in 137Cs activity concentrations in the soil up to 1700 kBq/kg [62] resulted in the enhanced diversity of cultivated bacteria compared to the reference area. However, the authors attributed these results not to the radioactive contamination level but to the high organic material content of the soil. The physical and chemical characteristics of the soil, rather than the 137Cs activity concentration, were the primary factors influencing the diversity and abundance of cultivated bacteria in the study [64].
Recent studies performed with NGS have revealed findings regarding the alpha diversity of bacterial communities in radioactively contaminated areas when compared to uncontaminated regions. The research by [65,66] demonstrated an increase in alpha diversity within bacterial communities in the contaminated zone, likely attributed to the relatively low activity concentration of 137Cs in the soil, which was insufficient to induce significant effects on bacteria (Table 1). Conversely, studies by [27,29] reported no alterations in bacterial diversity, with [27] noting a slight decrease specifically in areas with elevated activity concentrations of 137Cs and 134Cs exceeding 500 and 200 kBq/kg, respectively. Notably, [26] observed a significant reduction in the alpha diversity of bacterial communities in cases of more complex contamination, involving high activity concentrations of not only 137Cs, but of 90Sr, 241Am, and 154Eu in the soils of the Chernobyl exclusion zone. The use of culture-dependent methodologies has also provided insights into the effects of 137Cs contamination on bacterial communities following nuclear tests. Gu and colleagues [30] reported a decrease in bacterial diversity when high levels of 137Cs contamination were present, but in cases of moderate contamination, the alpha diversity was slightly increased. This tolerance of bacterial communities can be attributed to changes in their taxonomic structure and the dominance of species resistant to ionizing radiation, such as Geodermatophilus bullaregiensis [27] or representatives from the Prolixibacteraceae (Bacteroidota) and Methylococcaceae (Pseudomonadota) families [29].
The increase in the irradiation dosage from artificial radionuclides has led to a reduction in soil bacterial diversity and alterations in their taxonomic composition. The prevalence of specific phyla in contaminated areas is influenced by the soil characteristics, such as pH levels, moisture content, and organic carbon levels, which, in turn, are features of the geographical location of the research site. Predominantly observed phyla include Pseudomonadota, Acidobacteriota, and Actinomycetota in most instances [26,27,30,65]. Verrucomicrobiota representatives in contaminated soils have shown a tendency to decrease [26,65,87], while Chloroflexota have exhibited an increase [27,65].
Distinct operational taxonomic units (OTUs), identified down to the genus/species level, that dominate in contaminated areas are commonly associated with adverse environmental conditions [27,29,65,114]. These conditions include cold temperatures, low oxygen levels, acidity, alkalinity, salinity, and the presence of metals in soils. Examples of such OTUs include Geodermatophyllus bulgariensis [27,115], Truepera radiovictrix [27,116], and Rubrobacter taiwanensis [27].
In the Okuma area, which stands as one of the most heavily contaminated regions following the Fukushima accident, various microorganisms thriving in harsh environments have been identified [27]. These include the piezotolerant bacterium Marinilactibacillus piezotolerans [117], the volcanic inhabitant Rubrobacter spartanus [118], the nitrogen-fixing species Pleomorphomonas koreensis [119], and the selenate-respiring bacterium Thauera selenatis [120] (all species are mentioned in [27]).
2.2. Soils with Enhanced Levels of Naturally Occurring Radionuclides and Heavy Metals
The landscape and geochemical features of territories, mining activity (uranium, coal, phosphates, and rare-earth elements), oil extraction, and many other factors affect the concentrations of naturally occurring radionuclides (238U, 226Ra, 232Th, 40K, etc.) and associated heavy metals and metalloids in soils ([38,121,122,123,124,125] and others (Table 1)). The conditions of the increased background of natural radioactivity solely caused by the geochemical features of the underlying rock may be typical for some soil bacterial communities, but their structure may differ from that in reference territories. However, in cases when the increase in the natural radiation background is the result of anthropogenic activity, bacterial communities are forced to adapt to new unfavorable conditions, which leads to a change in their taxonomic structure and community composition.
Most available studies have focused on the state of microbial communities under the influence of elevated concentrations of naturally occurring radionuclides, which have entered the soil due to uranium mining and the disposal of uranium industry waste [19,67,68,69,70,71,72,74,75,76,77,79,81,84]. Much less research has been dedicated to the effect of exclusively natural soil radioactivity on bacterial communities [15,73,86,88], the consequences of nuclear industry plant [85,87] and oil extraction activities [35,78], as well as the results of laboratory experiments with the introduction of radioactive substances or materials containing uranium isotopes into the soil [90].
The research results on bacterial communities from areas with elevated levels of naturally occurring radionuclides and HMs, regardless of climatic conditions, soil pH, and the nitrogen, phosphorus, and organic carbon contents, predominantly show a significant decrease in alpha diversity compared to the nearby reference areas (Table 1). The bacterial diversity decreased less in the area with mostly RN contamination than in the neighboring areas contaminated with RN and HMs or only HMs (for example, [126,127]). However, there are exceptions. Thus, in studies of the bacterial communities of soils technogenically contaminated with U and HMs [84] and soils with increased natural radioactivity (238U, 232Th, and 40K) [15], no statistically significant changes in diversity were found in areas with different RN or HM concentrations in the soil. Significant changes in the taxonomic composition of bacterial communities at the phylum level are usually not recorded; however, statistically significant rearrangements at the level of lower taxa that are already adapted to live in an RN environment are common ([15,20,80,82,83] and others from Table 1).
Frequently, bacteria capable of changing the degree of the oxidation of elements dominate the composition of communities in contaminated territories (Table S1), for example, iron-reducing [73,80], capable of U(VI) reduction [73,80], and sulfate-reducing bacteria [80,128]. In a contaminated environment, bacteria that produce [82] and are resistant to antibiotics [15,79] and metals [15,79] thrive, as well as some halophytes [15,79]. As a result, in territories with elevated levels of naturally occurring radionuclides, Acidithiobacillus ferrooxidans and several Pseudomonas species have an advantage [67,68,70,83,84,86]. The bacterium A. ferrooxidans is known for its ability to inhabit extremely acidic conditions and oxidize Fe2+ [129], while representatives of the genus Pseudomonas are involved in the redox transformations of U(VI) and exhibit resistance to uranium and HMs [70,130,131].
2.3. Laboratory Experiments with External Irradiation of Soils
Of the studies investigating the influence of radioactivity on bacterial diversity, some inquiries delve into the repercussions of external gamma radiation. A direct comparison between laboratory findings and those derived from environmental settings, characterized by markedly lower irradiation doses, proves challenging. Nonetheless, insights gleaned from scenarios involving exceptionally high external irradiation doses highlight the mechanisms that allow bacteria to thrive in adverse conditions and try to separate them.
Research examining the impact of γ-radiation on the soil bacterial diversity and composition under laboratory conditions is predominantly centered on identifying radiotolerant species and genera that could be used for the bioremediation of radioactively contaminated sites. These studies range from doses as low as 5 Gy [92], investigating arbuscular mycorrhiza in Holcus lanatus, to doses as high as 100 kGy [100], simulating the Martian environment.
The taxonomic structure and composition of bacterial communities often change, and their diversity significantly decreases when soils are exposed to high doses of radiation [96,99,101,103]. In such cases, the eliminated species are frequently replaced by more tolerant bacteria, fungi, or microalgae [99,101]. However, in certain instances, such as hydrocarbon-polluted soils [96] or acid brown earth soils [95], higher doses up to 10 kGy did not result in a decrease in the soil bacterial diversity or even cause an increase in the case of irradiation up to 3 kGy of garden clayey soil [96].
The prevalence of Deinococcota [93,94,98,101,103] in desert bacterial communities exposed to high levels of external irradiation, often accompanied by a significant reduction in other taxonomic groups [102], represents a key characteristic of their taxonomic composition. Members of the Deinococcota phylum exhibit remarkable radiotolerance, with notable examples including the exceptionally resilient Deinococcus radiodurans, capable of surviving exposures exceeding 5 kGy [132]; Deinococcus ficus, which can withstand 3 kGy (equivalent to an absorbed dose of 18 kGy) [102]; and Deinococcus guangxiensis, exhibiting a 10% survival rate at 9.8 kGy [133].
Several representatives of the Bacteroidota phylum have been identified as exceptionally radioresistant bacteria, such as the genus Hymenobacter [102]. Within this genus, certain species exhibit remarkable tolerance to high levels of irradiation: H. taeanensis can withstand doses up to 3 kGy, H. swuensis up to 7.3 kGy, and H. xinjiangensis up to 8 kGy [134,135,136]. Inhabitants of the Taklimakan Desert [98] have been found to include the radioresistant [137] genera Rufibacter and antibiotic-resistant Pontibacter [138].
An increased number of certain members of the Pseudomonadota phylum have been observed in soils subjected to laboratory irradiation conditions [94,98]. This includes thermophilic and radioresistant Microvirga [98,139] and microaerophilic, antibiotic-resistant Lysobacter [98,140,141]. Within the Chloroflexota phylum, which includes hyperthermophilic species [142], there have been documented increases in certain cases [96,101]. Similarly, the Bacillota phylum [98,103] has shown an increase, encompassing thermophilic and halophilic anaerobic species such as Anaerosporobacter [103,143].
3. Mechanisms of Resistance and Adaptation in Bacterial Communities in Radioactively Contaminated Soil
In conditions of radioactive soil contamination or exposure to high doses of γ-radiation in a laboratory experiment, changes occur in the structure of ecologically trophic groups of soil microbial communities due to habitat modifications. The shift in the functional composition of the soil microorganisms reflects changes in the taxonomic profile of the community, the chemical composition of the soil, and the availability of essential and toxic elements. The change in the taxonomic structure of soil bacterial communities under radiation exposure may result in the change in the distribution of genes involved in different biological processes. Consequently, this triggers the restructuring of the existing metabolic pathways and the activation of new ones, ensuring the stable existence of the community in the environment contaminated by radionuclides [20,25,26,102]. It is expected that, in the structure of bacterial communities in radioactively contaminated soils, as a rule, bacteria with a greater resistance to radiation compared to other groups, as well as with a wide range of other properties, which allow them to exist in these unfavorable conditions, will gain an advantage or begin to dominate. For example, multiple-stress tolerance has been demonstrated for five radioresistant bacterial isolates obtained from various habitats [144].
In Table S1, a list of the genera and species of bacteria that the authors of the corresponding studies proposed as dominant in soils from radioactively contaminated sites or irradiated in the laboratory is presented. Usually, the species or genera mentioned as dominant are not characterized as radiotolerant. The main components of the contaminated sites discussed in this review are metals and radionuclides. Based on the mechanism proposed in [145,146,147,148] to provide bacterial tolerance to ionizing radiation and toxic compounds, a list of properties, including metal and antibiotic resistance, high DNA repair efficiency, resistance to low O2, the presence of antioxidative enzymes, thermophilicity, halotolerance, involvement in redox reactions, and others, was chosen. Based on this list ([11,70,98,115,116,117,118,119,120,128,130,131,134,137,138,139,140,141,143,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324] from Table S1) and the current literature available, an attempt was made to analyze the possible mechanisms that cause the beneficial properties of these bacteria in soils with an increased content of artificial and naturally occurring radionuclides. It should be noted that bacteria possessing one of the properties listed above can simultaneously be resistant to other environmental factors. This is due to the diversity of environmental factors in any natural habitat, especially in territories with extreme conditions.
In this section, we focused our attention on examining and discussing the functional characteristics of bacterial communities, primarily in radioactively contaminated soils, as the mechanisms of soil bacterial communities functioning under γ-ray exposure in laboratory studies are better examined and more evident. It should be noted that the survival of bacteria after exposure to high doses is possible due to the combination of the properties of the organisms ensuring the integrity of the genetic information, as proven in the studies of the extremely high radioresistance of D. radiodurans and other bacteria [147,325].
3.1. Resistance to Heavy Metals and Antibiotics
The tolerance of soil bacteria to toxic heavy metals and antibiotics shares several common mechanisms, including an ability to avoid the uptake of toxic compounds and to rapidly remove or isolate the absorbed molecules [145,146,326]. Furthermore, heavy metal contamination can contribute to the dissemination of antibiotic resistance through collaborative and indirect selection processes [85]. Typically, natural soil bacterial communities include species resistant to metals and/or antibiotics, such as representatives from the Staphylococcus, Acinetobacter, Pseudomonas, and Serratia genera (Table S1). These resistance genes are localized in both chromosomes (more specific) and in plasmid DNA (less specific, but capable of rapid horizontal gene transfer) [145,146,326].
Among the primary mechanisms of resistance to metals are accumulation in the cell wall, active transportation out of the cell, and intra/extracellular entrapment [145,327]. Additionally, certain bacteria have the capability to alter the oxidation state of elements, particularly transition metals like Fe, Mn, Cr, and others [145,327]. This ability is commonly found in bacteria that utilize redox reactions as an energy source [328], and the presence of such species can benefit the entire community by reducing the bioavailability of toxic elements, including radioactive ones like uranium [130,329,330,331]. Studies have shown an increase in the diversity of Fe-reducing bacterial OTUs in soils contaminated with both radioactivity and metals [76]. The analysis of various studies has revealed a prevalence of dominant chemotrophic bacteria in areas contaminated with artificial radionuclides [114], and more often in regions affected by naturally occurring radionuclides and metals [67,68,73,75,80,84,86].
The mechanisms of antibiotic resistance exhibit a greater diversity compared to resistance to toxic metals. Common strategies shared with various toxicants include the selective penetration of the cell wall and the active transportation of the toxicant out of the cell [146]. Specific to antibiotics, other mechanisms target protein structures, such as replacing sensitive targets with insensitive ones, inducing changes in proteins through point mutations or indels in coding genes, and increasing target production via enhanced expression or gene duplication [146].
The extensive diversity in antibiotic resistance mechanisms is a result of the prolonged co-evolution between antibiotic producers and their targets [332,333]. In recent decades, the hidden diversity stemming from this co-evolutionary arms race has gained momentum due to human antibiotic use [333]. The broader range of targets with specificities has led to a variety of antibiotic resistance mechanisms that are likely more sophisticated than those for heavy metal resistance, which, although numerous in the environment, are comparatively limited.
It is noteworthy that the prevalence of antibiotic-resistant and heavy metal-resistant bacterial groups appears to be comparable among species and genera that dominate in areas contaminated with both artificial and naturally occurring radionuclides. Bacteria such as Acinetobacter, Burkholderia, Streptomyces, Bacillus, and Staphylococcus, known for their antibiotic resistance [146,149,178,179], have been identified in soils contaminated with artificial radionuclides (refer to Table S1). These findings include only data on the cultivated species relevant for potential industrial applications involving the production of organic compounds and the development of remediation strategies for contaminated areas, with implications for public health and agriculture.
Conducting comprehensive studies to assess resistance to all metals and known antibiotics across all cultivated bacterial species is impractical. Moreover, the diversity of uncultivated bacteria significantly expands the pool of species capable of growing on artificial media. Therefore, the data presented in Table S1 material are incomplete, suggesting that the actual number of bacteria resistant to metals and antibiotics in radioactively contaminated environments may be higher. Oxidative stress has been linked to an increased frequency of antibiotic resistance genes in soil bacterial communities (for a comprehensive review, see [334]). Studies have reported heightened abundance and diversity of heavy metal and antibiotic resistance genes in regions affected by past nuclear weapon production activities [85]. Similar trends have been observed, with a simultaneous rise in the frequency of membrane transporter genes in soils contaminated with uranium [20,74].
