The Impact of Artificial Intelligence on Microbial Diagnosis
Abstract
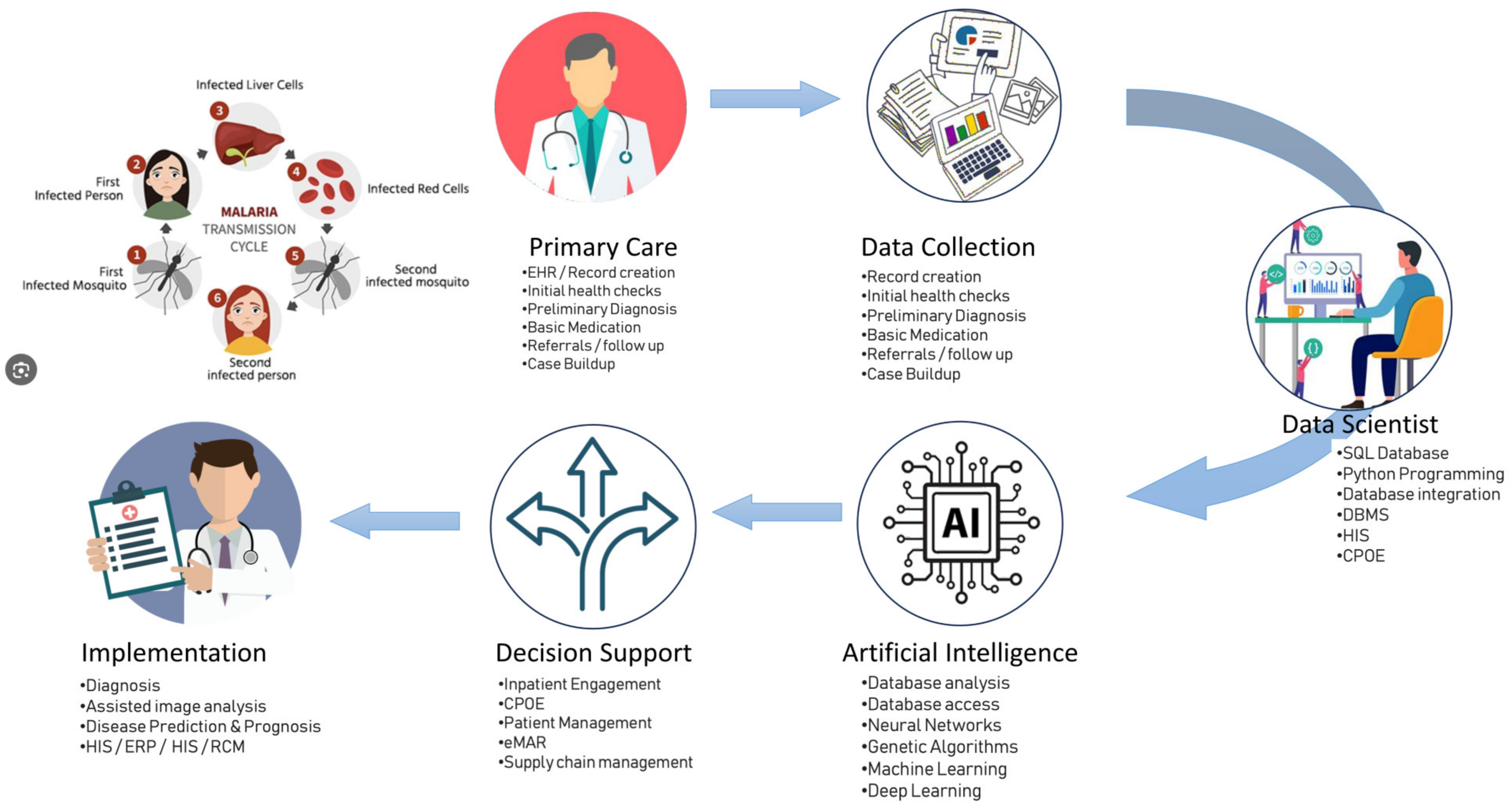
:1. Introduction
1.1. AI and Machine Learning in Human Health
1.2. AI Integration in Microbial Diagnosis
2. The Impact of AI and Convolutional Neural Networks on the Diagnosis of Various Infectious Diseases
2.1. SARS-CoV-2
2.2. Malaria
2.3. Mycobacteria
3. Impact of AI in Revolutionizing Diagnostic Microbiology
3.1. Whole-Slide Imaging and Microbial Cytopathology
3.2. Detection and Characterization of Infectious Diseases
3.3. AI-Enhanced Microscopes for Automated Microbial Classification
3.4. Revolutionizing Colony Counting
3.5. Advancements in AI Applications for Antimicrobial Susceptibility Testing
4. Conclusions: Challenges and Future Directions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in chemical and biological methods to identify microorganisms—From past to present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Barh, D. Artificial Intelligence in Precision Health: From Concept to Applications; Academic Press: London, UK, 2020. [Google Scholar]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.-M.; Zietz, M.; Hoffman, M.M.; et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 2018, 15, 20170387. [Google Scholar] [CrossRef] [PubMed]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Naik, N.; Hameed, B.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R. Legal and ethical consideration in artificial intelligence in healthcare: Who takes responsibility? Front. Surg. 2022, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Ahmed, S.; Aslam, M. Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation. Antibiotics 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G. The Role of ChatGPT, Generative Language Models, and Artificial Intelligence in Medical Education: A Conversation With ChatGPT and a Call for Papers. JMIR Med. Educ 2023, 9, e46885. [Google Scholar] [CrossRef] [PubMed]
- Alto, V. Modern Generative AI with ChatGPT and OpenAI Models: Leverage the Capabilities of OpenAI’s LLM for Productivity and Innovation with GPT3 and GPT4; Packt Publishing Ltd.: Birmingham, UK, 2023. [Google Scholar]
- Talaei-Khoei, A.; Yang, A.T.; Masialeti, M. How does incorporating ChatGPT within a firm reinforce agility-mediated performance? The moderating role of innovation infusion and firms’ ethical identity. Technovation 2024, 132, 102975. [Google Scholar] [CrossRef]
- Deo, R.C. Machine learning in medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Ragab, M.; Albukhari, A.; Alyami, J.; Mansour, R.F. Ensemble deep-learning-enabled clinical decision support system for breast cancer diagnosis and classification on ultrasound images. Biology 2022, 11, 439. [Google Scholar] [CrossRef]
- Kang, J.; Choi, Y.J.; Kim, I.-K.; Lee, H.S.; Kim, H.; Baik, S.H.; Kim, N.K.; Lee, K.Y. LASSO-based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2021, 53, 773. [Google Scholar] [CrossRef] [PubMed]
- Song, S.E.; Cho, K.R.; Cho, Y.; Kim, K.; Jung, S.P.; Seo, B.K.; Woo, O.H. Machine learning with multiparametric breast MRI for prediction of Ki-67 and histologic grade in early-stage luminal breast cancer. Eur. Radiol. 2022, 32, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Bulten, W.; Kartasalo, K.; Chen, P.-H.C.; Strom, P.; Pinckaers, H.; Nagpal, K.; Cai, Y.; Steiner, D.F.; van Boven, H.; Vink, R.; et al. Artificial intelligence for diagnosis and Gleason grading of prostate cancer: The PANDA challenge. Nat. Med. 2022, 28, 154–163. [Google Scholar] [CrossRef]
- Acs, B.; Rantalainen, M.; Hartman, J. Artificial intelligence as the next step towards precision pathology. J. Intern. Med. 2020, 288, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xie, L.; Han, J.; Guo, X. The application of deep learning in cancer prognosis prediction. Cancers 2020, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S.P. Artificial intelligence (AI) and internet of medical things (IoMT) assisted biomedical systems for intelligent healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Prezja, F.; Äyrämö, S.; Pölönen, I.; Ojala, T.; Lahtinen, S.; Ruusuvuori, P.; Kuopio, T. Improved accuracy in colorectal cancer tissue decomposition through refinement of established deep learning solutions. Sci. Rep. 2023, 13, 15879. [Google Scholar] [CrossRef] [PubMed]
