Insights into the Impact of Physicochemical and Microbiological Parameters on the Safety Performance of Deep Geological Repositories
Abstract
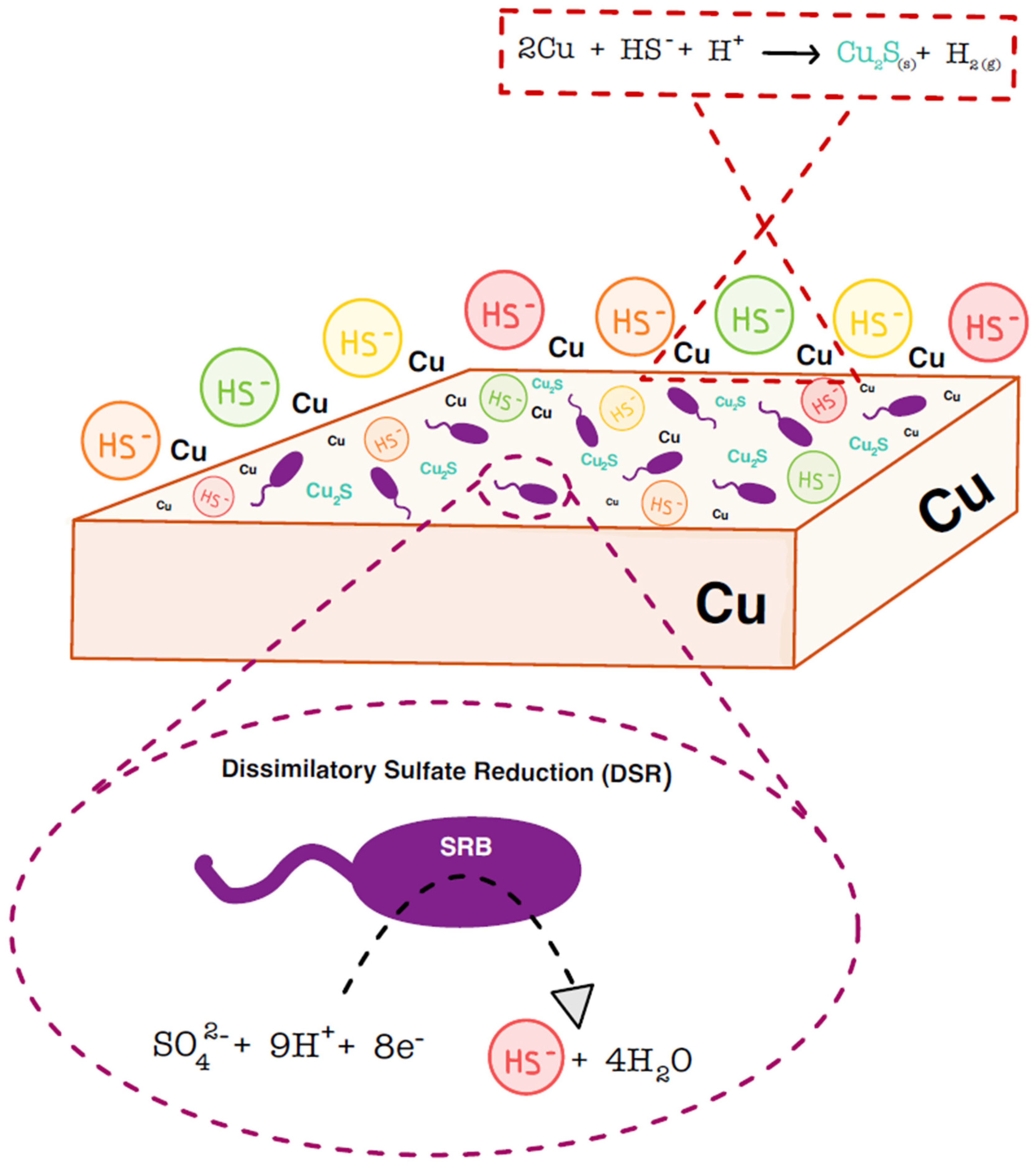
:1. Introduction
2. Effect of Radiation
2.1. Copper Corrosion
2.2. Bentonite Stability
2.3. Microbial Viability
3. Effect of Bentonite Dry Density and Microbial Activity on Bentonite Performance as an Engineered Barrier in DGRs
4. Effect of Radionuclides on the Diversity and Viability of Bentonite Microbial Communities
4.1. Uranium
4.2. Selenium
| Microorganism | Taxonomic Affiliation | Interaction Mechanism | Metal | Reference |
|---|---|---|---|---|
| Amycolatopsis ruanii | Actinomycetota (Bacteria) | Biomineralization | Uranium | [90] |
| Bacillus sphaericus | Bacillota (Bacteria) | Biosorption | Uranium | [94] |
| Bacillus sp. | Bacillota (Bacteria) | Biosorption and bioaccumulation | Uranium | [95] |
| Desulfovibrio vulgaris | Pseudomonadota (Bacteria) | Bioreduction | Uranium | [98] |
| Fusarium oxysporum | Ascomycota (Fungi) | Biomineralization | Uranium | [101] |
| Bacillus selenitireducens | Bacillota (Bacteria) | Bioreduction | Selenium | [103] |
| Shewanella oneidensis | Pseudomonadota (Bacteria) | Bioreduction | Selenium | [104] |
| Stenotrophomonas bentonitica | Pseudomonadota (Bacteria) | Bioreduction | Selenium | [105] |
| Pseudomonas seleniipraecipitans | Pseudomonadota (Bacteria) | Bioreduction | Selenium | [106] |
5. Copper Corrosion under Repository Conditions
6. Effect of Oxygen, Gasses, and Nutrients on the Microorganisms
7. Effect of Temperature
7.1. Impact of Temperature Evolution on Nuclear Repository Barriers
7.2. Impact of Temperature Evolution on Microorganisms
8. Limitations, Challenges, and Opportunities
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency (IAEA). Status and Trends in Spent Fuel and Radioactive Waste Management; Nuclear Energy Series No. NW-T-1.14; International Atomic Energy Agency: Vienna, Austria, 2022; ISSN 1995-7807. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/PUB1963_web.pdf (accessed on 1 January 2022).
- Xu, Y.; Zeng, Z.; Lv, H. Temperature dependence of apparent thermal conductivity of compacted bentonites as buffer material for high-level radioactive waste repository. Appl. Clay Sci. 2019, 174, 10–14. [Google Scholar] [CrossRef]
- Bajestani, M.S.; Nasir, O.; Oh, W.T. Properties of Bentonite-Based Sealing Materials during Hydration. Minerals 2023, 13, 1412. [Google Scholar] [CrossRef]
- García-Romero, E.; María Manchado, E.; Suárez, M.; García-Rivas, J. Spanish bentonites: A review and new data on their geology, mineralogy, and crystal chemistry. Minerals 2019, 9, 696. [Google Scholar] [CrossRef]
- Stroes-Gascoyne, S.; Hamon, C.J.; Maak, P.; Russell, S. The effects of the physical properties of highly compacted smectitic clay (bentonite) on the culturability of indigenous microorganisms. Appl. Clay Sci. 2010, 47, 155–162. [Google Scholar] [CrossRef]
- Engel, K.; Ford, S.E.; Binns, W.J.; Diomidis, N.; Slater, G.F.; Neufeld, J.D. Stable microbial community in compacted bentonite after 5 years of exposure to natural granitic groundwater. MSphere 2023, 8, e00048-23. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.V.; Pérez del Villar, L.; Martín, P.L.; Pelayo, M.; Fernández, A.M.; Garralón, A.; Cuevas, J.; Leguey, S.; Caballero, E.; Huertas, F.J.; et al. The study of Spanish clays for their use as sealing materials in nuclear waste repositories: 20 years of progress. J. Iber. Geol. 2006, 32, 15–36. [Google Scholar]
- 7º Plan General de Residuos Radiactivos. Ministerio para la transición ecológica y el reto demográfico. 2023. Available online: https://www.miteco.gob.es/es/energia/nuclear/residuos/plan-general.html (accessed on 27 December 2023).
