Screening the Protective Agents Able to Improve the Survival of Lactic Acid Bacteria Strains Subjected to Spray Drying Using Several Key Enzymes Responsible for Carbohydrate Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cultivation and Preparation of LAB Strains
2.3. Preparation of RSM Powders Containing LAB Strains
2.4. Conditions Setting for Spray Drying
2.5. Effects of RSM Protectants on the Viable Counts and Fermentation Activity of Selected LAB Strains
2.5.1. Effects on the Viable Counts of Selected LAB Strains
2.5.2. Effects on the Fermentation Activity of Selected LAB Strains
2.6. Enzymatic Activity Analysis
2.6.1. Samples Preparation and Determination of Protein Content
2.6.2. Determination of HK Activity
2.6.3. Determination of β-GAL Activity
2.6.4. Determination of PK Activity
2.6.5. Determination of LDH Activity
2.7. Effects of Spray Drying on Cell Membrane Property
2.7.1. Detection of Cell Membrane Fluidity
2.7.2. Detection of Cell Membrane Integrity
2.8. Morphological Observation of Spray-Dried Powder Containing LAB Strains
2.9. Effect of Spray Drying on the Gene Expression of Lb. acidophilus NCFM
2.10. Physiological Performances of Lb. acidophilus NCFM Cells Spray-Dried with Different Composite Protectants
2.10.1. Preparation of Spray-Dried Powder Containing Strain NCFM Encapsulated with 4 Composite Protectants
2.10.2. Effect of Composite Protectants on PK and LDH Activity of Strain NCFM
2.10.3. Effect of Four Composite Protectants on the Viable Counts of Strain NCFM
2.10.4. Effect of Composite Protectants on the Fermentation Activity of Strain NCFM
2.11. Statistical Analysis
3. Results
3.1. The Live Counts and Fermentation Activity of the Spray-Dried Selected LAB Strains in the Presence of RSM
3.1.1. Live Counts of Selected LAB Strains Subjected to Spray Drying
3.1.2. Fermentation Activity of the Selected LAB Strains Subjected to Spray Drying
3.2. The Micrographics and Membrane Properties of Selected LAB Strains
3.2.1. Microparticle Morphology of the Spray-Dried Powders Containing LAB Strains