3.2. Halotolerance
The ability to survive at high salt concentrations is due to the resistance to pronounced osmotic and oxidative stresses through ion homeostasis maintenance, the accumulation of osmolytes, and production of enzymes with stable activity in high-salt environments and thermotolerance [335,336]. But the strategy depends on the salt concentration [336]: Species, adapted to extremely high salt, accumulate inorganic ions intracellularly to balance the salt concentration in their environment. Low-salt or fluctuating salinity environments require inert compatible organic solute (osmolyte) accumulation to protect proteins from denaturation and the activation of proton pump and cation K+ and Na+ transport. Among the bacteria with the ability for halotolerance, Methylobacterium, Flavobacterium, Bacillus, Marinilactibacillus, Halomonas, Salinimicrobium, and others were found in radioactively contaminated soils (Table S1). In studies in which these bacteria have been identified, the habitat is not characterized by strong salinity, as in salt marshes. The dominant position of these bacteria in conditions of radioactive contamination can be explained by their high resistance to oxidative stress, accompanying halotolerance [336,337]. In some cases, it can be assumed that bacteria have entered the soil of radioactively contaminated areas from external sources, such as the deep-sea representative Marinilactibacillus piezotolerans [27], which could have been introduced as a result of a tsunami following an earthquake and causing the accident at the Fukushima Daiichi Nuclear Power Plant.
3.3. Redox Activity and Antioxidant Defense Systems
The ancestors of modern chemotrophic organisms were anaerobic. The evolutionary emergence of a system of antioxidant enzymes, such as catalase, superoxide dismutase, oxidase, and others, predates the presence of free oxygen in the Earth’s atmosphere [338]. This system laid the foundation for the evolution of mechanisms conferring tolerance to oxygen. It is known that most antioxidant enzymes in bacteria are encoded by single genes that make them suitable for horizontal gene transfers and duplications [339]. This feature provides, in short, from an evolutionary perspective, time to increase the fitness of an organism. Catalase- and oxidase-positive strains were isolated from desert soils exposed to 3 kGy [98]. In addition, many bacteria that dominate radioactively contaminated sites have such activity (Table S1). Also, the presence of catalase and/or oxidase activity may be associated with the ability to utilize redox reactions as an energy source, as seen in bacteria of the Aquabacterium, Halomonas, and Pseudomonas genera, or resistance to heavy metals and antibiotics, as observed in Acinetobacter, Staphylococcus, Ochrobactrum, Pseudomonas, Pseudolabrys, and Robiginitalea.
In addition to the enzymatic detoxification of reactive oxygen species, the ability to produce low-molecular-weight compounds with radioprotective and antioxidant properties, such as carotenoids, apparently plays a role in the formation of resistance to the effects of ionizing radiation. The majority of bacteria dominant in communities of radioactively contaminated areas (Table S1) are characterized by colonies colored yellow, orange, red, or pink, which may indicate the presence of carotenoids [340]—an important component of the antioxidant defense of prokaryotic cells [341]. Pigmented bacteria are common in extreme habitats, ranging from the cold deserts of Arctic and Antarctic regions to arid regions and saline lakes [98,340]. The antioxidant properties of carotenoids, providing protection from UV radiation, contribute to the survival of bacteria in other unfavorable conditions. Carotenoids have been found in bacterial isolates obtained from soils from the high background radiation area (HBRA) of the Chavara-Neendakara placer deposits (Kerala, India) [88] and the radioactive site of Misasa (Tottori, Japan) [340,341]. Also, pink-colored colonies of bacteria of the genus Methylobacterium were isolated from soils contaminated with radionuclides as a result of the Chernobyl accident [60]. The proportion of pigmented bacteria, including those with carotenoid presence, in the Bacillaceae family in the bottom sediments was significantly higher in samples from regions with a high radiation background, as a result of the Chernobyl nuclear power plant (ChNPP) accident, compared to areas not contaminated with radionuclides. Additionally, the increase in radiation background levels led to a significant growth (by 69.7%) in the proportion of bacilli capable of synthesizing multiple types of pigments [342]. Conversely, the loss of the ability to synthesize carotenoids due to mutation significantly reduced the radioresistance of Deinococcus radiodurans, as noted by [343].
3.4. Defense Mechanisms Associated with Thermophilicity
It is known that thermophilic bacteria can exhibit increased resistance to ionizing radiation. The evolution of prokaryotes began in the early Earth, when natural radioactivity, including the radiation of the early Sun and heavy elements, 232Th, 235U, and 238U, and 40K, was an intrinsic component of the natural environment [325]. It is not coincidental that resistance to ionizing radiation correlates with resistance to UV radiation and high temperatures, and the most radioresistant among modern bacteria belong to the group of hyperthermophilic organisms.
The majority of the radioactively contaminated sites studied are not associated with arid territories, where the soil heats up to high temperatures and the natural bacterial communities exhibit a wide variety of extremophiles. However, among the cultivated isolates from soil samples collected in the early years after the Chernobyl nuclear power plant accident, radioresistant bacteria of the genus Methylobacterium dominated [60], representatives of which have the ability to survive in conditions of high temperatures, UV radiation, high salinity, and drought [159]. Additionally, in the soils around the Fukushima nuclear power plant, the radio- and thermotolerant bacteria [115,116,177] Geodermatophillus bullgariensis, Truepera radiovictrix, and Rubrobacter taiwanensis [27] (Table S1) were registered among the dominant species. Thermophilic bacteria from the genera Alicyclobacillus and Bellilinea were found among the dominant species in areas with increased natural radioactivity [20,80].
Survival in such an inhospitable environment requires the presence of excellent antioxidant protection [147,148,344,345] and highly effective DNA repair mechanisms. An effective strategy to minimize oxidative damage for many bacteria is to thrive in environments with reduced oxygen levels. Interestingly, microaerophilic organisms are commonly found among both the most sensitive and the most resistant to ionizing radiation bacteria [148]. For example, among those found in radioactively contaminated soils, Gallionella and Flavobacterium bacteria can exist in microaerophilic conditions (Table S1). The acceleration of DNA damage repair is typical for a number of polyploid/merooligoploid bacteria common among Pseudonadota [346], like Methylobacterium and Pseudomonas, as shown in Table S1. In addition to enhanced antioxidant system activity, it can be achieved by increased nucleoid condensation [147] and the ability to assemble intact chromosomes from fragmented pieces. This latter ability is known for some hyperthermophilic bacteria, including radioresistant ones [147,346].
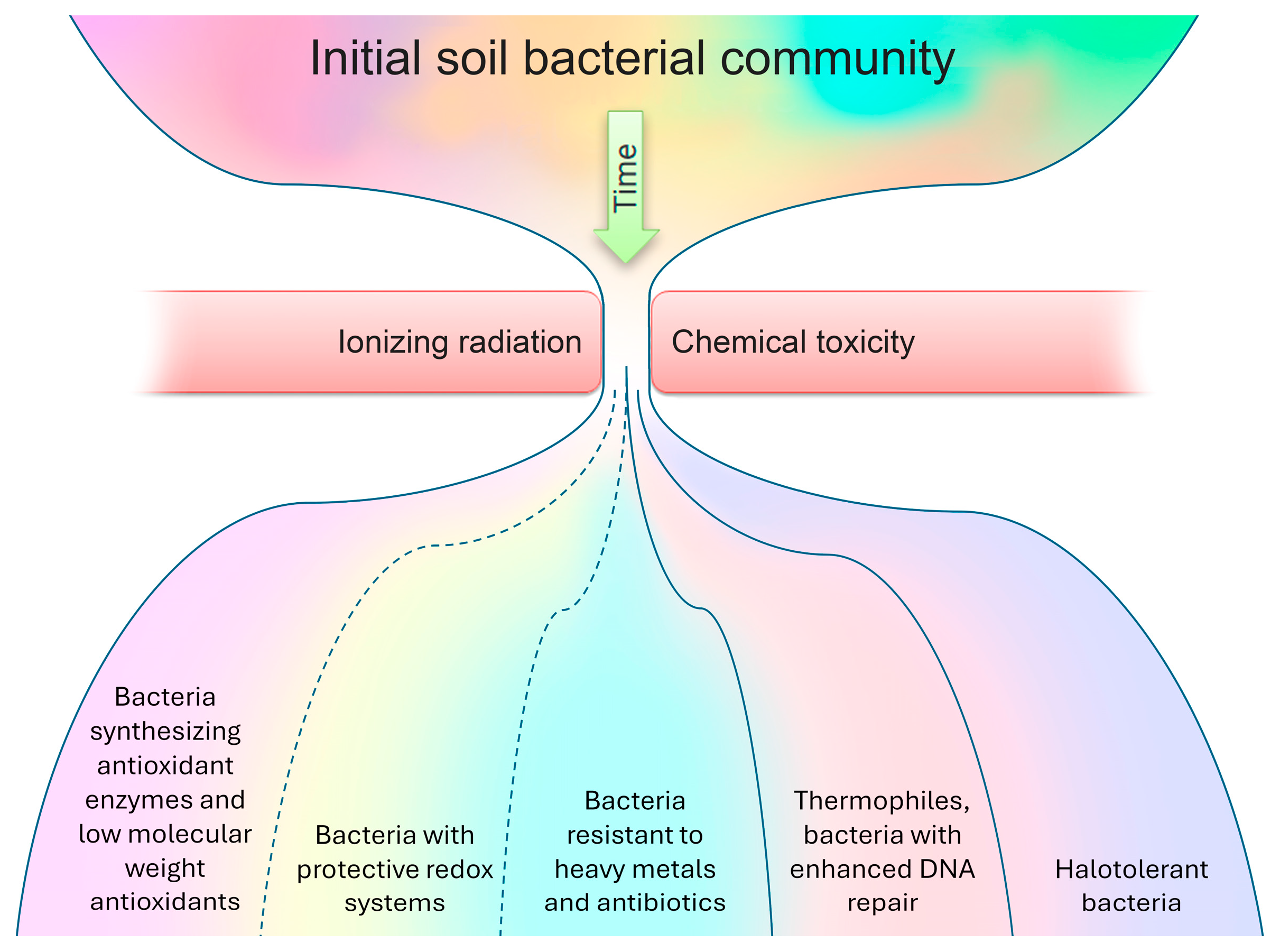
So, the proposed adaptation scheme (Figure 1) for the bacterial community in response to radioactive soil contamination is founded on comparing lists of dominant species (as presented in Table S1) with a compilation of the features that facilitate bacterial survival in contaminated environments. It has been observed that, while certain bacteria dominating contaminated areas may not be explicitly classified as radiotolerant, the presence of specific features enables their survival in the presence of metals and radionuclides. In radioactively contaminated communities, almost all dominated bacteria present had antioxidant enzymes (such as catalase, oxidase, or superoxide dismutase) or low-molecular-weight antioxidants (96%). Furthermore, a significant majority displayed resistance to metals and antibiotics (96%), alongside possessing protective redox systems (68%). Additionally, a substantial portion of the dominant bacterial species demonstrated halotolerance (72%), while a notable percentage were thermophiles with heightened DNA repair mechanisms (44%).
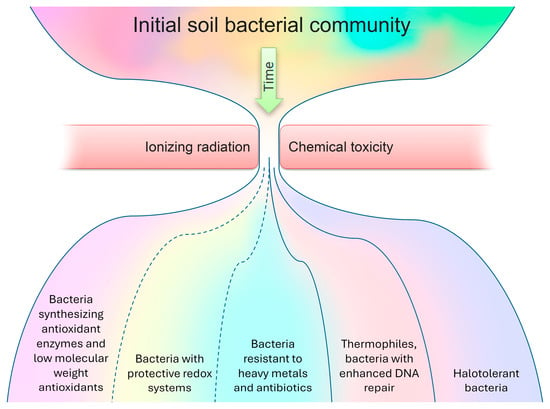
Figure 1.
The proposed adaptation scheme for a bacterial community in response to radioactive soil contamination.
4. Ionizing Radiation in the Forming of Soil Bacterial Communities
The review of the studies examining the impact of external irradiation exposure on soil revealed the remarkable radiotolerance of bacterial communities. The doses that induce significant changes in the taxonomic composition of soil bacterial communities at the phylum level are practically unattainable in landscapes affected by artificial or naturally occurring radionuclides. Adverse effects primarily manifest in the restructuring of the taxonomic structure and a decrease in diversity at high exposure levels. Consequently, the toxic exposure to radionuclides emerges as a key factor driving changes in community composition. The high prevalence of bacteria resistant to metals, antibiotics, and polycyclic aromatic hydrocarbons in radioactively contaminated soils support this hypothesis.
On the other hand, chronic exposure and the interaction of high-linear energy transfer radiation sources, such as uranium isotopes and certain decay products like 241Am, with biological macromolecules in naturally contaminated environments highlight the significance of ionizing radiation from radionuclides present in contaminated soils. Additionally, within irradiated communities, bacteria with highly efficient antioxidant systems and the ability to endure extreme conditions, leading to genome stabilization, have been identified. Both adaptation mechanisms can prove advantageous in the face of genotoxic and pro-oxidant exposures.
The physical and chemical characteristics of the soil, such as moisture, pH levels, granulometric composition, temperature, oxygen levels, organic matter content, NPK levels, dominant vegetation, among others, play a pivotal role in shaping the adaptation of bacterial communities in radioactively contaminated soils [27,62,65,85,87]. Furthermore, the selection process is significantly influenced by the spectrum of radionuclides present and the spatial heterogeneity of contamination [18,112,347]. Assessing the contribution of ionizing radiation to community-based biological effects is only possible under strictly controlled laboratory conditions. It is essential to note, however, that the response of organisms in natural and laboratory settings may differ significantly [348]. Despite these differences, some patterns in the taxonomic diversity of bacterial communities from radioactively contaminated areas have been observed, and certain structural differences between contaminated and reference communities have been distinguished for various radioecological situations.
To assess the long-term effects of radiation contamination, it would be appropriate to identify the functional potential of the genera/species of bacteria that thrive in the transformed community inhabiting the contaminated soil. This analysis could help to elucidate the causes of such changes and provide valuable insights into the resilience and adaptability of microbial communities in the face of environmental stressors. It would indeed be important to consider the main physical and chemical characteristics of the soil and their changes as a result of technogenic influence when assessing the long-term effects of radiation contamination. Additionally, understanding the influence of such changes on the formation of soil bacterial communities is crucial. To gain an in-depth understanding of these relationships, laboratory experiments on determining the influence of changes in the basic characteristics of the soil on the structure of communities against the background of the same level of radiation pollution would be highly beneficial.
When studying the communities inhabiting radioactively contaminated soils, it would be beneficial to focus on analyzing the whole complex of metabolic pathways represented in the community. This can be achieved by examining the frequency of occurrence of genes that confer resistance to contamination, which becomes more feasible with the enrichment of databases of annotated bacterial genomes and the enrichment of metataxonomic research data through the additional sequencing of amplicons of stability genes propagated by horizontal transfer or through the metagenomic analysis of genome-wide libraries.
5. Conclusions
Radioactive contamination has the potential to alter the taxonomic structure of soil bacterial communities, favoring tolerant representatives while decreasing the number of or eliminating sensitive ones. Exposure to external ionizing radiation in laboratory conditions has been shown to result in a significant decrease in diversity, with the dominance of separate highly radiotolerant taxonomic groups. The radioactive contamination of the soil is more likely to lead to a decrease in the alpha diversity and rearrangements in the taxonomic structure. In addition to radioresistance, bacteria that dominate in contaminated environments exhibit tolerance to metals and antibiotics, as well as the ability to produce low-molecular-weight antioxidants, halotolerance, and thermophilic characteristics, with an increased ability to maintain the stability of macromolecules, including DNA.
