Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors—A Review
Abstract
:1. Introduction
2. Trypanosoma cruzi
3. Vectors
3.1. Development, Attraction and Blood Ingestion
3.2. The Intestine of Triatomines, the Excretory System and the Fate of Blood
3.3. Immune System of Triatomines
4. The Microbiota of Triatomines
4.1. Infection Routes
4.2. Microbiota of Triatomines
4.3. Identification of Symbionts
4.4. Functions of Symbionts/Microbiota
4.5. Development of Symbionts/Bacteria in Triatomines
4.6. Intestinal Bacteriolysis
5. Interactions of Triatomines with Trypanosoma cruzi
5.1. Effects of the Vector on Trypanosoma cruzi—Development of the Parasite in the Vector
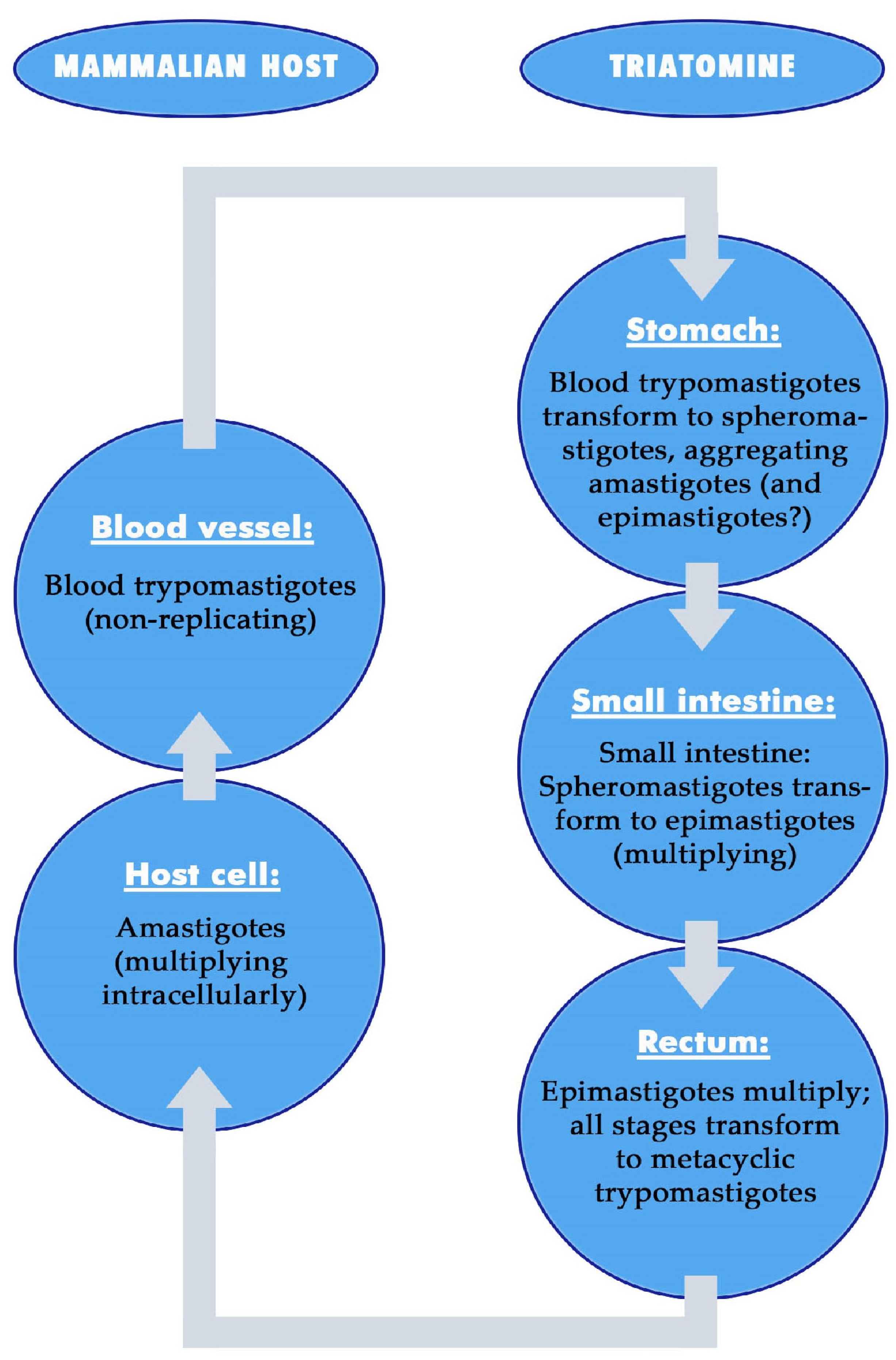
5.1.1. Development of Trypanosoma cruzi in the Stomach
5.1.2. Development of Trypanosoma cruzi in the Small Intestine
5.1.3. Development of Trypanosoma cruzi in the Rectum
5.2. Effects of Trypanosoma cruzi on Triatomines
5.2.1. Effects of Trypanosoma cruzi on Nymphs and Adults of Triatomines
5.2.2. Effects of Trypanosoma cruzi on the Behavior of Triatomines
5.2.3. Effects of Trypanosoma cruzi on Digestion and Immunity
6. Interaction of Trypanosoma cruzi and the Microbiota of Triatomines
6.1. Effects of the Microbiota on Trypanosoma cruzi and Competition of Both
6.2. Indirect Effects of Trypanosoma cruzi via Inducing Vector Immunity
6.3. Interactions of Trypanosoma cruzi with Wolbachia sp. and Symbionts
7. Suggestions for Future Research
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Chagas, C. Nova trypanozomiaze humana. Über eine neue Trypanosomiasis des Menschen. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218, plus 13 plates. [Google Scholar] [CrossRef]
- WHO. Chagas Disease (American Trypanosomiasis). Available online: http://www.who.int/health-topics/chagas-disease (accessed on 5 February 2024).
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions—A comprehensive review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Distribution of Cases of Chagas Disease, Based on Official Estimates, 2018. 2023. Available online: https://cdn.who.int/media/docs/default-source/ntds/chagas-disease/chagas-2018-cases.pdf?sfvrsn=f4e94b3b_2 (accessed on 5 February 2024).
- Jansen, A.M.; Roque, A.L.R.; Xavier, S.C.C. Trypanosoma cruzi enzootic cycle: General aspects, domestic and synanthropic hosts and reservoirs. In American Trypanosomiasis Chagas Disease, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–282. [Google Scholar] [CrossRef]
- Schaub, G.A. Kissing bugs. In Encyclopedia of Parasitology, 4th ed.; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1400–1403. [Google Scholar]
- PAHO. Chagas’disease. Epidemiol. Bull. PAHO 1982, 3, 1–5. [Google Scholar]
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- PAHO. World Chagas Disease Day 2021. Available online: http://www.paho.org/en/news/13-4-2021-70-people-chagas-dont-know-theyre-infected (accessed on 5 February 2024).
- da Rocha Siriano, L.; Marchiol, A.; Pereira Certo, M.; Cubides, J.C.; Forsyth, C.; Augusto de Sousa, F. Mandatory notification of chronic Chagas disease: Confronting the epidemiological silence in the State of Goiás, Brazil. Trop. Med. Infect. Dis. 2020, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Abasolo, R.; Gutiérrez-Cabrera, A.E.; Cruz-López, L.; Alavez-Rosas, D.; Benelli, G.; Córdoba-Aguilar, A. Chagas disease vector control strategies: Where we are and where should we go from here. Entomol. Gen. 2023, 43, 771–788. [Google Scholar] [CrossRef]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas disease in the United States: A public health approach. Clin. Microbiol. Rev. 2020, 33, e00023-19. [Google Scholar] [CrossRef]
- Schuster, J.P.; Schaub, G.A. Trypanosoma cruzi: Skin-penetration kinetics of vector-derived metacyclic trypomastigotes. Int. J. Parasitol. 2000, 30, 1475–1479. [Google Scholar] [CrossRef]
- Lumbreras, H.; Flores, W.; Escallón, A. Allergische Reaktionen auf Stiche von Reduviiden und ihre Bedeutung bei der Chagaskrankheit. Z. Trop. Med. 1959, 10, 6–19. (In German) [Google Scholar]
- Paddock, C.D.; McKerrow, J.H.; Hansell, E.; Foreman, K.W.; Hsieh, I.; Marshall, N. Identification, cloning, and recombinant expression of procalin, a major triatomine allergen. J. Immunol. 2001, 167, 2694–2699. [Google Scholar] [CrossRef]
- Waldeck, B.; Schaub, G.A. “Natural infections” with Trypanosoma cruzi via the skin of mice: Size of mouthparts of vectors and numbers of invading parasites. Parasitol. Res. 2022, 121, 2033–2041. [Google Scholar] [CrossRef]
- Steverding, D. The history of Chagas disease. Parasites Vectors 2014, 7, 317. [Google Scholar] [CrossRef]
- Meiser, C.K.; Schaub, G.A. Xenodiagnosis. In Parasitology Research Monographs. Nature Helps… How Plants and Other Organisms Contribute to Solve Health Problems; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1, pp. 273–299. [Google Scholar]
- Lana, M.; Chiari, C.A. Caracterização biológica comparativa das cepas Berenice e Berenice-78 de Trypanosoma cruzi isoladas da mesma paciente em diferentes períodos. Mem. Inst. Oswaldo Cruz 1986, 81, 247–253. (In Brazilian) [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.E.; Macedo, A.M.; Barnabé, C.; Freitas, J.M.; Chiari, E.; Veloso, V.M.; Carneiro, C.M.; Bahia, M.T.; Tafuri, W.L.; Lana, M. Further genetic characterization of the two Trypanosoma cruzi Berenice strains (Be-62 and Be-78) isolated from the first human case of Chagas disease (Chagas 1909). Acta Trop. 2006, 97, 239–246. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Schaub, G.A. Chagas´ disease, man. In Encyclopedia of Parasitology, 4th ed.; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 479–483. [Google Scholar]
- Bock, M.; Haberkorn, A.; Herlinger, H.; Mayer, K.H.; Petersen, S. The structure-activity relationship of 4-(5′-nitrofurfurylidene-amino)-tetrahydro-4H-1,4-thiazine-1,1-dioxides active against Trypanosoma cruzi. Arzneimittelforschung 1972, 22, 1564–1569. [Google Scholar]
- Gönnert, R. Nifurtimox: Causal treatment of Chagas’ disease. Arzneimittelforschung 1972, 22, 1563. [Google Scholar] [PubMed]
- Andrade, S.G.; Figueira, R.M. Estudo experimental sobre a ação terapêutica da droga Ro 7-1051 na infecção por diferentes cepas do Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 1977, 19, 335–341. (In Brazilian) [Google Scholar]
- Neal, R.A.; van Bueren, J. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 709–714. [Google Scholar] [CrossRef]
- Coura, J.R.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug. Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón-Figueira, J.C.; Martinez-Peinado, N.; Escabia, E.; Ros-Lucas, A.; Chatelain, E.; Scandale, I.; Gascon, J.; Pinazo, M.J.; Alonso-Padilla, J. State-of-the-art in the drug discovery pathway for Chagas disease: A framework for drug development and target validation. Res. Rep. Trop. Med. 2023, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- De Fuentes-Vicente, J.A.; Santos-Hernández, N.G.; Ruiz-Castillejos, C.; Espinoza-Medinilla, E.E.; Flores-Villegas, A.L.; de Alba-Alvarado, M.; Cabrera-Bravo, M.; Moreno-Rodríguez, A.; Vidal-López, D.G. What do you need to know before studying Chagas disease? A beginner’s guide. Trop. Med. Infect. Dis. 2023, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gaussmann, S.; Tippler, B.; Ott, J.; Popowicz, G.M.; Schliebs, W.; Sattler, M.; Erdmann, R.; Kalel, V.C. Novel trypanocidal inhibitors that block glycosome biogenesis by targeting PEX3-PEX19 interaction. Front. Cell. Dev. Biol. 2021, 9, 737159. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.S.; Gould, M.K.; Noeske, J.; Saldivia, M.; Jumani, R.S.; Ng, P.S.; René, O.; Chen, Y.-L.; Kaiser, M.; Ritchie, R.; et al. Cyanotriazoles are selective topoisomerase II poisons that rapidly cure trypanosome infections. Science 2023, 380, 1349–1356. [Google Scholar] [CrossRef]
- Schaub, G.A.; Lösch, P. Trypanosoma cruzi: Origin of metacyclic trypomastigotes in the urine of the vector Triatoma infestans. Exp. Parasitol. 1988, 65, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Teixeira, M.M.G.; Stevens, J.R. The evolution of Trypanosoma cruzi: The ‘bat seeding’ hypothesis. Trends Parasitol. 2012, 28, 136–141. [Google Scholar] [CrossRef]
- Messenger, L.A.; Miles, M.A. Evidence and importance of genetic exchange among field populations of Trypanosoma cruzi. Acta Trop. 2015, 151, 150–155. [Google Scholar] [CrossRef]
- Tomasini, N.; Diosque, P. Evolution of Trypanosoma cruzi: Clarifying hybridisations, mitochondrial introgressions and phylogenetic relationships between major lineages. Mem. Inst. Oswaldo Cruz 2015, 110, 403–413. [Google Scholar] [CrossRef]
- Schwabl, P.; Imamura, H.; Van den Broeck, F.; Costales, J.A.; Maiguashca-Sánchez, J.; Miles, M.A.; Andersson, B.; Grijalva, M.J.; Llewellyn, M.S. Meiotic sex in Chagas disease parasite Trypanosoma cruzi. Nat. Commun. 2019, 10, 3972. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef]
- Zingales, B.; Bartholomeu, D.C. Trypanosoma cruzi genetic diversity: Impact on transmission cycles and Chagas disease. Mem. Inst. Oswaldo Cruz 2022, 117, e210193. [Google Scholar] [CrossRef]
- Schaub, G.A.; Meiser, C.K.; Balczun, C. Interactions of Trypanosoma cruzi and triatomines. In Parasitology Research Monographs. Progress in Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 155–178. [Google Scholar]
