The Research of Antagonistic Endophytic Bacterium Bacillus velezensis CSUFT-BV4 for Growth Promotion and Induction of Resistance to Anthracnose in Camellia oleifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Strains and Plants
2.2. Molecular Biology Identification of CSUFT-BV4
2.3. Determination of the Biofilm Formation Ability of CSUFT-BV4
2.4. Determination of the Metabolites and Growth Promotion Characteristics of CSUFT-BV4
2.5. Determination of the Growth-Promoting Effect of CSUFT-BV4 on Different C. oleifera Cultivars
2.6. Antagonistic Activity of CSUFT-BV4 against Pathogens of C. oleifera Anthracnose In Vitro
2.7. Inhibitory Ability of CSUFT-BV4 on C. oleifera Anthracnose In Vivo
2.8. Variation in Defense Enzyme Activities in C. oleifera Leaves after Different Treatments
2.9. Data Analysis
3. Results
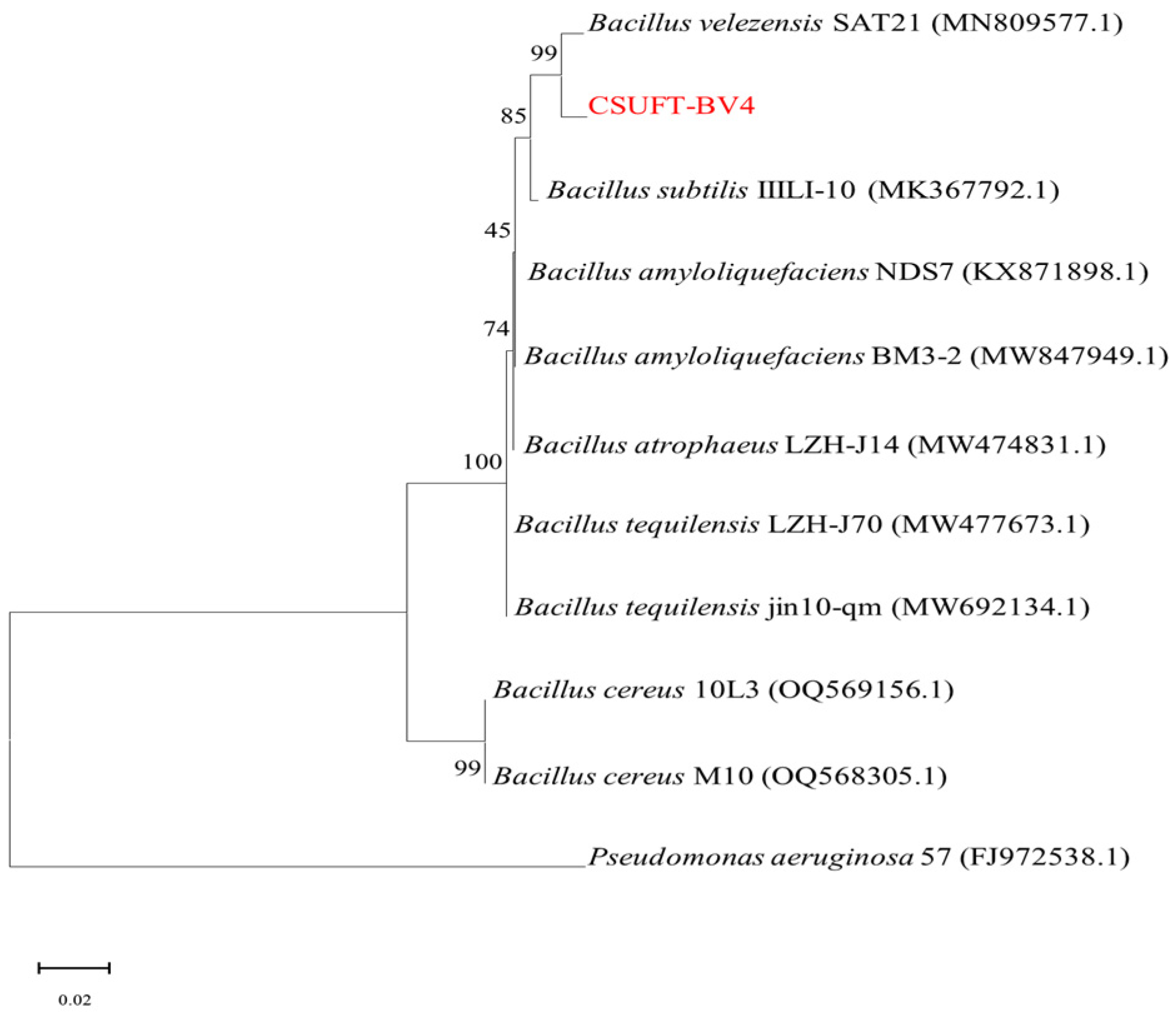
3.1. Molecular Biological Identification of CSUFT-BV4
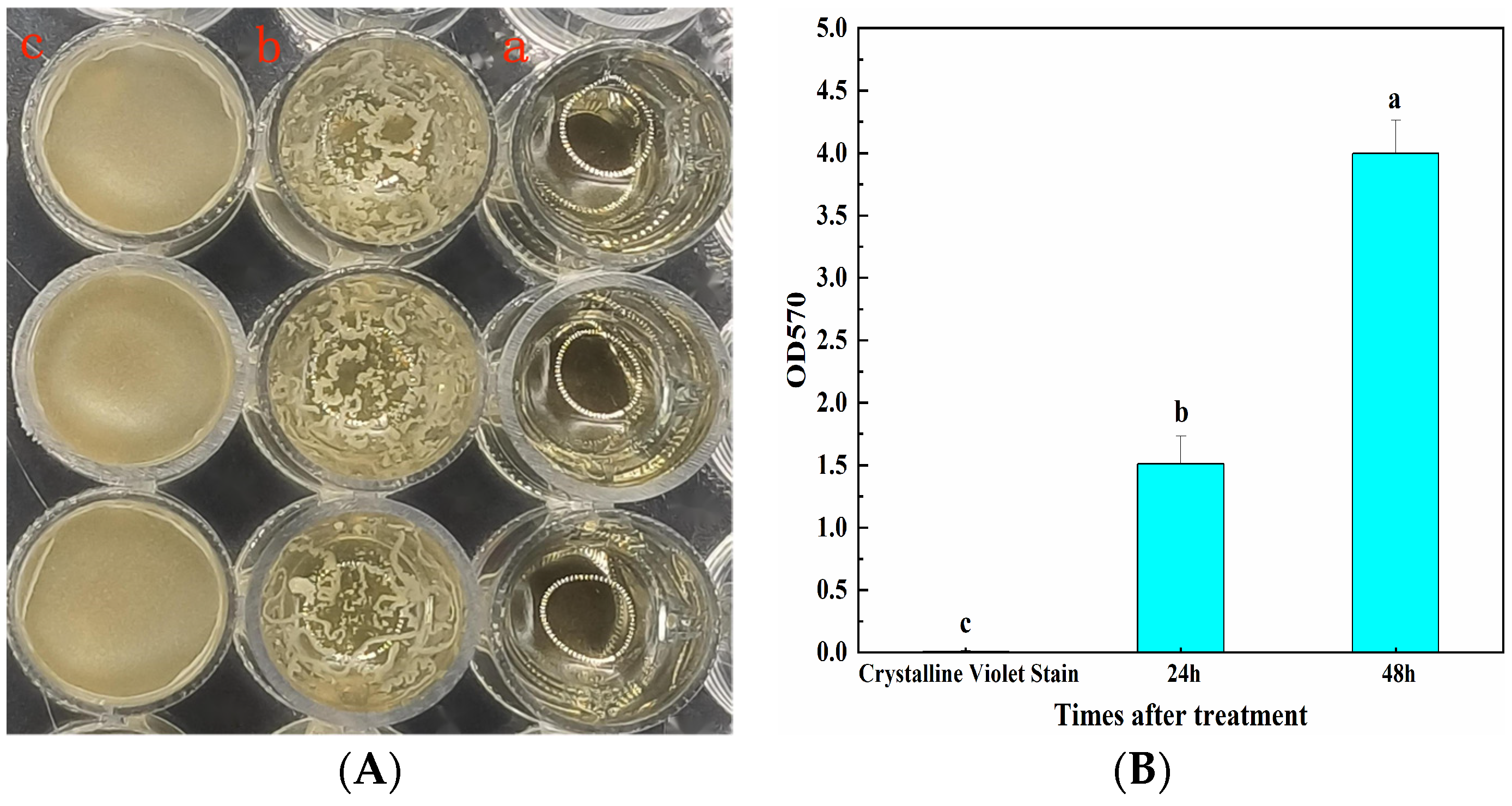
