Purpureocillium lilacinum SBF054: Endophytic in Phaseolus vulgaris, Glycine max, and Helianthus annuus; Antagonistic to Rhizoctonia solani; and Virulent to Euschistus heros
Abstract
:1. Introduction
2. Material and Methods
2.1. Origin of the Fungus
2.2. Morphological, Molecular, and Biochemical Characterization of the Strain
2.3. Production of Aerial Conidia, Blastospores, and Metabolites
2.4. Endophytic Effect on Bean, Soybean, and Sunflower Plants
2.5. Antagonism to Phytopathogenic Fungi
2.6. Mortality Bioassays against Insects of the Orders Lepidoptera, Coleoptera, and Hemiptera
2.7. Statistical Analysis
3. Results
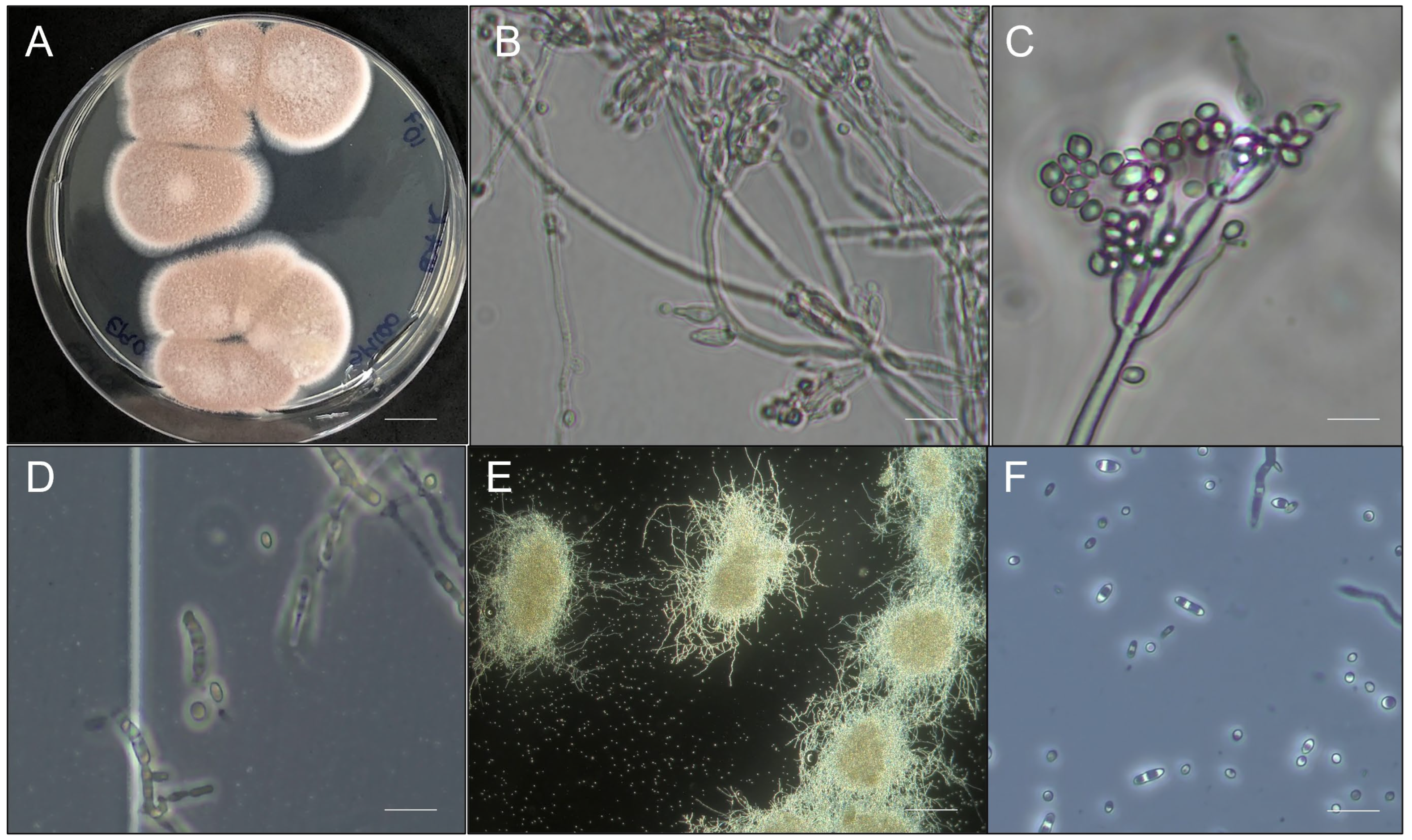
3.1. Morphological, Biochemical, and Molecular Characterization of the Strain