3.2.2. Cell Membrane Integrity of Selected LAB Strains
3.2.3. Cell Membrane Fluidity of the LAB Strains
3.3. The Enzyme Activity Related to Carbohydrate Utilization of LAB Strains
3.3.1. HK Activity
3.3.2. β-GAL Activity
3.3.3. PK Activity
3.3.4. LDH Activity
3.4. The Transcriptomics of Lb. acidophilus NCFM Subjected to Spray Drying in the Presence of RSM
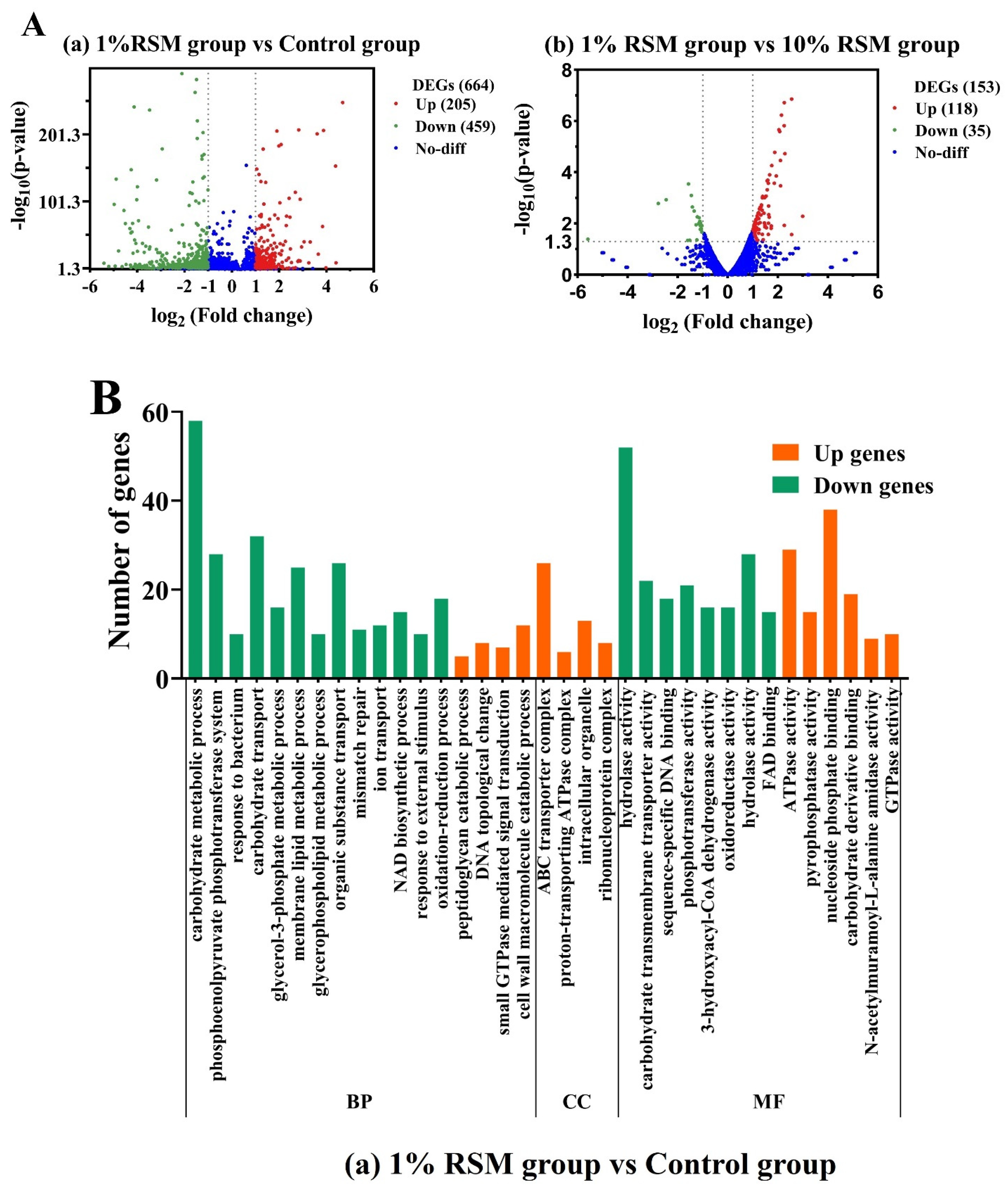
3.4.1. Identification of Differential Gene Expression (DEGs)
3.4.2. Annotation of the GO Database
3.4.3. KEGG Pathways
3.5. Effects of RSM-Based Composite Protective Agents on the Physiological Properties of Lb. acidophilus NCFM
3.5.1. Effects of RSM-Based Composite Protective Agents on PK and LDH Activity of Strain NCFM
3.5.2. Effect of RSM-Based Composite Protective Agents on the Viable Counts of Strain NCFM
3.5.3. Effect of RSM-Based Composite Protective Agent on the Fermentation Performance of Strain NCFM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Ma, C.; Song, X.; Zeng, J.; Zhang, L.; Gong, P. Spray drying co-encapsulation of lactic acid bacteria and lipids: A review. Trends Food Sci. Technol. 2022, 129, 134–143. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep milk: Physicochemical characteristics and relevance for functional food development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Huang, S.; Wang, J.; Fu, N.; Jeantet, R.; Chen, X.D. Effect of culturing lactic acid bacteria with varying skim milk concentration on bacteria survival during heat treatment. J. Food Eng. 2021, 294, 110396. [Google Scholar] [CrossRef]
- Yang, H.; Huang, P.; Hao, L.; Che, Y.; Dong, S.; Wang, Z.; Wu, C. Enhancing viability of dried lactic acid bacteria prepared by freeze drying and spray drying via heat preadaptation. Food Microbiol. 2023, 112, 104239. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tian, F.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Effects of cryoprotectants on viability of Lactobacillus reuteri CICC 6226. Appl. Microbiol. Biotechnol. 2011, 92, 609–616. [Google Scholar] [CrossRef]