- Werme, L. Design Premises for Canister for Spent Nuclear Fuel; Posiva SKB Technical Report TR-98-08; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1998. [Google Scholar]
- Bennett, D.G.; Gens, R. Overview of European concepts for high-level waste and spent fuel disposal with special reference waste container corrosion. J. Nucl. Mater. 2008, 379, 1–8. [Google Scholar] [CrossRef]
- Jonsson, M.; Emilsson, G.; Emilsson, L. Mechanical design analysis for the canister. Posiva SKB Rep. 2018, 4, 147. [Google Scholar]
- Abdelouas, A.; Alonso, U.; Bernier-Latmani, R.; Bosch, C.; Cherkouk, A.; Dobrev, D.; Fernández, A.M.; Finck, N.; Gaggiano, R.; Havlová, V.; et al. Initial State-of-the-Art of WP ConCorD. Final Version as of 17.08.2022 of Deliverable D15.1 of the HORIZON 2020 Project EURAD; EC Grant agreement no: 847593. 2022. Available online: https://www.ejp-eurad.eu/sites/default/files/2022-09/EURAD%20-%20D15.1%20ConCorD%20Initial%20SotA.pdf (accessed on 17 August 2022).
- Johnson, L.H.; Niemeyer, M.; Klubertanz, G.; Siegel, P.; Gribi, P. Calculations of the Temperature Evolution of a Repository for Spent Fuel, Vitrified High-Level Waste and Intermediate Level Waste in Opalinus Clay (No. NTB--01-04); National Cooperative for the Disposal of Radioactive Waste (NAGRA): Wettingen, Switzerland, 2002; Volume 80, pp. 1015–2636. [Google Scholar]
- Landolt, D.; Davenport, A.; Payer, A.; Shoesmitth, D. A Review of Materials and Corrosion Issues Regarding Canisters for Disposal of Spent Fuel and High-Level Waste in Opalinus Clay; NAGRA Technical Report 09-02; National Cooperative for the Disposal of Radioactive Waste (NAGRA): Wettingen, Switzerland, 2009. [Google Scholar]
- Pospiskova, I.; Dobrev, D.; Kouril, M.; Stoulil, J.; Novikova, D.; Kotnour, P.; Matal, O. Czech national programme and disposal canister concept. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. S1), 6–10. [Google Scholar] [CrossRef]
- Prachař, I.; Pospíšková, I.; Vokal, A.; Steinerova, L.; Vondrovic, L. DGR Development in the Czech Republic. Action Plan 2017–2025 (No. SURAO--112-2017); Radioactive Waste Repository Administration: Prague, Czech Republic, 2017. [Google Scholar]
- McMurry, J.; Dixon, D.A.; Garroni, J.D.; Ikeda, B.M.; Stroes-Gascoyne, S.; Baumgartner, P.; Melnyk, T.W. Evolution of a Canadian Deep Geologic Repository (No. 06819-REP-01200-10127-R00); Ontario Power Generation Report; Ontario Power Generation: Toronto, ON, Canada, February 2004. [Google Scholar]
- Guo, M. The Electrochemical and Corrosion Study of Copper for Nuclear Waste Containers under Deep Geological Disposal Conditions. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2020. [Google Scholar]
- Binns, W.J.; Behazin, M.; Briggs, S.; Keech, P.G. An overview of the Canadian nuclear waste corrosion program. Mater. Corros. 2023, 74, 1580–1586. [Google Scholar] [CrossRef]
- Dou, W.; Pu, Y.; Han, X.; Song, Y.; Chen, S.; Gu, T. Corrosion of Cu by a sulfate reducing bacterium in anaerobic vials with different headspace volumes. Bioelectrochemistry 2020, 133, 107478. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.S.; Behazin, M.; Binns, W.J.; Keech, P.G. An evaluation of corrosion processes affecting copper-coated nuclear waste containers in a deep geological repository. Prog. Mater. Sci. 2021, 118, 100766. [Google Scholar] [CrossRef]
- Mills, M.M.; Sanchez, A.C.; Boisvert, L.; Payne, C.B.; Ho, T.A.; Wang, Y. Understanding smectite to illite transformation at elevated (> 100° C) temperature: Effects of liquid/solid ratio, interlayer cation, solution chemistry and reaction time. Chem. Geol. 2022, 615, 121214. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Fernández-Cantos, M.V.; Gómez-Bolívar, J.; Eswayah, A.S.; Gardiner, P.H.E.; Pinel-Cabello, M.; Solari, P.L.; Merroun, M.L. Combined bioreduction and volatilization of SeVI by Stenotrophomonas bentonitica: Formation of trigonal selenium nanorods and methylated species. Sci. Total Environ. 2023, 858, 160030. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Morales-Hidalgo, M.; Povedano-Priego, C.; Jroundi, F.; Hidalgo-Iruela, J.; Cano-Cano, M.; Pérez-Muelas, E.; Merroun, M.L.; Martín-Sanchez, I. Unlocking the key role of bentonite fungal isolates in tellurium and selenium bioremediation and biorecovery: Implications in the safety of radioactive waste disposal. Sci. Total Environ. 2024, 912, 169242. [Google Scholar] [CrossRef]
- ENRESA. Available online: https://www.enresa.es/.
- Cátedra ENRESA. Available online: http://www.catedraenresauco.com/.
- Chapman, N. Geological disposal of radioactive wastes–concept, status and trends. J. Iber. Geol. 2006, 32, 7–14. [Google Scholar]
- Jonsson, M. Radiation Effects on Materials Used in Geological Repositories for Spent Nuclear Fuel. Int. Sch. Res. Not. 2012, 2012, 639520. [Google Scholar] [CrossRef]
- Soroka, I.; Chae, N.; Jonsson, M. On the mechanism of γ-radiation-induced corrosion of copper in water. Corros. Sci. 2021, 182, 109279. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1990. [Google Scholar]
- Björkbacka, Å.; Yang, M.; Gasparrini, C.; Leygraf, C.; Jonsson, M. Kinetics and mechanisms of reactions between H2O2 and copper and copper oxides. Dalton Trans. 2015, 44, 16045–16051. [Google Scholar] [CrossRef]
- Reed, D.T.; Konynenburg, R.A.V. Effect of ionizing radiation on moist air systems. MRS Online Proc. Libr. (OPL) 1987, 112, 393. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Hosseinpour, S.; Johnson, M.; Leygraf, C.; Jonsson, M. Radiation induced corrosion of copper for spent nuclear fuel storage. Radiat. Phys. Chem. 2013, 92, 80–86. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Johnson, C.M.; Leygraf, C.; Jonsson, M. Role of the oxide layer in radiation-induced corrosion of copper in anoxic water. J. Phys. Chem. C 2016, 120, 11450–11455. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Johnson, C.M.; Leygraf, C.; Jonsson, M. Radiation induced corrosion of copper in humid air and argon atmospheres. J. Electrochem. Soc. 2017, 164, C201. [Google Scholar] [CrossRef]
- Norrfors, K.K.; Björkbacka, Å.; Kessler, A.; Wold, S.; Jonsson, M. γ-radiation induced corrosion of copper in bentonite-water systems under anaerobic conditions. Radiat. Phys. Chem. 2018, 144, 8–12. [Google Scholar] [CrossRef]
- King, F.; Lilja, C.; Pedersen, K.; Pitkänen, P.; Vähänen, M. An Update of the State-of-the-Art Report on the Corrosion of Copper under Expected Conditions in a Deep Geologic Repository (No. SKB-TR--10-67); Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2010. [Google Scholar]
- Galamboš, M.; Daňo, M.; Rosskopfová, O.; Šeršeň, F.; Kufčáková, J.; Adamcová, R.; Rajec, P. Effect of gamma-irradiation on adsorption properties of Slovak bentonites. J. Radioanal. Nucl. Chem. 2012, 292, 481–492. [Google Scholar] [CrossRef]
- Allard, T.; Calas, G. Radiation effects on clay mineral properties. Appl. Clay Sci. 2009, 43, 143–149. [Google Scholar] [CrossRef]
- Strìček, I.; Šucha, V.; Uhlík, P. Gamma-irradiation effects on smectite properties. 4. Mid-European clay conference, Zakopane, Poland. Abstr. Mineral. Spec. Pap. 2008, 33, 157. [Google Scholar]
- Strìček, I. Mineral Stability of Bentonites in Barrier Conditions. Ph.D. Thesis, Comenius University in Bratislava, Bratislava, Slovakia, 2010. [Google Scholar]
- Plötze, M.; Kahr, G.; Stengele, R.H. Alteration of clay minerals—Gamma-irradiation effects on physico chemical properties. Appl. Clay Sci. 2003, 23, 195–202. [Google Scholar] [CrossRef]
- Allard, T.; Balan, E.; Calas, G.; Fourdrin, C.; Morichon, E.; Sorieul, S. Radiation-induced defects in clay minerals: A review. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 277, 112–120. [Google Scholar] [CrossRef]
- Sorieul, S.; Allard, T.; Wang, L.M.; Grambin-Lapeyre, C.; Lian, J.; Calas, G.; Ewing, R.C. Radiation-Stability of Smectite. Environ. Sci. Technol. 2008, 42, 8407–8411. [Google Scholar] [CrossRef]
- Holmboe, M.; Norrfors, K.K.; Jonsson, M.; Wold, S. Effect of γ-radiation on radionuclide retention in compacted bentonite. Radiat. Phys. Chem. 2011, 80, 1371–1377. [Google Scholar] [CrossRef]
- Gu, B.X.; Wang, L.M.; Minc, L.D.; Ewing, R.C. Temperature Effects on the Radiation Stability and Ion Exchange Capacity of Smectites. J. Nucl. Mater. 2001, 297, 345–354. [Google Scholar] [CrossRef]
- Chikkamath, S.; Manjanna, J.; Kabadagi, A.; Patil, D.; Tripathi, V.S.; Kar, A.S.; Tomar, B.S. Gamma (60Co) irradiation and thermal effect on redox behavior of interlayer iron in montmorillonite. Appl. Clay Sci. 2021, 200, 105893. [Google Scholar] [CrossRef]
- Van Gerwen, S.J.; Rombouts, F.M.; Van’t Riet, K.; Zwietering, M.H. A data analysis of the irradiation parameter D10 for bacteria and spores under various conditions. J. Food Prot. 1999, 62, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Černá, K.; Bartak, D.S. Online Resource: State of Art—Attachment I to the Work Report 2019, Project Limiting Factors for Survivability and Proliferation of Microorganisms Significant for Corrosion of Deep Geological Repository Barrier Systems (BioBen) Project Number: TAČR TK02010169; 2019. Available online: https://www.surao.cz/wp-content/uploads/2023/09/TZ552_2021.pdf (accessed on 17 August 2022).