- De Fuentes-Vicente, J.A.; Vidal-López, D.G.; Flores-Villegas, A.L.; Moreno-Rodríguez, A.; De Alba-Alvarado, M.C.; Salazar-Schettino, P.M.; Rodríguez-López, M.H.; Gutiérrez-Cabrera, A.E. Trypanosoma cruzi: A review of biological and methodological factors in Mexican strains. Acta Trop. 2019, 195, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Galvão, R.; Carcavallo, D.; da Silva Rocha, D.; Jurberg, J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa 2003, 202, 1–36. [Google Scholar] [CrossRef]
- Hashimoto, K.; Schofield, C.J. Elimination of Rhodnius prolixus in Central America. Parasites Vectors 2012, 5, 45. [Google Scholar] [CrossRef]
- Serrano, A.A.; Schenkman, S.; Yoshida, N.; Mehlert, A.; Richardson, J.M.; Ferguson, M.A. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J. Biol. Chem. 1995, 270, 27244–27253. [Google Scholar] [CrossRef]
- Schaub, G.A.; Vogel, P.; Balczun, C. Parasite-vector interactions. In Molecular Parasitology—Protozoan Parasites and Their Molecules; Walochnik, J., Duchêne, M., Eds.; Springer: Vienna, Austria, 2016; pp. 431–489. [Google Scholar]
- Salazar, R.; Castillo-Neyra, R.; Tustin, A.W.; Borrini-Mayorí, K.; Náquira, C.; Levy, M.Z. Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2015, 92, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Blakely, B.N.; Hanson, S.F.; Romero, A. Survival and transstadial persistence of Trypanosoma cruzi in the bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2018, 55, 742–746. [Google Scholar] [CrossRef]
- Galvão, C. Taxonomy. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 15–38. [Google Scholar]
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 123–520. [Google Scholar]
- De Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E. Kissing bugs (Triatominae). In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 953–970. [Google Scholar] [CrossRef]
- Schaub, G.A. An update on the knowledge of parasite-vector interactions of Chagas disease. Res. Rep. Trop. Med. 2021, 12, 63–76. [Google Scholar] [CrossRef]
- Schaub, G.A.; Mehlhorn, H. Insects. In Encyclopedia of Parasitology, 4th ed.; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1348–1357. [Google Scholar]
- Noireau, F.; Dujardin, J.P. Flight and nutritional status of sylvatic Triatoma sordida and Triatoma guasayana. Mem. Inst. Oswaldo Cruz 2001, 96, 385–389. [Google Scholar] [CrossRef]
- Sarquis, O.; Carvalho-Costa, F.A.; Oliveira, L.S.; Duarte, R.; D′Andrea, P.S.; de Oliveira, T.G.; Lima, M.M. Ecology of Triatoma brasiliensis in northeastern Brazil: Seasonal distribution, feeding resources, and Trypanosoma cruzi infection in a sylvatic population. J. Vector Ecol. 2010, 35, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.A.; Guarneri, A.A.; Lorenzo, M.G. Activity and shelter-related behavior in Rhodnius prolixus: The role of host odours. Acta Trop. 2019, 196, 150–154. [Google Scholar] [CrossRef]
- Schaub, G.A.; Lawrenz, A.; Stadler, A. “Living syringes”: Use of triatomines as blood samplers from small and wild animals. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2012, 18, 349–352. [Google Scholar]
- Lazzari, C.R. The behaviour of kissing bugs. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 215–238. [Google Scholar]
- Murillo-Solano, C.; López-Domínguez, J.; Gongora, R.; Rojas-Gulloso, A.; Usme-Ciro, J.; Perdomo-Balaguera, E.; Herrera, C.; Parra-Henao, G.; Dumonteil, E. Diversity and interactions among triatomine bugs, their blood feeding sources, gut microbiota and Trypanosoma cruzi in the Sierra Nevada de Santa Marta in Colombia. Sci. Rep. 2021, 11, 12306. [Google Scholar] [CrossRef] [PubMed]
- Wenk, P.; Lucic, S.; Betz, O. Functional anatomy of the hypopharynx and the salivary pump in the feeding apparatus of the assassin bug Rhodnius prolixus (Reduviidae, Heteroptera). Zoomorphology 2010, 129, 225–234. [Google Scholar] [CrossRef]
- Pontes, G.B.; Minoli, S.; Insaurralde, I.O.; de Brito Sánchez, M.G.; Barrozo, R. Bitter stimuli modulate the feeding decision of a blood-sucking insect via two sensory inputs. J. Exp. Biol. 2014, 217, 3708–3717. [Google Scholar] [CrossRef]
- Sant’Anna, M.R.V.; Soares, A.C.; Araújo, R.N.; Gontijo, N.F.; Pereira, M.H. Triatomines (Hemiptera, Reduviidae) blood intake: Physical constraints and biological adaptations. J. Insect Physiol. 2017, 97, 20–26. [Google Scholar] [CrossRef]
- Paim, R.M.M.; Nascimento, B.W.L.; Nascimento, A.M.D.; Pacheco, D.E.; Soares, A.C.; Araujo, R.N.; Sant’Anna, M.R.V.; Pessoa, G.C.D.; Gontijo, N.F.; Pereira, M.H. Functional aspects of salivary nitric oxide synthase of Rhodnius prolixus (Hemiptera, Reduviidae) and nitric oxide trafficking at the vector-host interface. Sci. Rep. 2017, 7, 16036. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Assumpção, T.C.; Francischetti, I.M. An insight into the sialomes of bloodsucking Heteroptera. Psyche 2012, 2012, 470436. [Google Scholar] [CrossRef]
- Montandon, C.E.; Barros, E.; Vidigal, P.M.; Mendes, M.T.; Anhê, A.C.; de Oliveira Ramos, H.J.; de Oliveira, C.J.; Mafra, C. Comparative proteomic analysis of the saliva of the Rhodnius prolixus, Triatoma lecticularia and Panstrongylus herreri triatomines reveals a high interespecific functional biodiversity. Insect Biochem. Mol. Biol. 2016, 71, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Santiago, P.B.; de Araújo, C.N.; Charneau, S.; Bastos, I.M.D.; Assumpção, T.C.F.; Queiroz, R.M.L.; Praça, Y.R.; Cordeiro, T.M.; Garcia, C.H.S.; da Silva, I.G.; et al. Exploring the molecular complexity of Triatoma dimidiata sialome. J. Proteom. 2018, 174, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, A.A.; Pereira, M.H.; Diotaiuti, L. Influence of the blood meal source on the development of Triatoma infestans, Triatoma brasiliensis, Triatoma sordida and Triatoma pseudomaculata (Heteroptera, Reduviidae). J. Med. Entomol. 2000, 37, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.L.; Genta, F.A. Blood digestion in triatomine insects. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 265–284. [Google Scholar]
- Wigglesworth, V.B. The Principles of Insect Physiology; Methuen: London, UK, 1941. [Google Scholar]
- Schaub, G.A. Trypanosoma cruzi: Quantitative studies of development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp. Parasitol. 1989, 68, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Kollien, A.H.; Schaub, G.A. The development of Trypanosoma cruzi in Triatominae. Parasitol. Today 2000, 16, 381–387. [Google Scholar] [CrossRef]
- Maddrell, S.H.P. The fastest fluid-secreting cell known: The upper Malpighian tubule cell of Rhodnius. BioEssays 1991, 13, 357–362. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The physiology of excretion in a blood-sucking insect, Rhodnius prolixus (Hemiptera, Reduviidae) II. Anatomy and histology of the excretory system. J. Exp. Biol. 1931, 8, 428–441. [Google Scholar] [CrossRef]
- Böker, C.A.; Schaub, G.A. Scanning electron microscopic studies of Trypanosoma cruzi in the rectum of its vector Triatoma infestans. Z. Parasitenkd. 1984, 70, 459–469. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The physiology of excretion in a blood-sucking insect, Rhodnius prolixus (Hemiptera; Reduviidae) I. Composition of the urine. J. Exp. Biol. 1931, 8, 411–427. [Google Scholar] [CrossRef]
- Mwangi, V.I.; Martinez, E.G.; Leda, R.L.; Catunda, M.E.S.L.A.; Dias, A.D.S.; Padron Antonio, Y.; Guerra, M.d.G.V.B. Resisting an invasion: A review of the triatomine vector (Kissing bug) defense strategies against a Trypanosoma sp. infection. Acta Trop. 2023, 238, 106745. [Google Scholar] [CrossRef] [PubMed]
- De Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lehane, M. Managing the blood meal. In The Biology of Blood Sucking Insects, 2nd ed.; Lehane, M., Ed.; Cambridge University Press: Cambridge, UK, 2005; pp. 84–115. [Google Scholar]
- Smit, J.D.; Guggenheim, R.; Bauer, P.G. Crystallized hemoglobin in Rhodnius prolixus after a blood meal on guinea-pig. Experientia 1983, 39, 1335–1338. [Google Scholar] [CrossRef]
- Balczun, C.; Siemanowski, J.; Pausch, J.K.; Helling, S.; Marcus, K.; Stephan, C.; Meyer, H.E.; Schneider, T.; Cizmowski, C.; Oldenburg, M.; et al. Intestinal aspartate proteases TiCatD and TiCatD2 of the haematophagous bug Triatoma infestans (Reduviidae): Sequence characterisation, expression pattern and characterisation of proteolytic activity. Insect Biochem. Mol. Biol. 2012, 42, 240–250. [Google Scholar] [CrossRef]
- Kollien, A.H.; Grospietsch, T.; Kleffmann, T.; Zerbst-Boroffka, I.; Schaub, G.A. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. J. Insect Physiol. 2001, 47, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Balczun, C.; Meiser, C.K.; Schaub, G.A. Triatomines as vectors of American Trypanosomiasis. In Parasitology Research Monographs. Arthropods as Vectors of Emerging Diseases; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 3, pp. 275–299. [Google Scholar]
