Decoding the Gut Microbiota–Gestational Diabetes Link: Insights from the Last Seven Years
Abstract
1. Introduction
1.1. The Human Microbiome
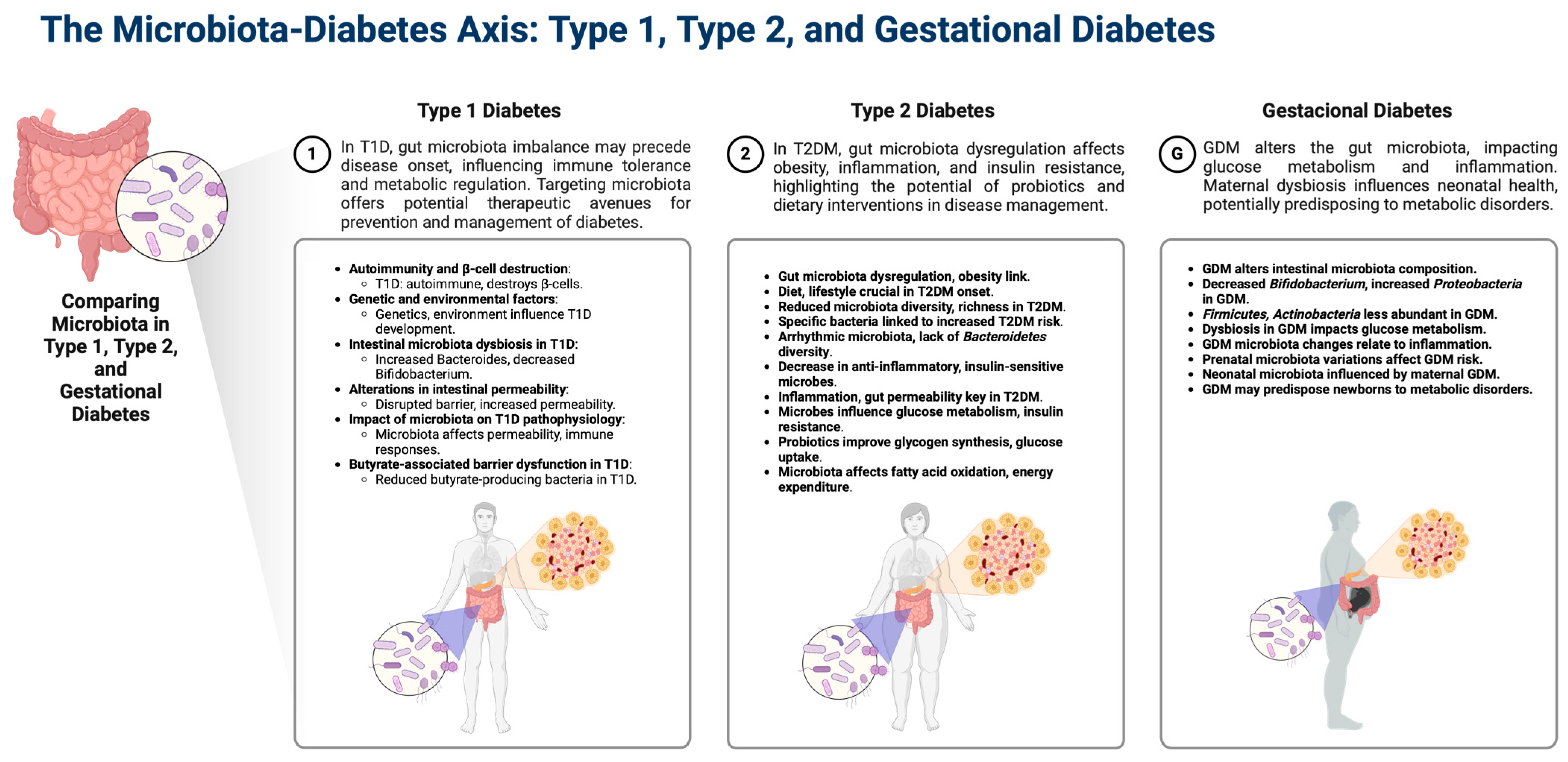
1.2. Gut Microbiota Dysbiosis in Diabetes
1.3. Gestational Diabetes
1.4. Pregnancy and Its Impact on Gut Microbiota Composition
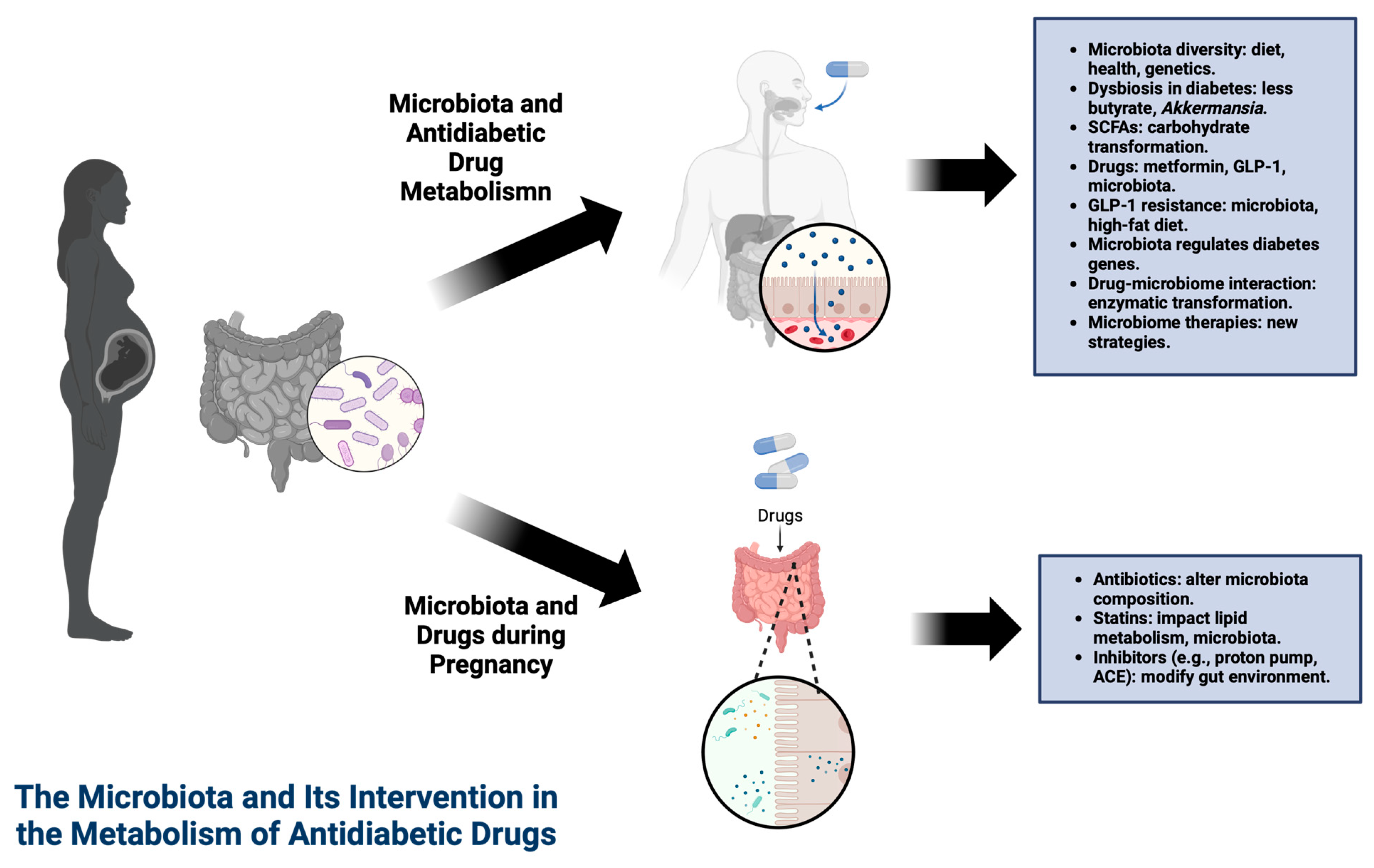
1.5. Drugs Used in Pregnancy Can Affect the Microbiota
1.6. The Microbiota and Its Intervention in the Metabolism of Antidiabetic Drugs
1.7. Use of Probiotics in Gestational Diabetes
1.8. Dietary Influences on Gut Microbiota and the Risk of Gestational Diabetes
1.9. Types of Dysbiosis in Gestational Diabetes
1.10. Effects on the Fetus
1.11. Future Perspectives
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Fuhler, G.M. The immune system and microbiome in pregnancy. Best. Pract. Res. Clin. Gastroenterol. 2020, 44, 101671. [Google Scholar] [CrossRef] [PubMed]
- Neri, C.; Serafino, E.; Morlando, M.; Familiari, A. Microbiome and Gestational Diabetes: Interactions with Pregnancy Outcome and Long-Term Infant Health. J. Diabetes Res. 2021, 2021, 9994734. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef] [PubMed]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 4650. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Rezazadah, A.; Farahbod, K.; Gerts, A.; Clunes, L.A.; Kollias, T.F. Type-2 Diabetes Mellitus and the Gut Microbiota: Systematic Review. Cureus 2023, 15, e49740. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Devi Rajeswari, V. Gestational diabetes mellitus—A metabolic and reproductive disorder. Biomed. Pharmacother. 2021, 143, 112183. [Google Scholar] [CrossRef] [PubMed]
- Committee, A.D.A.P.P. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S282–S294. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, gix058. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.J.; Romero, R.; Greenberg, J.M.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N.; Theis, K.R. Is there a placental microbiota? A critical review and re-analysis of published placental microbiota datasets. BMC Microbiol. 2023, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Li, Y.; Huang, S.; Zhang, L.; Cao, C.; Baker, P.N.; Tong, C.; Zheng, P.; Qi, H. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Q.; Huang, W.; Yan, Q.; Chen, Y.; Zhang, L.; Tian, Z.; Liu, T.; Yuan, X.; Liu, C.; et al. Gestational Diabetes Mellitus Is Associated with Reduced Dynamics of Gut Microbiota during the First Half of Pregnancy. mSystems 2020, 5. [Google Scholar] [CrossRef]
