Enhancing the Startup Rate of Microbial Methanogenic Systems through the Synergy of β-lactam Antibiotics and Electrolytic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Inoculation and Operation of Bioreactors
2.3. Illumina Sequencing and PICRUSt2 Analysis
3. Results
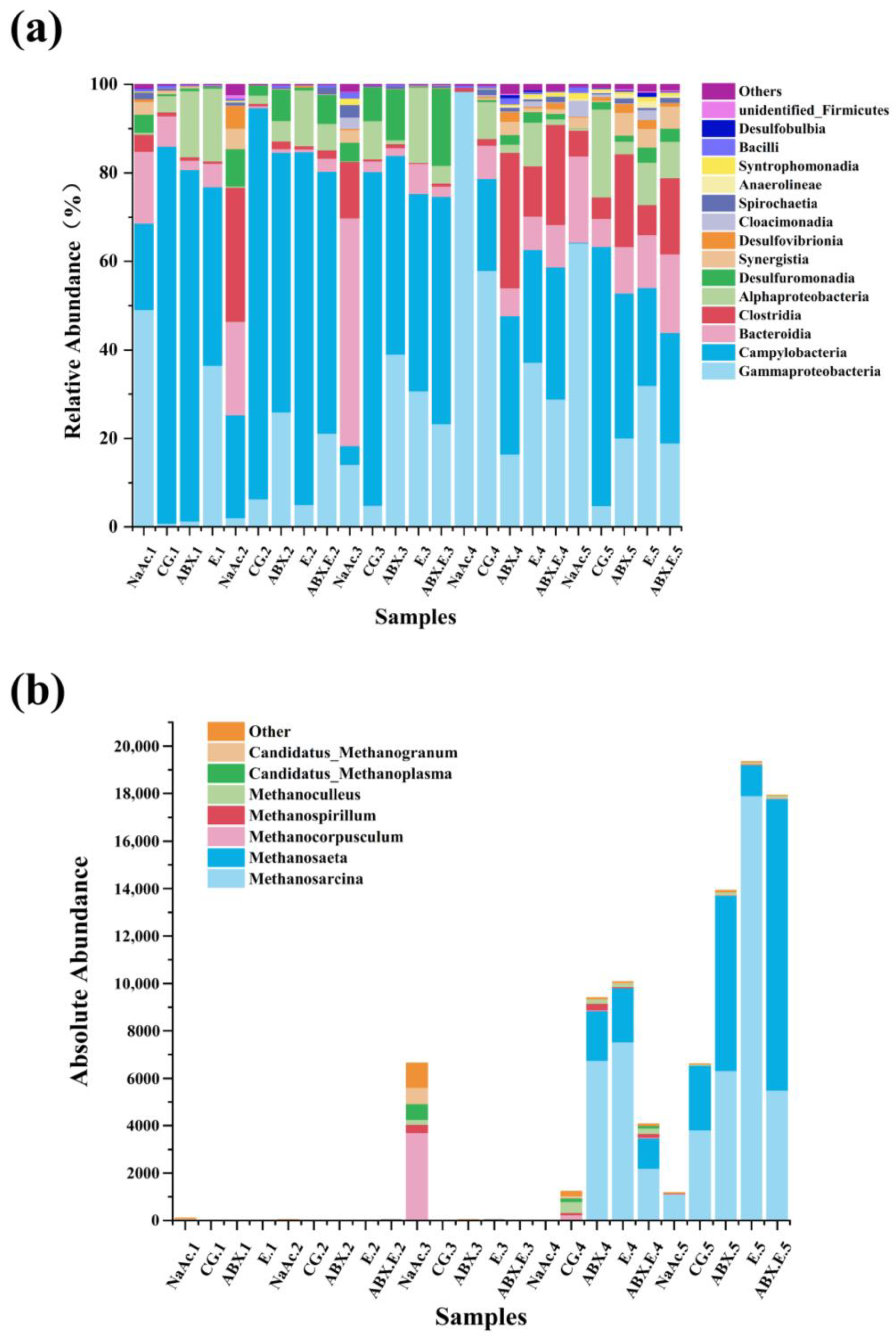
3.1. Evolution in the Composition and Structure of Methane-Producing Community
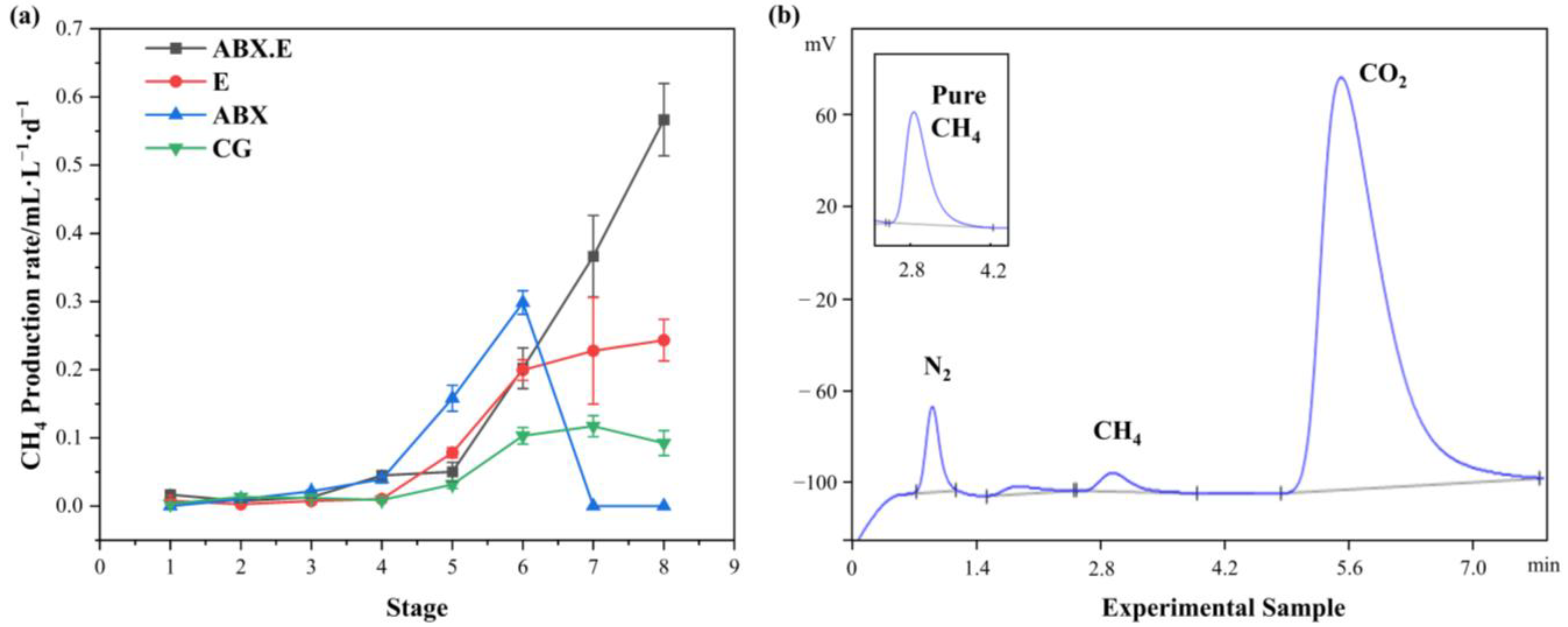
3.2. Methane-Production Performance
3.3. Function and Metabolic Pathway Analysis Based on PICRUSt2 Predictions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Cheng, H.; Li, E.; Pan, Z.; Cheng, F. Formation of caco3 hollow microspheres in carbonated distiller waste from solvay soda ash plants. Front. Chem. Sci. Eng. 2022, 16, 1659–1671. [Google Scholar] [CrossRef]
- Hasaka, S.; Sakamoto, S.; Fujii, K. The potential of digested sludge-assimilating microflora for biogas production from food processing wastes. Microorganisms 2023, 11, 2321. [Google Scholar] [CrossRef]
- Rosi, L.; Cenni, M.; Ciuffi, B.; Casini, D.; Rizzo, A.M.; Chiaramonti, D. Enhancing biogas production in anaerobic digestion by the addition of oxidized and non-oxidized biochars. Biomass Convers. Biorefinery 2024, 14, 5457–5468. [Google Scholar] [CrossRef]
- Łazarski, S.; Butarewicz, A.; Cichosz, M.; Kiełkowska, U. Study on the effect of dedicated microelement mixture (dmm) on the kick-off phase of the digester and stabilization of the methane fermentation process. Energies 2023, 16, 3763. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Xia, A.; Feng, D.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. Effects of oxytetracycline on mesophilic and thermophilic anaerobic digestion for biogas production from swine manure. Fuel 2023, 344, 128054. [Google Scholar] [CrossRef]
- Lauwers, A.M.; Heinen, W.; Gorris, L.G.M.; van der Drift, C. Variation of parameters affecting the start-up of methanogenic fluidized bed reactors. Biotechnol. Lett. 1989, 11, 907–912. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Méndez-Acosta, H.O.; Arreola-Vargas, J.; Gabriel-Barajas, J.E.; Aguilar-Mota, M.N.; Snell-Castro, R. New insights into microbial interactions and putative competitive mechanisms during the hydrogen production from tequila vinasses. Appl. Microbiol. Biotechnol. 2022, 106, 6861–6876. [Google Scholar] [CrossRef]