- Gama, M.D.V.F.; Alexandre, Y.D.N.; Pereira da Silva, J.M.; Castro, D.P.; Genta, F.A. Digestive α-L-fucosidase activity in Rhodnius prolixus after blood feeding: Effect of secretagogue and nutritional stimuli. Front. Physiol. 2023, 14, 1123414. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.S.; Gomes, B.; Oliveira, P.L.; Garcia, E.S.; Azambuja, P.; Genta, F.A. Characterization of the temporal pattern of blood protein digestion in Rhodnius prolixus: First description of early and late gut cathepsins. Front. Physiol. 2021, 11, 509310. [Google Scholar] [CrossRef]
- Rocha, L.L.; Neves, C.A.; Zanuncio, J.C.; Serrão, J.E. Digestive cells in the midgut of Triatoma vitticeps (Stal, 1859) in different starvation periods. Comptes Rendus Biol. 2010, 333, 405–415. [Google Scholar] [CrossRef]
- Rocha, L.L.; Neves, C.A.; Zanuncio, J.C.; Serrão, J.E. Endocrine and regenerative cells in the midgut of Chagas’ disease vector Triatoma vitticeps during different starvation periods. Folia Biol. 2014, 62, 259–267. [Google Scholar] [CrossRef]
- Kollien, A.H.; Waniek, P.J.; Nisbet, A.J.; Billingsley, P.F.; Schaub, G.A. Activity and sequence characterization of two cysteine proteases in the digestive tract of the reduviid bug Triatoma infestans. Insect Mol. Biol. 2004, 13, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.C.; Machado, E.M.M.; Garcia, E.S.; Azambuja, P. Trypanosoma cruzi: Effects of infection on cathepsin D activity in the midgut of Rhodnius prolixus. Exp. Parasitol. 2006, 112, 130–133. [Google Scholar] [CrossRef]
- Briegel, H. Physiological bases of mosquito ecology. J. Vector Ecol. 2003, 28, 1–11. [Google Scholar] [PubMed]
- Billingsley, P.F.; Downe, A.E.R. Ultrastructural changes in posterior midgut cells associated with blood feeding in adult female Rhodnius prolixus Stål (Heteroptera: Reduviidae). Can. J. Zool. 1983, 61, 2574–2586. [Google Scholar] [CrossRef]
- Billingsley, P.F.; Downe, A.E.R. Cellular localisation of aminopeptidase in the midgut of Rhodnius prolixus Stål (Hemiptera: Reduviidae) during blood digestion. Cell Tissue Res. 1985, 241, 421–428. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Whitten, M.M.A. Vector immunity. In SGM Symposium 63: Microbe-Vector Interactions in Vector Borne Diseases; Gillespie, S.H., Osborne, A., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 199–262. [Google Scholar]
- Müller, U.; Vogel, P.; Alber, G.; Schaub, G.A. The innate immune system of mammals and insects. In Contributions to Microbiology; Egesten, A., Schmidt, A., Herwald, H., Eds.; Karger: Basel, Switzerland, 2008; Volume 15, pp. 21–44. [Google Scholar]
- Salcedo-Porras, N.; Lowenberger, C. Immune system of triatomines. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 307–344. [Google Scholar]
- Ratcliffe, N.A.; Mello, C.B.; Castro, H.C.; Dyson, P.; Figueiredo, M. Immune reactions of vector insects to parasites and pathogens. Microorganisms 2024, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Whitten, M.; Sun, F.; Tew, I.; Schaub, G.A.; Soukou, C.; Nappi, A.; Ratcliffe, N. Differential modulation of Rhodnius prolixus nitric oxide activities following challenge with Trypanosoma rangeli, T. cruzi and bacterial cell wall components. Insect Biochem. Mol. Biol. 2007, 37, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Settembrini, B.P.; Coronel, M.F.; Nowicki, S.; Nighorn, A.J.; Villar, M.J. Distribution and characterization of nitric oxide synthase in the nervous system of Triatoma infestans (Insecta: Heteroptera). Cell Tissue Res. 2007, 328, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Genta, F.A.; Souza, R.S.; Garcia, E.S.; Azambuja, P. Phenoloxidases from Rhodnius prolixus: Temporal and tissue expression pattern and regulation by ecdysone. J. Insect Physiol. 2010, 56, 1253–1259. [Google Scholar] [CrossRef]
- Garcia, E.S.; Castro, D.P.; Figueiredo, M.B.; Azambuja, P. Immune homeostasis to microorganisms in the guts of triatomines (Reduviidae)—A review. Mem. Inst. Oswaldo Cruz 2010, 105, 605–610. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Lowenberger, C. The innate immune system of kissing bugs, vectors of Chagas disease. Dev. Comp. Immunol. 2019, 98, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Porras, N.; Noor, S.; Cai, C.; Oliveira, P.L.; Lowenberger, C. Rhodnius prolixus uses the peptidoglycan recognition receptor rpPGRP-LC/LA to detect Gram-negative bacteria and activate the IMD pathway. Curr. Res. Insect Sci. 2021, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Porras, N.; Oliveira, P.L.; Guarneri, A.A.; Lowenberger, C. A fat body transcriptome analysis of the immune responses of Rhodnius prolixus to artificial infections with bacteria. Parasites Vectors 2022, 15, 269. [Google Scholar] [CrossRef]
- Gumiel, M.; de Mattos, D.P.; Vieira, C.S.; Moraes, C.S.; Moreira, C.J.C.; Gonzalez, M.S.; Teixeira-Ferreira, A.; Waghabi, M.; Azambuja, P.; Carels, N. Proteome of the triatomine digestive tract: From catalytic to immune pathways; focusing on annexin expression. Front. Mol. Biosci. 2020, 7, 589435. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, A.D.; Lilia, J.P.; Jesús, M.B.; Henry, R.M. The IMD and Toll canonical immune pathways of Triatoma pallidipennis are preferentially activated by Gram-negative and Gram-positive bacteria, respectively, but cross-activation also occurs. Parasites Vectors 2022, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Borsatto, K.C.; Coronado, M.A.; Galvão, C.; Arni, R.K.; Alevi, K.C.C. Transcriptomics applied to the study of Chagas disease vectors. Am. J. Trop. Med. Hyg. 2022, 106, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Satyavathi, V.; Ghosh, R.; Subramanian, S. Long non-coding RNAs regulating immunity in insects. Noncoding RNA 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Moure, U.A.E.; Tan, T.; Sha, L.; Lu, X.; Shao, Z.; Yang, G.; Wang, Y.; Cui, H. Advances in the immune regulatory role of non-coding RNAs (miRNAs and lncRNAs) in insect-pathogen interactions. Front. Immunol. 2022, 13, 856457. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 2005, 26, 186–192. [Google Scholar] [CrossRef]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef]
- Carmona-Peña, S.P.; Vázquez-Chagoyán, J.C.; Castro, D.P.; Genta, F.A.; Contreras-Garduño, J. Benefits and costs of immune memory in Rhodnius prolixus against Trypanosoma cruzi. Microb. Pathog. 2022, 165, 105505. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, A.C.; da Silva, P.; Miranda, M.T.; Bianchi, A.G.; Miranda, A.; Ribolla, P.E.; Daffre, S. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J. Biol. Chem. 1999, 274, 25330–25334. [Google Scholar] [CrossRef] [PubMed]
- Meiser, C.K.; Piechura, H.; Werner, T.; Dittmeyer-Schäfer, S.; Meyer, H.E.; Warscheid, B.; Schaub, G.A.; Balczun, C. Kazal-type inhibitors in the stomach of Panstrongylus megistus (Triatominae, Reduviidae). Insect Biochem. Mol. Biol. 2010, 40, 345–353. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Genta, F.A.; Sorgine, M.H.F.; Raquel Logullo, R.; Mesquita, R.D.; Paiva-Silva, G.O.; Majerowicz, D.; Medeiros, M.; Koerich, L.; Terra, W.R.; et al. An insight into the transcriptome of the digestive tract of the bloodsucking bug, Rhodnius prolixus. PLoS Negl. Trop. Dis. 2014, 8, e2594. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, T.C.F.; Eaton, D.P.; Pham, V.M.; Francischetti, I.M.B.; Aoki, V.; Hans-Filho, G.; Rivitti, E.A.; Valenzuela, J.G.; Diaz, L.A.; Ribeiro, J.M.C. An insight into the sialotranscriptome of Triatoma matogrossensis, a kissing bug associated with fogo selvagem in South America. Am. J. Trop. Med. Hyg. 2012, 86, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A.; Gutiérrez-Cabrera, A.E.; Rojas-Wastavino, G.E.; Bucio-Torres, M.I.; Cabrera-Bravo, M. Immune defence mechanisms of triatomines against bacteria, viruses, fungi and parasites. Bull. Entomol. Res. 2015, 105, 523–532. [Google Scholar] [CrossRef]
- Soares, T.S.; Buarque, D.S.; Queiroz, B.R.; Gomes, C.M.; Braz, G.R.; Araújo, R.N.; Pereira, M.H.; Guarneri, A.A.; Tanaka, A.S. A Kazal-type inhibitor is modulated by Trypanosoma cruzi to control microbiota inside the anterior midgut of Rhodnius prolixus. Biochimie 2015, 112, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Buarque, D.S.; Gomes, C.M.; Araújo, R.N.; Pereira, M.H.; Ferreira, R.C.; Guarneri, A.A.; Tanaka, A.S. A new antimicrobial protein from the anterior midgut of Triatoma infestans mediates Trypanosoma cruzi establishment by controlling the microbiota. Biochimie 2016, 123, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Santiago, P.B.; Charneau, S.; Mandacaru, S.C.; Bentes, K.L.D.S.; Bastos, I.M.D.; de Sousa, M.V.; Ricart, C.A.O.; de Araújo, C.N.; Santana, J.M. Proteomic mapping of multifunctional complexes within triatomine saliva. Front. Cell. Infect. Microbiol. 2020, 10, 459. [Google Scholar] [CrossRef]
- Carvalho-Costa, T.M.; Tiveron, R.D.R.; Mendes, M.T.; Barbosa, C.G.; Nevoa, J.C.; Roza, G.A.; Silva, M.V.; Figueiredo, H.C.P.; Rodrigues, V.; Soares, S.C.; et al. Salivary and intestinal transcriptomes reveal differential gene expression in starving, fed and Trypanosoma cruzi-infected Rhodnius neglectus. Front. Cell. Infect. Microbiol. 2021, 11, 773357. [Google Scholar] [CrossRef]
- Praça, Y.R.; Santiago, P.B.; Charneau, S.; Mandacaru, S.C.; Bastos, I.M.D.; Bentes, K.L.D.S.; Silva, S.M.M.; da Silva, W.M.C.; da Silva, I.G.; de Sousa, M.V.; et al. An integrative sialomic analysis reveals molecules from Triatoma sordida (Hemiptera: Reduviidae). Front. Cell. Infect. Microbiol. 2022, 11, 798924. [Google Scholar] [CrossRef]