- The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Triviño, J.; Álvarez, D.; Cadavid, J.Á.; Alvarez, A.M. From gut to placenta: Understanding how the maternal microbiome models life-long conditions. Front. Endocrinol. 2023, 14, 1304727. [Google Scholar] [CrossRef] [PubMed]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022, 20, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Tang, L.; Li, S.; Liu, S.; He, J.; Li, P.; Wang, S.; Yang, M.; Zhang, L.; Lei, Y.; et al. Gut microbiota alters host bile acid metabolism to contribute to intrahepatic cholestasis of pregnancy. Nat. Commun. 2023, 14, 1305. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Molina-Vega, M.; Picón-César, M.J.; Gutiérrez-Repiso, C.; Fernández-Valero, A.; Lima-Rubio, F.; González-Romero, S.; Moreno-Indias, I.; Tinahones, F.J. Metformin action over gut microbiota is related to weight and glycemic control in gestational diabetes mellitus: A randomized trial. Biomed. Pharmacother. 2022, 145, 112465. [Google Scholar] [CrossRef]
- Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 1375. [Google Scholar] [CrossRef]
- Kyriachenko, Y.; Falalyeyeva, T.; Korotkyi, O.; Molochek, N.; Kobyliak, N. Crosstalk between gut microbiota and antidiabetic drug action. World J. Diabetes 2019, 10, 154–168. [Google Scholar] [CrossRef]
- Borse, S.P.; Chhipa, A.S.; Sharma, V.; Singh, D.P.; Nivsarkar, M. Management of Type 2 Diabetes: Current Strategies, Unfocussed Aspects, Challenges, and Alternatives. Med. Princ. Pract. 2021, 30, 109–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef] [PubMed]
- Induri, S.N.R.; Kansara, P.; Thomas, S.C.; Xu, F.; Saxena, D.; Li, X. The Gut Microbiome, Metformin, and Aging. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Maskarinec, G.; Raquinio, P.; Kristal, B.S.; Setiawan, V.W.; Wilkens, L.R.; Franke, A.A.; Lim, U.; Le Marchand, L.; Randolph, T.W.; Lampe, J.W.; et al. The gut microbiome and type 2 diabetes status in the Multiethnic Cohort. PLoS ONE 2021, 16, e0250855. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J.; et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Tercé, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017, 25, 1075–1090.e1075. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363, eaat9931. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Hang, J.; Liu, J.; Guo, F.; Ding, Y.; Li, M.; Nie, Q.; Lin, J.; Zhuo, Y.; et al. Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target. Science 2023, 381, eadd5787. [Google Scholar] [CrossRef] [PubMed]