In studies of the radioactive contamination of the soil, it is challenging to quantify the differential contribution of radioactive exposure and the chemical toxicity of radionuclides. Additionally, the bacterial community transformation following the introduction of radionuclides into the soil is influenced by the initial community composition and the physical and chemical features of the soil.
Thus, the systematization of the data presented in the literature can make a significant contribution to understanding the processes occurring in the bacterial communities of radioactively contaminated soils, as well as the main factors affecting the diversity and composition of prokaryotic soil communities during the radioactive pollution of their habitat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12040733/s1. Table S1: Comparison of lists of dominating bacteria from the reviewed studies and the characteristics that allow them to thrive under radioactive contamination.
Author Contributions
Conceptualization, A.R., E.B. and I.V.; methodology, E.B., A.R. and I.V.; validation, E.B., I.V. and A.R.; formal analysis, E.B., T.M., M.T., E.R. and A.R.; investigation, E.B., T.M., M.T., I.V., E.R. and A.R.; data curation, E.B., T.M., M.T., E.R. and A.R.; writing—original draft preparation, A.R., E.B. and T.M.; writing—review and editing, E.B., T.M., I.V. and A.R.; visualization, I.V.; supervision, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a Russian Science Foundation Grant No. 23-74-01125, https://rscf.ru/project/23-74-01125/ (accessed on 28 March 2024).
Data Availability Statement
Data are contained within the article and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and Death in the Soil Microbiome: How Ecological Processes Influence Biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Tecon, R.; Or, D. Biophysical Processes Supporting the Diversity of Microbial Life in Soil. FEMS Microbiol. Rev. 2017, 41, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.C.; Datta, M.; Sharma, V. Soil Microbes and Biofertilizers. In Soils in the Hindu Kush Himalayas: Management for Agricultural Land Use; Sharma, U.C., Datta, M., Sharma, V., Eds.; Geography of the Physical Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–144. ISBN 9783031114. [Google Scholar]
- Blagodatskaya, E.; Kuzyakov, Y. Active Microorganisms in Soil: Critical Review of Estimation Criteria and Approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Mukhtar, H.; Wunderlich, R.F.; Muzaffar, A.; Ansari, A.; Shipin, O.V.; Cao, T.N.-D.; Lin, Y.-P. Soil Microbiome Feedback to Climate Change and Options for Mitigation. Sci. Total Environ. 2023, 882, 163412. [Google Scholar] [CrossRef] [PubMed]
- Dobrovol’skaya, T.G.; Zvyagintsev, D.G.; Chernov, I.Y.; Golovchenko, A.V.; Zenova, G.M.; Lysak, L.V.; Manucharova, N.A.; Marfenina, O.E.; Polyanskaya, L.M.; Stepanov, A.L.; et al. The Role of Microorganisms in the Ecological Functions of Soils. Eurasian Soil Sci. 2015, 48, 959–967. [Google Scholar] [CrossRef]
- Rahman, Z. An Overview on Heavy Metal Resistant Microorganisms for Simultaneous Treatment of Multiple Chemical Pollutants at Co-Contaminated Sites, and Their Multipurpose Application. J. Hazard. Mater. 2020, 396, 122682. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.-C.; Uivarosi, V.; Andries, A. Recent Progress in Understanding the Molecular Mechanisms of Radioresistance in Deinococcus Bacteria. Extremophiles 2015, 19, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, Y.; He, J.; Qi, H.; Xiao, F.; He, S. Gene Regulation for the Extreme Resistance to Ionizing Radiation of Deinococcus radiodurans. Gene 2019, 715, 144008. [Google Scholar] [CrossRef]
- Kanekar, P.P.; Kanekar, S.P. Radiophilic, Radioresistant, and Radiotolerant Microorganisms. In Diversity and Biotechnology of Extremophilic Microorganisms from India; Springer Nature Singapore: Singapore, 2022; pp. 251–267. ISBN 9789811915. [Google Scholar]
- Popic, J.M.; Salbu, B.; Strand, T.; Skipperud, L. Assessment of Radionuclide and Metal Contamination in a Thorium Rich Area in Norway. J. Environ. Monit. 2011, 13, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Abdolmaleki, P.; Ghanati, F.; Behmanesh, M.; Payez, A. Modification of Catalase and MAPK in Vicia Faba Cultivated in Soil with High Natural Radioactivity and Treated with a Static Magnetic Field. J. Plant Physiol. 2014, 171, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Thørring, H.; Wærsted, F.M.; Raaness, A.; Skipperud, L.; Jensen, L.K. Elevated Natural Radioactivity in Undisturbed Forest and Mountain Areas of Arctic Norway—Local Geology, Soil Characteristics, and Transfer to Biota. J. Environ. Radioact. 2020, 222, 106291. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yong, J.; Liu, Q.; Chen, H.; Mao, P. Response of Soil Microbial Communities to Natural Radionuclides along Specific-Activity Gradients. Ecotoxicol. Environ. Saf. 2022, 246, 114156. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, M.A.; Khandaker, M.U.; Nwankwo, V.U.J.; Idris, A.M. The Status of Natural Radioactivity in Nigerian Environments. Radiat. Environ. Biophys. 2022, 61, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Geras’kin, S.A.; Evseeva, T.I.; Taskaev, A.I.; Maĭstrenko, T.A.; Mikhalik, B. The effects on non-human species inhabiting areas with enhanced level of natural radioactivity in the north of Russia. Radiats. Biol. Radioecol. 2007, 47, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.V.; Belykh, E.S.; Maystrenko, T.A.; Shadrin, D.M.; Pylina, Y.I.; Chadin, I.F.; Velegzhaninov, I.O. Genetic Analysis in Earthworm Population from Area Contaminated with Radionuclides and Heavy Metals. Sci. Total Environ. 2020, 723, 137920. [Google Scholar] [CrossRef] [PubMed]
- Radeva, G.; Kenarova, A.; Bachvarova, V.; Flemming, K.; Popov, I.; Vassilev, D.; Selenska-Pobell, S. Bacterial Diversity at Abandoned Uranium Mining and Milling Sites in Bulgaria as Revealed by 16S RRNA Genetic Diversity Study. Water Air Soil Pollut. 2013, 224, 1748. [Google Scholar] [CrossRef]
- Yan, X.; Luo, X.; Zhao, M. Metagenomic Analysis of Microbial Community in Uranium-Contaminated Soil. Appl. Microbiol. Biotechnol. 2016, 100, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Le Guernic, A.; Sanchez, W.; Bado-Nilles, A.; Palluel, O.; Turies, C.; Chadili, E.; Cavalié, I.; Delahaut, L.; Adam-Guillermin, C.; Porcher, J.-M.; et al. In Situ Effects of Metal Contamination from Former Uranium Mining Sites on the Health of the Three-Spined Stickleback (Gasterosteus aculeatus L.). Ecotoxicology 2016, 25, 1234–1259. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Mu, H.; Hu, N.; Sun, J.; Yin, J.; Dai, K.; Long, D.; Ding, D. Differential Expression of NPM, GSTA3, and GNMT in Mouse Liver Following Long-Term in Vivo Irradiation by Means of Uranium Tailings. Biosci. Rep. 2018, 38, BSR20180536. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging Health Risks and Underlying Toxicological Mechanisms of Uranium Contamination: Lessons from the Past Two Decades. Environ. Int. 2020, 145, 106107. [Google Scholar] [CrossRef] [PubMed]
- Bolsunovsky, A.; Borisov, R.; Melgunov, M. New Data on Mobility of Transuranium Elements in Sediments of the Yenisei River. J. Environ. Radioact. 2023, 270, 107285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; She, J.; Liu, J.; Zhang, Q.; Wei, X.; Huang, L.; Zeng, X.; Wang, J. Insight into Microbial Functional Genes’ Role in Geochemical Distribution and Cycling of Uranium: The Evidence from Covering Soils of Uranium Tailings Dam. J. Hazard. Mater. 2024, 461, 132630. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Hernandez, C.; Courbert, C.; Simonucci, C.; David, S.; Vogel, T.M.; Larose, C. Community Structure and Functional Genes in Radionuclide Contaminated Soils in Chernobyl and Fukushima. FEMS Microbiol. Lett. 2019, 366, fnz180. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Kumagai, A.; Hori, T.; Nanba, K.; Aoyagi, T.; Takasaki, M.; Katayama, Y. Direct Comparison of Bacterial Communities in Soils Contaminated with Different Levels of Radioactive Cesium from the First Fukushima Nuclear Power Plant Accident. Sci. Total Environ. 2021, 756, 143844. [Google Scholar] [CrossRef] [PubMed]
- Lypska, A.; Riabchenko, N.; Rodionova, N.; Burdo, O. Radiation-Induced Effects on Bone Marrow of Bank Voles Inhabiting the Chornobyl Exclusion Zone. Int. J. Radiat. Biol. 2022, 98, 1366–1375. [Google Scholar] [CrossRef]
- Videvall, E.; Burraco, P.; Orizaola, G. Impact of Ionizing Radiation on the Environmental Microbiomes of Chornobyl Wetlands. Environ. Pollut. 2023, 330, 121774. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, Z.; Wang, W.; Tang, Q.; Song, S.; Zhu, J.; Xie, Y.; Zhang, L. The Effects of Radiation Pollution on the Population Diversities and Metabolic Characteristics of Soil Microorganisms. Water Air Soil Pollut. 2014, 225, 2133. [Google Scholar] [CrossRef]
- Prăvălie, R. Nuclear Weapons Tests and Environmental Consequences: A Global Perspective. AMBIO 2014, 43, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, A. Radionuclide Contamination in Canada: A Scoping Review. Heliyon 2023, 9, e16602. [Google Scholar] [CrossRef]
- Feng, D.; Ji, M.; Liao, H.; Yang, F.; Zhou, X.; Pan, T.; Lu, C.; Luo, J.; Miao, Y. An Overview of Plutonium Isotopes in Soils, China: Distribution, Spatial Patterns, and Sources. Environ. Res. 2023, 216, 114677. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, A.L.; Tsvetnova, O.B.; Panikov, S.N. Changes in the Structure of the Microbial Community under the Influence of Oil and Radioactive Pollution. Eurasian Soil Sci. 2012, 45, 1169–1173. [Google Scholar] [CrossRef]
- Galitskaya, P.; Gumerova, R.; Ratering, S.; Schnell, S.; Blagodatskaya, E.; Selivanovskaya, S. Oily Waste Containing Natural Radionuclides: Does It Cause Stimulation or Inhibition of Soil Bacterial Community? J. Plant Nutr. Soil Sci. 2015, 178, 825–833. [Google Scholar] [CrossRef]
- Al Abdullah, J.; Al-Masri, M.S.; Amin, Y.; Awad, I.; Sheaib, Z. Chemical Fractionation of Radium-226 in NORM Contaminated Soil from Oilfields. J. Environ. Radioact. 2016, 165, 47–53. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, B.; McLaughlin, M.C.; Blotevogel, J.; Borch, T.; Warner, N.R. Oil & Gas Produced Water Retention Ponds as Potential Passive Treatment for Radium Removal and Beneficial Reuse. Environ. Sci. Process. Impacts 2021, 23, 501–518. [Google Scholar] [CrossRef] [PubMed]
- El Afifi, E.M.; Mansy, M.S.; Hilal, M.A. Radiochemical Signature of Radium-Isotopes and Some Radiological Hazard Parameters in TENORM Waste Associated with Petroleum Production: A Review Study. J. Environ. Radioact. 2023, 256, 107042. [Google Scholar] [CrossRef] [PubMed]
- Février, L.; Martin-Garin, A. Biogeochemical Behaviour of Anionic Radionuclides in Soil: Evidence for Biotic Interactions. Radioprotection 2005, 40, S79–S86. [Google Scholar] [CrossRef]
- Wilkins, M.J.; Livens, F.R.; Vaughan, D.J.; Beadle, I.; Lloyd, J.R. The Influence of Microbial Redox Cycling on Radionuclide Mobility in the Subsurface at a Low-level Radioactive Waste Storage Site. Geobiology 2007, 5, 293–301. [Google Scholar] [CrossRef]
- Prakash, D.; Gabani, P.; Chandel, A.K.; Ronen, Z.; Singh, O.V. Bioremediation: A Genuine Technology to Remediate Radionuclides from the Environment. Microb. Biotechnol. 2013, 6, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Newsome, L.; Morris, K.; Lloyd, J.R. The Biogeochemistry and Bioremediation of Uranium and Other Priority Radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Hosseini, S.A. The Effect of Microbial Biomass in Soil on Adsorption of Radio Cesium. IJSR 2014, 3, 239–240. [Google Scholar] [CrossRef]
- Seeprasert, P.; Yoneda, M.; Shimada, Y.; Matsui, Y. Tolerance and Accumulation of Cesium and Strontium in Saprothophic Fungi. J. Phys. Conf. Ser. 2015, 611, 012022. [Google Scholar] [CrossRef]
- Safonov, A.V.; Andryushchenko, N.D.; Ivanov, P.V.; Boldyrev, K.A.; Babich, T.L.; German, K.E.; Zakharova, E.V. Biogenic Factors of Radionuclide Immobilization on Sandy Rocks of Upper Aquifers. Radiochemistry 2019, 61, 99–108. [Google Scholar] [CrossRef]
- Lakshmi, P.K.; Abirami, S.; Meenakshi, S.; Usha, C.; Sakthieaswari, P.; Aarthy, K.; Gayathri, S.S.; Baby, S. Application of Deinococcus radiodurans for Bioremediation of Radioactive Wastes. In Microbes and Microbial Biotechnology for Green Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 717–732. ISBN 9780323904. [Google Scholar]
- Patel, R.; Mugunthan, J.; Singh, P.; Mukherjee, S.; Koka, R. Microbial Bioremediation and Biodegradation of Radioactive Waste Contaminated Sites. In Microbes and Microbial Biotechnology for Green Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 733–746. ISBN 9780323904. [Google Scholar]
- Yu, X.; Xiong, F.; Zhou, C.; Luo, Z.; Zhou, Z.; Chen, J.; Sun, K. Uranium Bioprecipitation Mediated by a Phosphate-Solubilizing Enterobacter sp. N1-10 and Remediation of Uranium-Contaminated Soil. Sci. Total Environ. 2024, 906, 167688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Shaw, G. Soil Contamination with Radionuclides and Potential Remediation. Chemosphere 2000, 41, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Wagh, S.; Yadav, S.; Patra, A.; Poswal, A.; Dharekar, A.A.; Pulhani, V.; Saradhi, I.V.; Kumar, A.V. Long-Term Behaviour of Uranium in Soil System: Understanding the Processes Controlling the Bioavailability. J. Hazard. Mater. Adv. 2022, 8, 100151. [Google Scholar] [CrossRef]
- Yasuda, H. Prediction of Long-Term Health Risk from Radiocesium Deposited on Ground with Consideration of Land-Surface Properties. Appl. Sci. 2021, 11, 4424. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.K.; Arae, H.; Sorimachi, A.; Hosoda, M.; Tokonami, S.; Ishikawa, T. Variability of Radiocaesium Inventory in Fukushima Soil Cores from One Site Measured at Different Times. Radiat. Prot. Dosim. 2015, 167, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Le, B.A.; Huynh, N.P.T.; Le, C.H. Natural Radionuclides in Rice Soils in the Mekong Delta Region, Vietnam: Health Risk, Transfer to Rice, and Long-Term Accumulation in Topsoil. Water Air Soil Pollut. 2021, 232, 354. [Google Scholar] [CrossRef]