- Huang, S.; Gaucher, F.; Cauty, C.; Jardin, J.; Le Loir, Y.; Jeantet, R.; Chen, X.D.; Jan, G. Growth in hyper-concentrated sweet whey triggers multi stress tolerance and spray drying survival in Lactobacillus casei BL23: From the molecular basis to new perspectives for sustainable probiotic production. Front. Microbiol. 2018, 9, 2548. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei NCDC297. LWT-Food Sci. Technol. 2017, 83, 50–58. [Google Scholar] [CrossRef]
- Lin, T.C.; Chen, B.Y.; Chen, C.Y.; Chen, Y.S.; Wu, H. Comparative analysis of spray-drying microencapsulation of Bifidobacterium adolescentis and Lactobacillus acidophilus cultivated in different growth media. J. Food Process Eng. 2019, 42, e13258. [Google Scholar] [CrossRef]
- Liu, H.; Gong, J.; Chabot, D.; Miller, S.; Cui, S.W.; Ma, J.; Zhong, F.; Wang, Q. Protection of heat-sensitive probiotic bacteria during spray-drying by sodium caseinate stabilized fat particles. Food Hydrocoll. 2015, 51, 459–467. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Cao, J.; Zhuang, R.; E, J.; Yao, C.; Wang, R.; Wang, J. Effects of the repair treatment on improving the heat resistance of Lactiplantibacillus plantarum LIP-1. Innov. Food Sci. Emerg. Technol. 2023, 84, 103251. [Google Scholar] [CrossRef]
- Mao, H.; Chen, X.D.; Fu, N. Exploring the integrity of cellular membrane and resistance to digestive juices of dehydrated lactic acid bacteria as influenced by drying kinetics. Food Res. Int. 2022, 157, 111395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yan, X.; Wu, J.; Weng, P.; Wu, Z. Effects of freeze drying in complex lyoprotectants on the survival, and membrane fatty acid composition of Lactobacillus plantarum L1 and Lactobacillus fermentum L2. Cryobiology 2022, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, F.; Peng, Z.; Liu, Z.; Huang, T.; Xiong, T. Protection effect of gelatin-xylooligosaccharides Maillard reaction products on spray-dried Limosilactobacillus fermentum and possible action mechanism. Food Biosci. 2023, 56, 103251. [Google Scholar] [CrossRef]