- Masulli, M.; Vitacolonna, E.; Fraticelli, F.; Della Pepa, G.; Mannucci, E.; Monami, M. Effects of probiotic supplementation during pregnancy on metabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2020, 162, 108111. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, Cd009951. [Google Scholar] [CrossRef] [PubMed]
- Pakmehr, A.; Ejtahed, H.S.; Shirzad, N.; Hemmatabadi, M.; Farhat, S.; Larijani, B. Preventive effect of probiotics supplementation on occurrence of gestational diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2022, 9, 1031915. [Google Scholar] [CrossRef]
- Shahriari, A.; Karimi, E.; Shahriari, M.; Aslani, N.; Khooshideh, M.; Arab, A. The effect of probiotic supplementation on the risk of gestational diabetes mellitus among high-risk pregnant women: A parallel double-blind, randomized, placebo-controlled clinical trial. Biomed. Pharmacother. 2021, 141, 111915. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. A Vegetarian Diet Is a Major Determinant of Gut Microbiota Composition in Early Pregnancy. Nutrients 2018, 10, 890. [Google Scholar] [CrossRef]
- Sugino, K.Y.; Hernandez, T.L.; Barbour, L.A.; Kofonow, J.M.; Frank, D.N.; Friedman, J.E. A maternal higher-complex carbohydrate diet increases bifidobacteria and alters early life acquisition of the infant microbiome in women with gestational diabetes mellitus. Front. Endocrinol. 2022, 13, 921464. [Google Scholar] [CrossRef]
- Mokkala, K.; Paulin, N.; Houttu, N.; Koivuniemi, E.; Pellonperä, O.; Khan, S.; Pietilä, S.; Tertti, K.; Elo, L.L.; Laitinen, K. Metagenomics analysis of gut microbiota in response to diet intervention and gestational diabetes in overweight and obese women: A randomised, double-blind, placebo-controlled clinical trial. Gut 2021, 70, 309–318. [Google Scholar] [CrossRef]
- Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Gaspar, B.S. Gut Microbiome Changes in Gestational Diabetes. Int. J. Mol. Sci. 2022, 23, 12839. [Google Scholar] [CrossRef]
- Su, M.; Nie, Y.; Shao, R.; Duan, S.; Jiang, Y.; Wang, M.; Xing, Z.; Sun, Q.; Liu, X.; Xu, W. Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS ONE 2018, 13, e0205695. [Google Scholar] [CrossRef]