- Conrad, R. Complexity of temperature dependence in methanogenic microbial environments. Front. Microbiol. 2023, 14, 1232946. [Google Scholar] [CrossRef]
- Alan, C.; Pascale, C. Optimization of biogas production during start-up with electrode-assisted anaerobic digestion. Chemosphere 2022, 302, 134739. [Google Scholar]
- Jiang, Q.; Zhang, C.; Wu, P.; Ding, P.; Zhang, Y.; Cui, M.-H.; Liu, H. Algae biochar enhanced methanogenesis by enriching specific methanogens at low inoculation ratio during sludge anaerobic digestion. Bioresour. Technol. 2021, 338, 125493. [Google Scholar] [CrossRef] [PubMed]
- Marquart, K.A.; Haller, B.R.; Paper, J.M.; Flynn, T.M.; Boyanov, M.I.; Shodunke, G.; Gura, C.; Jin, Q.; Kirk, M.F. Influence of ph on the balance between methanogenesis and iron reduction. Geobiology 2019, 17, 185–198. [Google Scholar] [CrossRef]
- Madigou, C.; Le Cao, K.-A.; Bureau, C.; Mazéas, L.; Déjean, S.; Chapleur, O. Ecological consequences of abrupt temperature changes in anaerobic digesters. Chem. Eng. J. 2019, 361, 266–277. [Google Scholar] [CrossRef]
- Lohani, S.P.; Shakya, S.; Gurung, P.; Dhungana, B.; Paudel, D.; Mainali, B. Anaerobic co-digestion of food waste, poultry litter and sewage sludge: Seasonal performance under ambient condition and model evaluation. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 3, 17. [Google Scholar] [CrossRef]
- Cai, G.; Zhu, G.; Zhou, M.; Lv, N.; Wang, R.; Li, C.; Li, J.; Pan, X. Syntrophic butyrate-oxidizing methanogenesis promoted by anthraquinone-2-sulfonate and cysteine: Distinct tendencies towards the enrichment of methanogens and syntrophic fatty-acid oxidizing bacteria. Bioresour. Technol. 2021, 332, 125074. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ko, J.H.; Zhou, L.; Gao, X.; Liu, Y.; Shi, X.; Xu, Q. Iron oxide alleviates acids stress by facilitating syntrophic metabolism between syntrophomonas and methanogens. Chemosphere 2020, 247, 125866. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Liu, H.; Zhang, W.; Li, J. Exogenous n-hexanoyl-l-homoserine lactone assists in upflow anaerobic sludge blanket recovery from acetate accumulation via aceticlastic methanogens enrichment. Bioresour. Technol. 2022, 346, 126600. [Google Scholar] [CrossRef]