3.2. The Biofilm Formation Ability of CSUFT-BV4
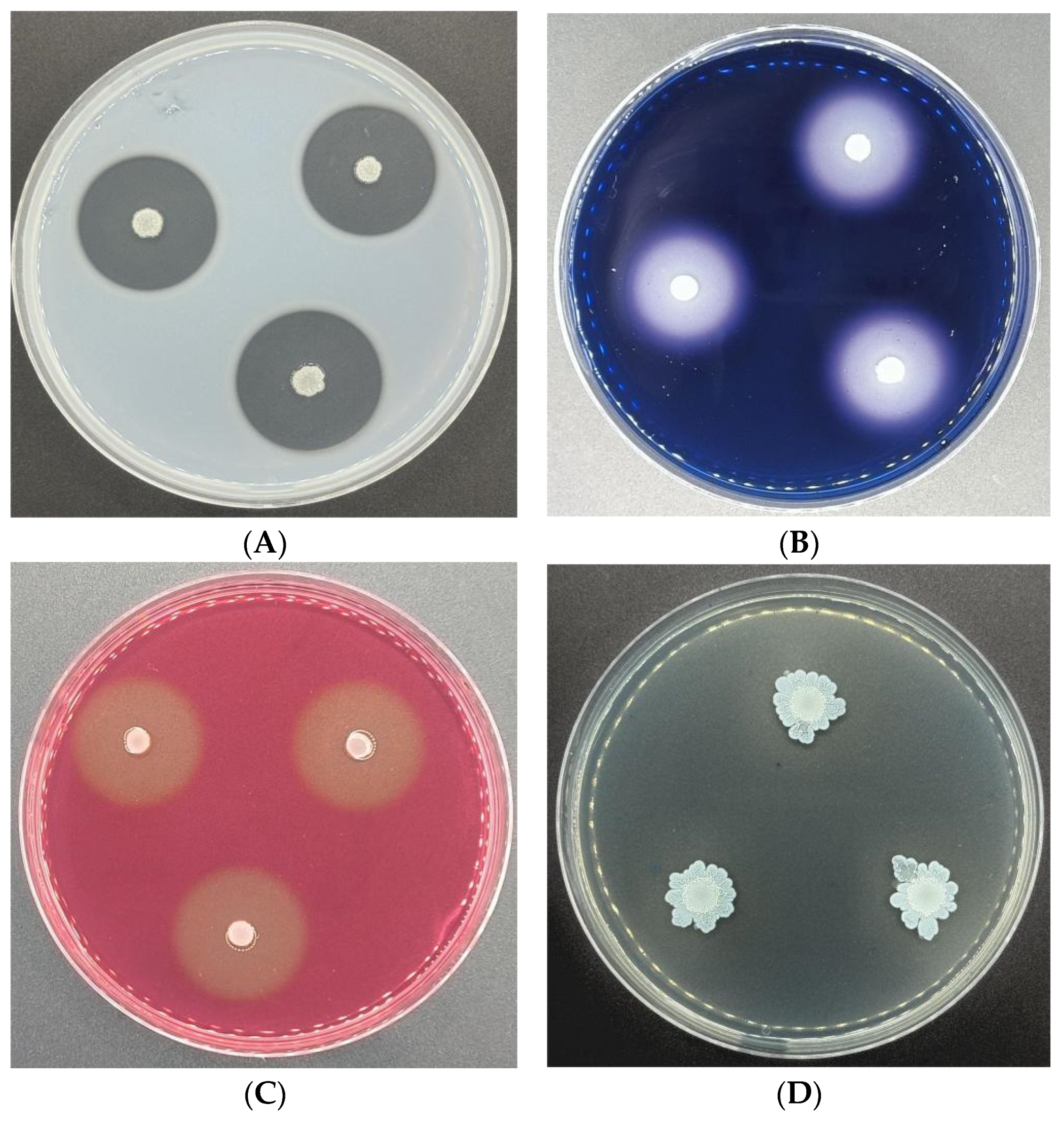
3.3. The Metabolic Substance Synthesized and Growth-Promoting Characteristics of CSUFT-BV4
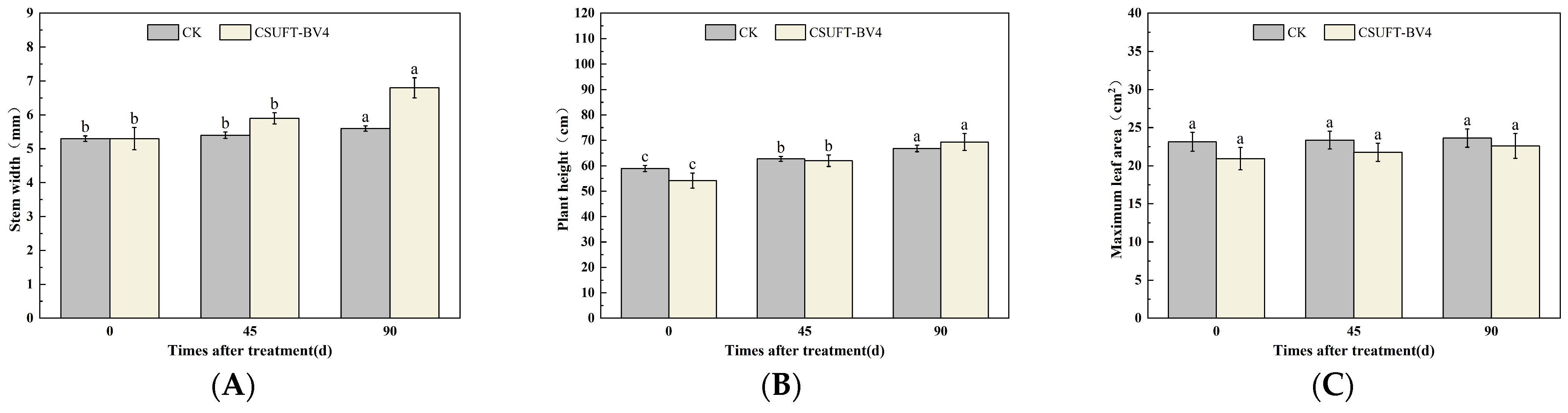
3.4. The Growth Promotion of “Huashuo” C. oleifera Cultivars under Different Inoculation Methods of CSUFT-BV4
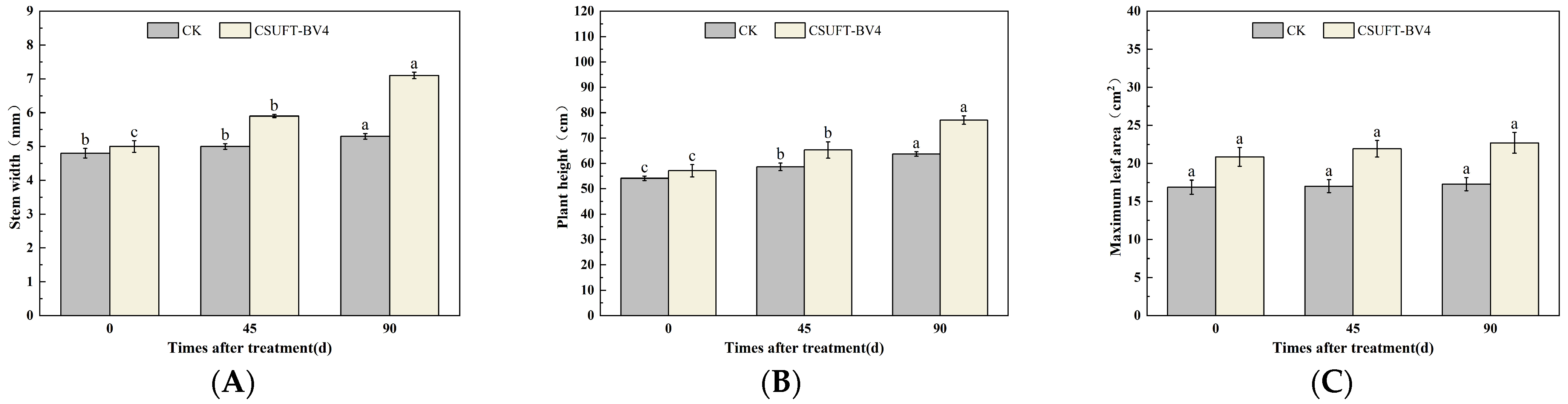
3.4.1. The Effect of Leaf Spray Method on the Growth of “Huashuo” C. oleifera Cultivars
3.4.2. The Effect of Root Irrigation Method on the Growth of “Huashuo” C. oleifera Cultivars
3.4.3. The Effect of Leaf Spray + Root Irrigation Method on the Growth of “Huashuo” C. oleifera Cultivars
3.5. The Growth Promotion of “Huajin” C. oleifera Cultivars under Different Inoculation Methods of CSUFT-BV4
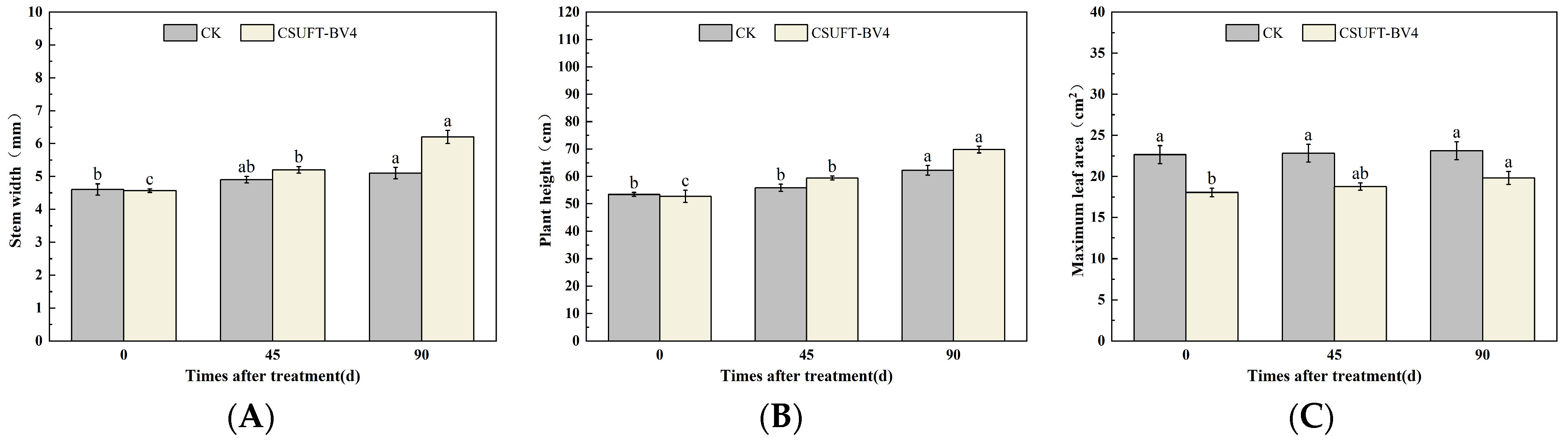
3.5.1. The Effect of Leaf Spray Method on the Growth of “Huajin” C. oleifera Cultivars
