Genomic Differences Associated with Resistance and Virulence in Pseudomonas aeruginosa Isolates from Clinical and Environmental Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Isolates
2.2. Antimicrobial Susceptibility Testing
2.3. MLST Typing
2.4. DNA Extraction and Sequencing
2.5. Identification of Genes Associated with Virulence and Antimicrobial Resistance
2.6. Comparison of the Genomes
3. Results
3.1. Antimicrobial Susceptibility Testing
3.2. Multilocus Sequence Typing (MLST)
3.3. General Characteristics of Genomes
3.4. Comparison of Genes Associated with Virulence
3.5. Comparison of Genes Associated with Antibiotic Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Martinez, L. Pseudomonas aeruginosa: Aportacion Al Conocimiento de Su Estructura y Al de Los Mecanismos Que Contribuyen a Su Resistencia a Los Antimicrobianos. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2007; pp. 7–9. [Google Scholar]
- Crone, S.; Vives-Flórez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martínez-García, E.; Rojas-Acosta, C.; Catalina Gomez-Puerto, M.; Calum, H.; et al. The Environmental Occurrence of Pseudomonas aeruginosa. APMIS 2020, 128, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, R.S. Pseudomonas: Molecular and Applied Biology; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319311982. [Google Scholar]
- Kenneth, R.; George, R. Microbiología Médica Sherris; Kenneth, R., George, R., Eds.; Quinta; Mac Graw Hill: Santa Fe, México, 2011; ISBN 9786071505545. [Google Scholar]
- Luján, D. Uso de Pseudomonas aeruginosa en biorremediación. BioTecnología 2019, 23, 32–42. [Google Scholar]
- Nain, Z.; Karim, M.M. Whole-Genome Sequence, Functional Annotation, and Comparative Genomics of the High Biofilm-Producing Multidrug-Resistant Pseudomonas aeruginosa MZ4A Isolated from Clinical Waste. Gene Rep. 2021, 22, 100999. [Google Scholar] [CrossRef]
- Vanegas, J.; Jiménez, J. Principales Características de La Genética Bacteriana de Pseudomonas aeruginosa Que Contribuyen Con Su Patogénesis y Resistencia. Hechos Microbiológicos 2014, 4, 98–105. [Google Scholar] [CrossRef]
- Paz, V.M.; Mangwani, S.; Martínez, A.; Álvarez, D.; Solano, S.G.; Vázquez, R. Pseudomonas aeruginosa: Patogenicidad y Resistencia Antimicrobiana En La Infección Urinaria. Rev. Chil. Infectología 2019, 36, 180–189. [Google Scholar] [CrossRef]
- Battle, S.E.; Meyer, F.; Rello, J.; Kung, V.L.; Hauser, A.R. Hybrid Pathogenicity Island PAGI-5 Contributes to the Highly Virulent Phenotype of a Pseudomonas aeruginosa Isolate in Mammals. J. Bacteriol. 2008, 190, 7130–7140. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Bilocq, F.; Pot, B.; Cornelis, P.; Zizi, M.; Van Eldere, J.; Deschaght, P.; Vaneechoutte, M.; Jennes, S.; Pitt, T.; et al. Pseudomonas aeruginosa Population Structure Revisited. PLoS ONE 2009, 4, e7740. [Google Scholar] [CrossRef] [PubMed]
- Zurita, J.; Sevillano, G.; Belen, M.; Paz, A.; Rizkallah, B. Pseudomonas aeruginosa Epidemic High-Risk Clones and Their Association with Multidrug-Resistant. Open Forum Infect. Dis. 2023, 10, 921–922. [Google Scholar] [CrossRef]
- del Barrio-Tofiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa Epidemic High-Risk Clones and Their Association with Horizontally-Acquired β-Lactamases: 2020 Update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef] [PubMed]
- Amábile, C.F. Antibiotics and Antibiotic Resistance in the Environment; Routledge: London, UK, 2015; ISBN 9781315679419. [Google Scholar]
- Slekovec, C.; Plantin, J.; Cholley, P.; Thouverez, M.; Talon, D.; Bertrand, X.; Hocquet, D. Tracking Down Antibiotic-Resistant Pseudomonas aeruginosa Isolates in a Wastewater Network. PLoS ONE 2012, 7, e49300. [Google Scholar] [CrossRef] [PubMed]