- Rani, P.S.; Agrawal, R. Effect on cellular membrane fatty acids in the stressed cells of Leuconostoc mesenteroides: A native probiotic lactic acid bacteria. Food Biotechnol. 2008, 22, 47–63. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, H.; Tong, X.; Bao, G.; Yang, S.; Wang, S.; Shen, C.; Li, Y. Comparative genomic analyses reveal carbohydrates-rich environment adaptability of Lentilactobacillus laojiaonis sp. nov. IM3328. Food Biosci. 2023, 53, 102737. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, S.; Sun, X.; Jie, Y.; Zhao, H.; Zhu, B.; Dziugan, P.; Zhang, B. A novel insight to screen the optimal spray-drying protectants and parameters for manufacturing lactic acid bacteria preparations. Dry. Technol. 2019, 38, 1843–1856. [Google Scholar] [CrossRef]
- Jing, E.; Zhang, J.; Ma, R.; Chen, Z.; Yao, C.; Wang, R.; Zhang, Q.; Ying, Y.; Li, J.; Wang, J. Study of the internal mechanism of L-glutamate for improving the survival rate of Lactiplantibacillus plantarum LIP-1 after freeze-drying. Innov. Food Sci. Emerg. Technol. 2023, 84, 103253. [Google Scholar] [CrossRef]
- Jing, E.; Chen, J.; Chen, Z.; Ma, R.; Zhang, J.; Yao, C.; Wang, R.; Zhang, Q.; Yang, Y.; Li, J.; et al. Effects of different initial pH values on freeze-drying resistance of Lactiplantibacillus plantarum LIP-1 based on transcriptomics and proteomics. Food Res. Int. 2021, 149, 110694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jiang, X.; Wang, T.; Zhang, B.; Zhao, H. Lyciumbarbarum polysaccharide (LBP): A novel prebiotics Candidate for Bifidobacterium and Lactobacillus. Front. Microbiol. 2018, 9, 1034. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.F.; Mao, X.; Wang, J.Y.; Duan, C.Y.; Wang, Z.J.; Liu, L.B. Growth and acid production of Lactobacillus delbrueckii ssp. bulgaricus ATCC 11842 in the fermentation of algal carcass. J. Dairy Sci. 2016, 99, 4243–4250. [Google Scholar] [CrossRef]