3.5.2. The Effect of Root Irrigation Method on the Growth of “Huajin” C. oleifera Cultivars
3.5.3. The Effect of Leaf Spraying + Root Irrigation Method on the Growth of “Huajin” C. oleifera Cultivars
3.6. Antagonistic Activities of CSUFT-BV4 against C. oleifera Anthracnose In Vitro
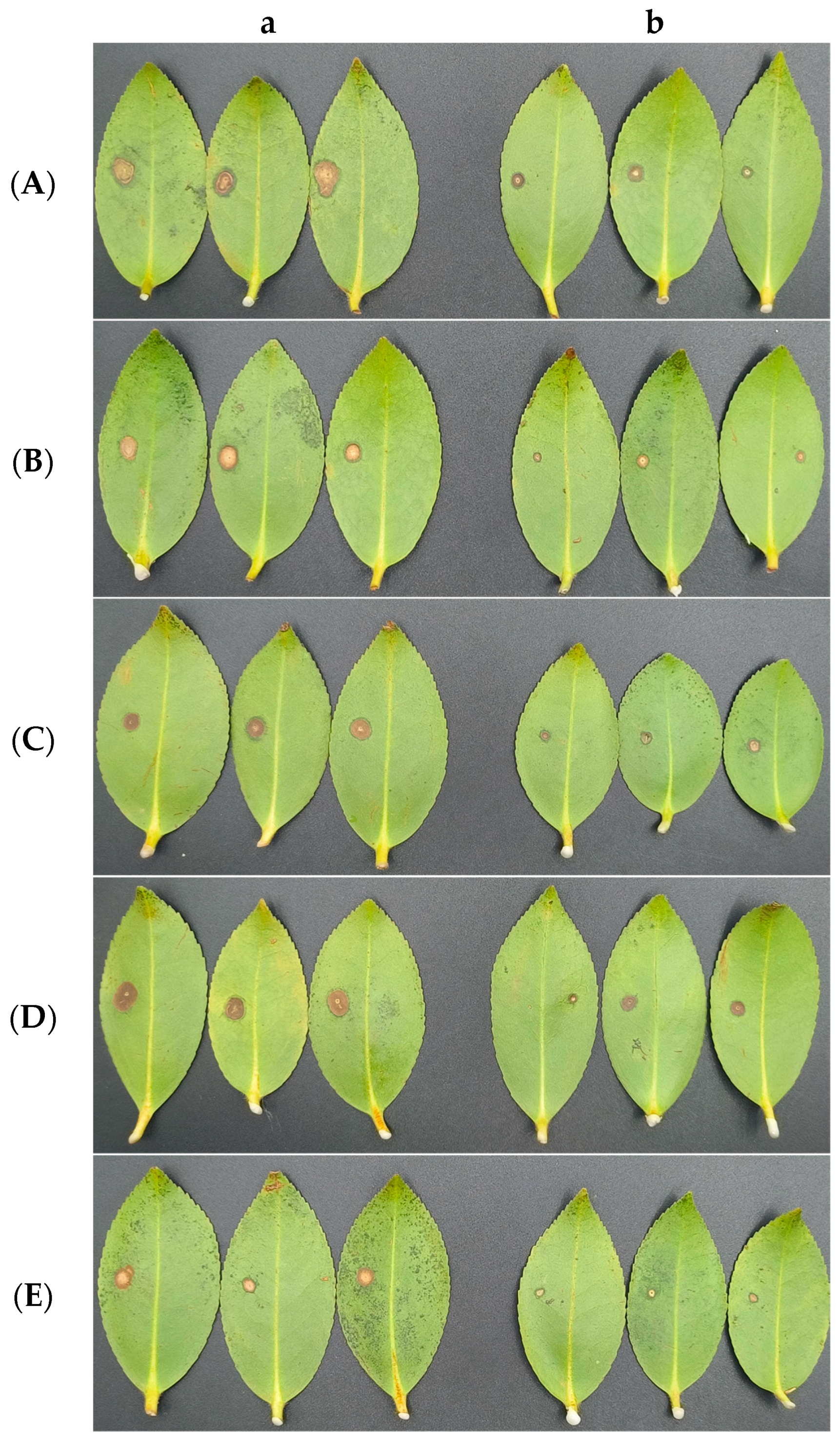
3.7. Antagonistic Activities of CSUFT-BV4 against C. oleifera Anthracnose In Vivo
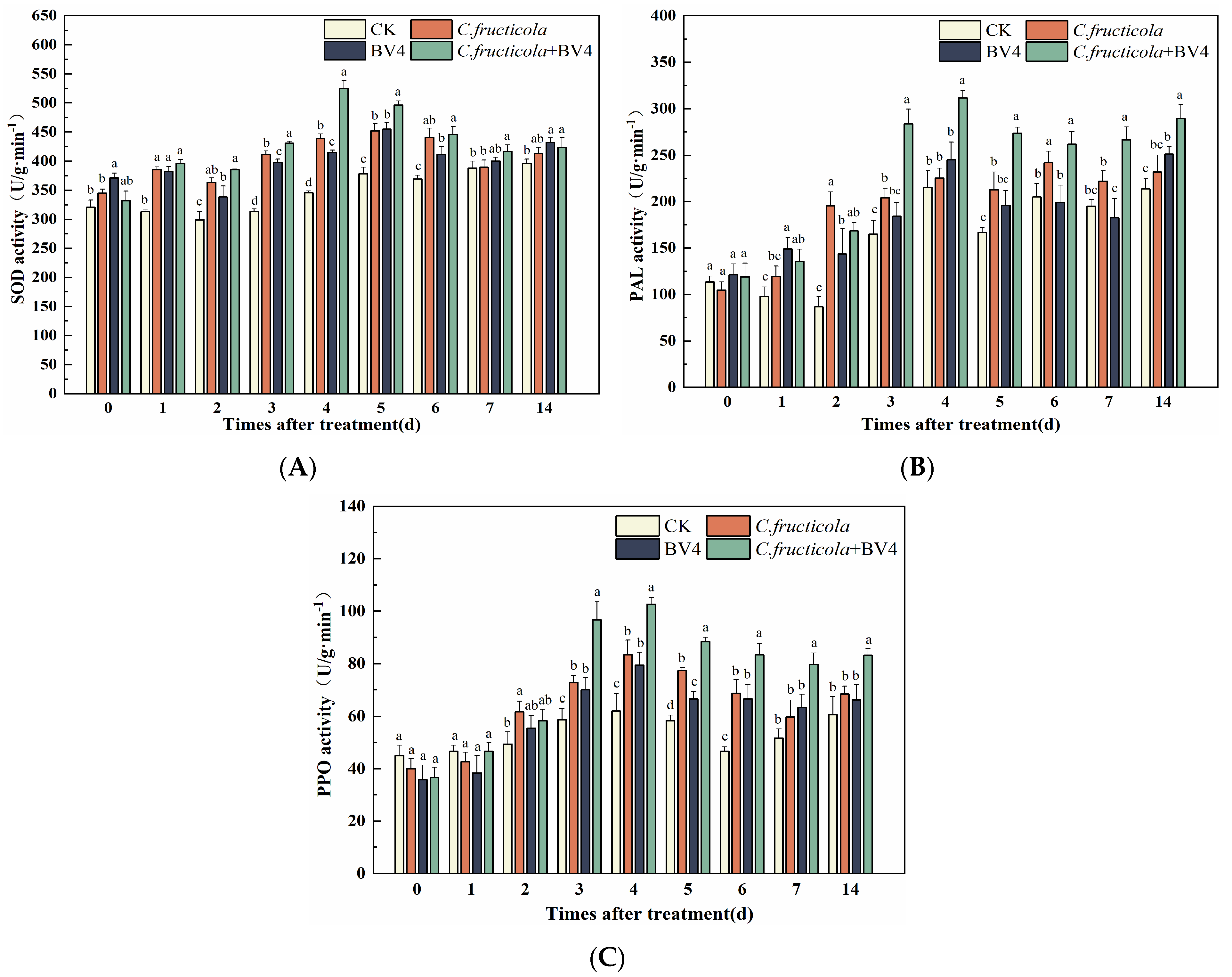
3.8. The Effect of CSUFT-BV4 on the Activity Level of C. oleifera Defense Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.L.; Zeng, G.Y.; Wang, G.C.; He, Y.H. Endophytic Antagonistic Growth-Promoting Bacteria Screening and Induction of Systemic Resistance to Root Rot of Camellia oleifera. Chin. J. Oil Crop Sci. Available online: https://kns.cnki.net/kcms/detail/42.1429.S.20231113.0950.001.html (accessed on 15 November 2023).