- Torsvik, V.; Goksøyr, J.; Daae, F.L. High Diversity in DNA of Soil Bacteria. Appl. Environ. Microbiol. 1990, 56, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Øvreås, L. Microbial Diversity and Function in Soil: From Genes to Ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the Classification of Cultured and Uncultured Bacteria and Archaea Using 16S RRNA Gene Sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.V. Metabarcoding and Metagenomics in Soil Ecology Research: Achievements, Challenges, and Prospects. Biol. Bull. Rev. 2021, 11, 40–53. [Google Scholar] [CrossRef]
- Nesme, J.; Achouak, W.; Agathos, S.N.; Bailey, M.; Baldrian, P.; Brunel, D.; Frostegård, Å.; Heulin, T.; Jansson, J.K.; Jurkevitch, E.; et al. Back to the Future of Soil Metagenomics. Front. Microbiol. 2016, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Rodriguez, J.; Martens-Habbena, W. Fine-Scale Evaluation of Two Standard 16S RRNA Gene Amplicon Primer Pairs for Analysis of Total Prokaryotes and Archaeal Nitrifiers in Differently Managed Soils. Front. Microbiol. 2023, 14, 1140487. [Google Scholar] [CrossRef] [PubMed]
- Romanovskaia, V.A.; Stoliar, S.M.; Malashenko, I.R.; Shatokhina, E.S. The effect of long-acting radiation on the diversity of heterotrophic bacteria in the soils of a 10-kilometer area around the Chernobyl Atomic Electric Power Station. Mikrobiolohichnyi Zhurnal 1996, 58, 3–12. [Google Scholar] [PubMed]
- Romanovskaia, V.A.; Sokolov, I.G.; Rokitko, P.V.; Chernaia, N.A. Ecological consequences of radioactive pollution for soil bacteria within the 10-km region around the Chernobyl Atomic Energy Station. Mikrobiologiia 1998, 67, 274–280. [Google Scholar] [PubMed]
- Chapon, V.; Piette, L.; Vesvres, M.-H.; Coppin, F.; Marrec, C.L.; Christen, R.; Theodorakopoulos, N.; Février, L.; Levchuk, S.; Martin-Garin, A.; et al. Microbial Diversity in Contaminated Soils along the T22 Trench of the Chernobyl Experimental Platform. Appl. Geochem. 2012, 27, 1375–1383. [Google Scholar] [CrossRef]
- Niedrée, B.; Berns, A.E.; Vereecken, H.; Burauel, P. Do Chernobyl-like Contaminations with 137Cs and 90Sr Affect the Microbial Community, the Fungal Biomass and the Composition of Soil Organic Matter in Soil? J. Environ. Radioact. 2013, 118, 21–29. [Google Scholar] [CrossRef]
- Ivanova, T.I.; Kuz’mina, N.P.; Sobakin, P.I. Impact of radioactive elements on microbial complexes in cryogenic soils of Yakutia. Biol. Bull. 2016, 43, 113–120. [Google Scholar] [CrossRef]
- Theodorakopoulos, N.; Février, L.; Barakat, M.; Ortet, P.; Christen, R.; Piette, L.; Levchuk, S.; Beaugelin-Seiller, K.; Sergeant, C.; Berthomieu, C.; et al. Soil Prokaryotic Communities in Chernobyl Waste Disposal Trench T22 Are Modulated by Organic Matter and Radionuclide Contamination. FEMS Microbiol. Ecol. 2017, 93, fix079. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Zhang, Z.-D.; Tang, Q.-Y.; Gu, M.-Y.; Zhang, L.-J.; Hou, M.; Sharon, A.; Yuan, H.-L. Effect of Ionizing Radiation on the Bacterial and Fungal Endophytes of the Halophytic Plant Kalidium Schrenkianum. Microorganisms 2021, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Selenska-Pobell, S.; Kampf, G.; Flemming, K.; Radeva, G.; Satchanska, G. Bacterial diversity in soil samples from two uranium waste piles as determined by rep-APD, RISA and 16S rDNA retrieval. Antonie Leeuwenhoek 2001, 79, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Osman, S.; Vaishampayan, P.A.; Andersen, G.L.; Stetler, L.D.; Sani, R.K. Microbial Diversity in Uranium Mining-Impacted Soils as Revealed by High-Density 16S Microarray and Clone Library. Microb. Ecol. 2010, 59, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Faleiro, M.L.; Chaves, S.; Tenreiro, R.; Santos, E.; Costa, M.C. Anaerobic Bio-Removal of Uranium (VI) and Chromium (VI): Comparison of Microbial Community Structure. J. Hazard. Mater. 2010, 176, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Islam, E.; Sar, P. Culture-dependent and -independent Molecular Analysis of the Bacterial Community within Uranium Ore. J. Basic Microbiol. 2011, 51, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.J.; Pereira, R.; Duarte, K.; Rocha-Santos, T.A.P.; Antunes, S.C.; Gonçalves, F.; Duarte, A.C.; Freitas, A.C. Sterols and Fatty Acid Biomarkers as Indicators of Changes in Soil Microbial Communities in a Uranium Mine Area. J. Environ. Sci. Health Part A 2011, 46, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Dhal, P.K.; Islam, E.; Kazy, S.K.; Sar, P. Culture-Independent Molecular Analysis of Bacterial Diversity in Uranium-Ore/-Mine Waste-Contaminated and Non-Contaminated Sites from Uranium Mines. 3 Biotech 2011, 1, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Mondani, L.; Benzerara, K.; Carrière, M.; Christen, R.; Mamindy-Pajany, Y.; Février, L.; Marmier, N.; Achouak, W.; Nardoux, P.; Berthomieu, C.; et al. Influence of Uranium on Bacterial Communities: A Comparison of Natural Uranium-Rich Soils with Controls. PLoS ONE 2011, 6, e25771. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nongkhlaw, M.; Acharya, C.; Joshi, S.R. Uranium (U)-Tolerant Bacterial Diversity from U Ore Deposit of Domiasiat in North-East India and Its Prospective Utilisation in Bioremediation. Microb. Environ. 2013, 28, 33–41. [Google Scholar] [CrossRef]
- Islam, E.; Paul, D.; Sar, P. Microbial Diversity in Uranium Deposits from Jaduguda and Bagjata Uranium Mines, India as Revealed by Clone Library and Denaturing Gradient Gel Electrophoresis Analyses. Geomicrobiol. J. 2014, 31, 862–874. [Google Scholar] [CrossRef]
- Sitte, J.; Löffler, S.; Burkhardt, E.-M.; Goldfarb, K.C.; Büchel, G.; Hazen, T.C.; Küsel, K. Metals Other than Uranium Affected Microbial Community Composition in a Historical Uranium-Mining Site. Environ. Sci. Pollut. Res. 2015, 22, 19326–19341. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Luo, X. Radionuclides Distribution, Properties, and Microbial Diversity of Soils in Uranium Mill Tailings from Southeastern China. J. Environ. Radioact. 2015, 139, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Galitskaya, P.; Biktasheva, L.; Saveliev, A.; Ratering, S.; Schnell, S.; Selivanovskaya, S. Response of Soil Microorganisms to Radioactive Oil Waste: Results from a Leaching Experiment. Biogeosciences 2015, 12, 3681–3693. [Google Scholar] [CrossRef]
- Suriya, J.; Chandra Shekar, M.; Nathani, N.M.; Suganya, T.; Bharathiraja, S.; Krishnan, M. Assessment of Bacterial Community Composition in Response to Uranium Levels in Sediment Samples of Sacred Cauvery River. Appl. Microbiol. Biotechnol. 2017, 101, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, B.; Chariton, A.A.; Harford, A.J.; Hose, G.C.; Greenfield, P.; Elbourne, L.D.H.; Oytam, Y.; Stephenson, S.; Midgley, D.J.; Paulsen, I.T. Effects of Uranium Concentration on Microbial Community Structure and Functional Potential. Environ. Microbiol. 2017, 19, 3323–3341. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.; Zhang, X.; Chaoliang, C.; Luo, X.; Zhang, Y. Comprehensive Evaluation of Soil Near Uranium Tailings, Beishan City, China. Bull. Environ. Contam. Toxicol. 2018, 100, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Streten, C.; Parry, D.L.; McGuinness, K.A.; Lu, P.; Gibb, K.S. Soil Uranium Concentration at Ranger Uranium Mine Land Application Areas Drives Changes in the Bacterial Community. J. Environ. Radioact. 2018, 189, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lusa, M.; Knuutinen, J.; Lindgren, M.; Virkanen, J.; Bomberg, M. Microbial Communities in a Former Pilot-Scale Uranium Mine in Eastern Finland—Association with Radium Immobilization. Sci. Total Environ. 2019, 686, 619–640. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, Q.; Chen, X.; Dong, F.; Chen, H.; Liu, M.; Ali, I. Speciation Distribution of Heavy Metals in Uranium Mining Impacted Soils and Impact on Bacterial Community Revealed by High-Throughput Sequencing. Front. Microbiol. 2019, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Oladeinde, A.; Kieran, T.J.; Finger, J.W.; Bayona-Vásquez, N.J.; Cartee, J.C.; Beasley, J.C.; Seaman, J.C.; McArthur, J.V.; Rhodes, O.E.; et al. Co-occurrence of Antibiotic, Biocide, and Heavy Metal Resistance Genes in Bacteria from Metal and Radionuclide Contaminated Soils at the Savannah River Site. Microb. Biotechnol. 2020, 13, 1179–1200. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; San Choi, Y.; Park, S.Y.; Kim, C.G. A Case Study on the Correlation between Radon and Multiple Geophysicochemical Properties of Soils in G Island, Korea, and Effects on the Bacterial Metabolic Behaviors. J. Environ. Radioact. 2020, 222, 106336. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, T.; Claesen, J.; Van Gompel, A.; Vanhoudt, N.; Mysara, M.; Williamson, A.; Leys, N.; Van Houdt, R.; Boon, N.; Mijnendonckx, K. Soil Microbial Community Structure and Functionality Changes in Response to Long-term Metal and Radionuclide Pollution. Environ. Microbiol. 2021, 23, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Sowptika, P.; Anilkumar, G. The Bacterial Population Inhabiting a Top-Ranking High Background Radiation Area: A First-Time Report. J. Environ. Biol. 2023, 44, 45–56. [Google Scholar] [CrossRef]
- Feng, G.; Yong, J.; Liu, Q.; Chen, H.; Hu, Y.; Mao, P. Remedial Effect and Operating Status of a Decommissioned Uranium Mill Tailings (UMT) Repository: A Micro-Ecological Perspective Based on Bacterial Community. J. Environ. Manag. 2023, 340, 117993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, H.; Wang, R.; Zhou, L.; Li, N.; He, Y.; Yang, X.; Lai, J.; Chen, K.; Zhu, W. Non-Targeted Metabolomics and 16s RDNA Reveal the Impact of Uranium Stress on Rhizosphere and Non-Rhizosphere Soil of Ryegrass. J. Environ. Radioact. 2023, 258, 107090. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiong, Z.; Xiang, P.; Zhou, L.; Zhang, T.; Wu, Q.; Zhao, C. Effects of Uranium Mining on Soil Bacterial Communities and Functions in the Qinghai-Tibet Plateau. Chemosphere 2024, 347, 140715. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.E.; West, H.M.; Chamberlain, P.M.; Parekh, N.R.; Beresford, N.A.; Crout, N.M.J. Effects of Gamma Irradiation on Holcus Lanatus (Yorkshire Fog Grass) and Associated Soil Microorganisms. J. Environ. Radioact. 2004, 74, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Rainey, F.A.; Ray, K.; Ferreira, M.; Gatz, B.Z.; Nobre, M.F.; Bagaley, D.; Rash, B.A.; Park, M.-J.; Earl, A.M.; Shank, N.C.; et al. Extensive Diversity of Ionizing-Radiation-Resistant Bacteria Recovered from Sonoran Desert Soil and Description of Nine New Species of the Genus Deinococcus Obtained from a Single Soil Sample. Appl. Environ. Microbiol. 2005, 71, 5225–5235. [Google Scholar] [CrossRef]
- Chanal, A.; Chapon, V.; Benzerara, K.; Barakat, M.; Christen, R.; Achouak, W.; Barras, F.; Heulin, T. The Desert of Tataouine: An Extreme Environment That Hosts a Wide Diversity of Microorganisms and Radiotolerant Bacteria. Environ. Microbiol. 2006, 8, 514–525. [Google Scholar] [CrossRef]
- McNamara, N.P.; Griffiths, R.I.; Tabouret, A.; Beresford, N.A.; Bailey, M.J.; Whiteley, A.S. The Sensitivity of a Forest Soil Microbial Community to Acute Gamma-Irradiation. Appl. Soil Ecol. 2007, 37, 1–9. [Google Scholar] [CrossRef]
- El-Sayed, W.S.; Ghanem, S. Bacterial Community Structure Change Induced by Gamma Irradiation in Hydrocarbon Contaminated and Uncontaminated Soils Revealed by PCR-Denaturing Gradient Gel Electrophoresis. Biotechnology 2008, 8, 78–85. [Google Scholar] [CrossRef]
- Shah, V.; Shah, S.; Mackey, H.; Kambhampati, M.; Collins, D.; Dowd, S.E.; Colichio, R.; McDonnell, K.T.; Green, T. Microbial Community in the Soil Determines the Forest Recovery Post-Exposure to Gamma Irradiation. Environ. Sci. Technol. 2013, 47, 11396–11402. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Z.; Luo, X.; Liu, M.; Huang, Q. Diversity of Ionizing Radiation-resistant Bacteria Obtained from the Taklimakan Desert. J. Basic Microbiol. 2015, 55, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, S.; Gong, X.; Chang, L.; Jia, S.; Yang, X.; Wu, D.; Zhang, X. Effects of Gamma Irradiation on Soil Biological Communities and C and N Pools in a Clay Loam Soil. Appl. Soil Ecol. 2016, 108, 352–360. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Manucharova, N.A.; Gorlenko, M.V.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Bulat, S.A. 100 KGy Gamma-Affected Microbial Communities within the Ancient Arctic Permafrost under Simulated Martian Conditions. Extremophiles 2017, 21, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Kerfahi, D.; Song, H.; Dong, K.; Seo, H.; Lim, S.; Srinivasan, S.; Kim, M.K.; Waldman, B.; Adams, J.M. Changes in Soil Taxonomic and Functional Diversity Resulting from Gamma Irradiation. Sci. Rep. 2019, 9, 7894. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Srinivasan, S.; Dong, K.; Ramasamy, D.; Waldman, B.; Adams, J.M. Community Ecology of Deinococcus in Irradiated Soil. Microb. Ecol. 2019, 78, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Al-Najjar, M.A.A.; Albokari, M.M. Shifts in Microbial Community Composition in Tannery-Contaminated Soil in Response to Increased Gamma Radiation. Ann. Microbiol. 2019, 69, 1567–1577. [Google Scholar] [CrossRef]
- Evrard, O.; Chartin, C.; Laceby, J.P.; Onda, Y.; Wakiyama, Y.; Nakao, A.; Cerdan, O.; Lepage, H.; Jaegler, H.; Vandromme, R.; et al. Radionuclide Contamination in Flood Sediment Deposits in the Coastal Rivers Draining the Main Radioactive Pollution Plume of Fukushima Prefecture, Japan (2011–2020). Earth Syst. Sci. Data 2021, 13, 2555–2560. [Google Scholar] [CrossRef]