- Qu, F.; Zhao, M.; Fang, Y.; Nishinari, K.; Phillips, G.; Wu, Z.; Chen, C. Effect of acidification on the protection of alginate-encapsulated probiotic based on emulsification/internal gelation. J. Sci. Food Agric. 2016, 96, 4358–4366. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Microencapsulation of Lactobacillus rhamnosus HN001 by spray drying and its evaluation under gastrointestinal and storage conditions. LWT-Food Sci. Technol. 2022, 153, 112485. [Google Scholar] [CrossRef]
- Zhang, C.; Gui, Y.; Chen, X.; Chen, D.; Guan, C.; Yin, B.; Pan, Z.; Gu, R. Transcriptional homogenization of Lactobacillus rhamnosus hsryfm 1301 under heat stress and oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2023, 51, 102337. [Google Scholar] [CrossRef]
- Yin, M.; Yuan, Y.; Chen, M.; Liu, F.; Saqib, M.N.; Chiou, B.S.; Zhong, F. The dual effect of shellac on survival of spray-dried Lactobacillus rhamnosus GG microcapsules. Food Chem. 2022, 389, 132999. [Google Scholar] [CrossRef] [PubMed]
- Khem, S.; Small, D.M.; May, B.K. The behaviour of whey protein isolate in protecting Lactobacillus plantarum. Food Chem. 2016, 190, 717–723. [Google Scholar] [CrossRef]
- Cui, S.; Hang, F.; Liu, X.; Xu, Z.; Liu, Z.; Zhao, J.; Zhang, H.; Chen, W. Effect of acids produced from carbohydrate metabolism in cryoprotectants on the viability of freeze-dried Lactobacillus and prediction of optimal initial cell concentration. J. Biosci. Bioeng. 2018, 125, 513–518. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J. Appl. Microbiol. 2004, 96, 1024–1039. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, M.; Li, Q.; Wang, T.; Zhang, B.; Zhao, H.; Fu, J. The beneficial effects of polysaccharide obtained from persimmon (Diospyros kaki L.) on the proliferation of Lactobacillus and gut microbiota. Int. J. Biol. Macromol. 2021, 182, 1874–1882. [Google Scholar] [CrossRef]
- McCoy, J.G.; Levin, E.J.; Zhou, M. Structural insight into the PTS sugar transporter EIIC. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 577–585. [Google Scholar] [CrossRef]