- Xia, Y.D.; Liu, J.A.; Wang, Z.K.; He, Y.; Tan, Q.; Du, Z.; Niu, A.; Liu, M.; Li, Z.; Sang, M.; et al. Antagonistic Activity and Potential Mechanisms of Endophytic Bacillus subtilis YL13 in Biocontrol of Camellia oleifera Anthracnose. Forests 2023, 14, 886. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Liao, W.J.; Zou, D.X.; Wu, Y.J.; Deng, Y. Biological Characteristics and Identification of Anthracnose Pathogen in Guangxi Camellia oleifera. J. Plant Prot. 2015, 42, 382–389. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.Y.; Xu, L.P.; Zhu, D.X. Identification of a new anthracnose pathogen of Camellia oleifera by multigene phylogenetic analysis. J. Plant Prot. 2014, 41, 602–607. [Google Scholar]
- Li, H.; Li, Y.; Jiang, S.Q.; Liu, J.A.; Zhou, G.Y. Identification of anthracnose pathogen of Camellia oleifera in Hunan Province. Sci. Silvae Sin. 2017, 53, 43–53. [Google Scholar] [CrossRef]
- Yu, J.X.; He, Z.; Li, M.; Wu, Y.; Peng, S.; Zhong, J.; Gao, B. Analysis of antagonistic substances of anthracnose antagonist bacterium P-14 in Camellia oleifera. For. Res. 2019, 32, 118–124. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.X.; Guo, Q.; Zhang, J.B.; Ruiz-Menjivar, J. The impact of agricultural chemical inputs on environment: Global evidence from informetric analysis and visualization. Int. J. Low-Carbon Technol. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Hartmann, A.; Proenca, D.N. Biological Control of Phytopathogens: Mechanisms and Applications. Pathogens 2023, 12, 783. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Wang, F.; Ying, J.B.; Liu, L.; Zhang, D.H.; Hong, Y.T.; Shen, D.Z. Identification and screening of the antagonistic endophytic Bacillus 6715 against anthracnose in Camellia oleifera in Dehongzhou. China J. West China For. Sci. 2021, 50, 131–138. [Google Scholar] [CrossRef]
- Meng, J.X.; Zhang, X.Y.; Han, X.S.; Fan, B. Application and Development of Biocontrol Agents in China. Pathogens 2022, 11, 1120. [Google Scholar] [CrossRef]
- James, E.K.; Olivares, F.L. Infection and Colonization of Sugar Cane and Other Graminaceous Plants by Endophytic Diazotrophs. Crit. Rev. Plant Sci. 1998, 17, 77–119. [Google Scholar] [CrossRef]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome Survey and Characterization of Endophytic Bacteria Exhibiting a Beneficial Effect on Growth and Development of Poplar Trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.K.; Liu, D.; Li, R.Y.; Wu, J.R.; Sheng, H. The effect of biofilm formation of Bacillus DZY6715 on its resistance to anthracnose of Camellia oleifera. Non-Wood For. Res. 2023, 41, 91–101. [Google Scholar] [CrossRef]
- Chen, S.Q.; Li, X.M.; Wen, M.X.; Sha, G.; Bo, N.; Wang, T. The Separation and Identification of Epiphytic Bacillus subtilis from Tea Tree and Its Bacteriostatic Characterization. Mol. Plant Breed. Available online: https://kns.cnki.net/kcms/detail/46.1068.S.20231204.1801.034.html (accessed on 5 December 2023).