- Wouters, B.G.; Begg, A.C. Irradiation-induced damage and the DNA damage response. Basic Clin. Radiobiol. 2009, 4, 11–26. [Google Scholar]
- Jung, K.W.; Lim, S.; Bahn, Y.S. Microbial radiation-resistance mechanisms. J. Microbiol. 2017, 55, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Mattimore, V.; Battista, J.R. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 1996, 178, 633–637. [Google Scholar] [CrossRef]
- Alper, T.; Howard-Flanders, P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature 1956, 178, 978–979. [Google Scholar] [CrossRef]
- Musilova, M.; Wright, G.; Ward, J.M.; Dartnell, L.R. Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology 2015, 15, 1076–1090. [Google Scholar] [CrossRef]
- Daly, M.J. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 2009, 7, 237–245. [Google Scholar] [CrossRef]
- Phillips, R.W.; Wiegel, J.; Berry, C.J.; Fliermans, C.; Peacock, A.D.; White, D.C.; Shimkets, L.J. Kineococcus radiotolerans sp. nov., a radiation-resistant, gram-positive bacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Bagwell, C.E.; Bhat, S.; Hawkins, G.M.; Smith, B.W.; Biswas, T.; Hoover, T.R.; Saunders, E.; Han, C.S.; Tsodikov, O.V.; Shimkets, L.J. Survival in nuclear waste, extreme resistance, and potential applications gleaned from the genome sequence of Kineococcus radiotolerans SRS30216. PLoS ONE 2008, 3, e3878. [Google Scholar] [CrossRef] [PubMed]
- Stroes-Gascoyne, S.; Lucht, L.M.; Borsa, J.; Delaney, T.L.; Haveman, S.A.; Hamon, C.J. Radiation resistance of the natural microbial population in buffer materials. MRS Online Proc. Libr. (OPL) 1994, 353, 345. [Google Scholar] [CrossRef]
- Haynes, H.M.; Pearce, C.I.; Boothman, C.; Lloyd, J.R. Response of bentonite microbial communities to stresses relevant to geodisposal of radioactive waste. Chem. Geol. 2018, 501, 58–67. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Steinmetz, H.J. Approaches to Disposal of Nuclear Waste. Energies 2022, 15, 7804. [Google Scholar] [CrossRef]
- Marsh, A.I.; Williams, L.G.; Lawrence, J.A. The important role and performance of engineered barriers in a UK geological disposal facility for higher activity radioactive waste. Prog. Nucl. Energy 2021, 137, 103736. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management—A Review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Svensson, D.; Dueck, A.; Nilsson, U.; Olsson, S.; Sandén, T.; Lydmark, S.; Jägerwall, S.; Pedersen, K.; Hansen, S. Alternative Buffer Material. Status of the Ongoing Laboratory Investigation of Reference Materials and Test Package 1; SKB Report TR-11-06; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2011. [Google Scholar]
- Porras, D.E.V.; Angélica, R.S.; Paz, S.P.A.D. Practical mineralogical quantification of bentonites supported for a PXRD calibrated hkl model. Braz. J. Geol. 2021, 51, e20200088. [Google Scholar] [CrossRef]
- Chen, B.; Peng, F.; Zhang, L.; Sun, D. Investigation on swelling characteristics of GMZ bentonite with different initial water contents. Ann. Nucl. Energy 2023, 181, 109565. [Google Scholar] [CrossRef]
- Pedersen, K.; Motamedi, M.; Karnland, O.; Sandén, T. Mixing and sulphate-reducing activity of bacteria in swelling, compacted bentonite clay under high-level radioactive waste repository conditions. J. Appl. Microbiol. 2000, 89, 1038–1047. [Google Scholar] [CrossRef]
- Rättö, M.; Itävaara, M. Microbial Activity in Bentonite Buffers: Literature Study; VTT Technology; VTT Technical Research Centre of Finland: Espoo, Finland, 2012. [Google Scholar]
- Martinez-Moreno, M.F.; Povedano-Priego, C.; Morales-Hidalgo, M.; Mumford, A.D.; Ojeda, J.J.; Jroundi, F.; Merroun, M.L. Impact of compacted bentonite microbial community on the clay mineralogy and copper canister corrosion: A multidisciplinary approach in view of a safe Deep Geological Repository of nuclear wastes. J. Hazard. Mater. 2023, 458, 131940. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, M.F.; Povedano-Priego, C.; Mumford, A.D.; Morales-Hidalgo, M.; Mijnendonckx, K.; Jroundi, F.; Ojeda, J.J.; Merroun, M.L. Microbial responses to elevated temperature: Evaluating bentonite mineralogy and copper canister corrosion within the long-term stability of deep geological repositories of nuclear waste. Sci. Total Environ. 2024, 915, 170149. [Google Scholar] [CrossRef] [PubMed]
- Posiva. Safety Functions, Performance Targets and Technical Design Requirements for a KBS-3V Repository—Conclusions and Recommendations from a Joint SKB and Posiva Working Group; Posiva SKB Rep. 01; Posiva: Eurajoki, Finland, 2017. [Google Scholar]
- Beaver, R.C.; Vachon, M.A.; Tully, C.S.; Engel, K.; Spasov, E.; Binns, W.J.; Noël, J.J.; Neufeld, J.D. Impact of dry density and incomplete saturation on microbial growth in bentonite clay for nuclear waste storage. J. Appl. Microbiol. 2024, 135, lxae053. [Google Scholar] [CrossRef] [PubMed]