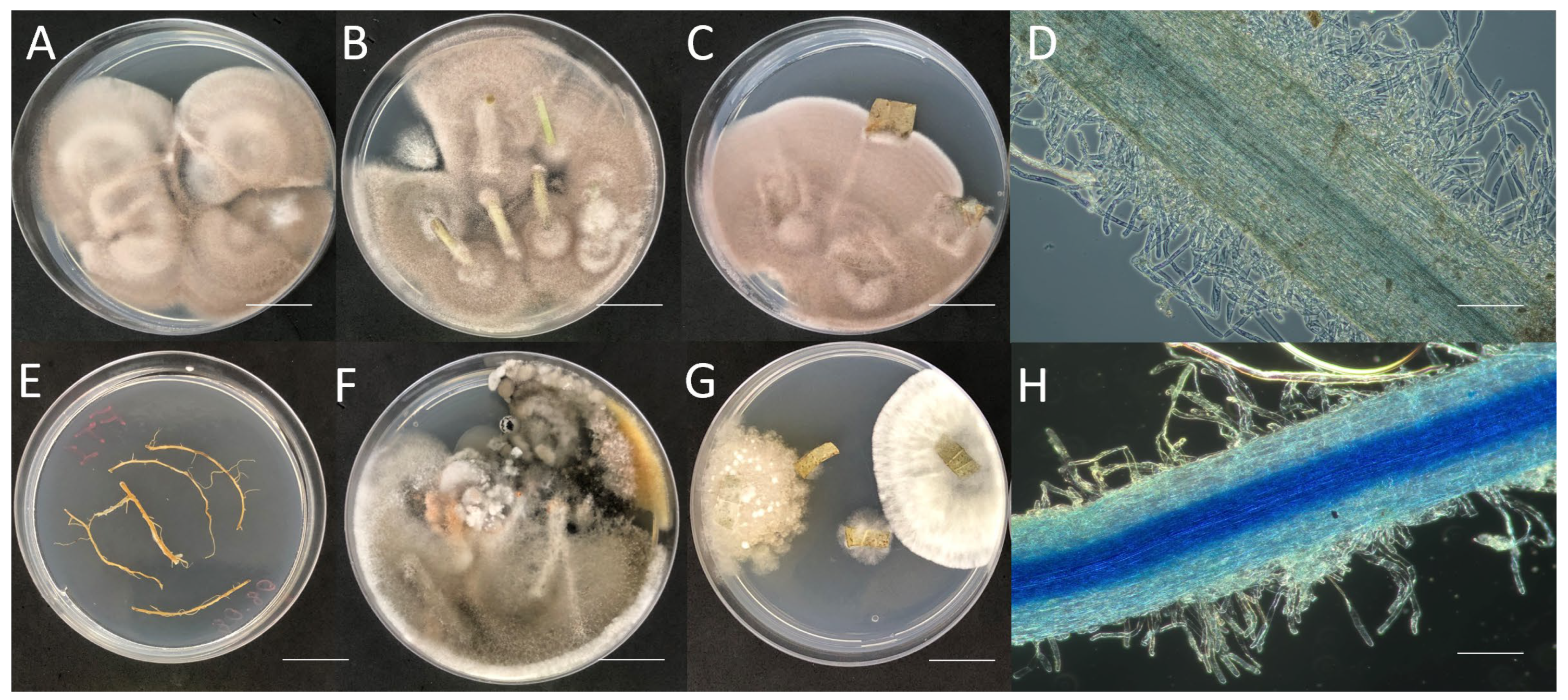
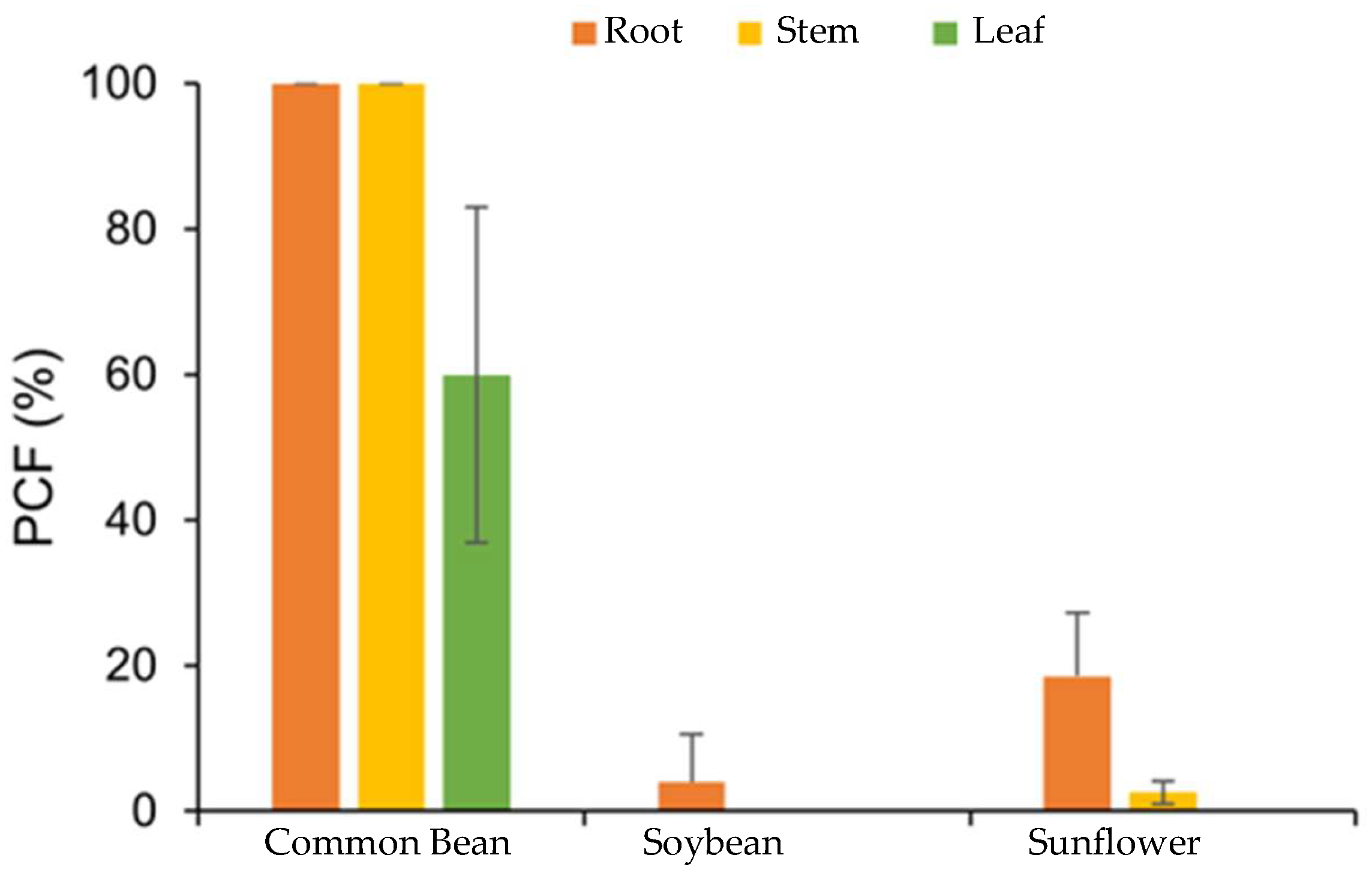
3.2. Purpureocillium lilacinum Endophytically Colonizes Common Bean Plants
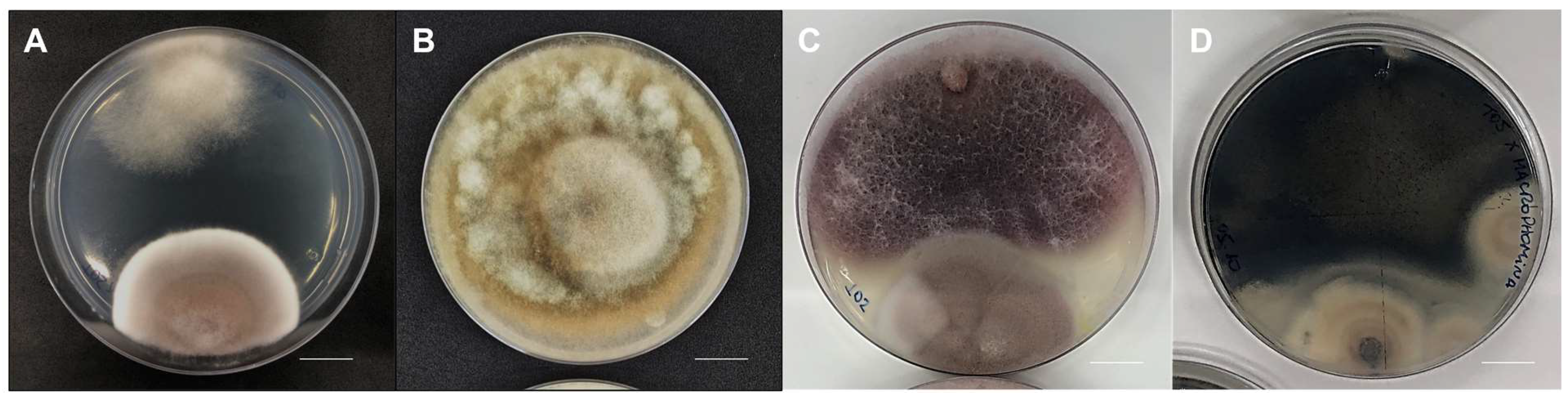
3.3. Antagonistic Activity against the Phytopathogen Rhizoctonia solani
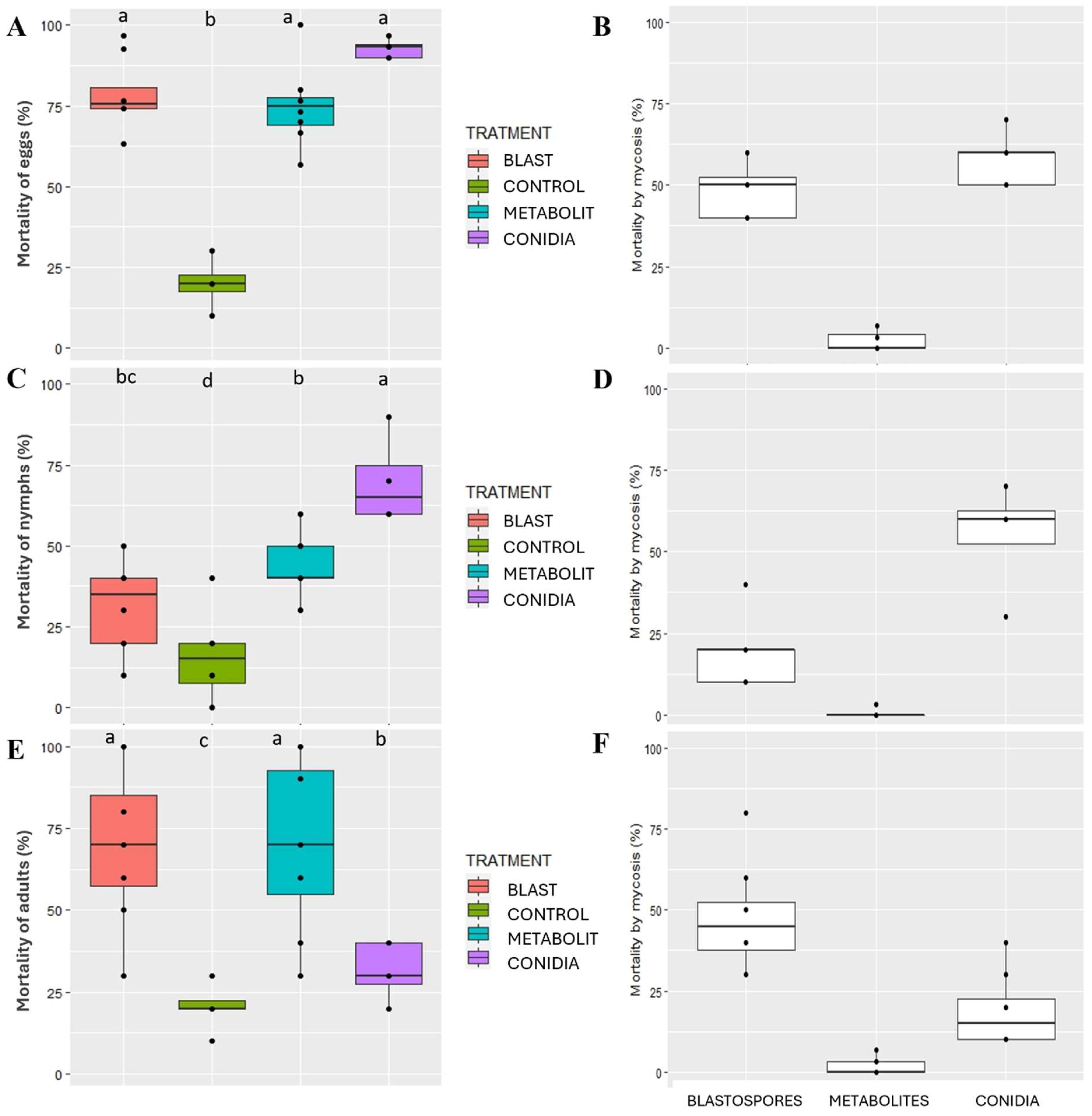
3.4. High Virulence of SBF054 against Euschistus heros
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministério da Agricultura, Pecuária e Abastecimento. Valor da Produção Agropecuária fecha 2022 em R$1,189 trilhão. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/valor-da-producao-agropecuaria-fecha-2022-em-r-1-189-trilhao (accessed on 26 September 2023).