- Xue, S. Colonization in Tomato and Antagonistic Bacterial Wilt Study of Bacillus amyloliquefaciens. Master’s Thesis, Hainan University, Hainan, China, 2017. [Google Scholar]
- Shahid, M.; Hameed, S.; Tariq, M.; Zafar, M.; Ali, A.; Ahmad, N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann. Microbiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Luo, C.P.; Zhou, H.F.; Zou, J.C.; Wang, X.Y.; Zhang, R.S.; Xiang, Y.P.; Chen, Z.Y. Bacillomycin L and surfactin contribute synergistically to the phenotypic features of Bacillus subtilis 916 and the biocontrol of rice sheath blight induced by Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2015, 99, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Song, W.X. Preliminary Studies on the Inhibitory Effect of Six Strains of Antagonistic Bacillus spp. on Soilborne Disease Pathogens and Their Biological Characterization. Master’s Thesis, Guangxi University, Guangxi, China, 2020. [Google Scholar] [CrossRef]
- Chen, L.; Heng, J.Y.; Qin, S.Y.; Bian, K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Z.Y.; Zhao, Q.Q.; Wang, L.H.; Ma, L. Inhibition of anthracnose pathogens of Camellia oleifera by biocontrol bacteria. J. Beijing For. Univ. 2020, 42, 107–116. [Google Scholar]
- Chen, S.; Wang, J.H.; Zhang, B.X.; Liu, X.L. The effectiveness of Bacillus velezensis against soybean root rot in pots and detection of defense enzyme activity. Mol. Plant Breed. 2022, 20, 6492–6500. [Google Scholar] [CrossRef]
- LI, T.T. Inhibition of Antibacterial Substance by Bacillus Atrophaeus 297 Against Susceptibility of Quorum Sensingsignal Molecule DKPs. Master’s Thesis, Shanxi Agricultural University, Shanxi, China, 2019. [Google Scholar] [CrossRef]
- Angelopoulou, D.J.; Naska, E.J.; Paplomatas, E.J.; Tjamos, S.E. Biological control agents (BCAs) of verticillium wilt: Influence of application rates and delivery method on plant protection, triggering of host defense mechanisms and rhizosphere populations of BCAs. Plant Pathol. 2014, 63, 1062–1069. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Pande, S.; Sharma, M.; Humayun, P.; Kiran, B.K.; Sandeep, D.; Vidya, M.S.; Deepthi, K.; Rupela, O. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011, 30, 1070–1078. [Google Scholar] [CrossRef]
- Wu, Q.L.; Dou, X.; Wang, Q.; Guan, Z.B.; Cai, Y.J.; Liao, X.R. Isolation of β-1,3-Glucanase-Producing Microorganisms from Poria cocos Cultivation Soil via Molecular Biology. Molecules 2018, 23, 1555. [Google Scholar] [CrossRef]
- Liu, N. The Separation of Microbial Fertilizer Strains Was Promoted by the Hydrolysis of Phosphorus and Potassium. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar] [CrossRef]
- Wang, J.J. Fertilizer-Functional Microbes Screen and the Study of Growth-Promoting Efficacy on Wheat. Master’s Thesis, Northwest A&F University, Shanxi, China, 2019. [Google Scholar]
- Cao, J.J.; Xiong, M.X.; Chao, Y.P.; Zhao, P.; Wang, Z. The isolation and identification of extremely saline-tolerant nitrogen-fixing bacteria and their nitrogen-fixing characteristics. Acta Microbiol. Sin. 2021, 61, 3483–3495. [Google Scholar] [CrossRef]
- Liang, P. Preliminary Experimental Study on Introduced “Hua” Series of Camellia oleifera in Liuzhou, Guangxi. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2019. [Google Scholar] [CrossRef]
- Zhang, F.H. Comparative Study on Fruit and Seed Development of 4 Camellia oleifera Cultivars. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2020. [Google Scholar] [CrossRef]
- Sang, X.N.; Liu, J.A.; Feng, F.S.; Zhou, G.Y. The Screening, Identification and Prevention of Endophytic Antagonistic Bacteria in Camellia oleifera. Chin. J. Biol. Control 2021, 37, 575–583. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.D.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.T.; Franco, C.M.M. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl. Environ. Microbiol. 2003, 69, 5603–5608. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.M.; Ismael, W.H.; Mahfouz, A.Y.; Daigham, G.E.; Attia, M.S. Efficacy of endophytic bacteria as promising inducers for enhancing the immune responses in tomato plants and managing Rhizoctonia root-rot disease. Sci. Rep. 2024, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Duan, T.F.; Jiang, C.C.; Wang, F. Screening of endophytic bacteria antagonizing anthracnose of Dangshan pear and analysis of their inhibitory effects. Food Sci. 2018, 39, 185–190. [Google Scholar] [CrossRef]