- Man, M.; Tong, H.; Srikanthan, N.; Usman, M.O.; Tully, C.S.; Noël, J.J.; Behazin, M.; Binns, W.J.; Keech, P.G.; Simpson, M.J. Analysis of natural organic matter chemistry in bentonite clay under compaction using different dry densities and duration. Appl. Geochem. 2024, 166, 105985. [Google Scholar] [CrossRef]
- Burzan, N.; Murad Lima, R.; Frutschi, M.; Janowczyk, A.; Reddy, B.; Rance, A.; Diomidis, N.; Bernier-Latmani, R. Growth and Persistence of an Aerobic Microbial Community in Wyoming Bentonite MX-80 Despite Anoxic in situ Conditions. Front. Microbiol. 2022, 13, 858324. [Google Scholar] [CrossRef] [PubMed]
- Maanoja, S.; Palmroth, M.; Salminen, L.; Lehtinen, L.; Kokko, M.; Lakaniemi, A.-M.; Auvinen, H.; Kiczka, M.; Muuri, E.; Rintala, J. The effect of compaction and microbial activity on the quantity and release rate of water-soluble organic matter from bentonites. Appl. Clay Sci. 2021, 211, 106192. [Google Scholar] [CrossRef]
- Povedano-Priego, C.; Jroundi, F.; Lopez-Fernandez, M.; Shrestha, R.; Spanek, R.; Martín-Sánchez, I.; Villar, M.V.; Ševců, A.; Dopson, M.; Merroun, M.L. Deciphering indigenous bacteria in compacted bentonite through a novel and efficient DNA extraction method: Insights into biogeochemical processes within the Deep Geological Disposal of nuclear waste concept. J. Hazard. Mater. 2021, 408, 124600. [Google Scholar] [CrossRef]
- Jalique, D.R.; Stroes-Gascoyne, S.; Hamon, C.J.; Priyanto, D.G.; Kohle, C.; Evenden, W.G.; Wolfaardt, G.M.; Grigoryan, A.A.; McKelvie, J.; Korber, D.R. Culturability and diversity of microorganisms recovered from an eight-year old highly-compacted, saturated MX-80 Wyoming bentonite plug. Appl. Clay Sci. 2016, 126, 245–250. [Google Scholar] [CrossRef]
- Bengtsson, A.; Pedersen, K. Microbial sulphide-producing activity in water saturated Wyoming MX-80, Asha and Calcigel bentonites at wet densities from 1500 to 2000 kgm−3. Appl. Clay Sci. 2017, 137, 203–212. [Google Scholar] [CrossRef]
- Johansson, A.J.; Lilja, C.; Sjögren, L.; Gordon, A.; Hallbeck, L.; Johansson, L. Insights from post-test examination of three packages from the MiniCan test series of copper-cast iron canisters for geological disposal of spent nuclear fuel: Impact of the presence and density of bentonite clay. Corros. Eng. Sci. Technol. 2017, 52, 54–60. [Google Scholar] [CrossRef]
- Gilmour, K.A.; Davie, C.T.; Gray, N. Survival and activity of an indigenous iron-reducing microbial community from MX80 bentonite in high temperature/low water environments with relevance to a proposed method of nuclear waste disposal. Sci. Total Environ. 2022, 814, 152660. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, H.; Bishop, M.E.; Zhang, J.; Wang, H.; Xie, S.; Wang, S.; Huang, L.; Eberl, D.D. Microbial reduction of structural iron in interstratified illite-smectite minerals by a sulfate-reducing bacterium. Geobiology 2012, 10, 150–162. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, H.; Liu, D.; Fischer, T.B.; Wang, S.; Huang, L. Microbial reduction of Fe(III) in illite–smectite minerals by methanogen Methanosarcina mazei. Chem. Geol. 2012, 292–293, 35–44. [Google Scholar] [CrossRef]
- Darda, S.A.; Gabbar, H.A.; Damideh, V.; Aboughaly, M.; Hassen, I. A comprehensive review on radioactive waste cycle from generation to disposal. J. Radioanal. Nucl. Chem. 2021, 329, 15–31. [Google Scholar] [CrossRef]
- Acharya, C. Microbial Bioremediation of Uranium: An Overview; BARC Newsletter: Mumbai, India, 2015; pp. 27–30. [Google Scholar]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in Pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef]
- Povedano-Priego, C.; Jroundi, F.; Solari, P.L.; Guerra-Tschuschke, I.; Abad-Ortega, M.D.M.; Link, A.; Vilchez-Vargas, R.; Merroun, M.L. Unlocking the bentonite microbial diversity and its implications in selenium bioreduction and biotransformation: Advances in deep geological repositories. J. Hazard. Mater. 2023, 445, 130557. [Google Scholar] [CrossRef] [PubMed]
- Flamíková, D.; Nečas, V. Assessment of the impact of selected changes in the deep geological repository model on its long-term safety. EPJ Web Conf. 2020, 225, 06012. [Google Scholar] [CrossRef]
- Gao, N.; Huang, Z.; Liu, H.; Hou, J.; Liu, X. Advances on the toxicity of uranium to different organisms. Chemosphere 2019, 237, 124548. [Google Scholar] [CrossRef]
- Banala, U.K.; Das, N.P.I.; Toleti, S.R. Microbial interactions with uranium: Towards an effective bioremediation approach. Environ. Technol. Innov. 2021, 21, 101254. [Google Scholar] [CrossRef]
- Lopez-Fernandez, M.; Jroundi, F.; Ruiz-Fresneda, M.A.; Merroun, M.L. Microbial interaction with and tolerance of radionuclides: Underlying mechanisms and biotechnological applications. Microb. Biotechnol. 2021, 14, 810–828. [Google Scholar] [CrossRef]