- Díaz-Garrido, P.; Cárdenas-Guerra, R.E.; Martínez, I.; Poggio, S.; Rodríguez-Hernández, K.; Rivera-Santiago, L.; Ortega-López, J.; Sánchez-Esquivel, S.; Espinoza, B. Differential activity on trypanosomatid parasites of a novel recombinant defensin type 1 from the insect Triatoma (Meccus) pallidipennis. Insect Biochem. Mol. Biol. 2021, 139, 103673. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.M.L.; Junqueira, A.C.; Celano, V.; Costa, G.L.; Coura, J.R. Fungal flora of the digestive tract of 5 species of triatomines vectors of Trypanosoma cruzi, Chagas 1909. Braz. J. Microbiol. 2001, 35, 288–291. [Google Scholar] [CrossRef]
- Moraes, A.M.L.; Figueiredo, A.R.; Junqueira, A.C.; Costa, G.L.; Aguiar, R.K.; Oliveira, P.C. Fungal flora of the digestive tract of Panstrongylus megistus (Reduviidae) used for experimental xenodiagnosis of Trypanosoma (Schizotripanum) cruzi Chagas 1909. Rev. Iberoam. Micol. 2001, 18, 79–82. [Google Scholar]
- Lima, M.S.; Laport, M.S.; Lorosa, E.S.; Jurberg, J.; dos Santos, K.R.N.; da Silva Neto, M.A.C.; Rachid, C.T.C.D.C.; Atella, G.C. Bacterial community composition in the salivary glands of triatomines (Hemiptera: Reduviidae). PLoS Negl. Trop. Dis. 2018, 12, e0006739. [Google Scholar] [CrossRef]
- Schaub, G.A. Intestinal bacteria/mutualistic symbionts of triatomines—A review. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2020, 22, 191–194. [Google Scholar]
- Guarneri, A.A.; Schaub, G.A. Interaction of triatomines with their bacterial microbiota and trypanosomes. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 345–386. [Google Scholar]
- Cardoso, M.A.; de Brito, T.F.; Brito, I.A.D.A.; Berni, M.A.; Coelho, V.L.; Pane, A. The neglected virome of triatomine insects. Front. Trop. Dis. 2022, 3, 828712. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Leulier, F.; Royet, J. Maintaining immune homeostasis in fly gut. Nat. Immunol. 2009, 10, 936–938. [Google Scholar] [CrossRef]
- Broderick, N.A.; Buchon, N.; Lemaitre, B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. mBio 2014, 5, e01117-14. [Google Scholar] [CrossRef]
- Douglas, A.E. The molecular basis of bacterial-insect symbiosis. J. Mol. Biol. 2014, 426, 3830–3837. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in disease-transmitting vectors. Nat. Rev. Microbiol. 2023, 21, 604–618. [Google Scholar] [CrossRef]
- Díaz-Albiter, H.M.; Ferreira, T.N.; Costa, S.G.; Rivas, G.B.; Gumiel, M.; Cavalcante, D.R.; Gonzalez, M.S.; Mello, C.B.; Dillon, V.M.; Vieira Bruno, R.; et al. Everybody loves sugar: First report of plant feeding in triatomines. Parasites Vectors 2016, 9, 114. [Google Scholar] [CrossRef]
- Páez-Rondón, O.; Aldana, E.; Dickens, J.; Otálora-Luna, F. Ethological description of a fixed action pattern in a kissing bug (Triatominae): Vision, gustation, proboscis extension and drinking of water and guava. J. Ethol. 2018, 36, 107–116. [Google Scholar] [CrossRef]
- Pal, E.; Allison, J.D.; Hurley, B.P.; Slippers, B.; Fourie, G. Life history traits of the Pentatomidae (Hemiptera) for the development of pest management tools. Forests 2023, 14, 861. [Google Scholar] [CrossRef]
- Schaub, G.A.; Jensen, C. Developmental time and mortality of the reduviid bug Triatoma infestans with differential exposure to coprophagic infections with Blastocrithidia triatomae (Trypanosomatidae). J. Invertebr. Pathol. 1990, 55, 17–27. [Google Scholar] [CrossRef]
- Schaub, G.A.; Böker, C.A.; Jensen, C.; Reduth, D. Cannibalism and coprophagy are modes of transmission of Blastocrithidia triatomae (Trypanosomatidae) between triatomines. J. Protozool. 1989, 36, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo Figueiras, A.N.; Kenigsten, A.; Lazzari, C.R. Aggregation in the haematophagous bug Triatoma infestans: Chemical signals and temporal pattern. J. Insect Physiol. 1994, 40, 311–316. [Google Scholar] [CrossRef]
- Lorenzo, M.G.; Lazzari, C.R. The spatial pattern of defaecation in Triatoma infestans and the role of faeces as a chemical mark of the refuge. J. Insect Physiol. 1996, 42, 903–907. [Google Scholar] [CrossRef]
- Vallejo, G.A.; Guhl, F.; Schaub, G.A. Triatominae—Trypanosoma cruzi/T rangeli: Vector-parasite interactions. Acta Trop. 2009, 110, 137–147. [Google Scholar] [CrossRef]
- Dumonteil, E.; Ramirez-Sierra, M.J.; Pérez-Carrillo, S.; Teh-Poot, C.; Herrera, C.; Gourbière, S.; Waleckx, E. Detailed ecological associations of triatomines revealed by metabarcoding and next-generation sequencing: Implications for triatomine behavior and Trypanosoma cruzi transmission cycles. Sci. Rep. 2018, 8, 4140. [Google Scholar] [CrossRef] [PubMed]
- Orantes, L.C.; Monroy, C.; Dorn, P.L.; Stevens, L.; Rizzo, D.M.; Morrissey, L.; Hanley, J.P.; Rodas, S.G.; Richards, B.; Wallin, K.F.; et al. Uncovering vector, parasite, blood meal and microbiome patterns from mixed-DNA specimens of the Chagas disease vector Triatoma dimidiata. PLoS Negl. Trop. Dis. 2018, 12, e0006730. [Google Scholar] [CrossRef]
- Kieran, T.J.; Arnold, K.M.H.; Thomas, J.C.; Varian, C.P.; Saldaña, A.; Calzada, J.E.; Glenn, T.C.; Gottdenker, N.L. Regional biogeography of microbiota composition in the Chagas disease vector Rhodnius pallescens. Parasites Vectors 2019, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Arias-Giraldo, L.M.; Muñoz, M.; Hernández, C.; Herrera, G.; Velásquez-Ortiz, N.; Cantillo-Barraza, O.; Urbano, P.; Ramírez, J.D. Species-dependent variation of the gut bacterial communities across Trypanosoma cruzi insect vectors. PLoS ONE 2020, 15, e0240916. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Pronovost, H.; Bierman, E.F.; Sanford, A.; Majeau, A.; Moore, R.; Herrera, C. Interactions among Triatoma sanguisuga blood feeding sources, gut microbiota and Trypanosoma cruzi diversity in southern Louisiana. Mol. Ecol. 2020, 29, 3747–3761. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.E.; Mitchell, E.A.; Zhang, Y.; Curtis-Robles, R.; Thapa, S.; Hamer, S.A.; Allen, M.S. Comparison of the bacterial gut microbiome of North American Triatoma spp. with and without Trypanosoma cruzi. Front. Microbiol. 2020, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, H.; Gao, M.; Huang, P.; Zhou, H.; Ma, Y.; Zhou, M.; Liang, J.; Yang, J.; Lv, Z. Dynamic of composition and diversity of gut microbiota in Triatoma rubrofasciata in different developmental stages and environmental conditions. Front. Cell. Infect. Microbiol. 2020, 10, 587708. [Google Scholar] [CrossRef]
- Eberhard, F.E.; Klimpel, S.; Guarneri, A.A.; Tobias, N.J. Exposure to Trypanosoma parasites induces changes in the microbiome of the Chagas disease vector Rhodnius prolixus. Microbiome 2022, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Gumpert, J. Untersuchungen über die Symbiose von Tieren mit Pilzen und Bakterien. X. Die Symbiose der Triatominen. 2. Infektion symbiontenfreier Triatominen mit symbiontischen und saprophytischen Mikroorganismen und gemeinsame Eigenschaften der symbiontischen Stämme. Z. Allg. Mikrobiol. 1962, 2, 290–302. (In German) [Google Scholar] [CrossRef]
- Rodríguez, J.; Pavía, P.; Montilla, M.; Puerta, C.J. Identifying triatomine symbiont Rhodococcus rhodnii as intestinal bacteria from Rhodnius ecuadoriensis (Hemiptera: Reduviidae) laboratory insects. Int. J. Trop. Insect Sci. 2011, 31, 34–37. [Google Scholar] [CrossRef]
- Eichler, S.; Schaub, G.A. Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp. Parasitol. 2002, 100, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.F. Rhodococcus triatomae sp nov, isolated from a blood-sucking bug. Int. J. Syst. Evol. Microbiol. 2005, 55, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Bordenstein, S.R. An introduction to phylosymbiosis. Proc. Biol. Sci. 2020, 287, 20192900. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Jiang, L.; Qiao, G.; Chen, J. Phylosymbiosis: The eco-evolutionary pattern of insect–symbiont interactions. Int. J. Mol. Sci. 2023, 24, 15836. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. Symbiotic bacteria in a blood-sucking insect, Rhodnius prolixus Stål (Hemiptera, Triatomidae). Parasitology 1936, 28, 284–289. [Google Scholar] [CrossRef]
- Eichler, S.; Schaub, G.A. The effects of aposymbiosis and of an infection with Blastocrithidia triatomae (Trypanosomatidae) on the tracheal system of the reduviid bugs Rhodnius prolixus and Triatoma infestans. J. Insect Physiol. 1998, 44, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Porras, N.; Umaña-Diaz, C.; Bitencourt, R.O.B.; Lowenberger, C. The role of bacterial symbionts in triatomines: An evolutionary perspective. Microorganisms. 2020, 8, 1438. [Google Scholar] [CrossRef]
- Hill, P.; Campbell, J.A.; Petrie, I.A. Rhodnius prolixus and its symbiotic actinomycete: A microbiological, physiological and behavioural study. Proc. R. Soc. Lond. B Biol. Sci. 1976, 194, 501–525. [Google Scholar]
- Watanabe, F.; Bito, T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Eppinger, M.; Bunk, B.; Johns, M.A.; Edirisinghe, J.N.; Kutumbaka, K.K.; Koenig, S.S.; Creasy, H.H.; Rosovitz, M.J.; Riley, D.R.; Daugherty, S.; et al. Genome sequences of the biotechnologically important Bacillus megaterium strains QM B1551 and DSM319. J. Bacteriol. 2011, 193, 4199–42213. [Google Scholar] [CrossRef]