- Mayz, J.C.; Manzi, L.V. Bacterias Hidrocarburoclásticas Del Género Pseudomonas En La Rizosfera de Samanea Saman (Jacq.) Merr. Rev. Colomb. Biotecnol. 2017, 19, 29–37. [Google Scholar] [CrossRef]
- Ordoñez Burbano, D.E.; Abella Medina, C.A.; Echeverry Tamayo, A.; Paz Lasprilla, L.M.; Benítez-Campo, N. Biodegradación de Hidrocarburos Alifáticos Saturados Por Microorganismos Aislados de Suelo Contaminado Con Derivados Del Petróleo. Rev. Ciencias 2019, 22, 33–44. [Google Scholar] [CrossRef]
- Kung, V.L.; Ozer, E.A.; Hauser, A.R. The Accessory Genome of Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2010, 74, 621. [Google Scholar] [CrossRef] [PubMed]
- McAulay, K.; Schuetz, A.N.; Fauntleroy, K.; Shen, L.; Merveille, Y.M.; Deroncelay, A.; Cole, N.; Fitzgerald, D.W.; Ocheretina, O. Multidrug-Resistant Pseudomonas aeruginosa in Healthcare Facilities in Port-Au-Prince, Haiti. J. Glob. Antimicrob. Resist. 2021, 25, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Curran, B.; Jonas, D.; Grundmann, H.; Pitt, T.; Dowson, C.G. Development of a Multilocus Sequence Typing Scheme for the Opportunistic Pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 2004, 42, 5644–5649. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting Chromosomal DNA Restriction Patterns Produced by Pulsed-Field Gel Electrophoresis: Criteria for Bacterial Strain Typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Gomila, M.; Del Carmen Gallegos, M.; Fernández-Baca, V.; Pareja, A.; Pascual, M.; Díaz-Antolín, P.; García-Valdés, E.; Lalucat, J. Genetic Diversity of Clinical Pseudomonas aeruginosa Isolates in a Public Hospital in Spain. BMC Microbiol. 2013, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S., II; Amy Mathers, F.J.; April Bobenchik, D.M.; Alexandra Lynn Bryson, D.; Shelley Campeau, D.; Sharon Cullen, D.K.; Tanis Dingle, R.; Marcelo Galas, F.F.; Humphries, R.M.; Thomas Kirn, F.J.; et al. CLSI M100-Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2024; p. 416. [Google Scholar]
- GitHub—Tseemann/Mlst: :Id: Scan Contig Files against PubMLST Typing Schemes. Available online: https://github.com/tseemann/mlst (accessed on 1 March 2023).
- Jolley, K.A.; Maiden, M.C.J. BIGSdb: Scalable Analysis of Bacterial Genome Variation at the Population Level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef]
- QIAGEN DNeasy Blood & Tissue Kits. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/genomic-dna/dneasy-blood-and-tissue-kit/ (accessed on 21 November 2022).
- MiSeqTM System Velocidad y Simplicidad Para La Resecuenciación Selectiva y La Secuenciación de Genomas Pequeños. 2021. pp. 1–5. Available online: https://www.illumina.com/content/dam/illumina-marketing/documents/products/datasheets/miseq-data-sheet-m-gl-00006-translations/miseq-system-specification-sheet-m-gl-00006-esp-view.pdf (accessed on 12 March 2022).
- Andrews, S. FastQC: Una Herramienta de Control de Calidad Para Datos de Secuencia de Alto Rendimiento. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 12 March 2022).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. bioRxiv 2016, 096412. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding Data and Analysis Capabilities. Nucleic Acids Res. 2020, 48, D606. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, D.A.; Assaf, R.; Aziz, R.K.; Brettin, T.; Bun, C.; Conrad, N.; Davis, J.J.; Dietrich, E.M.; Disz, T.; Gerdes, S.; et al. PATRIC as a Unique Resource for Studying Antimicrobial Resistance. Brief. Bioinform. 2019, 20, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- RStudio, E. The Popular Open-Source IDE from Posit. Available online: https://posit.co/products/open-source/rstudio/ (accessed on 27 February 2023).