- Neves, A.R.; Pool, W.A.; Castro, R.; Mingote, A.; Santos, F.; Kok, J.; Kuipers, O.P.; Santos, H. The α-Phosphoglucomutase of Lactococcus lactis is unrelated to the α-d-phosphohexomutase superfamily and is encoded by the essential gene pgmH. J. Biol. Chem. 2006, 281, 36864–36873. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, M.; Gogoi, P.; Kanaujia, S.P. Computational characterization of TTHA0379: A potential glycerophosphocholine binding protein of Ugp ATP-binding cassette transporter. Gene 2016, 592, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fu, N.; Duan, M.; Woo, M.W.; Selomulya, C.; Chen, X.D. The mechanisms of the protective effects of reconstituted skim milk during convective droplet drying of lactic acid bacteria. Food Res. Int. 2015, 76, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, N.; Woo, M.W.; Chen, X.D. Heat stability of Lactobacillus rhamnosus GG and its cellular membrane during droplet drying and heat treatment. Food Res. Int. 2018, 112, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2019, 104, 51–65. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Chen, W.; Wang, M.; Du, G.; Chen, J. A combined physiological and proteomic approach to reveal lactic-acid-induced alterations in Lactobacillus casei Zhang and its mutant with enhanced lactic acid tolerance. Appl. Microbiol. Biotechnol. 2011, 93, 707–722. [Google Scholar] [CrossRef]
- Chai, H.; Ham, J.; Kim, T.; Lim, D. Identifying ligand-binding specificity of the oligopeptide receptor OppA from Bifidobacterium longum KACC91563 by structure-based molecular modeling. Arab. J. Chem. 2022, 15, 104198. [Google Scholar] [CrossRef]
- Salvetti, E.; Fondi, M.; Fani, R.; Torriani, S.; Felis, G.E. Evolution of lactic acid bacteria in the order Lactobacillales as depicted by analysis of glycolysis and pentose phosphate pathways. Syst. Appl. Microbiol. 2013, 36, 291–305. [Google Scholar] [CrossRef]