- Wu, N.; Mo, H.; Mu, Q.; Liu, P.; Liu, G.; Yu, W. The Gut Mycobiome Characterization of Gestational Diabetes Mellitus and Its Association With Dietary Intervention. Front. Microbiol. 2022, 13, 892859. [Google Scholar] [CrossRef] [PubMed]
- Soderborg, T.K.; Carpenter, C.M.; Janssen, R.C.; Weir, T.L.; Robertson, C.E.; Ir, D.; Young, B.E.; Krebs, N.F.; Hernandez, T.L.; Barbour, L.A.; et al. Gestational Diabetes Is Uniquely Associated With Altered Early Seeding of the Infant Gut Microbiota. Front. Endocrinol. 2020, 11, 603021. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhang, L.; Wang, M.; Chen, Y.; Gu, S.; Wang, K.; Leng, J.; Gu, Y.; Xie, X. The Gut Microbiota in Women Suffering from Gestational Diabetes Mellitus with the Failure of Glycemic Control by Lifestyle Modification. J. Diabetes Res. 2019, 2019, 6081248. [Google Scholar] [CrossRef] [PubMed]
- Dualib, P.M.; Taddei, C.R.; Fernandes, G.; Carvalho, C.R.S.; Sparvoli, L.G.; Silva, I.T.; Mattar, R.; Ferreira, S.R.G.; Dib, S.A.; Almeida-Pititto, B. Gut Microbiota across Normal Gestation and Gestational Diabetes Mellitus: A Cohort Analysis. Metabolites 2022, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736.e733. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Zhang, Y.; Shan, C.; Zhang, Y.; Fang, K.; Xia, Y.; Shi, Z. Relationships between gut microbiota, plasma glucose and gestational diabetes mellitus. J. Diabetes Investig. 2021, 12, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, P.; Chu, S.; Gao, W.; Cui, S.; Guo, S.; Xu, Y.; Yuan, E.; Zhu, T.; You, J.; et al. Correlation Analysis between GDM and Gut Microbial Composition in Late Pregnancy. J. Diabetes Res. 2021, 2021, 8892849. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, S.; Feng, Y.; Song, Y.; Lv, N.; Liu, F.; Zhang, X.; Wang, S.; Wei, Y.; Li, S.; et al. Perturbations of gut microbiota in gestational diabetes mellitus patients induce hyperglycemia in germ-free mice. J. Dev. Orig. Health Dis. 2020, 11, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, M.; Zhang, J.; Sun, Z.; Ran, L.; Ban, Y.; Wang, B.; Hou, X.; Zhai, S.; Ren, L.; et al. Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E247–E253. [Google Scholar] [CrossRef]