- Lopez-Ordonez, T.; Flores-López, C.A.; Montejo-Lopez, R.; Cruz-Hernandez, A.; Conners, E.E. Cultivable bacterial diversity in the gut of the Chagas disease vector Triatoma dimidiata: Identification of possible bacterial candidates for a paratransgenesis approach. Front. Ecol. Evol. 2018, 5, 174. [Google Scholar] [CrossRef]
- Wang, Y.; Baumdicker, F.; Schweiger, P.; Kuenzel, S.; Staubach, F. Horizontal gene transfer-mediated bacterial strain variation affects host fitness in Drosophila. BMC Biol. 2021, 19, 187. [Google Scholar] [CrossRef]
- Tobias, N.J.; Eberhard, F.E.; Guarneri, A.A. Enzymatic biosynthesis of B-complex vitamins is supplied by diverse microbiota in the Rhodnius prolixus anterior midgut following Trypanosoma cruzi infection. Comput. Struct. Biotechnol. J. 2020, 18, 3395–3401. [Google Scholar] [CrossRef] [PubMed]
- Ochi, K. Phylogenetic analysis of mycolic acid-containing wall-chemotype IV actinomycetes and allied taxa by partial sequencing of ribosomal protein AT-L30. Int. J. Syst. Bacteriol. 1995, 45, 653–660. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.V.; Ferreira, M.D.L.; Pinheiro, A.C.; Saraiva, M.F.; de Almeida, M.V.; Valle, M.S. Synthesis and biological aspects of mycolic acids: An important target against Mycobacterium tuberculosis. Sci. World J. 2008, 8, 720–751. [Google Scholar] [CrossRef]
- Lanan, M.C.; Rodrigues, P.A.; Agellon, A.; Jansma, P.; Wheeler, D.E. A bacterial filter protects and structures the gut microbiome of an insect. ISME J. 2016, 10, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Meng, X.Y.; Kamagata, Y.; Fukatsu, T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009, 7, 2. [Google Scholar] [CrossRef]
- Ohbayashi, T.; Takeshita, K.; Kitagawa, W.; Nikoh, N.; Koga, R.; Meng, X.Y.; Tago, K.; Hori, T.; Hayatsu, M.; Asano, K.; et al. Insect’s intestinal organ for symbiont sorting. Proc. Natl. Acad. Sci. USA 2015, 112, E5179–E5188. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Ohbayashi, T.; Jang, S.; Mergaert, P. Burkholderia insecticola triggers midgut closure in the bean bug Riptortus pedestris to prevent secondary bacterial infections of midgut crypts. ISME J. 2020, 14, 1627–1638. [Google Scholar] [CrossRef]
- Oishi, S.; Moriyama, M.; Koga, R.; Fukatsu, T. Morphogenesis and development of midgut symbiotic organ of the stinkbug Plautia stali (Hemiptera: Pentatomidae). Zool. Lett. 2019, 5, 16. [Google Scholar] [CrossRef]
- Drews, M. Die Cardia von Triatoma infestans (Reduviidae; Hemiptera). Untersuchungen zu ihrer Ultrastruktur und zur Lokalisation Symbiontischer Bakterien. Diploma Thesis, University Freiburg, Freiburg, Germany, 1988. (In German). [Google Scholar]
- Vieira, C.S.; Waniek, P.J.; Castro, D.P.; Mattos, D.P.; Moreira, O.C.; Azambuja, P. Impact of Trypanosoma cruzi on antimicrobial peptide gene expression and activity in the fat body and midgut of Rhodnius prolixus. Parasites Vectors 2016, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.L.; Cury, J.C.; Gurgel-Gonçalves, R.; Bahia, A.C.; Monteiro, F.A. Field-collected Triatoma sordida from central Brazil display high microbiota diversity that varies with regard to developmental stage and intestinal segmentation. PLoS Negl. Trop. Dis. 2018, 12, e0006709. [Google Scholar] [CrossRef]
- Rodríguez-Ruano, S.M.; Škochová, V.; Rego, R.O.M.; Schmidt, J.O.; Roachell, W.; Hypša, V.; Nováková, E. Microbiomes of North American Triatominae: The grounds for Chagas disease epidemiology. Front. Microbiol. 2018, 9, 1167. [Google Scholar] [CrossRef]
- Meiser, C.K.; Pausch, J.K.; Schaub, G.A. Feeding-induced changes of bacteriolytic activity and of the pattern of bacteriolytic compounds in the stomach and small intestine of the haematophagous bug Triatoma infestans (Klug, 1834) (Reduviidae, Triatominae). Parasitologia 2022, 2, 13–26. [Google Scholar] [CrossRef]
- Vieira, C.S.; Waniek, P.J.; Mattos, D.P.; Castro, D.P.; Mello, C.B.; Ratcliffe, N.A.; Garcia, E.S.; Azambuja, P. Humoral responses in Rhodnius prolixus: Bacterial feeding induces differential patterns of antibacterial activity and enhances mRNA levels of antimicrobial peptides in the midgut. Parasites Vectors 2014, 7, 232. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Pereira, M.E.A. Midgut glycosidases of Rhodnius prolixus. Insect Biochem. 1984, 14, 103–108. [Google Scholar] [CrossRef]
- González-Rete, B.; Salazar-Schettino, P.M.; Bucio-Torres, M.I.; Córdoba-Aguilar, A.; Cabrera-Bravo, M. Activity of the prophenoloxidase system and survival of triatomines infected with different Trypanosoma cruzi strains under different temperatures: Understanding Chagas disease in the face of climate change. Parasites Vectors 2019, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Kollien, A.H.; Fechner, S.; Waniek, P.J.; Schaub, G.A. Isolation and characterization of a cDNA encoding for a lysozyme from the gut of the reduviid bug Triatoma infestans. Arch. Insect Biochem. Physiol. 2003, 53, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.A.C.; Waniek, P.J.; Stock, P.; Mayer, C.; Jansen, A.M.; Schaub, G.A. Sequence characterization and expression patterns of defensin and lysozyme encoding genes from the gut of the reduviid bug Triatoma brasiliensis. Insect Biochem. Mol. Biol. 2006, 36, 547–560. [Google Scholar] [CrossRef]
- Ursic-Bedoya, R.; Buchhop, J.; Joy, J.B.; Durvasula, R.; Lowenberger, C. Prolixicin: A novel antimicrobial peptide isolated from Rhodnius prolixus with differential activity against bacteria and Trypanosoma cruzi. Insect Mol. Biol. 2011, 20, 775–786. [Google Scholar] [CrossRef]
- Batista, K.K.S.; Vieira, C.S.; Figueiredo, M.B.; Costa-Latgé, S.G.; Azambuja, P.; Genta, F.A.; Castro, D.P. Influence of Serratia marcescens and Rhodococcus rhodnii on the humoral immunity of Rhodnius prolixus. Int. J. Mol. Sci. 2021, 22, 10901. [Google Scholar] [CrossRef]
- Laughton, A.M.; Garcia, J.R.; Gerardo, N.M. Condition-dependent alteration of cellular immunity by secondary symbionts in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 2016, 86, 17–24. [Google Scholar] [CrossRef]
- Meiser, C.K.; Klenner, L.; Balczun, C.; Schaub, G.A. Bacteriolytic activity in saliva of the haematophagous Triatoma infestans (Reduviidae) and novel characterization and expression site of a third lysozyme. Arch. Insect Biochem. Physiol. 2023, 113, e22013. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vargas, M.J.; Santibáñez-López, C.E.; Corzo, G. An insight into the triabin protein family of American hematophagous reduviids: Functional, structural and phylogenetic analysis. Toxins 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.V.; Munks, R.J.L.; Lehane, S.M.; Lehane, M.J. Association of midgut defensin with a novel serine protease in the blood-sucking fly Stomoxys calcitrans. Insect Mol. Biol. 2002, 11, 197–205. [Google Scholar] [CrossRef]
- Lemos, F.J.; Terra, W. Digestion of bacteria and the role of midgut lysozyme in some insect larvae. Comp. Biochem. Physiol. B 1991, 100, 265–268. [Google Scholar] [CrossRef]
- Regel, R.; Matioli, S.R.; Terra, W.R. Molecular adaptation of Drosophila melanogaster lysozymes to a digestive function. Insect Biochem. Mol. Biol. 1998, 28, 309–319. [Google Scholar] [CrossRef]
- Henriques, B.S.; Gomes, B.; da Costa, S.G.; Moraes, C.D.S.; Mesquita, R.D.; Dillon, V.M.; Garcia, E.S.; Azambuja, P.; Dillon, R.J.; Genta, F.A. Genome wide mapping of peptidases in Rhodnius prolixus: Identification of protease gene duplications, horizontally transferred proteases and analysis of peptidase A1 structures, with considerations on their role in the evolution of hematophagy in Triatominae. Front. Physiol. 2017, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, A.A. Infecting triatomines with trypanosomes. In Trypanosomatids: Methods and Protocols. Methods in Molecular Biology; Michels, P., Ginger, M., Zilberstein, D., Eds.; Humana: New York, NY, USA, 2020; Volume 2116, pp. 69–79. [Google Scholar] [CrossRef]
- Hölscher, C.; Mossmann, H.; Hartmann, R.; Schaub, G.A. Effects of the isolation methodology on protein profiles of blood trypomastigotes of Trypanosoma cruzi. Parasitology 2003, 126, 41–51. [Google Scholar] [CrossRef]
- Schaub, G.A. Direct transmission of Trypanosoma cruzi between vectors of Chagas’ disease. Acta Trop. 1988, 45, 11–19. [Google Scholar]
- da Silva, I.G.; da Silva, H.H.G. Suscetibilidade de 11 espécies de triatomíneos (Hemiptera; Reduviidae) à cepa Y de Trypanosoma cruzi (Kinetoplastida; Trypanosomatidae). Rev. Bras. Entomol. 1993, 37, 459–463. (In Brazilian) [Google Scholar]
- Noireau, F.; Diosque, P.; Jansen, A.M. Trypanosoma cruzi: Adaptation to its vectors and its hosts. Vet. Res. 2009, 40, 26. [Google Scholar] [CrossRef]
- Garcia, E.S.; Genta, F.A.; de Azambuja, P.; Schaub, G.A. Interactions between intestinal compounds of triatomines and Trypanosoma cruzi. Trends Parasitol. 2010, 26, 499–505. [Google Scholar] [CrossRef]
- Tustin, A.W.; Castillo-Neyra, R.; Tamayo, L.D.; Salazar, R.; Borini-Mayorí, K.; Levy, M.Z. Elucidating the mechanism of Trypanosoma cruzi acquisition by triatomine insects: Evidence from a large field survey of Triatoma infestans. Trop. Med. Infect. Dis. 2020, 5, 87. [Google Scholar] [CrossRef]