- Papagianni, M.; Avramidis, N.; Filiousis, G. Glycolysis and the regulation of glucose transport in Lactococcus lactis spp. lactis in batch and fed-batch culture. Microb. Cell Factories 2007, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ravanal, C.M.; Flores, M.; Pérez, E.; Aroca, F.; Cardemil, E. Saccharomyces cerevisiae phosphoenolpyruvate carboxykinase: Relevance of arginine70 for catalysis. Biochimie 2004, 86, 357–362. [Google Scholar] [CrossRef] [PubMed]
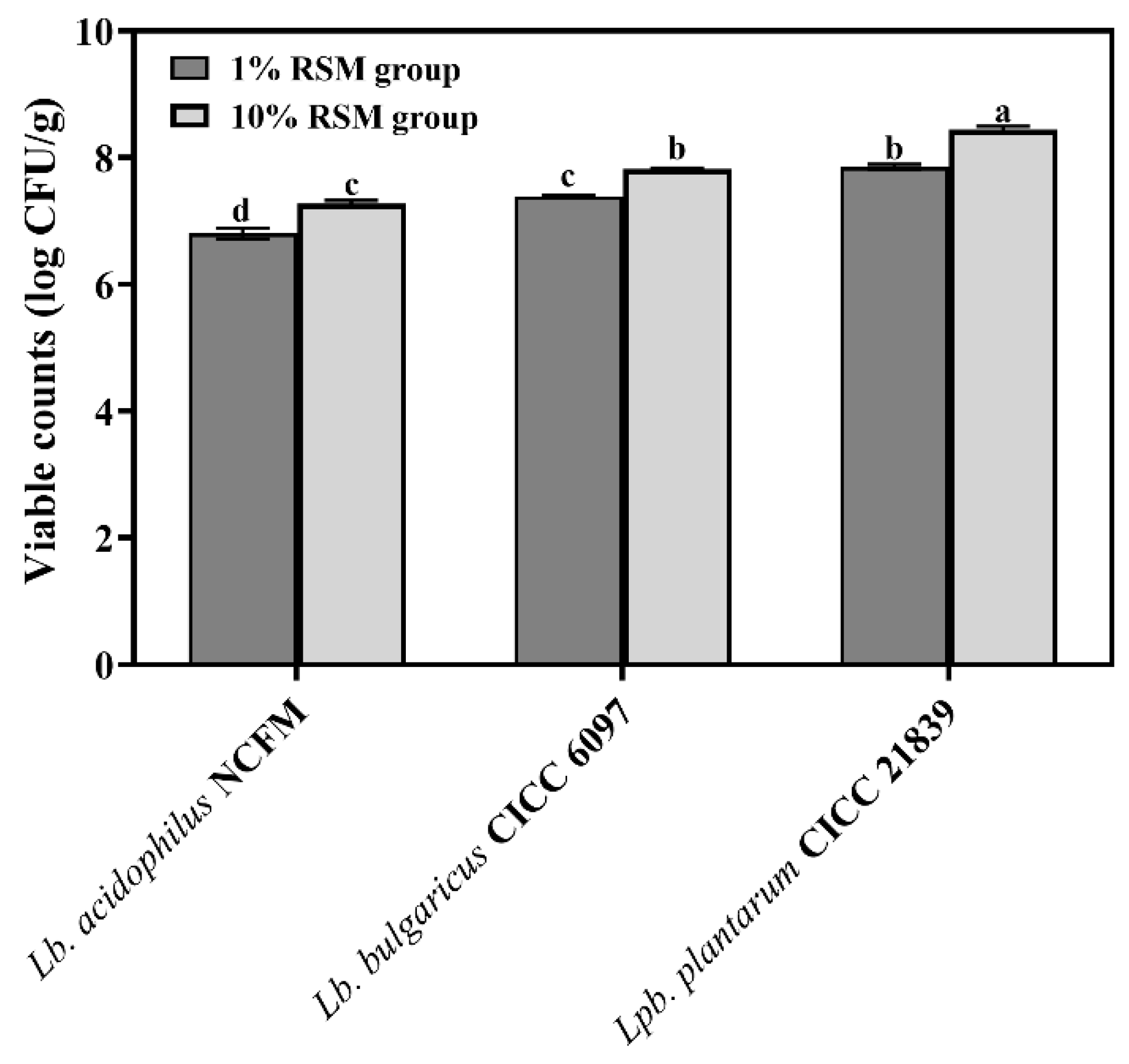

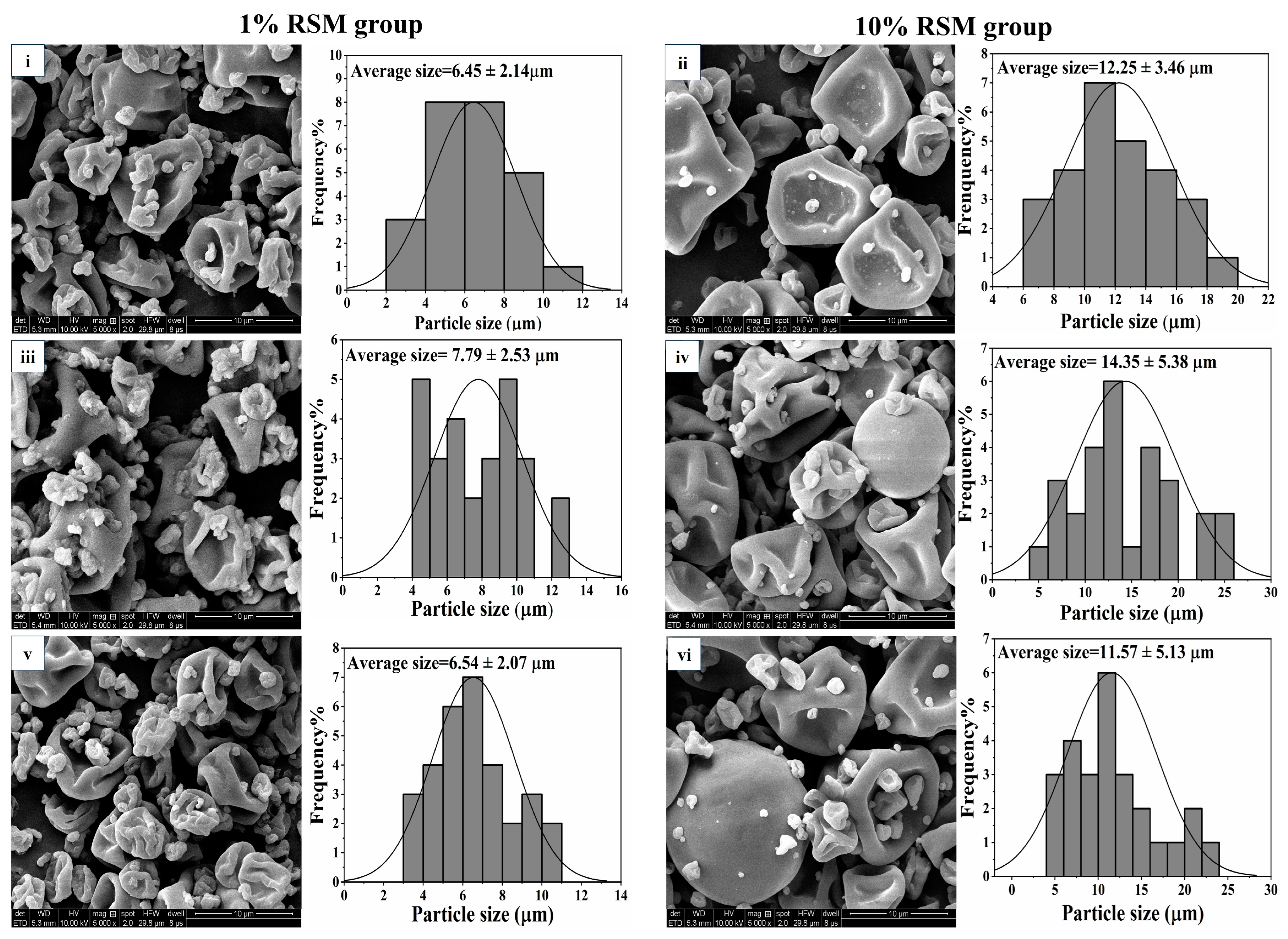

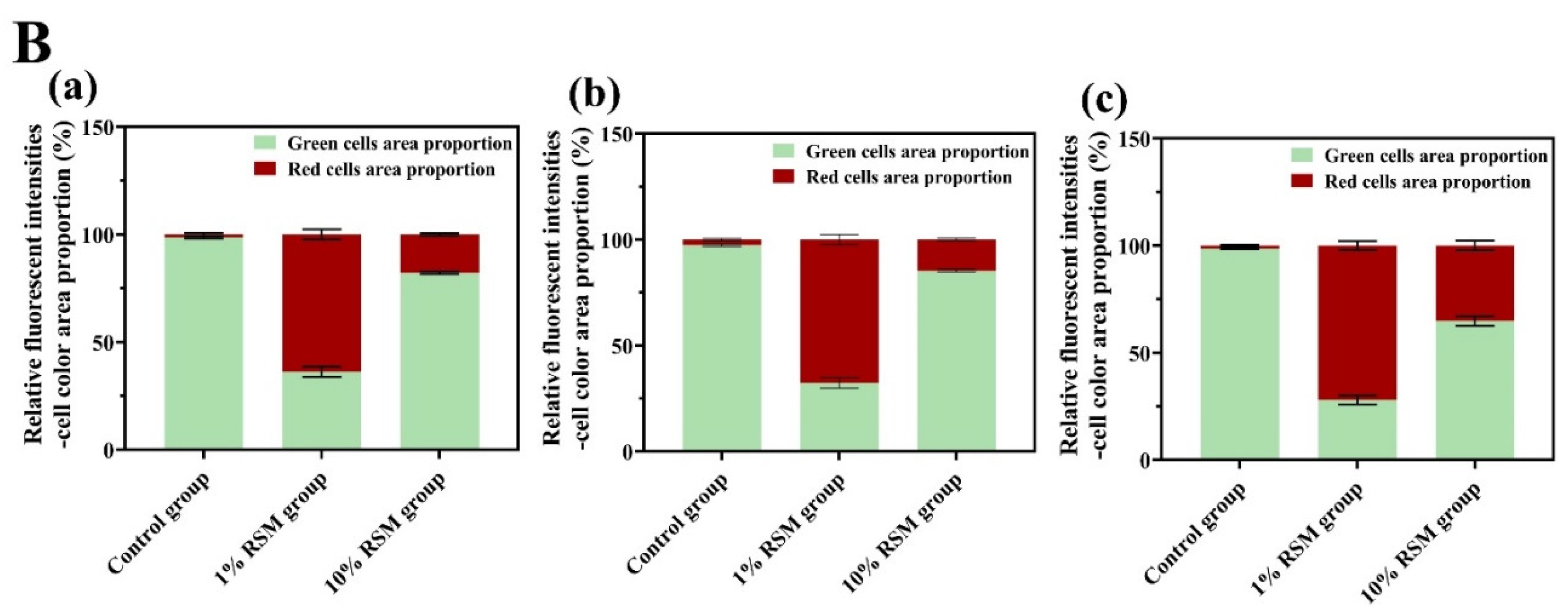
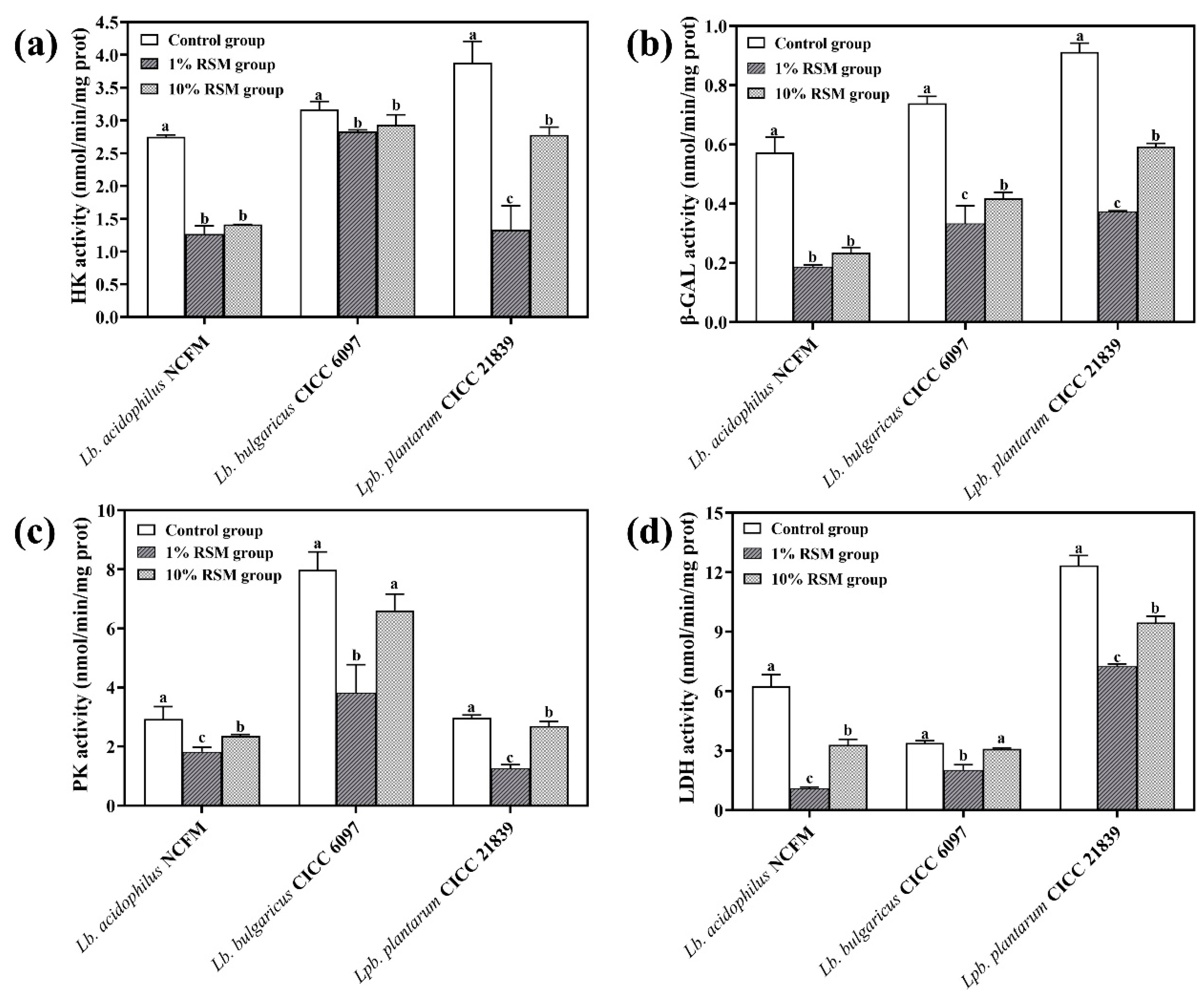


| Treatment | Lb. acidophilus NCFM | Lb. bulgaricus CICC 6097 | Lpb. plantarum CICC 21839 | |||
|---|---|---|---|---|---|---|
| p Value | η | p Value | η | p Value | η | |
| Control group | 0.295 ± 0.002 a | 3.579 ± 0.4 a | 0.290 ± 0.002 a | 3.419 ± 0.2 a | 0.289 ± 0.003 a | 3.392 ± 0.12 a |
| 1% RSM group | 0.320 ± 0.003 b | 4.451 ± 0.12 b | 0.318 ± 0.003 b | 4.491 ± 0.38 b | 0.362 ± 0.003 c | 7.352 ± 0.03 c |
| 10% RSM group | 0.318 ± 0.009 ab | 4.311 ± 0.33 ab | 0.309 ± 0.005 b | 4.093 ± 0.19 ab | 0.303 ± 0.005 b | 3.870 ± 0.21 b |
| Sample Group | Pathways | Related Enzymes (Genes) | |
|---|---|---|---|
| 1% RSM group vs. control group | Up-regulated | Ribosome; | 50S ribosomal protein (L34, L28, L30, L35, S21)/30S ribosomal protein (S14, L31, L33) |
| ABC transporters | ABC transporter ATP-binding protein (MdlB)/energy-coupling factor transporter ATPase/ABC transporter permease | ||
| Down-regulated | Phosphotransferase system (PTS) | PTS sugar transporter subunit IIA IIB IIC (crr, celA, celB)/PTS beta-glucoside transporter subunit BglF (scrA) | |
| Starch and sucrose metabolism | sucrose-6-phosphate hydrolase (scrB)/β-phosphoglucomutase (β-PGM) (pgmB) | ||
| Glycolysis | pyruvate synthase (pyk) | ||
| Cysteine metabolism | cystathionine beta-lyase (metC) | ||
| Pyruvate metabolism | fumarate reductase flavoprotein subunit (FrdA)/class II fumarate hydratase (fumC)/NAD-dependent malic enzyme (maeA)/L-lactate dehydrogenase (ldh); | ||
| Glycerolipid metabolism | dihydroxyacetone kinase subunit L (dhal, dhaK)/glycerol kinase GlpK (glpK) | ||