- Mokkala, K.; Houttu, N.; Vahlberg, T.; Munukka, E.; Rönnemaa, T.; Laitinen, K. Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol. 2017, 54, 1147–1149. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Comparative Studies of the Gut Microbiota in the Offspring of Mothers with and without Gestational Diabetes. Front. Cell Infect. Microbiol. 2020, 10, 536282. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Qi, C.; Yang, L.; Zhang, M.; Wang, H.; She, G.; Yu, R.; Miao, T.; Sun, J. A pregnancy complication-dependent change in SIgA-targeted microbiota during third trimester. Food Funct. 2020, 11, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, D.; Wang, Y.; Yuan, H.; Ning, X.; Rui, B.; Lei, Z.; Yuan, J.; Yan, J.; Li, M. The Intestinal Dysbiosis of Mothers with Gestational Diabetes Mellitus (GDM) and Its Impact on the Gut Microbiota of Their Newborns. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 3044534. [Google Scholar] [CrossRef]
- Ponzo, V.; Ferrocino, I.; Zarovska, A.; Amenta, M.B.; Leone, F.; Monzeglio, C.; Rosato, R.; Pellegrini, M.; Gambino, R.; Cassader, M.; et al. The microbiota composition of the offspring of patients with gestational diabetes mellitus (GDM). PLoS ONE 2019, 14, e0226545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balleza-Alejandri, L.R.; Peña-Durán, E.; Beltrán-Ramírez, A.; Reynoso-Roa, A.S.; Sánchez-Abundis, L.D.; García-Galindo, J.J.; Suárez-Rico, D.O. Decoding the Gut Microbiota–Gestational Diabetes Link: Insights from the Last Seven Years. Microorganisms 2024, 12, 1070. https://doi.org/10.3390/microorganisms12061070
Balleza-Alejandri LR, Peña-Durán E, Beltrán-Ramírez A, Reynoso-Roa AS, Sánchez-Abundis LD, García-Galindo JJ, Suárez-Rico DO. Decoding the Gut Microbiota–Gestational Diabetes Link: Insights from the Last Seven Years. Microorganisms. 2024; 12(6):1070. https://doi.org/10.3390/microorganisms12061070
Chicago/Turabian StyleBalleza-Alejandri, Luis Ricardo, Emiliano Peña-Durán, Alberto Beltrán-Ramírez, Africa Samantha Reynoso-Roa, Luis Daniel Sánchez-Abundis, Jesús Jonathan García-Galindo, and Daniel Osmar Suárez-Rico. 2024. "Decoding the Gut Microbiota–Gestational Diabetes Link: Insights from the Last Seven Years" Microorganisms 12, no. 6: 1070. https://doi.org/10.3390/microorganisms12061070
APA StyleBalleza-Alejandri, L. R., Peña-Durán, E., Beltrán-Ramírez, A., Reynoso-Roa, A. S., Sánchez-Abundis, L. D., García-Galindo, J. J., & Suárez-Rico, D. O. (2024). Decoding the Gut Microbiota–Gestational Diabetes Link: Insights from the Last Seven Years. Microorganisms, 12(6), 1070. https://doi.org/10.3390/microorganisms12061070


_Di_Marco.png)