- Mitsala, A.; Tsalikidis, C.; Pitiakoudis, M.; Simopoulos, C.; Tsaroucha, A.K. Artificial intelligence in colorectal cancer screening, diagnosis and treatment. A new era. Curr. Oncol. 2021, 28, 1581–1607. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.d.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Wang, C.; Dong, R.; Wang, X.; Lian, A.; Chi, C.; Ke, C.; Guo, L.; Liu, S.; Zhao, W.; Xu, G.; et al. Exhaled volatile organic compounds as lung cancer biomarkers during one-lung ventilation. Sci. Rep. 2014, 4, 7312. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Fijten, R.; Smolinska, A.; Dallinga, J.; Boumans, M.-L.; Stobberingh, E.; Boots, A.; Roekaerts, P.; Bergmans, D.; van Schooten, F.J. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci. Rep. 2015, 5, 17179. [Google Scholar] [CrossRef] [PubMed]
- Banday, K.M.; Pasikanti, K.K.; Chan, E.C.Y.; Singla, R.; Rao, K.V.S.; Chauhan, V.S.; Nanda, R.K. Use of urine volatile organic compounds to discriminate tuberculosis patients from healthy subjects. Anal. Chem. 2011, 83, 5526–5534. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Westenbrink, E.; McFarlane, M.J.; Harbord, R.; Chambers, S.; O’connell, N.; Bailey, C.; Nwokolo, C.U.; Bardhan, K.D.; Savage, R.; et al. Differentiating coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis–a pilot study. PLoS ONE 2014, 9, e107312. [Google Scholar] [CrossRef] [PubMed]
- Audrain, B.; Farag, M.A.; Ryu, C.-M.; Ghigo, J.-M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Smolinska, A.; van Berkel, J.J.B.N.; Fijten, R.R.R.; Stobberingh, E.E.; Boumans, M.L.L.; Moonen, E.J.; Wouters, E.F.M.; Dallinga, J.W.; Van Schooten, F.J. Identification of microorganisms based on headspace analysis of volatile organic compounds by gas chromatography–mass spectrometry. J. Breath Res. 2014, 8, 027106. [Google Scholar] [CrossRef]
- Rees, C.A.; Shen, A.; Hill, J. E Characterization of the Clostridium difficile volatile metabolome using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. B 2016, 1039, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.J.; Jeavons, T.H.; Nicholson, A.J.; Thornton, A.G. Development of specific tests for rapid detection of Escherichia coli and all species of Proteus in urine. J. Clin. Microbiol. 1977, 6, 195–201. [Google Scholar] [CrossRef]
- Muto-Fujita, A.; Takemoto, K.; Kanaya, S.; Nakazato, T.; Tokimatsu, T.; Matsumoto, N.; Kono, M.; Chubachi, Y.; Ozaki, K.; Kotera, M. Data integration aids understanding of butterfly–host plant networks. Sci. Rep. 2017, 7, 43368. [Google Scholar] [CrossRef]
- Libbrecht, M.W.; Noble, W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015, 16, 321–332. [Google Scholar] [CrossRef]
- Castelvecchi, D. Can we open the black box of AI? Nat. News 2016, 538, 20. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Palma, S.I.C.J.; Traguedo, A.P.; Porteira, A.R.; Frias, M.J.; Gamboa, H.; Roque, A.C.A. Machine learning for the meta-analyses of microbial pathogens’ volatile signatures. Sci. Rep. 2018, 8, 3360. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.-O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef]
- Zhang, T. An introduction to support vector machines and other kernel-based learning methods. AI Mag. 2001, 22, 103. [Google Scholar]
- Kim, J.I.; Maguire, F.; Tsang, K.K.; Gouliouris, T.; Peacock, S.J.; McAllister, T.A.; McArthur, A.G.; Beiko, R.G. Machine learning for antimicrobial resistance prediction: Current practice, limitations, and clinical perspective. Clin. Microbiol. Rev. 2022, 35, e00179-21. [Google Scholar] [CrossRef] [PubMed]
- Her, H.-L.; Wu, Y.-W. A pan-genome-based machine learning approach for predicting antimicrobial resistance activities of the Escherichia coli strains. Bioinformatics 2018, 34, i89–i95. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.C.; Kavvas, E.S.; Monk, J.M.; Palsson, B.O. Machine learning with random subspace ensembles identifies antimicrobial resistance determinants from pan-genomes of three pathogens. PLoS Comput. Biol. 2020, 16, e1007608. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Drancourt, M.; Colson, P.; Rolain, J.-M. Modern clinical microbiology: New challenges and solutions. Nat. Rev. Microbiol. 2013, 11, 574–585. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Wang, H.; Durant, T.J.; Mathison, B.A.; Sharp, S.E.; Kirby, J.E.; Long, S.W.; Rhoads, D.D. Applications of artificial intelligence in clinical microbiology diagnostic testing. Clin. Microbiol. Newsl. 2020, 42, 61–70. [Google Scholar] [CrossRef]
- Nayak, D.S.K.; Mahapatra, S.; Routray, S.P.; Sahoo, S.; Sahoo, S.K.; Fouda, M.M.; Singh, N.; Isenovic, E.R.; Saba, L.; Suri, J.S.; et al. aiGeneR 1.0: An Artificial Intelligence Technique for the Revelation of Informative and Antibiotic Resistant Genes in Escherichia coli. Front. Biosci.-Landmark 2024, 29, 82. [Google Scholar] [CrossRef]
- Samantray, D.; Tanwar, A.S.; Murali, T.S.; Brand, A.; Satyamoorthy, K.; Paul, B. A Comprehensive Bioinformatics Resource Guide for Genome-Based Antimicrobial Resistance Studies. OMICS A J. Integr. Biol. 2023, 27, 445–460. [Google Scholar] [CrossRef]