- Martínez, J.; Peñalver, A.; Baciu, T.; Artigues, M.; Danús, M.; Aguilar, C.; Borrull, F. Presence of Artificial Radionuclides in Samples from Potable Water and Wastewater Treatment Plants. J. Environ. Radioact. 2018, 192, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, T.; Hayashi, G.; Endo, S. Comparison of the Accident Process, Radioactivity Release and Ground Contamination between Chernobyl and Fukushima-1. J. Radiat. Res. 2015, 56 (Suppl. 1), i56–i61. [Google Scholar] [CrossRef] [PubMed]
- Beresford, N.A.; Fesenko, S.; Konoplev, A.; Skuterud, L.; Smith, J.T.; Voigt, G. Thirty Years after the Chernobyl Accident: What Lessons Have We Learnt? J. Environ. Radioact. 2016, 157, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H. An Assessment on the Environmental Contamination Caused by the Fukushima Accident. J. Environ. Manag. 2018, 206, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Onda, Y.; Taniguchi, K.; Yoshimura, K.; Kato, H.; Takahashi, J.; Wakiyama, Y.; Coppin, F.; Smith, H. Radionuclides from the Fukushima Daiichi Nuclear Power Plant in Terrestrial Systems. Nat. Rev. Earth Environ. 2020, 1, 644–660. [Google Scholar] [CrossRef]
- Hu, Q.-H.; Weng, J.-Q.; Wang, J.-S. Sources of Anthropogenic Radionuclides in the Environment: A Review. J. Environ. Radioact. 2010, 101, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Lukashenko, S.; Kabdyrakova, A.; Lind, O.C.; Gorlachev, I.; Kunduzbayeva, A.; Kvochkina, T.; Janssens, K.; De Nolf, W.; Yakovenko, Y.; Salbu, B. Radioactive Particles Released from Different Sources in the Semipalatinsk Test Site. J. Environ. Radioact. 2020, 216, 106160. [Google Scholar] [CrossRef] [PubMed]
- Panitskiy, A.; Syssoeva, Y.; Baigazy, S.; Kunduzbayeva, A.; Kenzhina, L.; Polivkina, Y.; Larionova, N.; Krivitskiy, P.; Aidarkhanova, A. Vertical Distribution of Radionuclides in Soil at the Semipalatinsk Test Site beyond Its Test Locations. PLoS ONE 2023, 18, e0278581. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sabour, M.F. Fate of Cesium and Strontium in Soil-to-Plant System (Overview). J. Radiat. Nucl. Appl. 2022, 7, 87–93. [Google Scholar] [CrossRef]
- Pareniuk, O.; Shavanova, K.; Laceby, J.P.; Illienko, V.; Tytova, L.; Levchuk, S.; Gudkov, I.; Nanba, K. Modification of 137Cs Transfer to Rape (Brassica Napus L.) Phytomass under the Influence of Soil Microorganisms. J. Environ. Radioact. 2015, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hezbri, K.; Ghodhbane-Gtari, F.; Del Carmen Montero-Calasanz, M.; Sghaier, H.; Rohde, M.; Schumann, P.; Klenk, H.-P.; Gtari, M. Description of Geodermatophilus Bullaregiensis sp. Nov. Antonie Leeuwenhoek 2015, 108, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.; Simões, C.; Nobre, M.F.; Pino, N.M.; Battista, J.R.; Silva, M.T.; Rainey, F.A.; da Costa, M.S. Truepera radiovictrix gen. nov., sp. nov., a New Radiation Resistant Species and the Proposal of Trueperaceae Fam. Nov. FEMS Microbiol. Lett. 2005, 247, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cao, J.; Fang, J.; Kato, C.; Cui, W. First Complete Genome Sequence of Marinilactibacilluspiezotolerans Strain 15R, a Marine Lactobacillus Isolated from Coal-Bearing Sediment 2.0 Kilometers below the Seafloor, Determined by PacBio Single-Molecule Real-Time Technology. Genome Announc. 2017, 5, e01625-16. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.S.; King, G.M.; Friesen, M.L. Rubrobacter Spartanus sp. nov., a Moderately Thermophilic Oligotrophic Bacterium Isolated from Volcanic Soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Im, W.-T.; Kim, S.-H.; Kim, M.K.; Ten, L.N.; Lee, S.-T. Pleomorphomonas koreensis sp. nov., a Nitrogen-Fixing Species in the Order Rhizobiales. Int. J. Syst. Evol. Microbiol. 2006, 56, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.S.; Debieux, C.M.; Dridge, E.J.; Splatt, P.; Wright, M. Biomineralization of Selenium by the Selenate-Respiring Bacterium Thauera Selenatis. Biochem. Soc. Trans. 2012, 40, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Mandujano-García, C.D.; Sosa, M.; Mantero, J.; Costilla, R.; Manjón, G.; García-Tenorio, R. Radiological Impact of Natural Radionuclides from Soils of Salamanca, Mexico. Appl. Radiat. Isot. 2016, 117, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Al-Khawlany, A.H.; Khan, A.R.; Pathan, J.M. Review on Studies in Natural Background Radiation. Radiat. Prot. Environ. 2018, 41, 215–222. [Google Scholar] [CrossRef]
- Abbasi, A.; Kurnaz, A.; Turhan, Ş.; Mirekhtiary, F. Radiation Hazards and Natural Radioactivity Levels in Surface Soil Samples from Dwelling Areas of North Cyprus. J. Radioanal. Nucl. Chem. 2020, 324, 203–210. [Google Scholar] [CrossRef]
- Belyaeva, O.; Movsisyan, N.; Pyuskyulyan, K.; Sahakyan, L.; Tepanosyan, G.; Saghatelyan, A. Yerevan Soil Radioactivity: Radiological and Geochemical Assessment. Chemosphere 2021, 265, 129173. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, R.A.; Silva, A.X.; Domingues, A.M.; Ribeiro, F.C.A.; Viglio, E.P. Relationships between Natural Radionuclides Activity Concentration in Soils of the State of Alagoas, Brazil and WRB/FAO Soil Classification, Types of Parental Rocks and Rainfall Variability. Appl. Radiat. Isot. 2022, 187, 110309. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Tong, H.; Zhang, X.; Jia, S.; Chen, M.; Liu, C. Heavy Metal Tolerance Genes Associated with Contaminated Sediments From an E-Waste Recycling River in Southern China. Front. Microbiol. 2021, 12, 665090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, T.; Zhou, L.; Lou, W.; Zeng, W.; Liu, T.; Yin, H.; Liu, H.; Liu, X.; Mathivanan, K.; et al. Soil Microbial Community Assembly Model in Response to Heavy Metal Pollution. Environ. Res. 2022, 213, 113576. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-Q.; Barkay, T. Microbial Mercury Transformations: Molecules, Functions and Organisms. Adv. Appl. Microbiol. 2022, 118, 31–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus Ferrooxidans and Its Potential Application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.D.; Krumholz, L.R. Uranium Reduction. Annu. Rev. Microbiol. 2006, 60, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Butiuc-Keul, A.; Carpa, R.; Podar, D.; Szekeres, E.; Muntean, V.; Iordache, D.; Farkas, A. Antibiotic Resistance in Pseudomonas spp. Through the Urban Water Cycle. Curr. Microbiol. 2021, 78, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, N.; Zhang, Y. The Radioresistant and Survival Mechanisms of Deinococcus radiodurans. Radiat. Med. Prot. 2023, 4, 70–79. [Google Scholar] [CrossRef]
- Sun, J.; Shen, P.; Chao, H.; Wu, B. Isolation and Identification of a New Radiation-Resistant Bacterium Deinococcus guangriensis sp. nov. and Analysis of Its Radioresistant Character. Wei Sheng Wu Xue Bao 2009, 49, 918–924. [Google Scholar] [PubMed]
- Zhang, Q.; Liu, C.; Tang, Y.; Zhou, G.; Shen, P.; Fang, C.; Yokota, A. Hymenobacter xinjiangensis sp. nov., a Radiation-Resistant Bacterium Isolated from the Desert of Xinjiang, China. Int. J. Syst. Evol. Microbiol. 2007, 57, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Srinivasan, S.; Lim, S.; Joe, M.; Lee, S.H.; Kwon, S.A.; Kwon, Y.J.; Lee, J.; Choi, J.J.; Lee, H.M.; et al. Hymenobacter swuensis sp. nov., a Gamma-Radiation-Resistant Bacteria Isolated from Mountain Soil. Curr. Microbiol. 2014, 68, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.-H.; Kim, M.-K.; Choe, H.N.; Lim, S. Hymenobacter taeanensis sp. nov., Radiation Resistant Bacterium Isolated from Coastal Sand Dune. Antonie Leeuwenhoek 2021, 114, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Srinivasan, S. Rufibacter radiotolerans sp. nov., a Novel Gamma-Radiation-Resistant Bacterium Isolated from Rice Field. Arch. Microbiol. 2021, 203, 347–353. [Google Scholar] [CrossRef]
- Joshi, M.N.; Sharma, A.C.; Pandya, R.V.; Patel, R.P.; Saiyed, Z.M.; Saxena, A.K.; Bagatharia, S.B. Draft Genome Sequence of Pontibacter sp. nov. BAB1700, a Halotolerant, Industrially Important Bacterium. J. Bacteriol. 2012, 194, 6329–6330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, R.; Chen, Y.; Xue, D.; Han, J.; Wang, J.; Dai, Q.; Lin, M.; Ke, X.; Zhang, W. Isolation and Identification of Microvirga Thermotolerans HR1, a Novel Thermo-Tolerant Bacterium, and Comparative Genomics among Microvirga Species. Microorganisms 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Panthee, S.; Hamamoto, H.; Paudel, A.; Sekimizu, K. Lysobacter Species: A Potential Source of Novel Antibiotics. Arch. Microbiol. 2016, 198, 839–845. [Google Scholar] [CrossRef]
- Bae, H.-S.; Im, W.-T.; Lee, S.-T. Lysobacter concretionis sp. nov., Isolated from Anaerobic Granules in an Upflow Anaerobic Sludge Blanket Reactor. Int. J. Syst. Evol. Microbiol. 2005, 55, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Spieck, E.; Spohn, M.; Wendt, K.; Bock, E.; Shively, J.; Frank, J.; Indenbirken, D.; Alawi, M.; Lücker, S.; Hüpeden, J. Extremophilic Nitrite-Oxidizing Chloroflexi from Yellowstone Hot Springs. ISME J. 2020, 14, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Lim, Y.W.; Yi, H.; Sekiguchi, Y.; Kamagata, Y.; Chun, J. Anaerosporobacter mobilis gen. nov., sp. nov., Isolated from Forest Soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Parmar, P.; Saraf, M. Radiation, Radionuclides and Bacteria: An in-Perspective Review. J. Environ. Radioact. 2017, 180, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal Tolerance Mechanisms in Bacteria: The Resistance Strategies Acquired by Bacteria That Can Be Exploited to ‘Clean-up’ Heavy Metal Contaminants from Water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic Resistance in Burkholderia Species. Drug Resist. Updates 2016, 28, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, P.; Rawat, S. Radiation Resistance in Thermophiles: Mechanisms and Applications. World J. Microbiol. Biotechnol. 2017, 33, 112. [Google Scholar] [CrossRef] [PubMed]
- Beblo, K.; Douki, T.; Schmalz, G.; Rachel, R.; Wirth, R.; Huber, H.; Reitz, G.; Rettberg, P. Survival of Thermophilic and Hyperthermophilic Microorganisms after Exposure to UV-C, Ionizing Radiation and Desiccation. Arch. Microbiol. 2011, 193, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Squire, M.S.; Townsend, H.A.; Actis, L.A. The Influence of Blue Light and the BlsA Photoreceptor on the Oxidative Stress Resistance Mechanisms of Acinetobacter Baumannii. Front. Cell. Infect. Microbiol. 2022, 12, 856953. [Google Scholar] [CrossRef] [PubMed]
- Albarracín, V.H.; Pathak, G.P.; Douki, T.; Cadet, J.; Borsarelli, C.D.; Gärtner, W.; Farias, M.E. Extremophilic Acinetobacter Strains from High-Altitude Lakes in Argentinean Puna: Remarkable UV-B Resistance and Efficient DNA Damage Repair. Orig. Life Evol. Biosph. 2012, 42, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Thornley, M.J. A Taxonomic Study of Acinetobacter and Related Genera. J. Gen. Microbiol. 1967, 49, 211–257. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.H.; Chaudhary, D.K.; Kim, J. Acinetobacter halotolerans sp. nov., a Novel Halotolerant, Alkalitolerant, and Hydrocarbon Degrading Bacterium, Isolated from Soil. Arch. Microbiol. 2017, 199, 701–710. [Google Scholar] [CrossRef]
- Kunakom, S.; Eustáquio, A.S. Burkholderia as a Source of Natural Products. J. Nat. Prod. 2019, 82, 2018–2037. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of Metabolic Activity, Beneficial and Pathogenic Aspects of Burkholderia spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Sedláček, I.; Holochová, P.; Busse, H.-J.; Koublová, V.; Králová, S.; Švec, P.; Sobotka, R.; Staňková, E.; Pilný, J.; Šedo, O.; et al. Characterisation of Waterborne Psychrophilic Massilia Isolates with Violacein Production and Description of Massilia antarctica Sp. Nov. Microorganisms 2022, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Holochová, P.; Mašlaňová, I.; Sedláček, I.; Švec, P.; Králová, S.; Kovařovic, V.; Busse, H.-J.; Staňková, E.; Barták, M.; Pantůček, R. Description of Massilia rubra sp. nov., Massilia aquatica sp. nov., Massilia mucilaginosa sp. nov., Massilia frigida sp. nov., and One Massilia Genomospecies Isolated from Antarctic Streams, Lakes and Regoliths. Syst. Appl. Microbiol. 2020, 43, 126112. [Google Scholar] [CrossRef] [PubMed]
- Grivalský, T.; Bučková, M.; Puškárová, A.; Kraková, L.; Pangallo, D. Water-Related Environments: A Multistep Procedure to Assess the Diversity and Enzymatic Properties of Cultivable Bacteria. World J. Microbiol. Biotechnol. 2016, 32, 42. [Google Scholar] [CrossRef] [PubMed]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A Survey of Methylobacterium Species and Strains Reveals Widespread Production and Varying Profiles of Cytokinin Phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Patt, T.E.; Cole, G.C.; Hanson, R.S. Methylobacterium, a New Genus of Facultatively Methylotrophic Bacteria. International J. Syst. Evol. Microbiol. 1976, 26, 226–229. [Google Scholar] [CrossRef]
- Green, P.N. Methylobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Good, N.M.; Fellner, M.; Demirer, K.; Hu, J.; Hausinger, R.P.; Martinez-Gomez, N.C. Lanthanide-Dependent Alcohol Dehydrogenases Require an Essential Aspartate Residue for Metal Coordination and Enzymatic Function. J. Biol. Chem. 2020, 295, 8272–8284. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Furuhata, K.; Matsumoto, A.; Koike, K.A.; Fukuyama, M.; Tabuchi, K. Phenotypic and Genetic Diversity of Chlorine-Resistant Methylobacterium Strains Isolated from Various Environments. Appl. Environ. Microbiol. 1995, 61, 2099–2107. [Google Scholar] [CrossRef]
- Romanovskaya, V.A.; Rokitko, P.V.; Mikheev, A.N.; Gushcha, N.I.; Malashenko, Y.R.; Chernaya, N.A. The Effect of G-Radiation and Desiccation on the Viability of the Soil Bacteria Isolated from the Alienated Zone around the Chernobyl Nuclear Power Plant. Microbiology 2002, 71, 608–613. [Google Scholar] [CrossRef]