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farm. Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- CONAB—Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Grãos; CONAB: Manaus, Brazil, 2022. [Google Scholar]
- Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Megharaj, M. Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance? Sci. Total Environ. 2019, 654, 177–189. [Google Scholar] [CrossRef]
- Sosa-Gómez, D.R.; Corrêa-Ferreira, B.S.; Kraemer, B.; Pasini, A.; Husch, P.E.; Vieira, C.E.D.; Reis Martinez, C.B.; Lopes, I.O.N. Prevalence, damage, management and insecticide resistance of stink bug populations (Hemiptera: Pentatomidae) in commodity crops. Agric. For. Entomol. 2020, 22, 99–118. [Google Scholar] [CrossRef]
- Moreira, F.M.; Cairo, P.A.R.; Nascimento, L.R.; Rosa, R.C.C.C.; Rocha, L.S.; Haddad, F. Optimal growth and N use efficiency enhancements by growth promoting rhizobacteria in seedlings banana under N2. Biocatal. Agric. Biotechnol. 2023, 50, 102734. [Google Scholar] [CrossRef]
- Agbessenou, A.; Akutse, K.S.; Yusuf, A.A.; Ekesi, S.; Subramanian, S.; Khamis, F.M. Endophytic fungi protect tomato and nightshade plants against Tuta absoluta (Lepidoptera: Gelechiidae) through a hidden friendship and cryptic battle. Sci. Rep. 2020, 10, 22195. [Google Scholar] [CrossRef] [PubMed]
- Bali, G.K.; Singh, S.K.; Maurya, D.K.; Wani, F.J.; Pandit, R.S. Morphological and molecular identifcation of the entomopathogenic fungus Purpureocillium lilacinum and its virulence against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) larvae and pupae. Egypt. J. Biol. Pest Control 2022, 32, 86. [Google Scholar]
- Moreira, F.M.; Cairo, P.A.R.; Borges, A.L.; Silva, L.D.S.; Haddad, F. Investigating the ideal mixture of soil and organic compound with Bacillus sp. and Trichoderma asperellum inoculations for optimal growth and nutrient content of banana seedlings. S. Afr. J. Bot. 2021, 137, 249–256. [Google Scholar] [CrossRef]
- Thi, H.N.; Le, K.Y.P.; Thien, N.D.T.; Nguyen, T.D.; Do, A.D. Insecticidal activity of isolated Purpureocillium lilacinum PL1 against whitefy, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) on cassava plantations in southern Viet Nam. Egypt. J. Biol. Pest Control 2023, 33, 44. [Google Scholar]
- Luangsa-Ard, J.; Houbraken, J.; Van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.R.; Zhang, C.C.; Fan, H.F.; Guo, Z.Y.; Yang, H.Y.; Chen, M.; Han, J.J.; Xu, J.; Zhang, K.Q.; et al. An efficient gene disruption system for the nematophagous fungus Purpureocillium lavendulum. Fungal Biol. 2019, 123, 274–282. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Q. Secondary Metabolites of Purpureocillium lilacinum. Molecules 2022, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- El-Marzoky, A.M.; Elnahal, A.S.M.; Jghef, M.M.; Abourehab, M.A.S.; El-Tarabily, K.A.; Ali, M.A.M.S. Purpureocillium lilacinum strain AUMC 10620 as a biocontrol agent against the citrus nematode Tylenchulus semipenetrans under laboratory and field conditions. Eur. J. Plant Pathol. 2023, 167, 59–76. [Google Scholar] [CrossRef]
- Isaac, G.S.; El-Deriny, M.M.; Tahaa, R.G. Efficacy of Purpureocillium lilacinum AUMC 10149 as biocontrol agent against root-knot nematode Meloidogyne incognita infecting tomato plant. Braz. J. Biol. 2024, 84, e253451. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.C.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Goffré, D.; Folgarait, P.J. Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. J. Invertebr. Pathol. 2015, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.R.; Lemos, R.N.S.; Rodrigues, A.A.C.; Batista Filho, A.; Oliveira, L.J.M.G.; Araújo, J.R.G. Occurrence of Purpureocillium lilacinum in citrus black fly nymphs. Rev. Bras. Frutic. 2018, 40, e-237. [Google Scholar] [CrossRef]