- Povedano-Priego, C.; Jroundi, F.; Lopez-Fernandez, M.; Sánchez-Castro, I.; Martin-Sánchez, I.; Huertas, F.J.; Merroun, M.L. Shifts in bentonite bacterial community and mineralogy in response to uranium and glycerol-2-phosphate exposure. Sci. Total Environ. 2019, 692, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cui, O.; Yu, Q.; Cui, O. Enrichment and remediation of uranium by microorganisms: A review. Open J. Environ. Biol. 2023, 8, 20–38. [Google Scholar] [CrossRef]
- Celik, F.; Camas, M.; Kyeremeh, K.; Sazak Camas, A. Microbial Sorption of Uranium Using Amycolatopsis sp. K47 Isolated from Uranium Deposits. Water Air Soil Pollut. 2018, 229, 112. [Google Scholar] [CrossRef]
- Lopez-Fernandez, M.; Vilchez-Vargas, R.; Jroundi, F.; Boon, N.; Pieper, D.; Merroun, M.L. Microbial community changes induced by uranyl nitrate in bentonite clay microcosms. Appl. Clay Sci. 2018, 160, 206–216. [Google Scholar] [CrossRef]
- Merroun, M.L.; Raff, J.; Rossberg, A.; Hennig, C.; Reich, T.; Selenska-Pobell, S. Complexation of Uranium by Cells and S-Layer Sheets of Bacillus sphaericus JG-A12. Appl. Environ. Microbiol. 2005, 71, 5532–5543. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, J.; Li, X.; Li, F.; Tu, H.; Sun, Q.; Liao, J.; Yang, J.; Yang, Y.; Liu, N. Biosorption and bioaccumulation behavior of uranium on Bacillus sp. dwc-2: Investigation by Box-Behenken design method. J. Mol. Liq. 2016, 221, 156–165. [Google Scholar] [CrossRef]
- Povedano-Priego, C.; Jroundi, F.; Lopez-Fernandez, M.; Morales-Hidalgo, M.; Martin-Sánchez, I.; Huertas, F.J.; Dopson, M.; Merroun, M.L. Impact of anoxic conditions, uranium(VI) and organic phosphate substrate on the biogeochemical potential of the indigenous bacterial community of bentonite. Appl. Clay Sci. 2022, 216, 106331. [Google Scholar] [CrossRef]
- Ben Ali Gam, Z.; Thioye, A.; Cayol, J.-L.; Joseph, M.; Fauque, G.; Labat, M. Characterization of Desulfovibrio salinus sp. nov., a slightly halophilic sulfate-reducing bacterium isolated from a saline lake in Tunisia. Int. J. Syst. Evol. Microbiol. 2018, 68, 715–720. [Google Scholar] [CrossRef]
- Zhou, C.; Vannela, R.; Hyun, S.P.; Hayes, K.F.; Rittmann, B.E. Growth of Desulfovibrio vulgaris When Respiring U(VI) and Characterization of Biogenic Uraninite. Environ. Sci. Technol. 2014, 48, 6928–6937. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Trivedi, D.; Atherton, N.; Lloyd, J.R. Microbial reduction of uraniumVI in sediments of different lithologies collected from Sellafield. Appl. Geochem. 2014, 51, 55–64. [Google Scholar] [CrossRef]
- Jroundi, F.; Descostes, M.; Povedano-Priego, C.; Sánchez-Castro, I.; Suvannagan, V.; Grizard, P.; Merroun, M.L. Profiling native aquifer bacteria in a uranium roll-front deposit and their role in biogeochemical cycle dynamics: Insights regarding in situ recovery mining. Sci. Total Environ. 2020, 721, 137758. [Google Scholar] [CrossRef] [PubMed]
- Povedano-Priego, C.; Jroundi, F.; Morales-Hidalgo, M.; Pinel-Cabello, M.; Peula-Ruiz, E.; Merroun, M.L.; Martin-Sánchez, I. Unveiling fungal diversity in uranium and glycerol-2-phosphate-amended bentonite microcosms: Implications for radionuclide immobilization within the Deep Geological Repository system. Sci. Total Environ. 2024, 908, 168284. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Goswami, L.; Lee, J.; Sonne, C.; Brown, R.J.C.; Kim, K.-H. Selenium in soil-microbe-plant systems: Sources, distribution, toxicity, tolerance, and detoxification. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2383–2420. [Google Scholar] [CrossRef]
- Wells, M.; McGarry, J.; Gaye, M.M.; Basu, P.; Oremland, R.S.; Stolz, J.F. Respiratory Selenite Reductase from Bacillus selenitireducens Strain MLS10. J. Bacteriol. 2019, 201, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-B.; Cheng, Y.-Y.; Wu, C.; Li, W.-W.; Li, N.; Yang, Z.-C.; Tong, Z.-H.; Yu, H.-Q. Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci. Rep. 2014, 4, 3735. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fresneda, M.A.; Martín, J.D.; Bolívar, J.G.; Cantos, M.V.F.; Bosch-Estévez, G.; Moreno, M.F.M.; Merroun, M.L. Green synthesis and biotransformation of amorphous Se nanospheres to trigonal 1D Se nanostructures: Impact on Se mobility within the concept of radioactive waste disposal. Environ. Sci. Nano J. 2018, 5, 2103–2116. [Google Scholar] [CrossRef]
- Hunter, W.J. Pseudomonas seleniipraecipitans Proteins Potentially Involved in Selenite Reduction. Curr. Microbiol. 2014, 69, 69–74. [Google Scholar] [CrossRef]
- Pinel-Cabello, M.; Chapon, V.; Ruiz-Fresneda, M.A.; Alpha-Bazin, B.; Berthomieu, C.; Armengaud, J.; Merroun, M.L. Delineation of cellular stages and identification of key proteins for reduction and biotransformation of Se(IV) by Stenotrophomonas bentonitica BII-R7. J. Hazard. Mater. 2021, 418, 126150. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Gomez-Bolivar, J.; Delgado-Martin, J.; Abad-Ortega, M.D.M.; Guerra-Tschuschke, I.; Merroun, M.L. The Bioreduction of Selenite under Anaerobic and Alkaline Conditions Analogous to Those Expected for a Deep Geological Repository System. Molecules 2019, 24, 3868. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Eswayah, A.S.; Romero-González, M.; Gardiner, P.H.E.; Solari, P.L.; Merroun, M.L. Chemical and structural characterization of SeIV biotransformations by Stenotrophomonas bentonitica into Se0 nanostructures and volatiles se species. Environ. Sci. Nano J. 2020, 7, 2140–2155. [Google Scholar] [CrossRef]