- Chistoserdova, L.; Chen, S.-W.; Lapidus, A.; Lidstrom, M.E. Methylotrophy in Methylobacterium Extorquens AM1 from a Genomic Point of View. J. Bacteriol. 2003, 185, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Pembroke, J.T. The Genus Ochrobactrum as Major Opportunistic Pathogens. Microorganisms 2020, 8, 1797. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, D.; Chatterjee, S. Malathion Biodegradation by a Psychrotolerant Bacteria ochrobactrum sp. M1D and Metabolic Pathway Analysis. Lett. Appl. Microbiol. 2021, 73, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Khan, M.H.; Misra, S.; Dixit, V.K.; Khare, P.; Srivastava, S.; Chauhan, P.S. Characterisation of Pseudomonas spp. and Ochrobactrum sp. Isolated from Volcanic Soil. Antonie Leeuwenhoek 2017, 110, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Hu, B.; Cai, J.; Zheng, P.; Azim, M.R.; Jilani, G.; Islam, E. Isolation of Ochrobactrum sp. QZ2 from Sulfide and Nitrite Treatment System. J. Hazard. Mater. 2009, 165, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.; Heckel, F.; Saha-Möller, C.R.; Taupp, M.; Meyer, J.-M.; Schreier, P. Opposite Enantioselectivities of Two Phenotypically and Genotypically Similar Strains of Pseudomonas Frederiksbergensis in Bacterial Whole-Cell Sulfoxidation. Appl. Environ. Microbiol. 2005, 71, 2199–2202. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial Soil Bacterium Pseudomonas Frederiksbergensis OS261 Augments Salt Tolerance and Promotes Red Pepper Plant Growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef]
- Kumar, R.; Acharya, V.; Mukhia, S.; Singh, D.; Kumar, S. Complete Genome Sequence of Pseudomonas Frederiksbergensis ERDD5:01 Revealed Genetic Bases for Survivability at High Altitude Ecosystem and Bioprospection Potential. Genomics 2019, 111, 492–499. [Google Scholar] [CrossRef]
- Macy, J.M.; Rech, S.; Auling, G.; Dorsch, M.; Stackebrandt, E.; Sly, L.I. Thauera selenatis Gen. Nov., sp. Nov., a Member of the Beta Subclass of Proteobacteria with a Novel Type of Anaerobic Respiration. Int. J. Syst. Bacteriol. 1993, 43, 135–142. [Google Scholar] [CrossRef]
- Wauters, G.; Charlier, J.; Janssens, M.; Delmée, M. Identification of Arthrobacter Oxydans, Arthrobacter luteolus sp. nov., and Arthrobacter albus sp. nov., Isolated from Human Clinical Specimens. J. Clin. Microbiol. 2000, 38, 2412–2415. [Google Scholar] [CrossRef] [PubMed]
- Asatiani, N.V.; Abuladze, M.K.; Kartvelishvili, T.M.; Bakradze, N.G.; Sapojnikova, N.A.; Tsibakhashvili, N.Y.; Tabatadze, L.V.; Lejava, L.V.; Asanishvili, L.L.; Holman, H.-Y. Effect of Chromium(VI) Action on Arthrobacter Oxydans. Curr. Microbiol. 2004, 49, 321–326. [Google Scholar] [CrossRef]
- Navarro-Llorens, J.M.; Drzyzga, O.; Perera, J. Genetic Analysis of Phenylacetic Acid Catabolism in Arthrobacter Oxydans CECT386. Arch. Microbiol. 2008, 190, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Wu, S.-H.; Lin, G.-H.; Lu, C.-P.; Lin, Y.-T.; Chang, W.-C.; Tsay, S.-S. Rubrobacter taiwanensis sp. nov., a Novel Thermophilic, Radiation-Resistant Species Isolated from Hot Springs. Int. J. Syst. Evol. Microbiol. 2004, 54, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, P.J.; Mao, Y.; He, M.; Sherman, D.H. Mitomycin Resistance in Streptomyces Lavendulae Includes a Novel Drug-Binding-Protein-Dependent Export System. J. Bacteriol. 1999, 181, 2507–2512. [Google Scholar] [CrossRef] [PubMed]
- Fiedoruk, K.; Drewnowska, J.M.; Mahillon, J.; Zambrzycka, M.; Swiecicka, I. Pan-Genome Portrait of Bacillus Mycoides Provides Insights into the Species Ecology and Evolution. Microbiol. Spectr. 2021, 9, e0031121. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus Mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Toffin, L.; Zink, K.; Kato, C.; Pignet, P.; Bidault, A.; Bienvenu, N.; Birrien, J.-L.; Prieur, D. Marinilactibacillus piezotolerans sp. nov., a Novel Marine Lactic Acid Bacterium Isolated from Deep Sub-Seafloor Sediment of the Nankai Trough. Int. J. Syst. Evol. Microbiol. 2005, 55, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.X.; Beiko, R.G.; Ragan, M.A. Lateral Transfer of Genes and Gene Fragments in Staphylococcus Extends beyond Mobile Elements. J. Bacteriol. 2011, 193, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, R.; Díez, R.M.; Domínguez-Bernal, G.; Orden, J.A.; Martínez-Pulgarín, S. Restoring Catalase Activity in Staphylococcus aureus subsp. Anaerobius Leads to Loss of Pathogenicity for Lambs. Vet. Res. 2010, 41, 41. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Irzykowska, L. Flavobacterium spp.—Characteristics, Occurrence, and Toxicity. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 938–942. ISBN 9780123847. [Google Scholar]
- Bernardet, J.; Bowman, J.P. Flavobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Králová, S. Role of Fatty Acids in Cold Adaptation of Antarctic Psychrophilic Flavobacterium spp. Syst. Appl. Microbiol. 2017, 40, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, P.; Chen, X.; Hugenholtz, P.; Janssen, P.H. Chthoniobacter flavus gen. nov., sp. nov., the First Pure-Culture Representative of Subdivision Two, Spartobacteria Classis Nov., of the Phylum Verrucomicrobia. Appl. Environ. Microbiol. 2004, 70, 5875–5881. [Google Scholar] [CrossRef] [PubMed]
- Lamprinou, V.; Hernández-Mariné, M.; Canals, T.; Kormas, K.; Economou-Amilli, A.; Pantazidou, A. Morphology and Molecular Evaluation of Iphinoe spelaeobios gen. nov., sp. nov. and Loriellopsis cavernicola gen. nov., sp. nov., Two Stigonematalean Cyanobacteria from Greek and Spanish Caves. Int. J. Syst. Evol. Microbiol. 2011, 61, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, R.; Johnson, D.B. Acidithiobacillus Ferrooxidans. Trends Microbiol. 2019, 27, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Harrold, Z.R.; Skidmore, M.L.; Hamilton, T.L.; Desch, L.; Amada, K.; van Gelder, W.; Glover, K.; Roden, E.E.; Boyd, E.S. Aerobic and Anaerobic Thiosulfate Oxidation by a Cold-Adapted, Subglacial Chemoautotroph. Appl. Environ. Microbiol. 2015, 82, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Z.-M.; Halachev, M.R.; Loman, N.J.; Constantinidou, C.; Pallen, M.J. Defining Bacterial Species in the Genomic Era: Insights from the Genus Acinetobacter. BMC Microbiol. 2012, 12, 302. [Google Scholar] [CrossRef]
- Rind, M.R.; Yasmin, A.; Raza, S.; Jatoi, W.A. Isolation, characterization and evolution of wild virulent strains of Agrobacterium for their potential transformation through use of potato discs. Pak. J. Bot. 2020, 52, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Kerpen, L.; Niccolini, L.; Licausi, F.; van Dongen, J.T.; Weits, D.A. Hypoxic Conditions in Crown Galls Induce Plant Anaerobic Responses That Support Tumor Proliferation. Front. Plant Sci. 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Tank, M.; Hara, E.; Tamaki, H.; Mori, K.; Takaichi, S.; Haruta, S.; Hanada, S. Aquabacterium pictum sp. nov., the First Aerobic Bacteriochlorophyll a-Containing Fresh Water Bacterium in the Genus Aquabacterium of the Class Betaproteobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.B.; Yee, N.; Young, L.Y. Draft Genome Sequence of Bosea sp. WAO an Arsenite and Sulfide Oxidizer Isolated from a Pyrite Rock Outcrop in New Jersey. Stand. Genom. Sci. 2018, 13, 6. [Google Scholar] [CrossRef]
- Bessarab, I.; Maszenan, A.M.; Haryono, M.A.S.; Arumugam, K.; Saw, N.M.M.T.; Seviour, R.J.; Williams, R.B.H. Comparative Genomics of Members of the Genus Defluviicoccus with Insights Into Their Ecophysiological Importance. Front. Microbiol. 2022, 13, 834906. [Google Scholar] [CrossRef] [PubMed]
- Sharak Genthner, B.R.; Devereux, R. Desulfomicrobium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Azabou, S.; Mechichi, T.; Patel, B.K.C.; Sayadi, S. Isolation and Characterization of a Mesophilic Heavy-Metals-Tolerant Sulfate-Reducing Bacterium Desulfomicrobium sp. from an Enrichment Culture Using Phosphogypsum as a Sulfate Source. J. Hazard. Mater. 2007, 140, 264–270. [Google Scholar] [CrossRef]
- Kiratisin, P.; Kowwigkai, P.; Pattanachaiwit, S.; Apisarnthanarak, A.; Leelaporn, A. Dyella Japonica Bacteremia in Hemodialysis Patient. Emerg. Infect. Dis. 2007, 13, 1266–1267. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.-H.; Yokota, A. Dyella japonica gen. nov., sp. nov., a Gamma-Proteobacterium Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2005, 55, 753–756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grimont, P.A.D.; Grimont, F. Enterobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic Resistance in Enterobacteriaceae: Mechanisms and Clinical Implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef] [PubMed]
- Morales-López, S.; A Yepes, J.; Prada-Herrera, J.C.; Torres-Jiménez, A. Enterobacteria in the 21st Century: A Review Focused on Taxonomic Changes. J. Infect. Dev. Ctries. 2019, 13, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A Halotolerant Enterobacter sp. Displaying ACC Deaminase Activity Promotes Rice Seedling Growth under Salt Stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Breeuwer, P.; Lardeau, A.; Peterz, M.; Joosten, H.M. Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 2003, 95, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.L.; Keffer, J.L.; Polson, S.W.; Chan, C.S. Gallionellaceae Pangenomic Analysis Reveals Insight into Phylogeny, Metabolic Flexibility, and Iron Oxidation Mechanisms. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Hallbeck, L.E.; Pedersen, K. Gallionella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; et al. Geobacter: The Microbe Electric’s Physiology, Ecology, and Practical Applications. Adv. Microb. Physiol. 2011, 59, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Dulay, H.; Tabares, M.; Kashefi, K.; Reguera, G. Cobalt Resistance via Detoxification and Mineralization in the Iron-Reducing Bacterium Geobacter sulfurreducens. Front. Microbiol. 2020, 11, 600463. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shao, Z. Aerobic Denitrification and Heterotrophic Sulfur Oxidation in the Genus Halomonas Revealed by Six Novel Species Characterizations and Genome-Based Analysis. Front. Microbiol. 2021, 12, 652766. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, R.H. Halomonas. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tsuji, A.; Takei, Y.; Nishimura, T.; Azuma, Y. Identification of New Halomonas Strains from Food-Related Environments. Microbes Environ. 2022, 37, ME21052. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, J. Comparative Genomics of Seven Genomes of Genus Idiomarina Reveals Important Halo Adaptations and Genes for Stress Response. 3 Biotech 2024, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Saw, J.H.; Lee, K.S.; Freitas, T.A.; Belisle, C.; Kawarabayasi, Y.; Donachie, S.P.; Pikina, A.; Galperin, M.Y.; Koonin, E.V.; et al. Genome Sequence of the Deep-Sea Gamma-Proteobacterium Idiomarina Loihiensis Reveals Amino Acid Fermentation as a Source of Carbon and Energy. Proc. Natl. Acad. Sci. USA 2004, 101, 18036–18041. [Google Scholar] [CrossRef] [PubMed]
- Donachie, S.P.; Hou, S.; Gregory, T.S.; Malahoff, A.; Alam, M. Idiomarina loihiensis sp. nov., a Halophilic Gamma-Proteobacterium from the Lō’ihi Submarine Volcano, Hawai’i. Int. J. Syst. Evol. Microbiol. 2003, 53, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Vinales, J.; Sackett, J.; Trutschel, L.; Amir, W.; Norman, C.; Leach, E.; Wilbanks, E.; Rowe, A. Physiologic, Genomic, and Electrochemical Characterization of Two Heterotrophic Marine Sediment Microbes from the Idiomarina Genus. Microorganisms 2022, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Brescia, F.; Pertot, I.; Puopolo, G. Lysobacter. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 313–338. ISBN 9780128234. [Google Scholar]
- Liu, J.-L.; Yao, J.; Zhu, X.; Zhou, D.-L.; Duran, R.; Mihucz, V.G.; Bashir, S.; Hudson-Edwards, K.A. Metagenomic Exploration of Multi-Resistance Genes Linked to Microbial Attributes in Active Nonferrous Metal(Loid) Tailings. Environ. Pollut. 2020, 273, 115667. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.-D.; Yang, J.-Y.; Gou, M. High-Throughput Sequencing Analysis of the Effects of Vanadium on Bacterial Community Structure in Purple Soil. Bull. Environ. Contam. Toxicol. 2023, 111, 59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Yu, F.B.; Blainey, P.C.; May, A.P.; Quake, S.R. Nucleic Acid Cleavage with a Hyperthermophilic Cas9 from an Uncultured Ignavibacterium. Proc. Natl. Acad. Sci. USA 2019, 116, 23100–23105. [Google Scholar] [CrossRef]
- Schleheck, D.; Tindall, B.J.; Rosselló-Mora, R.; Cook, A.M. Parvibaculum lavamentivorans gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear alkylbenzenesulfonate. Int. J. Syst. Evol. Microbiol. 2004, 54, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Young, C.-C.; Arun, A.B.; Shen, F.-T.; Jäckel, U.; Rosselló-Mora, R.; Lai, W.-A.; Rekha, P.D. Pseudolabrys taiwanensis gen. nov., sp. nov., an Alphaproteobacterium Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2006, 56, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Antonic, V.; Stojadinovic, A.; Zhang, B.; Izadjoo, M.J.; Alavi, M. Pseudomonas aeruginosa induces pigment production and enhances virulence in a white phenotypic variant of Staphylococcus aureus. Infect. Drug Resist. 2013, 6, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Lammers, L.N.; Pallud, C. High-affinity Amide-lanthanide Adsorption to Gram-positive Soil Bacteria. Environ. Microbiol. Rep. 2023, 15, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Young, C.C.; Ho, M.-J.; Arun, A.B.; Chen, W.-M.; Lai, W.-A.; Shen, F.-T.; Rekha, P.D.; Yassin, A.F. Pseudoxanthomonas spadix sp. nov., Isolated from Oil-Contaminated Soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Hiraishi, A. Rhodobium. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2015. [Google Scholar]
- Hiraishi, A.; Urata, K.; Satoh, T. A New Genus of Marine Budding Phototrophic Bacteria, Rhodobium gen. nov., Which Includes Rhodobium orientis sp. nov. and Rhodobium marinum Comb. Nov. Int. J. Syst. Bacteriol. 1995, 45, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Sandner-Miranda, L.; Vinuesa, P.; Cravioto, A.; Morales-Espinosa, R. The Genomic Basis of Intrinsic and Acquired Antibiotic Resistance in the Genus Serratia. Front. Microbiol. 2018, 9, 828. [Google Scholar] [CrossRef]