- Araújo, C.A.C.; Waniek, P.J.; Jansen, A.M. Development of a Trypanosoma cruzi (TcI) isolate in the digestive tract of an unfamiliar vector, Triatoma brasiliensis (Hemiptera, Reduviidae). Acta Trop. 2008, 107, 195–199. [Google Scholar] [CrossRef]
- Mejía-Jaramillo, A.M.; Peña, V.H.; Triana-Chávez, O. Trypanosoma cruzi: Biological characterization of lineages I and II supports the predominance of lineage I in Colombia. Exp. Parasitol. 2009, 121, 83–91. [Google Scholar] [CrossRef]
- Sandoval-Rodríguez, A.; Rojo, G.; López, A.; Ortiz, S.; Saavedra, M.; Botto-Mahan, C.; Cattan, P.E.; Solari, A. Comparing vector competence of Mepraia gajardoi and Triatoma infestans by genotyping Trypanosoma cruzi discrete typing units present in naturally infected Octodon degus. Acta Trop. 2019, 190, 119–122. [Google Scholar] [CrossRef]
- Fernandes, O.; Santos, S.; Junqueira, A.; Jansen, A.; Cupolillo, E.; Campbell, D.; Zingales, B.; Coura, J.R. Populational heterogeneity of Brazilian Trypanosoma cruzi isolates revealed by the mini-exon and ribosomal spacers. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. S1), 195–197. [Google Scholar] [CrossRef]
- Cortez, M.R.; Pinho, A.P.; Cuervo, P.; Alfaro, F.; Solano, M.; Xavier, S.C.C.; D’Andrea, P.S.; Fernandes, O.; Torrico, F.; Noireau, F.; et al. Trypanosoma cruzi (Kinetoplastida; Trypanosomatidae): Ecology of the transmission cycle in the wild environment of the Andean valley of Cochabamba; Bolivia. Exp. Parasitol. 2020, 114, 305–313. [Google Scholar] [CrossRef]
- Campos-Soto, R.; Ortiz, S.; Cordova, I.; Bruneau, N.; Botto-Mahan, C.; Solari, A. Interactions between Trypanosoma cruzi the Chagas disease parasite and naturally infected wild Mepraia vectors of Chile. Vector Borne Zoonotic Dis. 2016, 16, 165–171. [Google Scholar] [CrossRef]
- de Lana, M.; da Silveira Pinto, A.; Barnabé, C.; Quesney, V.; Noël, S.; Tibayrenc, M. Trypanosoma cruzi: Compared vectorial transmissibility of three major clonal genotypes by Triatoma infestans. Exp. Parasitol. 1998, 90, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.D.S.; de Lana, M.; Bastrenta, B.; Barnabé, C.; Quesney, V.; Noël, S.; Tibayrenc, M. Compared vectorial transmissibility of pure and mixed clonal genotypes of Trypanosoma cruzi in Triatoma infestans. Parasitol. Res. 1998, 84, 348–353. [Google Scholar] [CrossRef]
- Araújo, C.A.C.; Cabello, P.H.; Jansen, A.M. Growth behaviour of two Trypanosoma cruzi strains in single and mixed infections: In vitro and in the intestinal tract of the blood-sucking bug, Triatoma brasiliensis. Acta Trop. 2007, 101, 225–231. [Google Scholar] [CrossRef]
- Schaub, G.A.; Grünfelder, C.; Zimmermann, D.; Peters, W. Binding of lectin-gold conjugates by two Trypanosoma cruzi strains in ampullae and rectum of Triatoma infestans. Acta Trop. 1989, 46, 291–301. [Google Scholar] [CrossRef]
- Bauer, P.G. Electron microscopical studies on Trypanosoma cruzi and other microorganisms in the reduviid vector. Mem. Inst. Oswaldo Cruz 1984, 79 (Suppl), 25–32. [Google Scholar] [CrossRef]
- Urbina, J.A. Intermediary metabolism of Trypanosoma cruzi. Parasitol. Today 1994, 10, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.J.; Quevedo, Y.S.; Torres, A.M.; Gaitán Veloza, G.A.; Carranza Martínez, J.C.; Urrea-Montes, D.A.; Robello-Porto, C.; Vallejo, G.A. Comparative proteomic analysis of the hemolymph and salivary glands of Rhodnius prolixus and R. colombiensis reveals candidates associated with differential lytic activity against Trypanosoma cruzi I and T. cruzi II. PLoS Negl. Trop. Dis. 2024, 18. [Google Scholar] [CrossRef]
- Amino, R.; Martins, R.M.; Procopio, J.; Hirata, I.Y.; Juliano, M.A.; Schenkman, S. Trialysin, a novel pore-forming protein from saliva of hematophagous insects activated by limited proteolysis. J. Biol. Chem. 2002, 277, 6207–6213. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Kessler, R.L.; Lorenzo, M.G.; Paim, R.M.M.; Ferreira, L.L.; Probst, C.M.; Alves-Silva, J.; Guarneri, A.A. Colonization of Rhodnius prolixus gut by Trypanosoma cruzi involves an extensive parasite killing. Parasitology 2016, 143, 434–443. [Google Scholar] [CrossRef]
- Kessler, R.L.; Contreras, V.T.; Marliére, N.P.; Guarneri, A.A.; Silva, L.H.V.; Mazzarotto, G.A.; Batista, M.; Soccol, V.T.; Krieger, M.A.; Probst, C.M. Recently differentiated epimastigotes from Trypanosoma cruzi are infective to the mammalian host. Mol. Microbiol. 2017, 104, 712–736. [Google Scholar] [CrossRef]
- Dias, F.D.A.; Guerra, B.; Vieira, L.R.; Perdomo, H.D.; Gandara, A.C.; Amaral, R.J.; Vollu, R.E.; Gomes, S.A.; Lara, F.A.; Sorgine, M.H.; et al. Monitoring of the parasite load in the digestive tract of Rhodnius prolixus by combined qPCR analysis and imaging techniques provides new insights into the trypanosome life cycle. PLoS Negl. Trop. Dis. 2015, 9, e0004186. [Google Scholar] [CrossRef] [PubMed]
- Brack, C. Elektronenmikroskopische Untersuchungen zum Lebenszyklus von Trypanosoma cruzi unter besonderer Berückischtigung der Entwicklungsformen im Übertrager Rhodnius prolixus. Acta Trop. 1968, 25, 289–356. (In German) [Google Scholar] [PubMed]
- Brener, Z. A new aspect of Trypanosoma cruzi life-cycle in the invertebrate host. J. Protozool. 1972, 19, 23–27. [Google Scholar] [CrossRef]
- Tay, J. Evolución del Tripanosoma cruzi cepa mexicana en el huésped vertebrado, invertebrado e in vitro. Salud Publica Mex. 1980, 22, 513–520. [Google Scholar] [PubMed]
- Kollien, A.H.; Schmidt, J.; Schaub, G.A. Modes of association of Trypanosoma cruzi with the intestinal tract of the vector Triatoma infestans. Acta Trop. 1998, 70, 127–141. [Google Scholar] [CrossRef]
- Gutiérrez, L.S.; Burgos, C.; Bianchi, R.; Burgos, M.H. Immunocytochemical reaction of sera from Chagasic patients against Trypanosoma cruzi, intestine of Triatoma infestans and normal human heart. Medicina 1999, 59, 231–237. [Google Scholar]
- Kollien, A.H.; Schaub, G.A. The development of Trypanosoma cruzi (Trypanosomatidae) in the reduviid bug Triatoma infestans (Insecta): Influence of starvation. J. Eukaryot. Microbiol. 1998, 45, 59–63. [Google Scholar] [CrossRef]
- Schaub, G.A.; Lösch, P. Parasite/host-interrelationships of the trypanosomatids Trypanosoma cruzi and Blastocrithidia triatomae and the reduviid bug Triatoma infestans: Influence of starvation of the bug. Ann. Trop. Med. Parasitol. 1989, 83, 215–223. [Google Scholar] [CrossRef]
- Kleffmann, T.; Schmidt, J.; Schaub, G.A. Attachment of Trypanosoma cruzi epimastigotes to hydrophobic substrates and use of this property to separate stages and promote metacyclogenesis. J. Eukaryot. Microbiol. 1998, 45, 548–555. [Google Scholar] [CrossRef]
- Schmidt, J.; Kleffmann, T.; Schaub, G.A. Hydrophobic attachment of Trypanosoma cruzi to a superficial layer of the rectal cuticle in the bug Triatoma infestans. Parasitol. Res. 1998, 84, 527–536. [Google Scholar] [CrossRef]
- Povelones, M.L.; Holmes, N.A.; Povelones, M. A sticky situation: When trypanosomatids attach to insect tissues. PLoS Pathog. 2023, 19, e1011854. [Google Scholar] [CrossRef]
- Chiurillo, M.A.; Carlson, J.; Bertolini, M.S.; Raja, A.; Lander, N. Dual localization of receptor-type adenylate cyclases and cAMP response protein 3 unveils the presence of two putative signaling microdomains in Trypanosoma cruzi. mBio 2023, 14, e01064-23. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.D.L.M.; Balouz, V.; Centeno Cameán, C.; Cori, C.R.; Kashiwagi, G.A.; Gil, S.A.; Macchiaverna, N.P.; Cardinal, M.V.; Guaimas, F.; Lobo, M.M.; et al. Trypanosoma cruzi surface mucins are involved in the attachment to the Triatoma infestans rectal ampoule. PLoS Negl. Trop. Dis. 2019, 13, e0007418. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A. The effects of trypanosomatids on insects. Adv. Parasitol. 1992, 31, 255–319. [Google Scholar] [PubMed]
- Perlowagora-Szumlewicz, A.; Moreira, C.J.C. In vivo differentiation of Trypanosoma cruzi—1. Experimental evidence of the influence of vector species on metacyclogenesis. Mem. Inst. Oswaldo Cruz 1994, 89, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.D.F.P.; Guarneri, A.; Silber, A. The influence of environmental cues on the development of Trypanosoma cruzi in triatominae vector. Front. Cell. Infect. Microbiol. 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- González-Rete, B.; Gutiérrez-Cabrera, A.E.; De Fuentes-Vicente, J.A.; Salazar-Schettino, P.M.; Cabrera-Bravo, M.; Córdoba-Aguilar, A. Higher temperatures reduce the number of Trypanosoma cruzi parasites in the vector Triatoma pallidipennis. Parasites Vectors 2021, 14, 385. [Google Scholar] [CrossRef] [PubMed]
- Kleffmann, T. Mechanismen der Anheftung und Induktion der Metazyklogenese von Trypanosoma cruzi in Triatoma infestans. Ph.D. Thesis, Fakultät für Biologie und Biotechnologie, Ruhr-Universität Bochum, Bochum, Germany, 1999. (In German). [Google Scholar]
- Nogueira, N.; Bianco, C.; Cohn, Z. Studies on the selective lysis and purification of Trypanosoma cruzi. J. Exp. Med. 1975, 142, 224–229. [Google Scholar] [CrossRef]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef]