- Bianco, F.; Senol, H.; Papirio, S.; Zenk, H.; Kara, A.; Atasoy, S. Combined ultrasonic-hydrothermal pretreatment to improve the biomethane potential of hazelnut shell. Biomass Bioenergy 2022, 165, 106554. [Google Scholar] [CrossRef]
- Serrano, A.; Siles, J.A.; Martín, M.A.; Chica, A.; Estévez-Pastor, F.; Toro-Baptista, E. Improvement of anaerobic digestion of sewage sludge through microwave pre-treatment. J. Environ. Manag. 2016, 177, 231–239. [Google Scholar] [CrossRef]
- Tian, H.; Mancini, E.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Bioaugmentation strategy for overcoming ammonia inhibition during biomethanation of a protein-rich substrate. Chemosphere 2019, 231, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Sun, Y.; Zhang, Y.; Ren, X.; Lin, Z.; Cheng, S. Effect of start-up process using different electrochemical methods on the performance of CO2-reducing methanogenic biocathodes. Int. J. Hydrogen Energy 2020, 46, 3045–3055. [Google Scholar] [CrossRef]
- Mock, M.P.; Ochi, R.; Bieringer, M.; Bieringer, T.; Brotsack, R.; Leyer, S. Comparison of various reducing agents for methane production by methanothermobacter marburgensis. Microorganisms 2023, 11, 2533. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Sabel-Becker, B.; Holtmann, D. Enhanced electron uptake and methane production by corrosive methanogens during electromethanogenesis. Microorganisms 2022, 10, 2237. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, H.; Bai, Z.; Jiang, H.; Yang, F.; Sun, X.; Lu, Z.; Zhou, D. Soil exposure accelerates recovery of the gut microbiota in antibiotic-treated mice. Environ. Microbiol. Rep. 2021, 13, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Belakhov, V.V. Ecological aspects of application of tetraene macrolide antibiotic tetramycin in agriculture and food industry (a review). Russ. J. Gen. Chem. 2021, 91, 2858–2880. [Google Scholar] [CrossRef]
- Belakhov, V.V.; Yakovleva, E.P.; Kolodyaznaya, V.A.; Boikova, I.V. Imbricin, an antifungal antibiotic of non-medical application: Preparation, physicochemical properties, structural features, and industrial and agricultural uses (review). Russ. J. Gen. Chem. 2017, 87, 3220–3232. [Google Scholar] [CrossRef]