| 10% RSM group vs. 1% RSM group | Up-regulated | Pyrimidine metabolism | ribonucleoside-triphosphate reductase (nrdJ)/thioredoxin-disulfide reductase (trxB) |
| ABC transporters | glycerol-3-phosphate ABC transporter ATP-binding protein (ugpC) | ||
| Phosphotransferase system (PTS) | PTS lactose/cellobiose transporter subunit IIA (celC)/PTS sugar transporter subunit IIB, IIC (celA, celB) | ||
| Thiamine metabolism | phosphomethylpyrimidine kinase (thiD) | ||
| Pyruvate metabolism | acetate kinase (ackA) | ||
| Amino sugar and nucleotide sugar metabolism | glucosamine-6-phosphate deaminase (nagB)/nicotinic acid mononucleotide NaMN (nadD) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xie, S.; Xu, M.; Jiang, X.; Wang, Q.; Zhao, H.; Zhang, B. Screening the Protective Agents Able to Improve the Survival of Lactic Acid Bacteria Strains Subjected to Spray Drying Using Several Key Enzymes Responsible for Carbohydrate Utilization. Microorganisms 2024, 12, 1094. https://doi.org/10.3390/microorganisms12061094
Liu J, Xie S, Xu M, Jiang X, Wang Q, Zhao H, Zhang B. Screening the Protective Agents Able to Improve the Survival of Lactic Acid Bacteria Strains Subjected to Spray Drying Using Several Key Enzymes Responsible for Carbohydrate Utilization. Microorganisms. 2024; 12(6):1094. https://doi.org/10.3390/microorganisms12061094
Chicago/Turabian StyleLiu, Jing, Shanshan Xie, Mengfan Xu, Xiaoying Jiang, Qian Wang, Hongfei Zhao, and Bolin Zhang. 2024. "Screening the Protective Agents Able to Improve the Survival of Lactic Acid Bacteria Strains Subjected to Spray Drying Using Several Key Enzymes Responsible for Carbohydrate Utilization" Microorganisms 12, no. 6: 1094. https://doi.org/10.3390/microorganisms12061094
APA StyleLiu, J., Xie, S., Xu, M., Jiang, X., Wang, Q., Zhao, H., & Zhang, B. (2024). Screening the Protective Agents Able to Improve the Survival of Lactic Acid Bacteria Strains Subjected to Spray Drying Using Several Key Enzymes Responsible for Carbohydrate Utilization. Microorganisms, 12(6), 1094. https://doi.org/10.3390/microorganisms12061094