- Pérez-Morales, D.; Hernández, K.D.; Martínez, I.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Espinoza, B. Ultrastructural and physiological changes induced by different stress conditions on the human parasite Trypanosoma cruzi. Cell Stress Chaperones 2017, 22, 15–27. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Bourguignon, S.C.; Gonçalves, P.S.; Lechuga, G.C.; Provance, D.W. Metabolic alteration of Trypanosoma cruzi during differentiation of epimastigote to trypomastigote forms. Pathogens 2022, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Kollien, A.H.; Schaub, G.A. Trypanosoma cruzi in the rectum of the bug Triatoma infestans: Effects of blood ingestion by the starved vector. Am. J. Trop. Med. Hyg. 1998, 59, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A.; Böker, C.A. Colonization of the rectum of Triatoma infestans by Trypanosoma cruzi: Influence of starvation studied by scanning electron microscopy. Acta Trop. 1986, 43, 349–354. [Google Scholar] [PubMed]
- Loza-Murguía, M.; Noireau, F. Vectorial capacity of Triatoma guasayana (Wygodzinsky & Abalos) (Hemiptera: Reduviidae) compared with two other species of epidemic importance. Neotrop. Entomol. 2010, 39, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A. Does Trypanosoma cruzi stress its vector? Parasitol. Today 1989, 5, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A. Interactions of trypanosomatids and triatomines. Adv. Insect Physiol. 2009, 37, 177–242. [Google Scholar]
- Córdoba-Aguilar, A. Chagas bugs and Trypanosoma cruzi: Puppets and puppeteer? Acta Trop. 2020, 211, 105600. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Diotaiuti, L.; Gontijo, N.F.; Gontijo, A.F.; Pereira, M.H. Comparison of feeding behaviour of Triatoma infestans, Triatoma brasiliensis and Triatoma pseudomaculata in different hosts by electronic monitoring of the cibarial pump. J. Insect Physiol. 2000, 46, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Heger, T.J.; Guerin, P.M.; Eugster, W. Microclimatic factors influencing refugium suitability for Rhodnius prolixus. Physiol. Entomol. 2006, 31, 248–256. [Google Scholar] [CrossRef]
- Rolandi, C.; Schilman, P.E. The costs of living in a thermal fluctuating environment for the tropical haematophagous bug, Rhodnius prolixus. J. Therm. Biol. 2018, 74, 92–99. [Google Scholar] [CrossRef]
- Schofield, C.J. Density regulation of domestic populations of Triatoma infestans in Brazil. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Mc Cabe, A.; Yañez, F.; Pinto, R.; López, A.; Ortiz, S.; Muñoz-San Martin, C.; Botto-Mahan, C.; Solari, A. Survivorship of wild caught Mepraia spinolai nymphs: The effect of seasonality and Trypanosoma cruzi infection after feeding and fasting in the laboratory. Infect. Genet. Evol. 2019, 71, 197–204. [Google Scholar] [CrossRef]
- Estay-Olea, D.; Correa, J.P.; de Bona, S.; Bacigalupo, A.; Quiroga, N.; San Juan, E.; Solari, A.; Botto-Mahan, C. Trypanosoma cruzi could affect wild triatomine approaching behaviour to humans by altering vector nutritional status: A field test. Acta Trop. 2020, 210, 105574. [Google Scholar] [CrossRef]
- Sousa, G.; de Carvalho, S.S.; Atella, G.C. Trypanosoma cruzi affects Rhodnius prolixus lipid metabolism during acute infection. Front. Trop. Dis. 2021, 2, 737909. [Google Scholar] [CrossRef]
- Peterson, J.K.; Graham, A.L.; Dobson, A.P.; Chavez, O.T. Rhodnius prolixus life history outcomes differ when infected with different Trypanosoma cruzi I strains. Am. J. Trop. Med. Hyg. 2015, 93, 564–572. [Google Scholar] [CrossRef]
- Cordero-Montoya, G.; Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Vences-Blanco, M.O.; Rocha-Ortega, M.; Gutiérrez-Cabrera, A.E.; Rojas-Ortega, E.; Córdoba-Aguilar, A. The cost of being a killer’s accomplice: Trypanosoma cruzi impairs the fitness of kissing bugs. Parasitol. Res. 2019, 118, 2523–2529. [Google Scholar] [CrossRef]
- Eberhard, F.E.; Klimpel, S.; Guarneri, A.A.; Tobias, N.J. Metabolites as predictive biomarkers for Trypanosoma cruzi exposure in triatomine bugs. Comput. Struct. Biotechnol. J. 2021, 19, 3051–3057. [Google Scholar] [CrossRef]
- Cuevas, I.C.; Cazzulo, J.J.; Sánchez, D.O. gp63 homologues in Trypanosoma cruzi: Surface antigens with metalloprotease activity and a possible role in host cell infection. Infect. Immun. 2003, 71, 5739–5749. [Google Scholar] [CrossRef]
- Lazzari, C.R.; Pereira, M.H.; Lorenzo, M.G. Behavioural biology of Chagas disease vectors. Mem. Inst. Oswaldo Cruz 2013, 108, 34–47. [Google Scholar] [CrossRef]
- May-Concha, I.J.; Escalante-Talavera, M.J.; Dujardin, J.P.; Waleckx, E. Does Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida: Trypanosomatidae) modify the antennal phenotype of Triatoma dimidiata (Latreille, 1811) (Hemiptera: Triatominae)? Parasites Vectors 2022, 15, 466. [Google Scholar] [CrossRef]
- De Bona, S.; Correa, J.P.; San Juan, E.; Estay-Olea, D.; Quiroga, N.; Bacigalupo, A.; Araya-Donoso, R.; Botto-Mahan, C. Opportunistic or selective? Stage-dependent feeding behavior in a wild vector of Chagas disease. Int. J. Parasitol. 2023, 53, 55–64. [Google Scholar] [CrossRef]
- Ramírez-González, M.G.; Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Gutiérrez-Cabrera, A.E.; Rojas Ortega, E.; Córdoba-Aguilar, A. Zombie bugs? Manipulation of kissing bug behavior by the parasite Trypanosoma cruzi. Acta Trop. 2019, 200, 105177. [Google Scholar] [CrossRef]
- Verly, T.; Costa, S.; Lima, N.; Mallet, J.; Odêncio, F.; Pereira, M.; Moreira, C.J.C.; Britto, C.; Pavan, M.G. Vector competence and feeding-excretion behavior of Triatoma rubrovaria (Blanchard, 1843) (Hemiptera: Reduviidae) infected with Trypanosoma cruzi TcVI. PLoS Negl. Trop. Dis. 2020, 14, e0008712. [Google Scholar] [CrossRef]
- Depickère, S.; Ramírez-Ávila, G.M.; Deneubourg, J.-L. Alteration of the aggregation and spatial organization of the vector of Chagas disease, Triatoma infestans, by the parasite Trypanosoma cruzi. Sci. Rep. 2019, 9, 17432. [Google Scholar] [CrossRef] [PubMed]
- Alavez-Rosas, D.; Gutiérrez-Cabrera, A.E.; Cruz-López, L.; Córdoba-Aguilar, A. Lessons to be popular: The chemical basis of aggregation in Trypanosoma cruzi-infected and non-infected Chagasic bugs. R. Soc. Open Sci. 2024, 11, 231271. [Google Scholar] [CrossRef]
- Ouali, R.; Vieira, L.R.; Salmon, D.; Bousbata, S. Rhodnius prolixus hemolymph immuno-physiology: Deciphering the systemic immune response triggered by Trypanosoma cruzi establishment in the vector using quantitative proteomics. Cells 2022, 11, 1449. [Google Scholar] [CrossRef]
- Buarque, D.S.; Spindola, L.M.; Martins, R.M.; Braz, G.R.; Tanaka, A.S. Tigutcystatin, a cysteine protease inhibitor from Triatoma infestans midgut expressed in response to Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 2011, 413, 241–247. [Google Scholar] [CrossRef]
- Buarque, D.S.; Braz, G.R.C.; Martins, R.M.; Tanaka-Azevedo, A.M.; Gomes, C.M.; Oliveira, F.A.A.; Schenkman, S.; Tanaka, A.S. Differential expression profiles in the midgut of Triatoma infestans infected with Trypanosoma cruzi. PLoS ONE 2013, 8, e61203. [Google Scholar] [CrossRef]
- Pausch, J.K. Characterization of Intestinal Antibacterial Factors of Triatoma infestans (Reduviidae, Insecta) and Their Interaction with Trypanosoma cruzi (Trypanosomatidae, Kinetoplastida). Ph.D. Thesis, Fakultät für Biologie und Biotechnologie, Ruhr-Universität Bochum, Bochum, Germany, 2012. [Google Scholar]
- Ursic-Bedoya, R.J.; Nazzari, H.; Cooper, D.; Triana, O.; Wolff, M.; Lowenberger, C. Identification and characterization of two novel lysozymes from Rhodnius prolixus, a vector of Chagas disease. J. Insect Physiol. 2008, 54, 593–603. [Google Scholar] [CrossRef]
- Waniek, P.J.; Jansen, A.M.; Araújo, C.A.C. Trypanosoma cruzi infection modulates the expression of Triatoma brasiliensis def1 in the midgut. Vector-Borne Zoonotic Dis. 2011, 1, 845–847. [Google Scholar] [CrossRef]
- Reynoso-Ducoing, O.A.; González-Rete, B.; Díaz, E.; Candelas-Otero, F.N.; López-Aviña, J.A.; Cabrera-Bravo, M.; Bucio-Torres, M.I.; Torres-Gutiérrez, E.; Salazar-Schettino, P.M. Expression of proteins, glycoproteins, and transcripts in the guts of fasting, fed, and Trypanosoma cruzi-infected triatomines: A systematic review. Pathogens 2023, 12, 1124. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Garrido, P.; Sepúlveda-Robles, O.; Martínez-Martínez, I.; Espinoza, B. Variability of defensin genes from a Mexican endemic Triatominae: Triatoma (Meccus) pallidipennis (Hemiptera: Reduviidae). Biosci. Rep. 2018, 38, BSR20180988, Erratum in Biosci. Rep. 2020, 40, BSR-20180988_COR. [Google Scholar] [CrossRef] [PubMed]
- da Mota, F.F.; Castro, D.P.; Vieira, C.S.; Gumiel, M.; de Albuquerque, J.P.; Carels, N.; Azambuja, P. In vitro trypanocidal activity, genomic analysis of isolates, and in vivo transcription of type VI secretion system of Serratia marcescens belonging to the microbiota of Rhodnius prolixus digestive tract. Front. Microbiol. 2019, 9, 3205. [Google Scholar] [CrossRef] [PubMed]
- Gumiel, M.; da Mota, F.F.; de Sousa Rizzo, V.; Sarquis, O.; De Castro, D.P.; Lima, M.M.; de Souza Garcia, E.; Carels, N.; Azambuja, P. Characterization of the microbiota in the guts of Triatoma brasiliensis and Triatoma pseudomaculata infected by Trypanosoma cruzi in natural conditions using culture independent methods. Parasites Vectors 2015, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Cordon-Rosales, C.; Durvasula, R.V. Bacterial symbionts of the triatominae and their potential use in control of Chagas disease transmission. Annu. Rev. Entomol. 2002, 47, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, R.V.; Sundaram, R.K.; Kirsch, P.; Hurwitz, I.; Crawford, C.V.; Dotson, E.; Beard, C.B. Genetic transformation of a corynebacterial symbiont from the Chagas disease vector Triatoma infestans. Exp. Parasitol. 2008, 119, 94–98. [Google Scholar] [CrossRef]