- Peng, R.; Du, C.; Hu, A.; Li, Q.; Zhang, J.; Zhang, W.; Sun, F. Fabrication of core–shell type poly(nipam)-encapsulated citral and its application on bamboo as an anti-molding coating. RSC Adv. 2021, 11, 36884–36894. [Google Scholar] [CrossRef]
- Saady, N.M.C.; Sivaraman, S.; Venkatachalam, P.; Zendehboudi, S.; Zhang, Y.; Palma, R.Y.; Shanmugam, S.R.; Espinoza, J.E.R. Effect of veterinary antibiotics on methane yield from livestock manure anaerobic digestion: An analytical review of the evidence. Rev. Environ. Sci. Bio/Technol. 2024, 23, 133–161. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. B-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef]
- Aziz, A.; Sengar, A.; Basheer, F.; Farooqi, I.H.; Isa, M.H. Anaerobic digestion in the elimination of antibiotics and antibiotic-resistant genes from the environment—A comprehensive review. J. Environ. Chem. Eng. 2021, 10, 106423. [Google Scholar] [CrossRef]
- Zubair, M.; Li, Z.; Zhu, R.; Wang, J.; Liu, X.; Liu, X. The antibiotics degradation and its mechanisms during the livestock manure anaerobic digestion. Molecules 2023, 28, 4090. [Google Scholar] [CrossRef]
- Ghaderikia, A.; Taskin, B.; Yilmazel, Y.D. Start-up strategies of electromethanogenic reactors for methane production from cattle manure. Waste Manag. 2023, 159, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmit, A.J.; Ryder, E.F.; Kerby, J.L.; Murdoch, B.; Donovan, S.; Grandgenett, N.F.; Cook, R.E.; Siriwardana, C.; Morgan, W.; Pauley, M.; et al. Community development, implementation, and assessment of a niblse bioinformatics sequence similarity learning resource. PLoS ONE 2021, 16, e0257404. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. Picrust2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Terlesky, K.C.; Nelson, M.J.; Ferry, J.G. Isolation of an enzyme complex with carbon monoxide dehydrogenase activity containing corrinoid and nickel from acetate-grown methanosarcina thermophila. J. Bacteriol. 1986, 168, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Szuhaj, M.; Kakuk, B.; Wirth, R.; Rákhely, G.; Kovács, K.L.; Bagi, Z. Regulation of the methanogenesis pathways by hydrogen at transcriptomic level in time. Appl. Microbiol. Biotechnol. 2023, 107, 6315–6324. [Google Scholar] [CrossRef] [PubMed]