- Sunuwar, J.; Azad, R.K. A machine learning framework to predict antibiotic resistance traits and yet unknown genes underlying resistance to specific antibiotics in bacterial strains. Brief. Bioinform. 2021, 22, bbab179. [Google Scholar] [CrossRef]
- Rodrigues, C.; Groves, H. Community-acquired pneumonia in children: The challenges of microbiological diagnosis. J. Clin. Microbiol. 2018, 56, e01318-17. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Pokharel, K. Standard operating procedure for specimen collection, packaging and transport for diagnosis of SARS-COV-2. JNMA J. Nepal Med. Assoc. 2020, 58, 627. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.M.; Somoskövi, Á.; Gutierrez, C.; Lee, E.; Paramasivan, C.N.; Abimiku, A. Laboratory diagnosis of tuberculosis in resource-poor countries: Challenges and opportunities. Clin. Microbiol. Rev. 2011, 24, 314–350. [Google Scholar] [CrossRef]
- Peri, A.M.; Stewart, A.; Hume, A.; Irwin, A.; A Harris, P.N. New microbiological techniques for the diagnosis of bacterial infections and sepsis in ICU including point of care. Curr. Infect. Dis. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Ruddy, M.; McHugh, T.D.; Dale, J.W.; Banerjee, D.; Maguire, H.; Wilson, P.; Drobniewski, F.; Butcher, P.; Gillespie, S.H. Estimation of the rate of unrecognized cross-contamination with Mycobacterium tuberculosis in London microbiology laboratories. J. Clin. Microbiol. 2002, 40, 4100–4104. [Google Scholar] [CrossRef]
- Agarwal, R. Quality-improvement measures as effective ways of preventing laboratory errors. Lab. Med. 2014, 45, e80–e88. [Google Scholar] [CrossRef]
- Kazancigil, M.A. Big medical data, cloud computing, and artificial intelligence for improving diagnosis in healthcare. In Big Data Analytics for Healthcare; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–150. [Google Scholar]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94. [Google Scholar] [CrossRef]
- Agbehadji, I.E.; Awuzie, B.O.; Ngowi, A.B.; Millham, R.C. Review of big data analytics, artificial intelligence and nature-inspired computing models towards accurate detection of COVID-19 pandemic cases and contact tracing. Int. J. Environ. Res. Public Health 2020, 17, 5330. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alhumaid, S.; Al Mutair, A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Al Bshabshe, A.; Sulaiman, T.; AlFonaisan, M.K.; et al. Application of artificial intelligence in combating high antimicrobial resistance rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef]
- Májek, P.; Lüftinger, L.; Beisken, S.; Rattei, T.; Materna, A. Genome-wide mutation scoring for machine-learning-based antimicrobial resistance prediction. Int. J. Mol. Sci. 2021, 22, 13049. [Google Scholar] [CrossRef]
- Behara, K.; Bhero, E.; Agee, J.T.; Gonela, V. Artificial intelligence in medical diagnostics: A review from a South African context. Sci. Afr. 2022, 17, e01360. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Bagabir, S.A.; Ibrahim, N.K.; Bagabir, H.A.; Ateeq, R.H. Covid-19 and Artificial Intelligence: Genome sequencing, drug development and vaccine discovery. J. Infect. Public Health 2022, 15, 289–296. [Google Scholar] [CrossRef]
- Chiu, H.-Y.R.; Hwang, C.-K.; Chen, S.-Y.; Shih, F.-Y.; Han, H.-C.; King, C.-C.; Gilbert, J.R.; Fang, C.-C.; Oyang, Y.-J. Machine learning for emerging infectious disease field responses. Sci. Rep. 2022, 12, 328. [Google Scholar] [CrossRef]
- Pham, T.-H.; Qiu, Y.; Zeng, J.; Xie, L.; Zhang, P. A deep learning framework for high-throughput mechanism-driven phenotype compound screening and its application to COVID-19 drug repurposing. Nat. Mach. Intell. 2021, 3, 247–257. [Google Scholar] [CrossRef]
- Ong, E.; Wong, M.U.; Huffman, A.; He, Y. COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front. Immunol. 2020, 11, 1581. [Google Scholar] [CrossRef]
- Ong, E.; Cooke, M.F.; Huffman, A.; Xiang, Z.; Wong, M.U.; Wang, H.; Seetharaman, M.; Valdez, N.; He, Y. Vaxign2: The second generation of the first Web-based vaccine design program using reverse vaccinology and machine learning. Nucleic Acids Res. 2021, 49, W671–W678. [Google Scholar] [CrossRef]
- Dutta, D.; Naiyer, S.; Mansuri, S.; Soni, N.; Singh, V.; Bhat, K.H. COVID-19 diagnosis: A comprehensive review of the RT-qPCR method for detection of SARS-CoV-2. Diagnostics 2022, 12, 1503. [Google Scholar] [CrossRef]
- Alouani, D.J.; Rajapaksha, R.R.; Jani, M.; Rhoads, D.D.; Sadri, N. Specificity of SARS-CoV-2 real-time PCR improved by deep learning analysis. J. Clin. Microbiol. 2021, 59, e00461-20. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.-S.; Lee, D.-I.; Jeong, S.; Kang, G.-H.; Jang, Y.S.; Kim, W.; Choi, H.Y.; Kim, J.G.; Choi, S.-H. The application of a deep learning system developed to reduce the time for RT-PCR in COVID-19 detection. Sci. Rep. 2022, 12, 1234. [Google Scholar] [CrossRef]