- King, F. Nuclear waste canister materials: Corrosion behavior and long-term performance in geological repository systems. In Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste; Woodhead Publishing: Sawston, UK, 2017; pp. 365–408. [Google Scholar] [CrossRef]
- Hall, D.S.; Keech, P.G. An overview of the Canadian corrosion program for the long-term management of nuclear waste. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. S1), 2–5. [Google Scholar] [CrossRef]
- King, F.; Hall, D.S.; Keech, P.G. Nature of the near-field environment in a deep geological repository and the implications for the corrosion behaviour of the container. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. S1), 25–30. [Google Scholar] [CrossRef]
- Hultquist, G. Hydrogen evolution in corrosion of copper in pure water. Corros. Sci. 1986, 26, 173–177. [Google Scholar] [CrossRef]
- Little, B.J.; Hinks, J.; Blackwood, D.J. Microbially influenced corrosion: Towards an interdisciplinary perspective on mechanisms. Int. Biodeterior. Biodegrad. 2020, 154, 105062. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Stackebrandt, E.; Hamilton, W.A. Energy metabolism and phylogenetic diversity of sulphate-reducing bacteria. In Sulphate-Reducing Bacteria; Cambridge University Press: Cambridge, UK, 2007; pp. 1–38. [Google Scholar]
- Huttunen-Saarivirta, E.; Rajala, P.; Carpén, L. Corrosion behaviour of copper under biotic and abiotic conditions in anoxic groundwater: Electrochemical study. Electrochim. Acta 2016, 203, 350–365. [Google Scholar] [CrossRef]
- Marshall, M.H.; Simpson, M.J. State of Science Review: Natural Organic Matter in Clays and Groundwater; NWMO TR-2014-05; Nuclear Waste Management Organization: Toronto, ON, Canada, 2014. [Google Scholar]
- King, F.; Kolář, M. Lifetime predictions for nuclear waste disposal containers. Corrosion 2019, 75, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.; Chen, J.; Guo, M.; Ramamurthy, S.; Shoesmith, D.W.; Noël, J.J. Comments on E. Huttunen-Saarivirta et al., “Kinetic Properties of the Passive Film on Copper in the Presence of Sulfate-Reducing Bacteria” [J. Electrochem. Soc., 165, C450 (2018)]. J. Electrochem. Soc. 2019, 166, Y13. [Google Scholar] [CrossRef]
- SKB. Supplementary Information on Canister Integrity Issues; Technical Report SKB TR-19-15; SKB: Stockholm, Sweden, 2019; 135p. [Google Scholar]
- Qin, Z.; Daljeet, R.; Ai, M.; Farhangi, N.; Noël, J.J.; Ramamurthy, S.; Shoesmith, D.; King, F.; Keech, P. The active/passive conditions for copper corrosion under nuclear waste repository environment. Corros. Eng. Sci. Technol. 2017, 52 (Suppl. S1), 45–49. [Google Scholar] [CrossRef]
- Smith, J.M.; Wren, J.C.; Odziemkowski, M.; Shoesmith, D.W. The electrochemical response of preoxidized copper in aqueous sulfide solutions. J. Electrochem. Soc. 2007, 154, C431. [Google Scholar] [CrossRef]
- West, J.M.; McKinley, I.G.; Stroes-Gascoyne, S. Microbial effects on waste repository materials. Radioact. Environ. 2002, 2, 255–277. [Google Scholar]
- Giroud, N.; Tomonaga, Y.; Wersin, P.; Briggs, S.; King, F.; Vogt, T.; Diomidis, N. On the fate of oxygen in a spent fuel emplacement drift in Opalinus Clay. Appl. Geochem. 2018, 97, 270–278. [Google Scholar] [CrossRef]
- Wersin, P.; Johnson, L.H.; McKinley, I.G. Performance of the bentonite barrier at temperatures beyond 100 °C: A critical review. Phys. Chem. Earth Parts A/B/C 2007, 32, 780–788. [Google Scholar] [CrossRef]
- Grandia, F.; Domènech, C.; Arcos, D.; Duro, L. Assessment of the Oxygen Consumption in the Backfill. Geochemical Modelling in a Saturated Backfill (No. SKB-R--06-106); Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2006. [Google Scholar]
- Crusset, D.; Deydier, V.; Necib, S.; Gras, J.M.; Combrade, P.; Feron, D.; Burger, E. Corrosion of carbon steel components in the French high-level waste program: Evolution of disposal concept and selection of materials. Corros. Eng. Sci. Technol. 2017, 52, 17–24. [Google Scholar] [CrossRef]
- Lapuerta, S.; Bérerd, N.; Moncoffre, N.; Millard-Pinard, N.; Jaffrézic, H.; Crusset, D.; Féron, D. The Influence of Relative Humidity on Iron Corrosion under Proton Irradiation. J. Nucl. Mater. 2008, 375, 80–85. [Google Scholar] [CrossRef]
- Wendling, J.; Justinavicius, D.; Sentis, M.; Amaziane, B.; Bond, A.; Calder, N.J.; Treille, E. Gas transport modelling at different spatial scales of a geological repository in clay host rock. Environ. Earth Sci. 2019, 78, 221. [Google Scholar] [CrossRef]