- Panyasiri, C.; Supothina, S.; Veeranondha, S.; Chanthaket, R.; Boonruangprapa, T.; Vichai, V. Control efficacy of entomopathogenic fungus Purpureocillium lilacinum against chili thrips (Scirtothrips dorsalis) on chili plant. Insects 2022, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef] [PubMed]
- Girardi, N.S.; Sosa, A.L.; Etcheverry, M.G.; Passone, M.A. In vitro characterization bioassays of the nematophagous fungus Purpureocillium lilacinum: Evaluation on growth, extracellular enzymes, mycotoxins and survival in the surrounding agroecosystem of tomato. Fungal Biol. 2022, 126, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Z.; Lin, R.; Li, E.; Mao, Z.; Ling, J.; Yang, Y.; Yin, W.; Xie, B. Biosynthesis of antibiotic leucinostatins in bio-control fungus Purpureocillium lilacinum and their inhibition on Phytophthora revealed by genome mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Elsebai, M.F. Activity of Purpureocillium lilacinum filtrates on biochemical characteristics of Sclerotinia sclerotiorum and induction of defense responses in common bean. Eur. J. Plant Pathol. 2019, 155, 39–52. [Google Scholar] [CrossRef]
- Momose, I.; Onodera, T.; Doi, H.; Adachi, H.; Iijima, M.; Yamazaki, Y.; Sawa, R.; Kubota, Y.; Igarashi, M.; Kawada, M. Leucinostatin Y: A peptaibiotic produced by the entomoparasitic fungus Purpureocillium lilacinum 40-H-28. J. Nat. Prod. 2019, 82, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.B.R.Z.; Alves-Pereira, A.; Prado, R.C.; Zucchi, M.I.; Delalibera Júnior, I. Metarhizium species in soil from Brazilian biomes: A study of diversity, distribution, and association with natural and agricultural environments. Fungal Ecol. 2019, 41, 289–300. [Google Scholar] [CrossRef]
- Shin, T.Y.; Choi, J.B.; Bae, S.M.; Koo, H.N.; Woo, S.D. Study on selective media for isolation of entomopathogenic fungi. Int. J. Ind. Entomol. 2010, 20, 7–12. [Google Scholar]
- Samson, R.A. Paecilomyces and some allied Hyphomycetes. Stud. Mycol. 1974, 6, 1–119. [Google Scholar]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Adam, M.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Extraction of Fungal Chitinase Enzyme. In Plant-Microbe Interactions; Springer Protocols Handbooks; Humana: New York, NY, USA, 2021. [Google Scholar]
- Orlandelli, R.C.; Almeida, T.T.; Alberto, R.N.; Polonio, J.C.; Azevedo, J.L.; Pamphile, J.A. Antifungal and proteolytic activities of endophytic fungi isolated from Piper hispidum Sw. Braz. J. Microbiol. 2015, 46, 359–366. [Google Scholar] [CrossRef]
- Cuzzi, C.; Link, S.; Vilani, A.; Onofre, S.B. Extracellular enzymes produced by endophytic fungi isolated from Baccharis dracunculifolia D. C. (Asteraeceae). Glass Sci. Technol. 2011, 4, 47–57. [Google Scholar]
- Lamichhane, J.R.; Varvaro, L. A new medium for the detection of fluorescent pigment production by Pseudomonas. Plant Pathol. 2013, 62, 624–632. [Google Scholar] [CrossRef]
- Rajawat, M.V.S.; Singh, S.; Tyagi, S.P.; Saxena, A.K. A Modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere 2016, 26, 768–773. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef]
- Hawar, S.N. Extracellular enzyme of endophytic fungi isolated from Ziziphus spina leaves as medicinal plant. Int. J. Biomater. 2022, 2022, 2135927. [Google Scholar] [CrossRef]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate method for detection of phisher lipase activity in Candida albicans. Sabouraudia J. Med. Vet. Mycol. 1982, 20, 7–14. [Google Scholar] [CrossRef]