- Özbilge, E.; Sanlidag, T.; Ozbilge, E.; Baddal, B. Artificial Intelligence-Assisted RT-PCR Detection Model for Rapid and Reliable Diagnosis of COVID-19. Appl. Sci. 2022, 12, 9908. [Google Scholar] [CrossRef]
- Villarreal-González, R.; Acosta-Hoyos, A.J.; Garzon-Ochoa, J.A.; Galán-Freyle, N.J.; Amar-Sepúlveda, P.; Pacheco-Londoño, L.C. Anomaly identification during polymerase chain reaction for detecting SARS-CoV-2 using artificial intelligence trained from simulated data. Molecules 2020, 26, 20. [Google Scholar] [CrossRef]
- Cabrera Alvargonzález, J.; Larrañaga Janeiro, A.; Pérez Castro, S.; Martínez Torres, J.; Martínez Lamas, L.; Daviña Nuñez, C. Proof of concept of the potential of a machine learning algorithm to extract new information from conventional SARS-CoV-2 rRT-PCR results. Sci. Rep. 2023, 13, 7786. [Google Scholar] [CrossRef]
- Beduk, D.; de Oliveira Filho, J.I.; Beduk, T.; Harmanci, D.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; et al. ‘All In One’ SARS-CoV-2 variant recognition platform: Machine learning-enabled point of care diagnostics. Biosens. Bioelectron. X 2022, 10, 100105. [Google Scholar] [CrossRef]
- Tschoellitsch, T.; Dünser, M.; Böck, C.; Schwarzbauer, K.; Meier, J. Machine learning prediction of SARS-CoV-2 polymerase chain reaction results with routine blood tests. Lab. Med. 2021, 52, 146–149. [Google Scholar] [CrossRef]
- Brinati, D.; Campagner, A.; Ferrari, D.; Locatelli, M.; Banfi, G.; Cabitza, F. Detection of COVID-19 infection from routine blood exams with machine learning: A feasibility study. J. Med. Syst. 2020, 44, 135. [Google Scholar] [CrossRef]
- Yang, H.S.; Hou, Y.; Vasovic, L.V.; Steel, P.A.; Chadburn, A.; Racine-Brzostek, S.E. Routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. Clin. Chem. 2020, 66, 1396–1404. [Google Scholar] [CrossRef]
- Rocca, M.F.; Zintgraff, J.C.; Dattero, M.E.; Santos, L.S.; Ledesma, M.; Vay, C.; Prieto, M.; Benedetti, E.; Avaro, M.; Russo, M.; et al. A combined approach of MALDI-TOF mass spectrometry and multivariate analysis as a potential tool for the detection of SARS-CoV-2 virus in nasopharyngeal swabs. J. Virol. Methods 2020, 286, 113991. [Google Scholar] [CrossRef]
- Le, A.T.; Wu, M.; Khan, A.; Phillips, N.; Rajpurkar, P.; Garland, M.; Magid, K.; Sibai, M.; Huang, C.; Sahoo, M.K.; et al. Targeted plasma metabolomics combined with machine learning for the diagnosis of severe acute respiratory syndrome virus type 2. Front. Microbiol. 2023, 13, 1059289. [Google Scholar] [CrossRef]
- Rosado, J.; Pelleau, S.; Cockram, C.; Merkling, S.H.; Nekkab, N.; Demeret, C.; Meola, A.; Kerneis, S.; Terrier, B.; Fafi-Kremer, S.; et al. Multiplex assays for the identification of serological signatures of SARS-CoV-2 infection: An antibody-based diagnostic and machine learning study. Lancet Microbe 2021, 2, e60–e69. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Mpesiana, T.A. Covid-19: Automatic detection from X-ray images utilizing transfer learning with convolutional neural networks. Phys. Eng. Sci. Med. 2020, 43, 635–640. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, V.; Vaishali; Kaur, M. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution–based convolutional neural networks. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1379–1389. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.-C.; Diao, K.-Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef]
- Jia, L.-L.; Zhao, J.-X.; Pan, N.-N.; Shi, L.-Y.; Zhao, L.-P.; Tian, J.-H.; Huang, G. Artificial intelligence model on chest imaging to diagnose COVID-19 and other pneumonias: A systematic review and meta-analysis. Eur. J. Radiol. Open 2022, 9, 100438. [Google Scholar] [CrossRef]
- Tzeng, I.-S.; Hsieh, P.-C.; Su, W.-L.; Hsieh, T.-H.; Chang, S.-C. Artificial Intelligence-assisted chest X-ray for the diagnosis of COVID-19: A systematic review and meta-analysis. Diagnostics 2023, 13, 584. [Google Scholar] [CrossRef]
- Chang, S.; Ge, C.; Zhang, L.; Xie, L.; Kong, R.; Zhang, H. COVID-19 imaging-based AI research-a literature review. Curr. Med. Imaging 2022, 18, 496–508. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Zhang, L.; Xie, L. Diagnostic performance of corona virus disease 2019 chest computer tomography image recognition based on deep learning: Systematic review and meta-analysis. Medicine 2022, 101, e31346. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Zhou, B.; Sohn, J.J.; Zhou, J.; Jacob, J.T.; Higgins, K.A.; Bradley, J.D.; Liu, T. Review of machine learning in lung ultrasound in COVID-19 pandemic. J. Imaging 2022, 8, 65. [Google Scholar] [CrossRef]