- Liu, J.F.; Wu, Y.; Cai, C.Z.; Ni, H.Y.; Cao, X.L.; Pu, H.; Song, S.-B.; Pu, S.-Y.; Skoczylas, F. Investigation into water retention and gas permeability of Opalinus clay. Environ. Earth Sci. 2018, 77, 213. [Google Scholar] [CrossRef]
- Croisé, J.; Mayer, G.; Talandier, J.; Wendling, J. Impact of water consumption and saturation-dependent corrosion rate on hydrogen generation and migration from an intermediate-level radioactive waste repository. Transp. Porous Media 2011, 90, 59–75. [Google Scholar] [CrossRef]
- Perko, J.; Weetjens, E. Thermohydraulic analysis of gas generation in a disposal facility for vitrified high-level radioactive waste in boom clay. Nucl. Technol. 2011, 174, 401–410. [Google Scholar] [CrossRef]
- Enssle, C.P.; Brommundt, J.; Kaempfer, T.U.; Mayer, G.; Wendling, J. Full-scale 3D modelling of a nuclear waste repository in the Callovo-Oxfordian clay. Part 2: Thermo-hydraulic two-phase transport of water, hydrogen, 14C and 129I. In Clays in Natural and Engineered Barriers for Radioactive Waste Confinement, Special Publications; Norris, S., Bruno, J., Cathelineau, M., Delage, P., Fairhurst, C., Gaucher, E.C., Höhn, E.H., Kalinichev, A., Lalieux, P., Sellin, P., Eds.; Geological Society: London, UK, 2014; Volume 400, pp. 469–481. [Google Scholar]
- O’Brien, K.E.; Rainham, D.; O’Beirne-Ryan, A.M. Using field analogue soil column experiments to quantify radon-222 gas migration and transport through soils and bedrock of Halifax, Nova Scotia, Canada. Environ. Earth Sci. 2014, 72, 2607–2620. [Google Scholar] [CrossRef]
- Libert, M.; Bildstein, O.; Esnault, L.; Jullien, M.; Sellier, R. Molecular hydrogen: An abundant energy source for bacterial activity in nuclear waste repositories. Phys. Chem. Earth Parts A/B/C 2011, 36, 1616–1623. [Google Scholar] [CrossRef]
- Vikman, M.; Marjamaa, K.; Nykyri, M.; Small, J.S.; Miettinen, H.; Heikinheimo, L.; Haavisto, T.; Itävaara, M. The biogeochemistry of gas generation from low-level nuclear waste: Microbiological characterization during 18 years study under in situ conditions. Appl. Geochem. 2019, 105, 55–67. [Google Scholar] [CrossRef]
- Peitzsch, M.; Kremer, D.; Kersten, M. Microfungal alkylation and volatilization of selenium adsorbed by goethite. Environ. Sci. Technol. 2010, 44, 129–135. [Google Scholar] [CrossRef]
- Poller, A.; Mayer, G.; Darcis, M.; Smith, P. Modelling of Gas Generation in Deep Geological Repositories after Closure (No. NTB--16-04); National Cooperative for the Disposal of Radioactive Waste (NAGRA): Wettingen, Switzerland, 2016. [Google Scholar]
- Lopez-Fernandez, M.; Broman, E.; Simone, D.; Bertilsson, S.; Dopson, M. Statistical analysis of community RNA transcripts between organic carbon and geogas-fed continental deep biosphere groundwaters. MBio 2019, 10, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Rajala, P.; Bomberg, M. Geomicrobes: Life in terrestrial deep subsurface, volume II. Front. Microbiol. 2023, 14, 1169127. [Google Scholar] [CrossRef] [PubMed]
- Mehrshad, M.; Lopez-Fernandez, M.; Sundh, J.; Bell, E.; Simone, D.; Buck, M.; Bernier-Latmani, R.; Bertilsson, E.; Dopson, M. Energy efficiency and biological interactions define the core microbiome of deep oligotrophic groundwater. Nat. Commun. 2021, 12, 4253. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.C.; Kieft, T.L.; Kuloyo, O.; Linage-Alvarez, B.; Van Heerden, E.; Lindsay, M.R.; Guo, L.; Perlman, D.H.; Kyin, S.; Shwe, H.H.; et al. An oligotrophic deep-subsurface community dependent on syntrophy is dominated by sulfur-driven autotrophic denitrifiers. Proc. Natl. Acad. Sci. USA 2016, 113, E7927–E7936. [Google Scholar] [CrossRef] [PubMed]
- Nazina, T.N.; Luk’yanova, E.A.; Zakharova, E.V.; Ivoilov, V.S.; Poltaraus, A.B.; Kalmykov, S.N.; Belyaev, S.S.; Zubkov, A.A. Distribution and activity of microorganisms in the deep repository for liquid radioactive waste at the Siberian chemical combine. Microbiology 2006, 75, 727–738. [Google Scholar] [CrossRef]
- Kale, R.C.; Ravi, K. A review on the impact of thermal history on compacted bentonite in the context of nuclear waste management. Environ. Technol. Innov. 2021, 23, 101728. [Google Scholar] [CrossRef]
- Tan, Ö.; Yılmaz, L.; Zaimoğlu, A.S. Variation of some engineering properties of clays with heat treatment. Mater. Lett. 2004, 58, 1176–1179. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, W.; Qian, H. Effects of high temperature thermal treatment on the physical properties of clay. Environ. Earth Sci. 2016, 75, 610. [Google Scholar] [CrossRef]
- Lingnau, B.E.; Graham, J.; Yarechewski, D.; Tanaka, N.; Gray, M.N. Effects of temperature on strength and compressibility of sand-bentonite buffer. Eng. Geol. 1996, 41, 103–115. [Google Scholar] [CrossRef]
- Estabragh, A.R.; Khosravi, F.; Javadi, A.A. Effect of thermal history on the properties of bentonite. Environ. Earth Sci. 2016, 75, 657. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. Stability of bentonites in salt solutions: II. Potassium chloride solution—Initial step of illitization. Appl. Clay Sci. 2010, 49, 98–107. [Google Scholar] [CrossRef]