- Ansari, M.A.; Butt, T.M. Effect of successive subculturing on stability, virulence, conidial yield, germination, and shelf life of entomopathogenic fungi. J. Appl. Microbiol. 2011, 110, 1460–1469. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jaronski, S.T. Production of microsclerotia of the fungal entomopathogenic Metarhizium anisopliae and their potential for use as a biocontrol agent for soil-inhabiting insects. Mycol. Res. 2009, 113, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, B. Development of Drying Methods and Evaluation of Blastospores Efficacy of Beauveria bassiana, Cordyceps fumosorosea, and Metarhizium rileyi against Euschistus heros and Spodoptera spp. Master’s Thesis, University of São Paulo, Piracicaba, Brazil, 2020. [Google Scholar]
- Efstathiadou, E.; Ntatsi, G.; Savvas, D.; Tampakaki, A.P. Genetic characterization at the species and symbiovar level of indigenous rhizobial isolates nodulating Phaseolus vulgaris in Greece. Sci. Rep. 2021, 11, 8674. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research: Canberra, Australia, 1996. [Google Scholar]
- Pineda-Suazo, D.; Montero-Vargas, J.M.; Ordaz-Ortiz, J.J.; Vázquez-Marrufo, G. Growth inhibition of phytopathogenic fungi and oomycetes by basidiomycete Irpex lacteus and Identification of its antimicrobial extracellular metabolites. Pol. J. Microbiol. 2021, 70, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.R.L.; Torres, A.F.; Truzi, C.C.; Vieira, N.F.; Vacari, A.M.; Bortoli, S.A. Artificial corn-based diet for rearing Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Nobre, S.D.N.; Oliveira Neto, O.B.; Schmidt, F.G.V.; Dias, S.; Lauman, R.; Sá, M.F.G.; Sujii, E.R. Parâmetros bionômicos do bicudo-do-algodoeiro (Anthonomus grandis) criado em dieta artificial para a realização de bioensaios. Bol. Pesqui. Desenvolv. 2002, 1, 1–20. [Google Scholar]
- Martins, E.S.; Praça, L.B.; Dumas, V.F.; Silva-Werneck, J.O.; Sone, E.H.; Waga, I.C.; Berry, C.; Monnerat, R.G. Characterization of Bacillus thuringiensis isolates toxic to cotton boll weevil (Anthonomus grandis). Biol. Control 2007, 40, 65–68. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Borges, M.; Laumann, R.A.; Sujii, E.R.; Mayon, P.; Caulfield, J.C.; Midega, C.A.O.; Khan, Z.R.; Pickett, J.A.; Birkett, M.A.; et al. Semiochemicals from herbivory induced cotton plants enhance the foraging behavior of the cotton boll weevil, Anthonomus grandis. J. Chem. Ecol. 2012, 38, 1528–1538. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1952, 18, 265–267. [Google Scholar] [CrossRef]
- Wang, G.; Huang, W.; Zhou, G.; Mayes, M.A.; Zhou, J. Modeling the processes of soil moisture in regulating microbial and carbon-nitrogen cycling. J. Hydrol. 2020, 585, 124777. [Google Scholar] [CrossRef]
- Kumar, V.; Nautiyal, C.S. Endophytes modulate plant genes: Present status and future perspectives. Curr. Microbiol. 2023, 80, 353. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Komivi, S.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal endophytes: Beyond herbivore management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.; Jamian, S.; Saad, N.; Abdullah, S.; Hata, E.M.; Jalinas, J.; Ismail, S.I. Inoculation and colonization of the entomopathogenic fungi, Isaria javanica and Purpureocillium lilacinum, in tomato plants, and their effect on seedling growth, mortality and adult emergence of Bemisia tabaci (Gennadius). PLoS ONE 2023, 18, e0285666. [Google Scholar] [CrossRef]
- Rahman, M.T.; Rubayet, M.T.; Bhuiyan, M.K.A. Integrated management of rhizoctonia root rot disease of soybean caused by Rhizoctonia solani. Nippon J. Environ. Sci. 2020, 1, 1018. [Google Scholar] [CrossRef]