- Xia, Y.D.; Liu, J.N.; Chen, C.; Mo, X.L.; Tan, Q.; He, Y.; Wang, Z.K.; Yin, J.; Zhou, G.Y. The Multifunctions and Future Prospects of Endophytes and Their Metabolites in Plant Disease Management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef]
- Klein, M.N.; Kupper, K.C. Biofilm production by Aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol. 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Hmidet, N.; Ali, N.E.; Zouari-Fakhfakh, N.; Haddar, A.; Nasri, M.; Sellemi-Kamoun, A. Chicken feathers: A complex substrate for the co-production of α-amylase and proteases by B. licheniformis NH1. J. Ind. Microbiol. Biotechnol. 2010, 37, 983–990. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.H.; Zu, X.; Yu, X.Y.; Zhu, H.J.; Li, X.J.; Zhong, J.; Liu, E.M. Efficacy of Plant Growth-Promoting Bacteria Bacillus cereus YN917 for Biocontrol of Rice Blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef] [PubMed]
- Medison, R.G.; Jiang, J.W.; Medison, M.B.; Tan, L.T.; Kayange, C.D.M.; Sun, Z.X.; Zhou, Y. Evaluating the potential of Bacillus licheniformis YZCUO202005 isolated from lichens in maize growth promotion and biocontrol. Heliyon 2023, 9, e20204. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Kusajima, M.; Shima, S.; Fujita, M.; Minamisawa, K.; Che, F.S.; Yamakawa, H.; Nakashita, H. Involvement of ethylene signaling in Azospirillum sp B510-induced disease resistance in rice. Biosci. Biotechnol. Biochem. 2018, 82, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Farzand, A.; Heng, Z.; Khan, I.U.; Moosa, A.; Zubair, M.; Na, Y.; Ying, S.; Tang, C.M. Antagonistic Potential of Novel Endophytic Bacillus Strains and Mediation of Plant Defense against Verticillium Wilt in Upland Cotton. Plants 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Tan, Y.M.; Peng, Q.J.; Xiao, Y.; Xie, J.F.; Li, Z.; Ding, H.X.; Pan, H.; Wei, L.F. Biocontrol potential of endophytic bacterium Bacillus altitudinis GS-16 against tea anthracnose caused by Colletotrichum gloeosporioides. Peerj 2024, 12, e16761. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Yang, F.; Xie, X.; Goodwin, P.H.; Deng, X.X.; Tian, B.M.; Yang, L.R. Evaluation and Genome Analysis of Bacillus subtilis YB-04 as a Potential Biocontrol Agent Against Fusarium Wilt and Growth Promotion Agent of Cucumber. Front. Microbiol. 2022, 13, 885430. [Google Scholar] [CrossRef]












| Metabolic Substances | CSUFT-BV4 |
|---|---|
| Protease | + |
| Amylase | + |
| Cellulase | + |
| β-1,3-glucanase | + |
| Growth-Promoting Characteristics | CSUFT-BV4 |
|---|---|
| Indoleacetic acid production | + |
| Nitrogen fixation | + |
| Organic phosphorus solubilizing | + |
| Inorganic phosphorus solubilizing | + |
| Potassium solubilizing | − |
| Siderophore secretion | − |
| Strain | Inhibition Rate (%) | |||
|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Average | |
| CK | ||||
| C. fructicola | 74.2 | 72.8 | 72.6 | 73.2 ± 0.71 a |
| C. gloeosporioides | 70.7 | 72.6 | 71.3 | 71.5 ± 0.82 ab |
| C. siamense | 70.0 | 68.1 | 68.2 | 68.8 ± 0.89 b |
| C. camelliae | 71.3 | 72.0 | 66.4 | 69.9 ± 2.49 ab |
| C. kahawae | 72.5 | 71.9 | 69.6 | 71.4 ± 1.26 ab |
| Strain | Inhibition Rate (%) | |||
|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Average | |
| CK | 0 | |||
| C. fructicola | 58.8% | 63.3% | 62.5% | 61.6% ± 1.95 a |
| C. gloeosporioides | 60.0% | 62.5% | 60.0% | 60.8% ± 1.18 a |
| C. siamense | 62.0% | 54.0% | 58.3% | 58.1% ± 3.27 a |
| C. camelliae | 62.5% | 50.8% | 52.9% | 55.4% ± 5.1 a |
| C. kahawae | 61.0% | 62.0% | 55.6% | 59.5% ± 2.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Miao, X.; Xia, Y.; Chen, X.; Liu, J.; Zhou, G. The Research of Antagonistic Endophytic Bacterium Bacillus velezensis CSUFT-BV4 for Growth Promotion and Induction of Resistance to Anthracnose in Camellia oleifera. Microorganisms 2024, 12, 763. https://doi.org/10.3390/microorganisms12040763
He Y, Miao X, Xia Y, Chen X, Liu J, Zhou G. The Research of Antagonistic Endophytic Bacterium Bacillus velezensis CSUFT-BV4 for Growth Promotion and Induction of Resistance to Anthracnose in Camellia oleifera. Microorganisms. 2024; 12(4):763. https://doi.org/10.3390/microorganisms12040763
Chicago/Turabian StyleHe, Yuan, Xinyu Miao, Yandong Xia, Xingzhou Chen, Junang Liu, and Guoying Zhou. 2024. "The Research of Antagonistic Endophytic Bacterium Bacillus velezensis CSUFT-BV4 for Growth Promotion and Induction of Resistance to Anthracnose in Camellia oleifera" Microorganisms 12, no. 4: 763. https://doi.org/10.3390/microorganisms12040763