- Ohazuruike, L.; Lee, K.J. A comprehensive review on clay swelling and illitization of smectite in natural subsurface formations and engineered barrier systems. Nucl. Eng. Technol. 2023, 55, 1495–1506. [Google Scholar] [CrossRef]
- Payer, J.H.; Finsterle, S.; Apps, J.A.; Muller, R.A. Corrosion performance of engineered barrier system in deep horizontal drillholes. Energies 2019, 12, 1491. [Google Scholar] [CrossRef]
- Stoulil, J.; Pavlova, L.; Kouřil, M. Localised corrosion of stainless steels 316L and 2205 in synthetic bentonite pore water and bentonite slurry. Acta Metall. Slovaca 2019, 25, 24–32. [Google Scholar] [CrossRef]
- Schlegel, M.L.; Necib, S.; Daumas, S.; Labat, M.; Blanc, C.; Foy, E.; Linard, Y. Corrosion at the carbon steel-clay borehole water interface under anoxic alkaline and fluctuating temperature conditions. Corros. Sci. 2018, 136, 70–90. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Martinez-Moreno, M.F.; Povedano-Priego, C.; Morales-Hidalgo, M.; Jroundi, F.; Merroun, M.L. Impact of microbial processes on the safety of deep geological repositories for radioactive waste. Front. Microbiol. 2023, 14, 1134078. [Google Scholar] [CrossRef]
- Bartak, D.; Bedrníková, E.; Kašpar, V.; Říha, J.; Hlaváčková, V.; Večerník, P.; Šachlová, K.; Černá, K. Survivability and proliferation of microorganisms in bentonite with implication to radioactive waste geological disposal: Strong effect of temperature and negligible effect of pressure. World J. Microbiol. Biotechnol. 2024, 40, 41. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, A.A.; Jalique, D.R.; Medihala, P.; Stroes-Gascoyne, S.; Wolfaardt, G.M.; McKelvie, J.; Korber, D.R. Bacterial diversity and production of sulfide in microcosms containing uncompacted bentonites. Heliyon 2018, 4, e00722. [Google Scholar] [CrossRef] [PubMed]
- Masurat, P.; Eriksson, S.; Pedersen, K. Evidence of indigenous sulphate-reducing bacteria in commercial Wyoming bentonite MX-80. Appl. Clay Sci. 2010, 47, 51–57. [Google Scholar] [CrossRef]
- Laskowska, E.; Kuczyńska-Wiśnik, D. New insight into the mechanisms protecting bacteria during desiccation. Curr. Genet. 2020, 66, 313–318. [Google Scholar] [CrossRef] [PubMed]
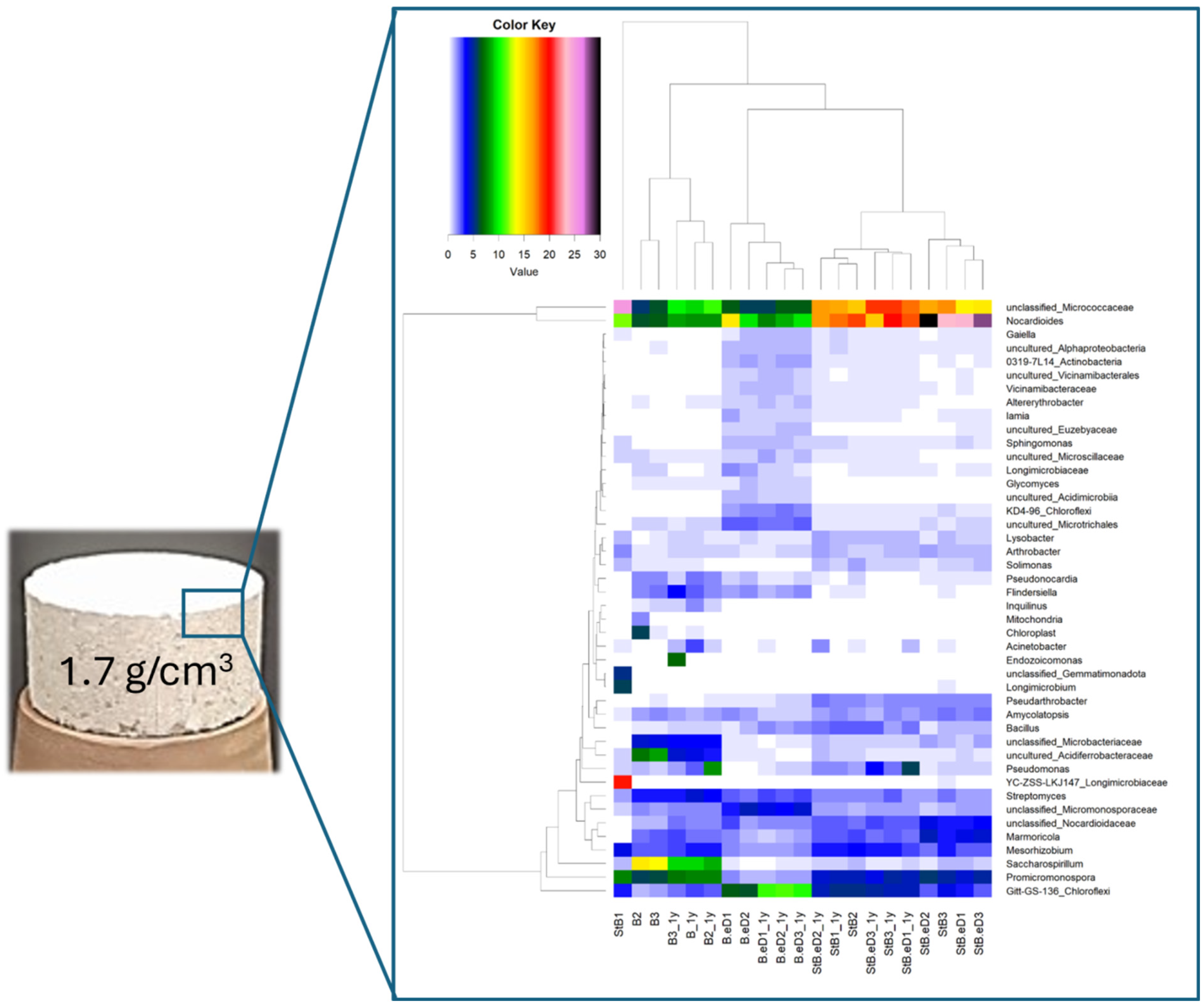
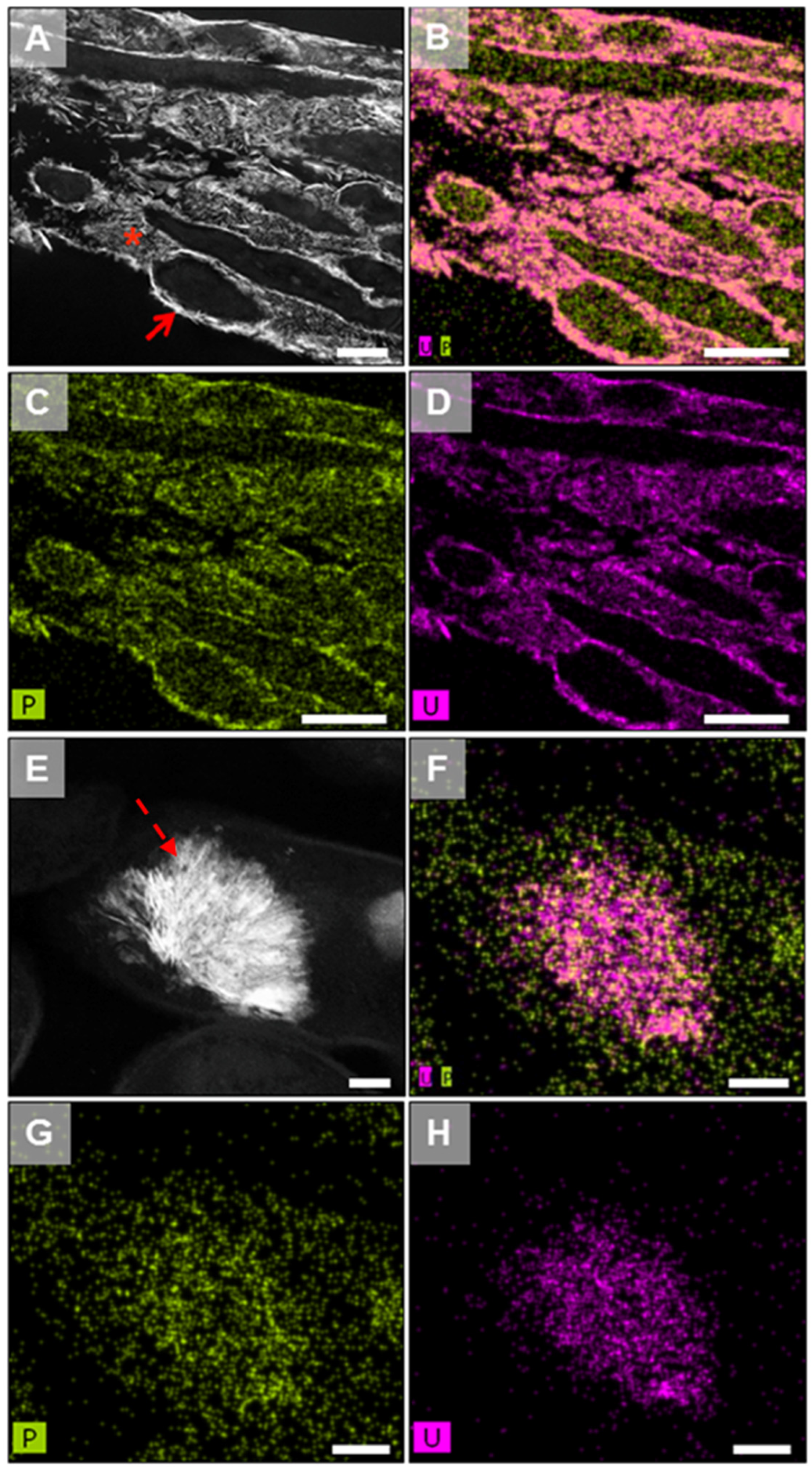
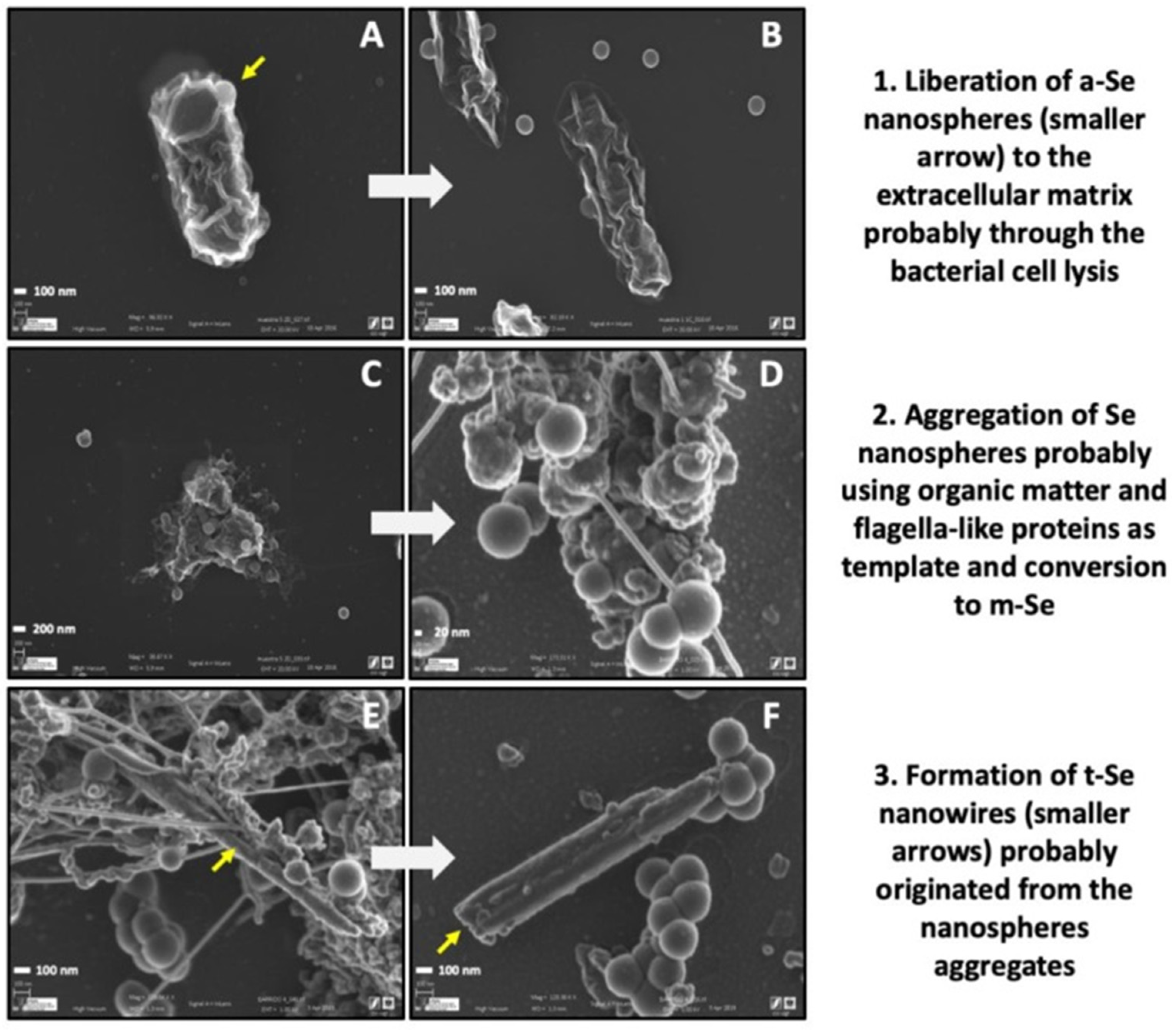
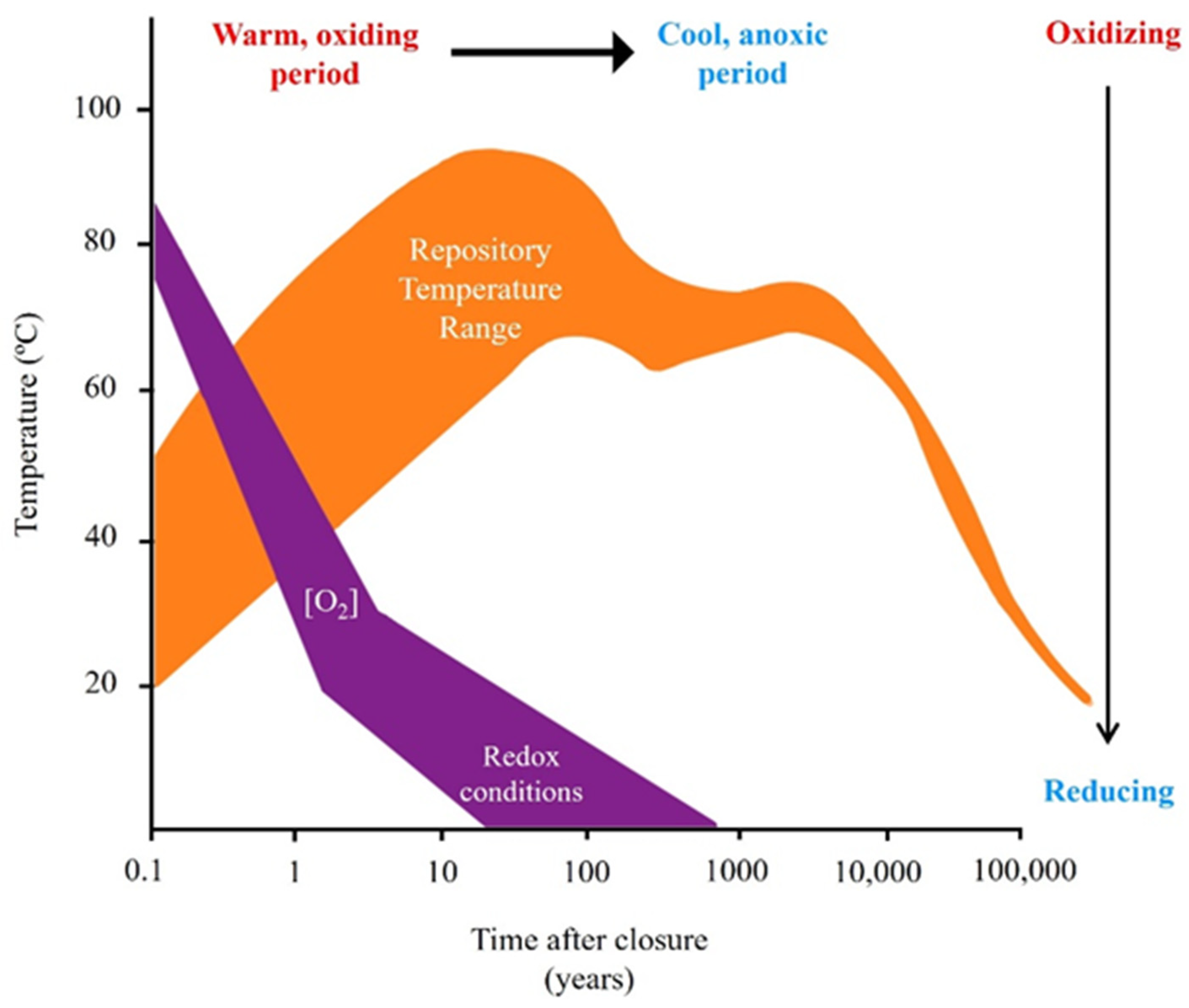
| Country | Company | Canister | Buffer | Buffer Density (g/cm3) | Host Rock | Absorbed Dose at the Surface (Gy/h) | Temperature at the Surface (°C) | References |
|---|---|---|---|---|---|---|---|---|
| Spain | ENRESA | Carbon steel | Bentonite | 1.65 | Clay/Granite | Not determined | <100 | [7,8] |
| Finland | POSIVA | Copper + cast iron | Bentonite | 1.55 | Crystalline | 0.33 | ~90 | [9,10,11] |
| Sweden | SKB | Copper + cast iron | Bentonite | 1.6 | Crystalline | 0.2 | ~90 | [10,12] |
| Switzerland | NAGRA | Carbon steel | Bentonite | >1.45 | Opalinus clay | <0.035 | <150 | [10,13,14] |
| France | ANDRA | Carbon steel | none | - | Granite | <10 | ~90 | [10,12] |
| Czech Republic | SÚRAO | Carbon steel | Bentonite | 1.4 | Crystalline | 0.3 | <95 | [12,15,16] |
| Belgium | ONDRAF-NIRAS | Carbon steel | Cement/c | - | Boom clay | 25 | ~95 | [10,12] |
| Canada | NWMO | Carbon steel coated with copper | Bentonite | 1.6 | Crystalline/sedimentary | 2 | <100 | [17,18,19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Hidalgo, M.; Povedano-Priego, C.; Martinez-Moreno, M.F.; Ruiz-Fresneda, M.A.; Lopez-Fernandez, M.; Jroundi, F.; Merroun, M.L. Insights into the Impact of Physicochemical and Microbiological Parameters on the Safety Performance of Deep Geological Repositories. Microorganisms 2024, 12, 1025. https://doi.org/10.3390/microorganisms12051025
Morales-Hidalgo M, Povedano-Priego C, Martinez-Moreno MF, Ruiz-Fresneda MA, Lopez-Fernandez M, Jroundi F, Merroun ML. Insights into the Impact of Physicochemical and Microbiological Parameters on the Safety Performance of Deep Geological Repositories. Microorganisms. 2024; 12(5):1025. https://doi.org/10.3390/microorganisms12051025
Chicago/Turabian StyleMorales-Hidalgo, Mar, Cristina Povedano-Priego, Marcos F. Martinez-Moreno, Miguel A. Ruiz-Fresneda, Margarita Lopez-Fernandez, Fadwa Jroundi, and Mohamed L. Merroun. 2024. "Insights into the Impact of Physicochemical and Microbiological Parameters on the Safety Performance of Deep Geological Repositories" Microorganisms 12, no. 5: 1025. https://doi.org/10.3390/microorganisms12051025
APA StyleMorales-Hidalgo, M., Povedano-Priego, C., Martinez-Moreno, M. F., Ruiz-Fresneda, M. A., Lopez-Fernandez, M., Jroundi, F., & Merroun, M. L. (2024). Insights into the Impact of Physicochemical and Microbiological Parameters on the Safety Performance of Deep Geological Repositories. Microorganisms, 12(5), 1025. https://doi.org/10.3390/microorganisms12051025