- Ozsahin, I.; Sekeroglu, B.; Musa, M.S.; Mustapha, M.T.; Ozsahin, D.U. Review on diagnosis of COVID-19 from chest CT images using artificial intelligence. Comput. Math. Methods Med. 2020, 2020, 9756518. [Google Scholar] [CrossRef]
- Abayomi-Alli, O.O.; Damaševičius, R.; Maskeliūnas, R.; Misra, S. An ensemble learning model for COVID-19 detection from blood test samples. Sensors 2022, 22, 2224. [Google Scholar] [CrossRef]
- Nachtigall, F.M.; Pereira, A.; Trofymchuk, O.S.; Santos, L.S. Detection of SARS-CoV-2 in nasal swabs using MALDI-MS. Nat. Biotechnol. 2020, 38, 1168–1173. [Google Scholar] [CrossRef]
- Costa, M.M.; Martin, H.; Estellon, B.; Dupé, F.-X.; Saby, F.; Benoit, N. Exploratory study on application of MALDI-TOF-MS to detect SARS-CoV-2 infection in human saliva. J. Clin. Med. 2022, 11, 295. [Google Scholar] [CrossRef]
- Cobre, A.d.F.; Surek, M.; Stremel, D.P.; Fachi, M.M.; Borba, H.H.L.; Tonin, F.S.; Pontarolo, R. Diagnosis and prognosis of COVID-19 employing analysis of patients’ plasma and serum via LC-MS and machine learning. Comput. Biol. Med. 2022, 146, 105659. [Google Scholar]
- Ikponmwoba, E.; Ukorigho, O.; Moitra, P.; Pan, D.; Gartia, M.R.; Owoyele, O. A machine learning framework for detecting COVID-19 infection using surface-enhanced Raman scattering. Biosensors 2022, 12, 589. [Google Scholar] [CrossRef]
- Venkatesan, P. The future of malaria control in light of RTS, S. Lancet Microbe 2022, 3, e251. [Google Scholar] [CrossRef]
- Fambirai, T.; Chimbari, M.J.; Ndarukwa, P. Global Cross-Border Malaria Control Collaborative Initiatives: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 12216. [Google Scholar] [CrossRef]
- Loddo, A.; Fadda, C.; Di Ruberto, C. An empirical evaluation of convolutional networks for malaria diagnosis. J. Imaging 2022, 8, 66. [Google Scholar] [CrossRef]
- Kassim, Y.M.; Yang, F.; Yu, H.; Maude, R.J.; Jaeger, S. Diagnosing malaria patients with Plasmodium falciparum and vivax using deep learning for thick smear images. Diagnostics 2021, 11, 1994. [Google Scholar] [CrossRef]
- Rajaraman, S.; Silamut, K.; Hossain, A.; Ersoy, I.; Maude, R.J.; Jaeger, S.; Thoma, G.R.; Antani, S.K. Understanding the learned behavior of customized convolutional neural networks toward malaria parasite detection in thin blood smear images. J. Med. Imaging 2018, 5, 034501. [Google Scholar] [CrossRef]
- Horning, M.P.; Delahunt, C.B.; Bachman, C.M.; Luchavez, J.; Luna, C.; Hu, L.; Jaiswal, M.S.; Thompson, C.M.; Kulhare, S.; Janko, S.; et al. Performance of a fully-automated system on a WHO malaria microscopy evaluation slide set. Malar. J. 2021, 20, 110. [Google Scholar] [CrossRef]
- Loh, D.R.; Yong, W.X.; Yapeter, J.; Subburaj, K.; Chandramohanadas, R. A deep learning approach to the screening of malaria infection: Automated and rapid cell counting, object detection and instance segmentation using Mask R-CNN. Comput. Med. Imaging Graph. 2021, 88, 101845. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.; Costa, M.G.F.; Barbosa, M.d.G.V.; Costa Filho, C.F.F. A new approach for malaria diagnosis in thick blood smear images. Biomed. Signal Process. Control 2022, 78, 103931. [Google Scholar] [CrossRef]
- Oliveira, A.d.S.; Costa, M.G.F.; Barbosa, M.d.G.V.; Filho, C.F.F.C. Performance analysis of deep learning algorithms in diagnosis of malaria disease. Diagnostics 2023, 13, 534. [Google Scholar] [CrossRef] [PubMed]
- Sengar, N.; Burget, R.; Dutta, M.K. A vision transformer based approach for analysis of plasmodium vivax life cycle for malaria prediction using thin blood smear microscopic images. Comput. Methods Programs Biomed. 2022, 224, 106996. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Rinehart, M.T.; Walzer, K.A.; Chi, J.-T.A.; Wax, A. Automated detection of P. falciparum using machine learning algorithms with quantitative phase images of unstained cells. PLoS ONE 2016, 11, e0163045. [Google Scholar] [CrossRef] [PubMed]
- Holmström, O.; Linder, N.; Ngasala, B.; Mårtensson, A.; Linder, E.; Lundin, M.; Moilanen, H.; Suutala, A.; Diwan, V.; Lundin, J. Point-of-care mobile digital microscopy and deep learning for the detection of soil-transmitted helminths and Schistosoma haematobium. Glob. Health Action 2017, 10 (Suppl. 3), 1337325. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Pathak, V.; Xi, H.; Chaware, A.; Cooke, C.; Kim, K.; Xu, S.; Li, Y.; Dunn, T.; Konda, P.C.; et al. Increasing a microscope’s effective field of view via overlapped imaging and machine learning. Opt. Express 2022, 30, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, F.; Fante, K.A.; Aliy, M. Malaria parasite detection in thick blood smear microscopic images using modified YOLOV3 and YOLOV4 models. BMC Bioinform. 2021, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.D.; Prats, C.; Espasa, M.; Serrat, F.Z.; Sales, C.M.; Silgado, A.; Codina, D.L.; Arruda, M.E.; i Prat, J.G.; Albuquerque, J. The malaria system microapp: A new, mobile device-based tool for malaria diagnosis. JMIR Res. Protoc. 2017, 6, e6758. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Poostchi, M.; Yu, H.; Zhou, Z.; Silamut, K.; Yu, J.; Maude, R.J.; Jaeger, S.; Antani, S. Deep learning for smartphone-based malaria parasite detection in thick blood smears. IEEE J. Biomed. Health Inform. 2019, 24, 1427–1438. [Google Scholar] [CrossRef]