- Grimont, F.; Grimont, P.A.D. The Genus Serratia. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 219–244. ISBN 9780387254. [Google Scholar]
- Kates, M.; Hagen, P.O. Influence of temperature on fatty acid composition of psychrophilic and mesophilic Serratia species. Can. J. Biochem. 1964, 42, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Hafidi, M.; Allaoui, A.; Biskri, L. Halotolerant Endophytic Bacterium Serratia Rubidaea ED1 Enhances Phosphate Solubilization and Promotes Seed Germination. Agriculture 2021, 11, 224. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.Q.; Zhi, X.Y.; Wang, X.; Dong, J.; Chen, Y.; Lai, R.; Li, W.J. Description of Sinobacter flavus gen. nov., sp. nov., and proposal of Sinobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2008, 58, 184–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From Diversity and Genomics to Functional Role in Environmental Remediation and Plant Growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, T.; Cui, X.; Xu, Y.; Hu, S.; Zhao, Y.; Zhang, W.; Liu, G.; Zhang, G. Sphingomonas radiodurans sp. nov., a novel radiation-resistant bacterium isolated from the north slope of Mount Everest. Int. J. Syst. Evol. Microbiol. 2022, 72, 005312. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chatterjee, S.; Mandal, N.C. Stenotrophomonas. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 427–442. ISBN 9780128234. [Google Scholar]
- Wang, Y.; He, T.; Shen, Z.; Wu, C. Antimicrobial Resistance in Stenotrophomonas spp. Microbiol. Spectr. 2018, 6, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, W.; Qadir, S.; Ahmad, M.; Rafiq, M.; Hasan, F.; Tehan, R.; McPhail, K.L.; Shah, A.A. Ectoine: A Compatible Solute in Radio-halophilic Stenotrophomonas sp. WMA-LM19 Strain to Prevent Ultraviolet-induced Protein Damage. J. Appl. Microbiol. 2018, 125, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas Maltophilia BJ01 Induces Salt Tolerance by Modulating Physiology and Biochemical Activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 568289. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Huang, J.J.; Hua, B.; Champagne, P. Nitrogen Removal Bacterial Strains, MSNA-1 and MSD4, with Wide Ranges of Salinity and PH Resistances. Bioresour. Technol. 2020, 310, 123309. [Google Scholar] [CrossRef] [PubMed]
- Vasileva-Tonkova, E.; Romanovskaya, V.; Gladka, G.; Gouliamova, D.; Tomova, I.; Stoilova-Disheva, M.; Tashyrev, O. Ecophysiological properties of cultivable heterotrophic bacteria and yeasts dominating in phytocenoses of Galindez Island, maritime Antarctica. World J. Microbiol. Biotechnol. 2014, 30, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Takano, T.; Liu, S. Isolation and Characterization of Novel Bacterial Taxa from Extreme Alkali-Saline Soil. World J. Microbiol. Biotechnol. 2012, 28, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Mochizuki, J.; Fukui, M. Sulfuriferula nivalis sp. nov., a Sulfur Oxidizer Isolated from Snow and Emended Description of Sulfuriferula Plumbiphila. Int. J. Syst. Evol. Microbiol. 2020, 70, 3273–3277. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Roepke, E.W.; Hua, A.A.; Flood, B.E.; Bailey, J.V. Complete Genome Sequence of Sulfuriferula sp. Strain AH1, a Sulfur-Oxidizing Autotroph Isolated from Weathered Mine Tailings from the Duluth Complex in Minnesota. Genome Announc. 2017, 5, e00673-17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Zeyad, M.T.; Choudhary, P.; Paul, S.; Chakdar, H.; Singh Rajawat, M.V. Chapter 26—Thiobacillus. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Camargo, F.A.O.; Bento, F.M.; Okeke, B.C.; Frankenberger, W.T. Hexavalent Chromium Reduction by an Actinomycete, Arthrobacter Crystallopoietes ES 32. Biol. Trace Element Res. 2004, 97, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.; França, L.; Rainey, F.A.; Schumann, P.; Nobre, M.F.; da Costa, M.S. Gaiella occulta gen. nov., sp. nov., a Novel Representative of a Deep Branching Phylogenetic Lineage within the Class Actinobacteria and Proposal of Gaiellaceae Fam. Nov. and Gaiellales Ord. Nov. Syst. Appl. Microbiol. 2011, 34, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiao, E.; Krumins, V.; Häggblom, M.M.; Dong, Y.; Pu, Z.; Li, B.; Wang, Q.; Xiao, T.; Li, F. Rhizosphere Microbial Response to Multiple Metal(Loid)s in Different Contaminated Arable Soils Indicates Crop-Specific Metal-Microbe Interactions. Appl. Environ. Microbiol. 2018, 84, e00701-18. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. Genus Kitasatospora, Taxonomic Features and Diversity of Secondary Metabolites. J. Antibiot. 2017, 70, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Sung, K.C.; Mo, H.K.; Son, D.Y.; Nam, J.S.; Chun, J.; Bae, K.S. Kitasatospora cheerisanensis sp. nov., a New Species of the Genus Kitasatospora That Produces an Antifungal Agent. Int. J. Syst. Bacteriol. 1999, 49 Pt 2, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.-R.; Malik, A.; Kim, S.B. Genome Based Characterization of Kitasatospora sp. MMS16-BH015, a Multiple Heavy Metal Resistant Soil Actinobacterium with High Antimicrobial Potential. Gene 2020, 733, 144379. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, S.; Bhattacharya, A.; Schumann, P.; Dastager, S.G.; Tang, S.-K.; Li, W.-J.; Chakrabarti, T. Microbacterium immunditiarum sp. nov., an Actinobacterium Isolated from Landfill Surface Soil, and Emended Description of the Genus Microbacterium. Int. J. Syst. Evol. Microbiol. 2012, 62, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mansur, M.C.P.P.; Cardoso-Rurr, J.S.; Silva, J.V.M.A.; de Souza, G.R.; Cardoso, V.d.S.; Mansoldo, F.R.P.; Pinheiro, Y.; Schultz, J.; Lopez Balottin, L.B.; da Silva, A.J.R.; et al. Carotenoids from UV-Resistant Antarctic microbacterium sp. LEMMJ01. Sci. Rep. 2019, 9, 9554. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Nakamura, K. Microlunatus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Yoon, J.-H.; Park, Y.-H. The Genus Nocardioides. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 1099–1113. ISBN 9780387254. [Google Scholar]
- Rosas-Díaz, J.; Escobar-Zepeda, A.; Adaya, L.; Rojas-Vargas, J.; Cuervo-Amaya, D.H.; Sánchez-Reyes, A.; Pardo-López, L. Paenarthrobacter sp. GOM3 Is a Novel Marine Species with Monoaromatic Degradation Relevance. Front. Microbiol. 2021, 12, 713702. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Zhang, J.; Gao, L.; Tian, Q. Biodegradation of Alicyclic Amines by a Newly Isolated Hypersaline Tolerant Strain Paenarthrobacter sp. TYUT067. Water Sci. Technol. 2021, 83, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, S.Y.; Shamim, K.; Vaigankar, D.C.; Naik, M.M.; Dubey, S.K. Rapid Arsenite Oxidation by Paenarthrobacter nicotinovorans Strain SSBW5: Unravelling the Role of GlpF, AioAB and AioE Genes. Arch. Microbiol. 2023, 205, 333. [Google Scholar] [CrossRef]
- Zitouni, A.; Boudjella, H.; Lamari, L.; Badji, B.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Nocardiopsis and Saccharothrix Genera in Saharan Soils in Algeria: Isolation, Biological Activities and Partial Characterization of Antibiotics. Res. Microbiol. 2005, 156, 984–993. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Hesketh, A.; Frangou, N.; Mossialos, D.; Van De Peer, Y.; Oliver, S.G.; Amoutzias, G.D. A Panoramic View of the Genomic Landscape of the Genus Streptomyces. Microb. Genom. 2023, 9, 001028. [Google Scholar] [CrossRef] [PubMed]
- De Lima Procópio, R.E.; Da Silva, I.R.; Martins, M.K.; De Azevedo, J.L.; De Araújo, J.M. Antibiotics Produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef]
- Kämpfer, P. Streptomyces. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Sahin, N. Isolation and Characterization of Mesophilic, Oxalate-Degrading Streptomyces from Plant Rhizosphere and Forest Soils. Naturwissenschaften 2004, 91, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Tatar, D.; Guven, K.; Spröer, C.; Klenk, H.-P.; Sahin, N. Streptomyces iconiensis sp. nov. and Streptomyces smyrnaeus sp. nov., Two Halotolerant Actinomycetes Isolated from a Salt Lake and Saltern. Int. J. Syst. Evol. Microbiol. 2014, 64, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Pornpukdeewattana, S.; Jindaprasert, A.; Massa, S. Alicyclobacillus Spoilage and Control—A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 108–122. [Google Scholar] [CrossRef]
- Sokołowska, B.; Połaska, M.; Dekowska, A. Alicyclobacillus—Still Current Issues in the Beverage Industry. In Safety Issues in Beverage Production; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Chang, S.-S.; Kang, D.-H. Alicyclobacillus spp. in the Fruit Juice Industry: History, Characteristics, and Current Isolation/Detection Procedures. Crit. Rev. Microbiol. 2004, 30, 55–74. [Google Scholar] [CrossRef]
- Correa-Llantén, D.N.; Amenábar, M.J.; Muñoz, P.A.; Monsalves, M.T.; Castro, M.E.; Blamey, J.M. Alicyclobacillus sp. Strain CC2, a Thermo-Acidophilic Bacterium Isolated from Deception Island (Antarctica) Containing a Thermostable Superoxide Dismutase Enzyme. Adv. Polar Sci. 2014, 25, 92–96. [Google Scholar]
- Kim, M.G.; Lee, J.-C.; Park, D.-J.; Li, W.-J.; Kim, C.-J. Alicyclobacillus tengchongensis sp. nov., a Thermo-Acidophilic Bacterium Isolated from Hot Spring Soil. J. Microbiol. 2014, 52, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, G.G.O.; Lopes, V.R.; Romano, T.; Camara, M.C. Clostridium. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 477–491. [Google Scholar]
- Spigaglia, P. Recent Advances in the Understanding of Antibiotic Resistance in Clostridium difficile Infection. Ther. Adv. Infect. 2016, 3, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Kuever, J.; Rainey, F.A.; Widdel, F. Desulfobulbus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zondervan, N.A.; Martins Dos Santos, V.A.P.; Suarez-Diez, M.; Saccenti, E. Phenotype and Multi-Omics Comparison of Staphylococcus and Streptococcus Uncovers Pathogenic Traits and Predicts Zoonotic Potential. BMC Genom. 2021, 22, 102. [Google Scholar] [CrossRef]
- Gutierrez, T.; Rhodes, G.; Mishamandani, S.; Berry, D.; Whitman, W.B.; Nichols, P.D.; Semple, K.T.; Aitken, M.D. Polycyclic Aromatic Hydrocarbon Degradation of Phytoplankton-Associated Arenibacter spp. and Description of Arenibacter algicola sp. nov., an Aromatic Hydrocarbon-Degrading Bacterium. Appl. Environ. Microbiol. 2014, 80, 618–628. [Google Scholar] [CrossRef]
- Nedashkovskaya, O.I. Arenibacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Wang, Y.; Yu, W.; Han, F. Expression and Characterization of a Cold-Adapted, Thermotolerant and Denaturant-Stable GH5 Endoglucanase Celal_2753 That Withstands Boiling from the Psychrophilic Bacterium Cellulophaga algicola IC166T. Biotechnol. Lett. 2016, 38, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, K.-H.; Lee, S.-Y.; Oh, T.-K.; Yoon, J.-H. Cellulophaga geojensis sp. nov., a Member of the Family Flavobacteriaceae Isolated from Marine Sand. Int. J. Syst. Evol. Microbiol. 2012, 62, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Nedashkovskaya, O.I.; Suzuki, M.; Lysenko, A.M.; Snauwaert, C.; Vancanneyt, M.; Swings, J.; Vysotskii, M.V.; Mikhailov, V.V. Cellulophaga pacifica sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Nedashkovskaya, O.I.; Kim, S.B. Pontibacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Cho, J.-C.; Giovannoni, S.J. Robiginitalea biformata gen. nov., sp. nov., a Novel Marine Bacterium in the Family Flavobacteriaceae with a Higher G+C Content. Int. J. Syst. Evol. Microbiol. 2004, 54, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Manh, H.D.; Matsuo, Y.; Katsuta, A.; Matsuda, S.; Shizuri, Y.; Kasai, H. Robiginitalea myxolifaciens sp. nov., a Novel Myxol-Producing Bacterium Isolated from Marine Sediment, and Emended Description of the Genus Robiginitalea. Int. J. Syst. Evol. Microbiol. 2008, 58, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Nedashkovskaya, O.I.; Vancanneyt, M.; Kim, S.B.; Han, J.; Zhukova, N.V.; Shevchenko, L.S. Salinimicrobium marinum sp. nov., a Halophilic Bacterium of the Family Flavobacteriaceae, and Emended Descriptions of the Genus Salinimicrobium and Salinimicrobium catena. Int. J. Syst. Evol. Microbiol. 2010, 60, 2303–2306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-G.; Cui, X.-L.; Zhang, Y.-Q.; Li, W.-J.; Wang, Y.-X.; Kim, C.-J.; Lim, J.-M.; Xu, L.-H.; Jiang, C.-L. Salinimicrobium terrae sp. nov., Isolated from Saline Soil, and Emended Description of the Genus Salinimicrobium. Int. J. Syst. Evol. Microbiol. 2008, 58, 2501–2504. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, D.O.; Brulla, W.J.; Krumholz, L.R.; Bryant, M.P. Syntrophus buswellii gen. nov., sp. nov.: A Benzoate Catabolizer from Methanogenic Ecosystems. Int. J. Syst. Bacteriol. 1984, 34, 216–217. [Google Scholar] [CrossRef]
- Galushko, A.; Kuever, J. Smithella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kuever, J.; Rainey, F.A.; Widdel, F. Desulfovibrio. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Chamkh, F.; Sproer, C.; Lemos, P.C.; Besson, S.; El Asli, A.-G.; Bennisse, R.; Labat, M.; Reis, M.; Qatibi, A.-I. Desulfovibrio marrakechensis sp. nov., a 1,4-Tyrosol-Oxidizing, Sulfate-Reducing Bacterium Isolated from Olive Mill Wastewater. Int. J. Syst. Evol. Microbiol. 2009, 59, 936–942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cáceres, P.F.F.; Vélez, L.P.; Junca, H.; Moreno-Herrera, C.X. Theobroma cacao L. Agricultural Soils with Natural Low and High Cadmium (Cd) in Santander (Colombia), Contain a Persistent Shared Bacterial Composition Shaped by Multiple Soil Variables and Bacterial Isolates Highly Resistant to Cd Concentrations. Curr. Res. Microb. Sci. 2021, 2, 100086. [Google Scholar] [CrossRef] [PubMed]