- Jose, C.; Klein, N.; Wyss, S.; Fieck, A.; Hurwitz, I.; Durvasula, R. Recombinant Arthrobacter β-1, 3-glucanase as a potential effector molecule for paratransgenic control of Chagas disease. Parasites Vectors 2013, 6, 65. [Google Scholar] [CrossRef]
- Taracena, M.L.; Oliveira, P.L.; Almendares, O.; Umaña, C.; Lowenberger, C.; Dotson, E.M.; Paiva-Silva, G.O.; Pennington, P.M. Genetically modifying the insect gut microbiota to control Chagas disease vectors through systemic RNAi. PLoS Negl. Trop. Dis. 2015, 9, e0003358. [Google Scholar] [CrossRef] [PubMed]
- Whitten, M.M.; Facey, P.D.; Del Sol, R.; Fernández-Martínez, L.T.; Evans, M.C.; Mitchell, J.J.; Bodger, O.G.; Dyson, P.J. Symbiont-mediated RNA interference in insects. Proc. Biol. Sci. 2016, 283, 20160042. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Furtado Pacheco, J.P.; Dyson, P.; Castro, H.C.; Gonzalez, M.S.; Azambuja, P.; Mello, C.B. Overview of paratransgenesis as a strategy to control pathogen transmission by insect vectors. Parasites Vectors 2022, 15, 112. [Google Scholar] [CrossRef]
- Jensen, C.; Schaub, G.A. Development of Blastocrithidia triatomae (Trypanosomatidae) in Triatoma infestans after vitamin B-supplementation of the blood-diet of the bug. Eur. J. Protistol. 1991, 27, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005, 21, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Teotônio, I.M.S.N.; Dias, N.; Hagström-Bex, L.; Nitz, N.; Francisco, A.F.; Hecht, M. Intestinal microbiota—A modulator of the Trypanosoma cruzi-vector-host triad. Microb. Pathog. 2019, 137, 103711. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Silva, E.; Morais, L.H.; Clarke, G.; Savino, W.; Peixoto, C. Targeting the gut microbiota in Chagas disease: What do we know so far? Front. Microbiol. 2020, 11, 585857. [Google Scholar] [CrossRef]
- Castro, D.P.; Moraes, C.S.; Gonzalez, M.S.; Ratcliffe, N.A.; Azambuja, P.; Garcia, E.S. Trypanosoma cruzi immune response modulation decreases microbiota in Rhodnius prolixus gut and is crucial for parasite survival and development. PLoS ONE 2012, 7, e36591. [Google Scholar] [CrossRef]
- Batista, K.K.S.; Vieira, C.S.; Florentino, E.B.; Caruso, K.F.B.; Teixeira, P.T.P.; Moraes, C.D.S.; Genta, F.A.; de Azambuja, P.; de Castro, D.P. Nitric oxide effects on Rhodnius prolixus’s immune responses, gut microbiota and Trypanosoma cruzi development. J Insect Physiol. 2020, 126, 104100. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.P.; Moraes, C.S.; Gonzalez, M.S.; Ribeiro, I.M.; Tomassini, T.C.B.; Azambuja, P.; Garcia, E.S. Physalin B inhibits Trypanosoma cruzi infection in the gut of Rhodnius prolixus by affecting the immune system and microbiota. J. Insect Physiol. 2012, 58, 1620–1625. [Google Scholar] [CrossRef]
- Araújo, C.A.C.; Pacheco, J.P.F.; Waniek, P.J.; Geraldo, R.B.; Sibajev, A.; Dos Santos, A.L.; Evangelho, V.G.O.; Dyson, P.J.; Azambuja, P.; Ratcliffe, N.A.; et al. A rhamnose-binding lectin from Rhodnius prolixus and the impact of its silencing on gut bacterial microbiota and Trypanosoma cruzi. Dev. Comp. Immunol. 2021, 114, 103823. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortés, J.G.; García-Contreras, R.; Bucio-Torres, M.I.; Cabrera-Bravo, M.; López-Jácome, L.E.; Franco-Cendejas, R.; Vences-Blanco, M.O.; Salazar-Schettino, P.M. Bacteria cultured from the gut of Meccus pallidipennis (Hemiptera: Reduviidae), a triatomine species endemic to Mexico. Med. Vet. Entomol. 2021, 35, 478–483. [Google Scholar] [CrossRef]
- Eichler, S. Interaktionen von Triatominen mit Ihren Symbionten und Trypanosomatiden. Ph.D. Thesis, Fakultät für Biologie und Biotechnologie, Ruhr-Universität Bochum, Bochum, Germany, 1998. (In German). [Google Scholar]
- Waltmann, A.; Willcox, A.C.; Balasubramanian, S.; Mayori, K.B.; Guerrero, S.M.; Sanchez, R.S.; Roach, J.; Pino, C.C.; Gilman, R.H.; Bern, C.; et al. Hindgut microbiota in laboratory-reared and wild Triatoma infestans. PLoS Negl. Trop. Dis. 2019, 13, e0007383. [Google Scholar] [CrossRef]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef]
- Espino, C.I.; Gómez, T.; González, G.; do Santos, M.F.; Solano, J.; Sousa, O.; Moreno, N.; Windsor, D.; Ying, A.; Vilchez, S.; et al. Detection of Wolbachia bacteria in multiple organs and feces of the triatomine insect Rhodnius pallescens (Hemiptera; Reduviidae). Appl. Environ. Microbiol. 2009, 75, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Mühlpfordt, H. Der Einfluß der Darmsymbionten von Rhodnius prolixus auf Trypanosoma cruzi. Z. Tropenmed. Parasitol. 1959, 10, 314–327. (In German) [Google Scholar]
- Mesquita, R.D.; Vionette-Amaral, R.J.; Lowenberger, C.; Rivera-Pomar, R.; Monteiro, F.A.; Minx, P.; Spieth, J.; Carvalho, A.B.; Panzera, F.; Lawson, D.; et al. Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc. Natl. Acad. Sci. USA 2015, 112, 14936–14941, Erratum in Proc. Natl. Acad. Sci. USA 2016, 113, E1415–E1416. [Google Scholar] [CrossRef] [PubMed]
- Brener, Z. The behavior of slender and stout forms of Trypanosoma cruzi in the blood-stream of normal and immune mice. Ann. Trop. Med. Parasitol. 1969, 63, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Saavedra, L.; Vallejo, G.A.; Guhl, F.; Messenger, L.A.; Ramírez, J.D. Transcriptional remodeling during metacyclogenesis in Trypanosoma cruzi I. Virulence 2020, 11, 969–980. [Google Scholar] [CrossRef] [PubMed]
- García-Huertas, P.; Cuesta-Astroz, Y.; Araque-Ruiz, V.; Cardona-Castro, N. Transcriptional changes during metacyclogenesis of a Colombian Trypanosoma cruzi strain. Parasitol. Res. 2023, 122, 625–634. [Google Scholar] [CrossRef]
- Ouali, R.; Vieira, L.R.; Salmon, D.; Bousbata, S. Early post-prandial regulation of protein expression in the midgut of Chagas disease vector Rhodnius prolixus highlights new potential targets for vector control strategy. Microorganisms 2021, 9, 804. [Google Scholar] [CrossRef]
- Kwakye-Nuako, G.; Middleton, C.E.; McCall, L.I. Small molecule mediators of host-T. cruzi-environment interactions in Chagas disease. PLoS Pathog. 2024, 20, e1012012. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Karafova, A.; Kamysz, W.; Schenkman, S.; Pelle, R.; McGwire, B.S. Secreted trypanosome cyclophilin inactivates lytic insect defense peptides and induces parasite calcineurin activation and infectivity. J. Biol. Chem. 2013, 288, 8772–8784. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, I. Yeast-insect associations: It takes guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef]
| Country | DTUs [43] | Triatomines [44,45] | |||||
|---|---|---|---|---|---|---|---|
| TcI | TcII | TcIII | TcIV | TcV | TcVI | ||
| Argentina | + | + | + | + | + | T. infestans, T. guasayana, T. patagonica, T. sordida | |
| Belize | + | + | T. dimidiata, R. pallescens | ||||
| Bolivia | + | + | + | + | + | + | T. infestans, T. sordida |
| Brazil | + | + | + | + | + | + | T. infestans, T. brasiliensis, T. sordida, P. geniculatus, P. megistus |
| Chile | + | + | + | + | + | T. infestans | |
| Colombia | + | + | + | + | + | + | T. dimidiata, R. prolixus, P. geniculatus |
| Costa Rica | + | T. dimidiata,P. geniculatus | |||||
| Ecuador | + | + | + | + | + | T. dimidiata, T. carrioni, R. prolixus, P. rufotuberculatus, P. geniculatus | |
| El Salvador | + | + | + | T. dimidiata | |||
| French Guiana | + | R. prolixus | |||||
| Guyana | + | R. prolixus | |||||
| Guatemala | + | T. dimidiata | |||||
| Honduras | + | + | + | + | T. dimidiata | ||
| Mexico | + | + | + | + | + | + | T. dimidiata, T. protracta, T. barberi, M. longipennis, M. pallidipennis |
| Nicaragua | + | T. dimidiata, P. geniculatus | |||||
| Panama | + | + | + | + | T. dimidiata, R. pallescens, P. geniculatus | ||
| Paraguay | + | + | + | + | + | T. infestans, T. sordida, P. geniculatus | |
| Peru | + | + | + | + | T. infestans, P. chinai, P. geniculatus, R. ecuadoriensis | ||
| Suriname | + | R. prolixus,P. geniculatus | |||||
| Uruguay | + | + | + | T. infestans, T. sordida, P. geniculatus, P. megistus | |||
| USA | + | + | + | + | + | T. sanguisuga, T. lecticularia, T. protracta | |
| Venezuela | + | + | + | + | + | + | T. dimidiata, T. maculata, R. prolixus, P. rufotuberculatus, P. geniculatus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaub, G.A. Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors—A Review. Microorganisms 2024, 12, 855. https://doi.org/10.3390/microorganisms12050855
Schaub GA. Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors—A Review. Microorganisms. 2024; 12(5):855. https://doi.org/10.3390/microorganisms12050855
Chicago/Turabian StyleSchaub, Günter A. 2024. "Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors—A Review" Microorganisms 12, no. 5: 855. https://doi.org/10.3390/microorganisms12050855
APA StyleSchaub, G. A. (2024). Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors—A Review. Microorganisms, 12(5), 855. https://doi.org/10.3390/microorganisms12050855