- Rosado, L.; Da Costa, J.M.C.; Elias, D.; Cardoso, J.S. Mobile-based analysis of malaria-infected thin blood smears: Automated species and life cycle stage determination. Sensors 2017, 17, 2167. [Google Scholar] [CrossRef]
- Yu, H.; Yang, F.; Rajaraman, S.; Ersoy, I.; Moallem, G.; Poostchi, M.; Palaniappan, K.; Antani, S.; Maude, R.J.; Jaeger, S. Malaria Screener: A smartphone application for automated malaria screening. BMC Infect. Dis. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Sri, N.T.; Sahithi, P.; Lavanya, P.; Amrin, S. Deep learning for smart phone-based malaria parasite detection in thick blood smears. Turk. J. Comput. Math. Educ. (TURCOMAT) 2023, 14, 672–676. [Google Scholar]
- World Health Organization. World Health Organization Global Tuberculosis Report 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 18 May 2024).
- Xiong, Y.; Ba, X.; Hou, A.; Zhang, K.; Chen, L.; Li, T. Automatic detection of Mycobacterium tuberculosis using artificial intelligence. J. Thorac. Dis. 2018, 10, 1936. [Google Scholar] [CrossRef]
- Ibrahim, A.U.; Guler, E.; Guvenir, M.; Suer, K.; Serte, S.; Ozsoz, M. Automated detection of Mycobacterium tuberculosis using transfer learning. J. Infect. Dev. Ctries. 2021, 15, 678–686. [Google Scholar] [CrossRef]
- Panicker, R.O.; Kalmady, K.S.; Rajan, J.; Sabu, M.K. Automatic detection of tuberculosis bacilli from microscopic sputum smear images using deep learning methods. Biocybern. Biomed. Eng. 2018, 38, 691–699. [Google Scholar] [CrossRef]
- El-Melegy, M.; Mohamed, D.; ElMelegy, T. (Eds.) Automatic detection of tuberculosis bacilli from microscopic sputum smear images using faster r-cnn, transfer learning and augmentation. In Pattern Recognition and Image Analysis: 9th Iberian Conference, IbPRIA 2019, Madrid, Spain, 1–4 July 2019, Proceedings, Part I 9; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- CostaFilho, C.F.; Levy, P.C.; Xavier, C.M.; Costa, M.G.; Fujimoto, L.B.; Salem, J. (Eds.) Mycobacterium tuberculosis recognition with conventional microscopy. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: New York, NY, USA, 2012. [Google Scholar]
- Kuok, C.; Horng, M.; Liao, Y.; Chow, N.; Sun, Y. An effective and accurate identification system of Mycobacterium tuberculosis using convolution neural networks. Microsc. Res. Tech. 2019, 82, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Wu, U.; Seigh, L.; LoPresti, E.; Yeh, F.-C.; Salgia, P.; Michelow, P.; Hazelhurst, S.; Chen, W.-Y.; Hartman, D.; et al. Artificial intelligence–based screening for Mycobacteria in whole-slide images of tissue samples. Am. J. Clin. Pathol. 2021, 156, 117–128. [Google Scholar] [CrossRef]
- Yang, M.; Nurzynska, K.; Walts, A.E.; Gertych, A. A CNN-based active learning framework to identify mycobacteria in digitized Ziehl-Neelsen stained human tissues. Comput. Med. Imaging Graph. 2020, 84, 101752. [Google Scholar] [CrossRef]
- Horvath, L.; Hänselmann, S.; Mannsperger, H.; Degenhardt, S.; Last, K.; Zimmermann, S.; Burckhardt, I. Machine-assisted interpretation of auramine stains substantially increases through-put and sensitivity of microscopic tuberculosis diagnosis. Tuberculosis 2020, 125, 101993. [Google Scholar] [CrossRef]
- Nurzynska, K.; Li, D.; Walts, A.E.; Gertych, A. Multilayer outperforms single-layer slide scanning in AI-based classification of whole slide images with low-burden acid-fast mycobacteria (AFB). Comput. Methods Programs Biomed. 2023, 234, 107518. [Google Scholar] [CrossRef]
- Burns, B.L.; Rhoads, D.D.; Misra, A. The Use of Machine Learning for Image Analysis Artificial Intelligence in Clinical Microbiology. J. Clin. Microbiol. 2023, 61, e02336-21. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Marletta, S.; Brunelli, M.; Mescoli, C.; Parisi, A.; Barresi, V.; Parwani, A.; Neil, D.; Scarpa, A.; et al. Diagnostic concordance between whole slide imaging and conventional light microscopy in cytopathology: A systematic review. Cancer Cytopathol. 2020, 128, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Marletta, S.; Treanor, D.; Eccher, A.; Pantanowitz, L. Whole-slide imaging in cytopathology: State of the art and future directions. Diagn. Histopathol. 2021, 27, 425–430. [Google Scholar] [CrossRef]