- Balch, W.E.; Wolfe, R.S. Specificity and biological distribution of coenzyme m (2-mercaptoethanesulfonic acid). J. Bacteriol. 1979, 137, 256–263. [Google Scholar] [CrossRef]
- White, R.H. Biosynthesis of the 7-mercaptoheptanoic acid subunit of component b [(7-mercaptoheptanoyl)threonine phosphate] of methanogenic bacteria. Biochemistry 2002, 28, 860–865. [Google Scholar] [CrossRef]





| Parameters | Inoculums | NaAc Culture Medium | CO2 Culture Medium |
|---|---|---|---|
| pH | 7.5 | 7.0 | 7.0 |
| TS 1 (mg/L) | 130 | - | - |
| VS 2 (mg/L) | 70 | - | - |
| TCOD 3 (mg/L) | 4100 | 4410 | 510 |
| SCOD 4 (mg/L) | 2680 | 4410 | 510 |
| TP 5 (mg/L) | 138 | 165 | 162 |
| TN 6 (mg/L) | 2100 | 1550 | 1500 |
| NH4+-N 7 (mg/L) | 940 | 290 | 285 |
| Abbreviation | Carbon Source | Antibiotics | Electrolytic Cell |
|---|---|---|---|
| NaAc | CH3COONa | × | × |
| CG | CO2 | × | × |
| ABX.E | CO2 | √ | √ |
| ABX | CO2 | √ | × |
| E | CO2 | × | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhe, Y.; Cheng, H.; Cheng, F.; Song, H.; Pan, Z. Enhancing the Startup Rate of Microbial Methanogenic Systems through the Synergy of β-lactam Antibiotics and Electrolytic Cells. Microorganisms 2024, 12, 734. https://doi.org/10.3390/microorganisms12040734
Zhe Y, Cheng H, Cheng F, Song H, Pan Z. Enhancing the Startup Rate of Microbial Methanogenic Systems through the Synergy of β-lactam Antibiotics and Electrolytic Cells. Microorganisms. 2024; 12(4):734. https://doi.org/10.3390/microorganisms12040734
Chicago/Turabian StyleZhe, Yuting, Huaigang Cheng, Fangqin Cheng, Huiping Song, and Zihe Pan. 2024. "Enhancing the Startup Rate of Microbial Methanogenic Systems through the Synergy of β-lactam Antibiotics and Electrolytic Cells" Microorganisms 12, no. 4: 734. https://doi.org/10.3390/microorganisms12040734
APA StyleZhe, Y., Cheng, H., Cheng, F., Song, H., & Pan, Z. (2024). Enhancing the Startup Rate of Microbial Methanogenic Systems through the Synergy of β-lactam Antibiotics and Electrolytic Cells. Microorganisms, 12(4), 734. https://doi.org/10.3390/microorganisms12040734