- Puthusseri, R.M.; Nair, H.P.; Johny, T.K.; Bhat, S.G. Insights into the Response of Mangrove Sediment Microbiomes to Heavy Metal Pollution: Ecological Risk Assessment and Metagenomics Perspectives. J. Environ. Manag. 2021, 298, 113492. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, X.; Sun, J.; Ma, Y. A Degeneration Gradient of Poplar Trees Contributes to the Taxonomic, Functional, and Resistome Diversity of Bacterial Communities in Rhizosphere Soils. Int. J. Mol. Sci. 2021, 22, 3438. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhu, D.; Zhang, Y.; Yang, Z.; Hu, Y.; Song, C.; Yang, W.; Chang, X. Pseudomonas Chlororaphis IRHB3 Assemblies Beneficial Microbes and Activates JA-Mediated Resistance to Promote Nutrient Utilization and Inhibit Pathogen Attack. Front. Microbiol. 2024, 15, 1328863. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, Y.; Ichikawa, N. The Class Holophagaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Yamada, T.; Imachi, H.; Ohashi, A.; Harada, H.; Hanada, S.; Kamagata, Y.; Sekiguchi, Y. Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., Strictly Anaerobic, Filamentous Bacteria of the Phylum Chloroflexi Isolated from Methanogenic Propionate-Degrading Consortia. Int. J. Syst. Evol. Microbiol. 2007, 57, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Kulichevskaya, I.S.; Ivanova, A.A.; Detkova, E.N.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Dedysh, S.N. Planctomicrobium piriforme gen. nov., sp. nov., a Stalked Planctomycete from a Littoral Wetland of a Boreal Lake. Int. J. Syst. Evol. Microbiol. 2015, 65, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yan, Z.; Liu, N.; Zhang, X.; Xu, C. Synergy of Cellulase Systems between Acetivibrio Thermocellus and Thermoclostridium Stercorarium in Consolidated-Bioprocessing for Cellulosic Ethanol. Microorganisms 2022, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vanderhaeghen, S.; Feiler, W.; Angelov, A.; Baudrexl, M.; Zverlov, V.; Liebl, W. Characterization of Two α-l-Arabinofuranosidases from Acetivibrio Mesophilus and Their Synergistic Effect in Degradation of Arabinose-Containing Substrates. Microorganisms 2021, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Hu, L.; Zhang, D.; Qian, Y.; Long, Y.; Shen, D.; Fang, C.; Yao, J.; Liu, J. Drivers and Ecological Consequences of Arsenite Detoxification in Aged Semi-Aerobic Landfill. J. Hazard. Mater. 2021, 420, 126597. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.A.; Vos, P.D. Bacillus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Heng, S.; Sutheeworapong, S.; Prommeenate, P.; Cheevadhanarak, S.; Kosugi, A.; Pason, P.; Waeonukul, R.; Ratanakhanokchai, K.; Tachaapaikoon, C. Complete Genome Sequence of Halocella sp. Strain SP3-1, an Extremely Halophilic, Glycoside Hydrolase- and Bacteriocin-Producing Bacterium Isolated from a Salt Evaporation Pond. Microbiol. Resour. Announc. 2019, 8, e01696-18. [Google Scholar] [CrossRef] [PubMed]
- Simankova, M.V.; Chernych, N.A.; Osipov, G.A.; Zavarzin, G.A. Halocella cellulolytica gen. nov., sp. nov., a New Obligately Anaerobic, Halophilic, Cellulolytic Bacterium. Syst. Appl. Microbiol. 1993, 16, 385–389. [Google Scholar] [CrossRef]
- Lomans, B.P.; Leijdekkers, P.; Wesselink, J.J.; Bakkes, P.; Pol, A.; van der Drift, C.; den Camp, H.J. Obligate Sulfide-Dependent Degradation of Methoxylated Aromatic Compounds and Formation of Methanethiol and Dimethyl Sulfide by a Freshwater Sediment Isolate, Parasporobacterium paucivorans gen. nov., sp. nov. Appl. Environ. Microbiol. 2001, 67, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-W.; Kim, B.-Y.; Song, J.; Weon, H.-Y.; Schumann, P.; Tindall, B.J.; Stackebrandt, E.; Fritze, D. Sporosarcina koreensis sp. nov. and Sporosarcina soli sp. nov., Isolated from Soil in Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Janarthine, S.R.S.; Eganathan, P. Plant Growth Promoting of Endophytic Sporosarcina aquimarina SjAM16103 isolated from the Pneumatophores of Avicennia marina L. Int. J. Microbiol. 2012, 2012, 532060. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Barrios, R.; Rubiano-Labrador, C.; Navarro-Narvaez, D.; Escobar-Galarza, J.; González, D.; Mira, S.; Moreno, D.; Contreras, A.; Miranda-Castro, W. Perchlorate-Reducing Bacteria from Antarctic Marine Sediments. Environ. Monit. Assess. 2022, 194, 654. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Nagano, H.; Ando, A.; Shima, J.; Ogawa, J. Modulation of Fatty Acid Composition and Growth in Sporosarcina Species in Response to Temperatures and Exogenous Branched-Chain Amino Acids. Appl. Microbiol. Biotechnol. 2017, 101, 5071–5080. [Google Scholar] [CrossRef] [PubMed]
- Polkade, A.V.; Ramana, V.V.; Joshi, A.; Pardesi, L.; Shouche, Y.S. Rufibacter immobilis sp. nov., Isolated from a High-Altitude Saline Lake. Int. J. Syst. Evol. Microbiol. 2015, 65, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Buczolits, S.; Busse, H. Hymenobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Chung, A.P.; Lopes, A.; Nobre, M.F.; Morais, P.V. Hymenobacter perfusus sp. nov., Hymenobacter flocculans sp. nov. and Hymenobacter metalli sp. nov. Three New Species Isolated from an Uranium Mine Waste Water Treatment System. Syst. Appl. Microbiol. 2010, 33, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Chhetri, G.; Kim, J.; Kim, I.; Seo, T. Pontibacter cellulosilyticus sp. nov., a Carboxymethyl Cellulose-Hydrolysing Bacterium Isolated from Coastal Water. Int. J. Syst. Evol. Microbiol. 2021, 71, 005058. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-N.; Jiang, L.; Osman, G.; Chu, M.; Gu, M.-Y.; Tang, Q.-Y.; Zhu, Y.-L.; Zhu, J.; Zhang, Z.-D. Pontibacter kalidii sp. nov., Isolated from Rhizosphere Soil of Kalidium Foliatum. Int. J. Syst. Evol. Microbiol. 2023, 73, 006087. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Park, Y.; Lee, S.E.; Han, J.H.; Cha, I.-T.; Lee, K.; Lee, B.-H.; Kim, M.K. Pontibacter pudoricolor sp. nov., and Pontibacter russatus sp. nov. Radiation-Resistant Bacteria Isolated from Soil. Antonie Leeuwenhoek 2020, 113, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dai, W.; Qiu, C.; Yang, Z.; Zhang, Y.; Zhou, M.; Zhang, L.; Fang, C.; Gao, Q.; Yang, Q.; et al. Unraveling Adaptation of Pontibacter korlensis to Radiation and Infertility in Desert through Complete Genome and Comparative Transcriptomic Analysis. Sci. Rep. 2015, 5, 10929. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Baek, J.H.; Jeong, S.E.; Hao, L.; Jeon, C.O. Microvirga terrae sp. nov., Isolated from Soil. Curr. Microbiol. 2023, 80, 42. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Qu, Z.; Zhou, J. Simultaneous Cobalt(III)-Histidine Reduction and Aerobic Denitrification by Paracoccus versutus LYM. Bioresour. Technol. 2020, 310, 123404. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.K.; Liu, T.; Awasthi, M.K.; Zhang, Z. Evaluation of Biochar Amendment on Heavy Metal Resistant Bacteria Abundance in Biosolids Compost. Bioresour. Technol. 2020, 306, 123114. [Google Scholar] [CrossRef] [PubMed]
- Salam, L.B.; Obayori, O.S. Functional Characterization of the ABC Transporters and Transposable Elements of an Uncultured paracoccus sp. Recovered from a Hydrocarbon-Polluted Soil Metagenome. Folia Microbiol. 2023, 68, 299–314. [Google Scholar] [CrossRef]
- Pal, U.; Bachmann, D.; Blank, L.M.; Tiso, T. Draft Genome Sequence and Annotation of the Halotolerant Carotenoid-Producing Strain Paracoccus bogoriensis BOG6 T. Microbiol. Resour. Announc. 2023, 12, e00133-23. [Google Scholar] [CrossRef] [PubMed]
- Arya, C.K.; Maurya, S.; Ramanathan, G. Insight into the Metabolic Pathways of paracoccus sp. Strain DMF: A Non-Marine Halotolerant Methylotroph Capable of Degrading Aliphatic Amines/Amides. Environ. Sci. Pollut. Res. 2023, 30, 125947–125964. [Google Scholar] [CrossRef] [PubMed]
- Didari, M.; Bagheri, M.; Amoozegar, M.A.; Bouzari, S.; Babavalian, H.; Tebyanian, H.; Hassanshahian, M.; Ventosa, A. Diversity of Halophilic and Halotolerant Bacteria in the Largest Seasonal Hypersaline Lake (Aran-Bidgol-Iran). J. Environ. Health Sci. Eng. 2020, 18, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jia, Y.; Wang, Q.; Yan, F.; Wu, M.; Li, X.; Fang, W.; Xu, F.; Liu, H.; Qiu, Z. Responsive Change of Crop-Specific Soil Bacterial Community to Cadmium in Farmlands Surrounding Mine Area of Southeast China. Environ. Res. 2022, 214, 113748. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Guo, Z.; Fan, L.; Hu, Z.; Wu, W. Diversity and antimicrobial activities of actinomycetes from pesticide-contaminated spots in Shandong Peninsula. Wei Sheng Wu Xue Bao 2012, 52, 435–441. [Google Scholar] [PubMed]
- Wu, C.; Wang, X.; Shao, Z. Diversity of oil-degrading bacteria isolated form the Indian Ocean sea surface. Wei Sheng Wu Xue Bao 2010, 50, 1218–1225. [Google Scholar] [PubMed]
- Tian, B.; Sun, Z.; Shen, S.; Wang, H.; Jiao, J.; Wang, L.; Hu, Y.; Hua, Y. Effects of Carotenoids from Deinococcus radiodurans on Protein Oxidation. Lett. Appl. Microbiol. 2009, 49, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Reddy, G.S.N.; Sengupta, S.; Shivaji, S. Deinococcus indicus sp. nov., an Arsenic-Resistant Bacterium from an Aquifer in West Bengal, India. Int. J. Syst. Evol. Microbiol. 2004, 54, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ershov, B. Natural Radioactivity and Chemical Evolution on the Early Earth: Prebiotic Chemistry and Oxygenation. Molecules 2022, 27, 8584. [Google Scholar] [CrossRef]
- Voica, D.M.; Bartha, L.; Banciu, H.L.; Oren, A. Heavy Metal Resistance in Halophilic Bacteria and Archaea. FEMS Microbiol. Lett. 2016, 363, fnw146. [Google Scholar] [CrossRef] [PubMed]
- Laneva, O.D. Mechanisms of Bacteria Resistance to Heavy Metals. Mikrobiolohichnyi Zhurnal 2009, 7, 54–56. [Google Scholar]
- Lloyd, J.R. Microbial Reduction of Metals and Radionuclides. FEMS Microbiol. Rev. 2003, 27, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, F.; Rashidi, A.; Jahani, S. Isolation and Identification of Native Sulfuroxidizing Bacterium Capable of Uranium Extraction. Prog. Biol. Sci. 2015, 5, 207–221. [Google Scholar] [CrossRef]
- Kolhe, N.; Zinjarde, S.; Acharya, C. Responses Exhibited by Various Microbial Groups Relevant to Uranium Exposure. Biotechnol. Adv. 2018, 36, 1828–1846. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, C.D.; Roebbert, Y.; Schippers, A.; Weyer, S. U Mobilization and Associated U Isotope Fractionation by Sulfur-Oxidizing Bacteria. Front. Microbiol. 2023, 14, 1190962. [Google Scholar] [CrossRef] [PubMed]
- Waglechner, N.; Culp, E.J.; Wright, G.D. Ancient Antibiotics, Ancient Resistance. EcoSal Plus 2021, 9, eESP-0027-2020. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic Resistance Genes in Bacteria: Occurrence, Spread, and Control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Bombaywala, S.; Purohit, H.J.; Dafale, N.A. Mobility of Antibiotic Resistance and Its Co-Occurrence with Metal Resistance in Pathogens under Oxidative Stress. J. Environ. Manag. 2021, 297, 113315. [Google Scholar] [CrossRef] [PubMed]
- Kunte, H.J. Osmoregulation in Bacteria: Compatible Solute Accumulation and Osmosensing. Environ. Chem. 2006, 3, 94. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, Adaptation, and Their Role in Decontamination of Hypersaline Environments. World J. Microbiol. Biotechnol. 2016, 32, 135. [Google Scholar] [CrossRef] [PubMed]
- Zamanzadeh-Nasrabadi, S.M.; Mohammadiapanah, F.; Hosseini-Mazinani, M.; Sarikhan, S. Salinity Stress Endurance of the Plants with the Aid of Bacterial Genes. Front. Genet. 2023, 14, 1049608. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, K.U.; Joulian, C.; Ingvorsen, K. Oxygen Tolerance of Sulfate-Reducing Bacteria in Activated Sludge. Environ. Sci. Technol. 2004, 38, 2038–2043. [Google Scholar] [CrossRef]
- Khademian, M.; Imlay, J.A. How Microbes Evolved to Tolerate Oxygen. Trends Microbiol. 2021, 29, 428–440. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Isolation, Characterization, and Diversity of Novel Radiotolerant Carotenoid-Producing Bacteria. Methods Mol. Biol. 2012, 892, 21–60. [Google Scholar] [CrossRef]
- Asker, D.; Beppu, T.; Ueda, K. Unique Diversity of Carotenoid-Producing Bacteria Isolated from Misasa, a Radioactive Site in Japan. Appl. Microbiol. Biotechnol. 2007, 77, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kharkhota, M.; Hrabova, H.; Kharchuk, M.; Ivanytsia, T.; Mozhaieva, L.; Poliakova, A.; Avdieieva, L. Chromogenicity of Aerobic Spore-Forming Bacteria of the Bacillaceae Family Isolated from Different Ecological Niches and Physiographic Zones. Braz. J. Microbiol. 2022, 53, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Crucial Roles of Carotenoids as Bacterial Endogenous Defense System for Bacterial Radioresistance of Deinococcus radiodurans. bioRxiv 2021. [Google Scholar] [CrossRef]
- Omelchenko, M.V.; Wolf, Y.I.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Daly, M.J.; Koonin, E.V.; Makarova, K.S. Comparative Genomics of Thermus Thermophilus and Deinococcus radiodurans: Divergent Routes of Adaptation to Thermophily and Radiation Resistance. BMC Evol. Biol. 2005, 5, 57. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Amils, R.; Wagner, D.; McGenity, T. Life and Applications of Extremophiles. Environ. Microbiol. 2011, 13, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, V.; Zerulla, K.; Lange, C.; Soppa, J. Quantification of Ploidy in Proteobacteria Revealed the Existence of Monoploid, (Mero-)Oligoploid and Polyploid Species. PLoS ONE 2011, 6, e16392. [Google Scholar] [CrossRef] [PubMed]
- Njinga, R.L.; Ogundele, T.L.; Adebayo, A.S.; Olatunji, M.A.; Olufemi, A.P.; Olowookere, C.J.; Aladeniyi, K.; Pereira, A.; Arogunjo, M.A.; Tshivhase, V.M. Distribution Dynamics and Descriptive Statistical Analysis of Radionuclides in the Farmland Soils near Mining Areas in Southwestern Nigeria. Environ. Geochem. Health 2023, 45, 3617–3636. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are Radiosensitivity Data Derived from Natural Field Conditions Consistent with Data from Controlled Exposures? A Case Study of Chernobyl Wildlife Chronically Exposed to Low Dose Rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).