- Franklin, M.M.; Schultz, F.A.; Tafoya, M.A.; Kerwin, A.A.; Broehm, C.J.; Fischer, E.G.; Gullapalli, R.R.; Clark, D.P.; Hanson, J.A.; Martin, D.R. A deep learning convolutional neural network can differentiate between Helicobacter pylori gastritis and autoimmune gastritis with results comparable to gastrointestinal pathologists. Arch. Pathol. Lab. Med. 2022, 146, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, C.; Kostopoulos, S.; Glotsos, D.; Kalatzis, I.; Asvestas, P.; Ravazoula, P.; Michail, G.; Cavouras, D.; Sakellaropoulos, G. Assessment of HPV Risk Type in H&E-stained Biopsy Specimens of the Cervix by Microscopy Image Analysis. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 702–710. [Google Scholar] [PubMed]
- Smith, K.P.; Kang, A.D.; Kirby, J.E. Automated interpretation of blood culture gram stains by use of a deep convolutional neural network. J. Clin. Microbiol. 2018, 56, e01521-17. [Google Scholar] [CrossRef]
- Sandle, T. Ready for the count? Back-to-basics review of microbial colony counting. J. GxP Compliance 2020, 24, 1–10. [Google Scholar]
- Sandle, T. Automated, Digital Colony Counting: Qualification and Data Integrity. J. GXP Compliance 2018, 22, 1–10. [Google Scholar]
- Zhang, J.; Li, C.; Rahaman, M.; Yao, Y.; Ma, P.; Zhang, J.; Zhao, X.; Jiang, T.; Grzegorzek, M. A comprehensive review of image analysis methods for microorganism counting: From classical image processing to deep learning approaches. Artif. Intell. Rev. 2022, 55, 2875–2944. [Google Scholar] [CrossRef]
- Bury, J.; Griffin, J. Digital pathology. In Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier: Amsterdam, The Netherlands, 2018; p. 476. [Google Scholar]
- Rahmani, A.M.; Azhir, E.; Ali, S.; Mohammadi, M.; Ahmed, O.H.; Ghafour, M.Y.; Ahmed, S.H.; Hosseinzadeh, M. Artificial intelligence approaches and mechanisms for big data analytics: A systematic study. PeerJ Comput. Sci. 2021, 7, e488. [Google Scholar] [CrossRef]
- DeYoung, B.; Morales, M.; Giglio, S. Microbiology 2.0–A “behind the scenes” consideration for artificial intelligence applications for interpretive culture plate reading in routine diagnostic laboratories. Front. Microbiol. 2022, 13, 2898. [Google Scholar] [CrossRef]
- Alouani, D.J.; Ransom, E.M.; Jani, M.; Burnham, C.-A.; Rhoads, D.D.; Sadri, N. Deep convolutional neural networks implementation for the analysis of urine culture. Clin. Chem. 2022, 68, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Han, L. Bioinformatics: Advancing biomedical discovery and innovation in the era of big data and artificial intelligence. Innov. Med. 2023, 1, 100012. [Google Scholar] [CrossRef]
- Vasala, A.; Hytonen, V.P.; Laitinen, O.H. Modern tools for rapid diagnostics of antimicrobial resistance. Front. Cell. Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Falk, T.; Mai, D.; Bensch, R.; Çiçek, Ö.; Abdulkadir, A.; Marrakchi, Y. U-Net: Deep learning for cell counting, detection, and morphometry. Nat. Methods 2019, 16, 67–70. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Dellière, S.; Rodriguez, C.; Birgand, G.; Lescure, F.-X.; Fourati, S.; Ruppé, E. Machine learning in the clinical microbiology laboratory: Has the time come for routine practice? Clin. Microbiol. Infect. 2020, 26, 1300–1309. [Google Scholar] [CrossRef]
- Rhoads, D.D. Computer vision and artificial intelligence are emerging diagnostic tools for the clinical microbiologist. J. Clin. Microbiol. 2020, 58, e00511-20. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’Hom, G.; Faverjon, F.; Rochais, Y.; Greub, G. Laboratory automation in clinical bacteriology: What system to choose? Clin. Microbiol. Infect. 2016, 22, 217–235. [Google Scholar] [CrossRef]
- Feucherolles, M.; Nennig, M.; Becker, S.L.; Martiny, D.; Losch, S.; Penny, C.; Cauchie, H.-M.; Ragimbeau, C. Combination of MALDI-TOF mass spectrometry and machine learning for rapid antimicrobial resistance screening: The case of Campylobacter spp. Front. Microbiol. 2022, 12, 804484. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial intelligence in disease diagnosis: A systematic literature review, synthesizing framework and future research agenda. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 8459–8486. [Google Scholar] [CrossRef]
- Mirbabaie, M.; Stieglitz, S.; Frick, N.R.J. Artificial intelligence in disease diagnostics: A critical review and classification on the current state of research guiding future direction. Health Technol. 2021, 11, 693–731. [Google Scholar] [CrossRef]