- Marti, G.A.; Lastra, C.C.L.; Pelizza, S.A.; García, J.J. Isolation of Paecilomyces lilacinus (Thom) Samson (Ascomycota: Hypocreales) from the Chagas disease vector, Triatoma infestans Klug (Hemiptera: Reduviidae) in an endemic area in Argentina. Mycopathologia 2006, 162, 369–372. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Bueno, A.F.; Silva, F.A.C. Insetos que atacam vagens e grãos. In Soja: Manejo Integrado de Insetos e Outros Artrópodes-Praga; Hoffmann-Campo, C.B., Corrêa-Ferreira, B.S., Moscardi, F., Eds.; Embrapa: Brasília, Brazil, 2012; pp. 335–420. [Google Scholar]
- Roggia, S.; Utiamada, C.; Hirose, E.; Stoetzer, A.; Avila, C.; Kischel, E.; Marzarotto, F.O.; Tomquelski, G.V.; Guedes, J.V.C.; Arnemann, J.A.; et al. Eficiência de Inseticidas no Controle do Percevejo Marrom (Euschistus Heros) em Soja, na Safra 2013/14: Resultados Sumarizados de Ensaios Cooperativos; Embrapa Soja: Londrina, Brasil, 2018; pp. 1–22. [Google Scholar]
- Grewal, G.K.; Joshi, N.; Suneja, Y. Pathogenicity of Metarhizium rileyi (Farlow) Kepler, S.A. Rehner and Humber isolates against Spodoptera litura (Fabricius) and their extracellular enzymatic activities. Egypt. J. Biol. Pest Control 2021, 31, 59. [Google Scholar] [CrossRef]
- Petrisor, C.; Stoian, G. The role of hydrolytic enzymes produced by entomopathogenic fungi in pathogenesis of insects mini review. Rom. J. Plant Prot. 2017, 10, 66–72. [Google Scholar]
- Dietsch, R.; Jakobs-Schönwandt, D.; Grünberger, G.; Patel, A. Desiccation-tolerant fungal blastospores: From production to application. Curr. Res. Biotechnol. 2021, 3, 323–339. [Google Scholar] [CrossRef]


| Characteristics | SBF054 |
|---|---|
| Solubilization | |
| Potassium | + |
| Phosphorus | - |
| Synthesis | |
| Organic acids | + |
| Siderophores | - |
| Enzymes | |
| Chitinase | + |
| Protease | + |
| Lipase | + |
| Cellulase | + |
| Amylase | - |
| Alkaline phosphatase | + |
| Enzymes | Degradation Index | ||||
|---|---|---|---|---|---|
| 02 Days | 04 Days | 06 Days | 08 Days | 10 Days | |
| Protease | 0.75 ± 0.02 | 0.83 ± 0.01 | 0.80 ± 0.02 | 0.78 ± 0.01 | 0.70 ± 0.02 |
| Lipase | 1 | 0.97 ± 0.01 | 0.85 ± 0.03 | 0.84 ± 0.02 | 0.83 ± 0.02 |
| Chitinase | 1 | 1 | 1 | 1 | 0.97 ± 0.01 |
| CV (%) | 13.75 | 7.84 | 8.63 | 8.82 | 13.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, F.M.; Machado, T.I.; Torres, C.A.R.; Souza, H.R.d.; Celestino, M.F.; Silva, M.A.; Gomes, G.C.; Cunha, B.B.d.R.; Santos, P.d.L.B.d.; Carvalho Filho, M.R.d.; et al. Purpureocillium lilacinum SBF054: Endophytic in Phaseolus vulgaris, Glycine max, and Helianthus annuus; Antagonistic to Rhizoctonia solani; and Virulent to Euschistus heros. Microorganisms 2024, 12, 1100. https://doi.org/10.3390/microorganisms12061100
Moreira FM, Machado TI, Torres CAR, Souza HRd, Celestino MF, Silva MA, Gomes GC, Cunha BBdR, Santos PdLBd, Carvalho Filho MRd, et al. Purpureocillium lilacinum SBF054: Endophytic in Phaseolus vulgaris, Glycine max, and Helianthus annuus; Antagonistic to Rhizoctonia solani; and Virulent to Euschistus heros. Microorganisms. 2024; 12(6):1100. https://doi.org/10.3390/microorganisms12061100
Chicago/Turabian StyleMoreira, Flávia Melo, Túlio Iglésias Machado, Caio Augusto Rosado Torres, Hebert Ribeiro de Souza, Matheus Felipe Celestino, Marco Antônio Silva, Giovana Cidade Gomes, Breno Beda dos Reis Cunha, Pedro de Luca Buffon dos Santos, Magno Rodrigues de Carvalho Filho, and et al. 2024. "Purpureocillium lilacinum SBF054: Endophytic in Phaseolus vulgaris, Glycine max, and Helianthus annuus; Antagonistic to Rhizoctonia solani; and Virulent to Euschistus heros" Microorganisms 12, no. 6: 1100. https://doi.org/10.3390/microorganisms12061100