- Arora, G.; Joshi, J.; Mandal, R.S.; Shrivastava, N.; Virmani, R.; Sethi, T. Artificial intelligence in surveillance, diagnosis, drug discovery and vaccine development against COVID-19. Pathogens 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Gerke, S.; Minssen, T.; Cohen, G. Ethical and legal challenges of artificial intelligence-driven healthcare. In Artificial Intelligence in Healthcare; Academic Press: Cambridge, MA, USA, 2020; pp. 295–336. [Google Scholar]
- Williamson, S.M.; Prybutok, V. Balancing Privacy and Progress: A Review of Privacy Challenges, Systemic Oversight, and Patient Perceptions in AI-Driven Healthcare. Appl. Sci. 2024, 14, 675. [Google Scholar] [CrossRef]



| Study | AI Model/Method | Performance (Sensitivity and Specificity) |
|---|---|---|
| Alouani et al. [71] | Deep learning model (qPCRdeepNet) | Improved specificity of RT-PCR test for SARS-CoV-2 through deep convolutional neural network analysis of fluorescent readings |
| Lee et al. [72] | Deep learning model (LSTM) | Shortened RT-PCR diagnosis time using raw fluorescence data in each cycle, integrated with patient clinical characteristics, blood test results, and chest CT imaging data |
| AI-based detection system [73] | AI-based detection and classification system | Automated categorization of RT-PCR data (positive, weak-positive, negative, or re-run) |
| Villarreal-González et al. [74] | Various ML models | Detection of atypical RT-PCR profiles, reducing false positives |
| Alvargonzález et al. [75] | ML algorithm based on Ct values | Distinguishable patterns in Ct values aiding detection of SARS-CoV-2 variants |
| Beduk et al. [76] | Dense Neural Network algorithm | Detection of SARS-CoV-2 variants using laser-scribed graphene sensors coupled with gold nanoparticles biosensing platform |
| Tschoellitsch et al. [77] | Random Forest algorithm | Prediction of RT-PCR test results using routine blood test data |
| Brinati et al. [78] | ML classification models using hemato-chemical values | Detection of COVID-19 infection with high accuracy and sensitivity using routine blood exam data |
| Yang et al. [79] | ML model | Identification of useful routine blood parameters for COVID-19 diagnosis |
| Abayomi-Alli et al. [93] | Ensemble learning approach with 15 supervised ML algorithms | Effective detection of COVID-19 using routine laboratory blood test results |
| MALDI-TOF-MS combined with ML [22] | MALDI-TOF-MS combined with ML algorithms | Detection of COVID-19 protein profiles in nasopharyngeal swab samples |
| Rocca et al. [80] | LC/MS-MS combined with ML | Discrimination of SARS-CoV-2-positive and negative patients through targeted plasma metabolomics |
| Rosado et al. [82] | Various ML approaches | Identification of serological signatures of SARS-CoV-2 infection |
| Detection in saliva, nasal swabs, plasma, and serum samples [94,95,96,97] | Various techniques (MALDI-TOF-MS, SERS, LC-MS, MALDI-MS) | Detection of SARS-CoV-2 in various biological samples |
| Apostolopoulos et al. [84] | Deep learning with transfer learning | Extraction of COVID-19 biomarkers from CX-R images |
| Singh et al. [85] | Convolutional Neural Networks with multi-objective MODE | Efficient classification of COVID-19-infected patients using chest CT images |
| Mei et al. [86] | AI algorithms integrating chest CT findings with clinical data | Rapid diagnosis of COVID-19 positive patients by integrating chest CT findings with clinical symptoms, exposure history, and laboratory testing |
| Systematic review [87,88] | Various deep learning models | High performance of deep learning models in interpreting chest CT scans for COVID-19 diagnosis |
| Wang et al. [91] | Deep learning models (Resnet, Densenet, VGG, Mobilenet, etc.) | Pooled sensitivity, specificity, AUROC, and diagnostic odds ratio for COVID-19 diagnosis using chest CT scans |
| Ozsahin et al. [92] | AI techniques for CT imaging | High sensitivity, specificity, precision, accuracy, AUROC, and F1 score for COVID-19 diagnosis using CT scans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsulimani, A.; Akhter, N.; Jameela, F.; Ashgar, R.I.; Jawed, A.; Hassani, M.A.; Dar, S.A. The Impact of Artificial Intelligence on Microbial Diagnosis. Microorganisms 2024, 12, 1051. https://doi.org/10.3390/microorganisms12061051
Alsulimani A, Akhter N, Jameela F, Ashgar RI, Jawed A, Hassani MA, Dar SA. The Impact of Artificial Intelligence on Microbial Diagnosis. Microorganisms. 2024; 12(6):1051. https://doi.org/10.3390/microorganisms12061051
Chicago/Turabian StyleAlsulimani, Ahmad, Naseem Akhter, Fatima Jameela, Rnda I. Ashgar, Arshad Jawed, Mohammed Ahmed Hassani, and Sajad Ahmad Dar. 2024. "The Impact of Artificial Intelligence on Microbial Diagnosis" Microorganisms 12, no. 6: 1051. https://doi.org/10.3390/microorganisms12061051