- YaRrr! The Pirate’s Guide to R. Available online: https://bookdown.org/ndphillips/YaRrr/logistic-regression-with-glmfamily-binomial.html (accessed on 1 March 2023).
- Chapter 7 Understanding ANOVA in R|Data Analysis in R. Available online: https://bookdown.org/steve_midway/DAR/understanding-anova-in-r.html (accessed on 1 March 2023).
- Alikhan, N. BRIG 0.95 Manual. Available online: https://sourceforge.net/projects/brig/ (accessed on 26 April 2022).
- European Committee on Antimicrobial Susceptibility Testing Eucast: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 2 April 2024).
- Wilderman, P.J.; Vasil, A.I.; Johnson, Z.; Wilson, M.J.; Cunliffe, H.E.; Lamont, I.L.; Vasil, M.L. Characterization of an Endoprotease (PrpL) Encoded by a PvdS-Regulated Gene in Pseudomonas aeruginosa. Infect. Immun. 2001, 69, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.H.; Tetu, S.G.; Larouche, A.; Elbourne, L.; Tremblay, S.; Ren, Q.; Dodson, R.; Harkins, D.; Shay, R.; Watkins, K.; et al. Complete Genome Sequence of the Multiresistant Taxonomic Outlier Pseudomonas aeruginosa PA7. PLoS ONE 2010, 5, 8842. [Google Scholar] [CrossRef] [PubMed]
- Rocchetta, H.L.; Burrows, L.L.; Lam, J. Genetics of O-Antigen Biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 1999, 63, 523–553. [Google Scholar] [CrossRef]
- Shirai, T.; Akagawa, M.; Makino, M.; Ishii, M.; Arai, A.; Nagasawa, N.; Sada, M.; Kimura, R.; Okayama, K.; Ishioka, T.; et al. Molecular Evolutionary Analyses of the Pseudomonas-Derived Cephalosporinase Gene. Microorganisms 2023, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Del Campo, R.; Perenguez, M.; Blanco, V.M.; Rodríguez-Baños, M.; Perez, F.; Maya, J.J.; Rojas, L.; Cantón, R.; Arias, C.A.; et al. Dissemination of High-Risk Clones of Extensively Drug-Resistant Pseudomonas aeruginosa in Colombia. Antimicrob. Agents Chemother. 2015, 59, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Mulet, X.; Cabot, G.; Ocampo-Sosa, A.A.; Domínguez, M.A.; Zamorano, L.; Juan, C.; Tubau, F.; Rodríguez, C.; Moyà, B.; Peña, C.; et al. Biological Markers of Pseudomonas aeruginosa Epidemic High-Risk Clones. Antimicrob. Agents Chemother. 2013, 57, 5527–5535. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Mulet, X.; López, C.; Juan, C. The Increasing Threat of Pseudomonas aeruginosa High-Risk Clones. Drug Resist. Updat. 2015, 21–22, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.M.; Cortés, J.A.; Leal, A.L.; Meneses, A.; Meléndez, M.P.; De Grebo, N. Resistance to Antibiotics in Pseudomonas aeruginosa in Colombian Hospitals. Rev. Chil. Infectol. 2013, 30, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.S.; Cubbon, M.D.; Karunaharan, R.N.; Pope, C.F.; Planche, T.D. Multidrug-Resistant Pseudomonas aeruginosa Outbreaks in Two Hospitals: Association with Contaminated Hospital Waste-Water Systems. J. Hosp. Infect. 2012, 82, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Pitondo-Silva, A.; Martins, V.V.; Fernandes, A.F.T.; Stehling, E.G. High Level of Resistance to Aztreonam and Ticarcillin in Pseudomonas aeruginosa Isolated from Soil of Different Crops in Brazil. Sci. Total Environ. 2014, 473–474, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Olga, P.; Apostolos, V.; Alexis, G.; George, V.; Athena, M. Antibiotic Resistance Profiles of Pseudomonas aeruginosa Isolated from Various Greek Aquatic Environments. FEMS Microbiol. Ecol. 2016, 92, fiw042. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.; Cabezas, A.F.; Ferauds, M.; Castillo, J.E.; Caicedo, L.D. Antimicrobial Resistance Patterns and Genotypic Diversity between Clinical and Water Systems Isolates of Pseudomonas aeruginosa in Cali, Colombia. Trop. Biomed. 2020, 37, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas Genomes: Diverse and Adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.; Vijay, A.K.; Kohli, G.S.; Rice, S.A.; Willcox, M. Comparative Genomics of Clinical Strains of Pseudomonas aeruginosa Strains Isolated from Different Geographic Sites. Sci. Rep. 2018, 8, 15668. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.C.; Sifri, C.D.; Goumnerov, B.C.; Rahme, L.G.; Ausubel, F.M.; Calderwood, S.B. Identification of Virulence Genes in a Pathogenic Strain of Pseudomonas aeruginosa by Representational Difference Analysis. J. Bacteriol. 2002, 184, 952–961. [Google Scholar] [CrossRef]
- Wolfgang, M.C.; Kulasekara, B.R.; Liang, X.; Boyd, D.; Wu, K.; Yang, Q.; Miyada, C.G.; Lory, S. Conservation of Genome Content and Virulence Determinants among clinicaland Environmental Isolates of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2003, 100, 8484. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, S.L.; Hancock, R.E.W. Pseudomonas aeruginosa: New Insights into Pathogenesis and Host Defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.W.; Khan, A.U. Updates on the Pathogenicity Status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.; Ragno, M.; Elsen, S.; Reboud, E.; Golovkine, G.; Bouillot, S.; Huber, P.; Lory, S.; Faudry, E.; Attrée, I. Pseudomonas aeruginosa Pore-Forming Exolysin and Type IV Pili Cooperate To Induce Host Cell Lysis. mBio 2017, 8, e02250-16. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, K.; Johnson, M.; Reid, T.E.; Springer, T.I. Utilizing in Silico and in Vitro Methods to Identify Possible Binding Sites of a Novel Ligand against Pseudomonas aeruginosa Phospholipase Toxin ExoU. Biochem. Biophys. Rep. 2022, 29, 101188. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, A.; Momiyama, K.; Kimura, T.; Akiyama, K.; Inoue, K.; Naito, Y.; Kinoshita, M.; Shimizu, M.; Kato, H.; Shime, N.; et al. An Outbreak of Fluoroquinolone-Resistant Pseudomonas aeruginosa ST357 Harboring the ExoU Gene. J. Infect. Chemother. 2018, 24, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Alva, P.P.; Raj, J.M.; Karunasagar, I.; Premanath, R. Environmental and Clinical Pseudomonas aeruginosa Isolates with Pooled Presence of exo S, exo U, exo T and exo Y Genes. J. Pure Appl. Microbiol. 2018, 12, 1119–1124. [Google Scholar] [CrossRef]
- Kulasekara, B.R.; Kulasekara, H.D.; Wolfgang, M.C.; Stevens, L.; Frank, D.W.; Lory, S. Acquisition and Evolution of the ExoU Locus in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 4037–4050. [Google Scholar] [CrossRef]
- Kirienko, N.V.; Kirienko, D.R.; Larkins-Ford, J.; Wählby, C.; Ruvkun, G.; Ausubel, F.M. Pseudomonas aeruginosa Disrupts Caenorhabditis Elegans Iron Homeostasis, Causing a Hypoxic Response and Death. Cell Host Microbe 2013, 13, 406–416. [Google Scholar] [CrossRef]
- Xie, F.; Dai, S.; Zhao, Y.; Huang, P.; Yu, S.; Ren, B.; Wang, Q.; Ji, Z.; Alterovitz, G.; Zhang, Q.; et al. Generation of Fluorinated Amychelin Siderophores against Pseudomonas aeruginosa Infections by a Combination of Genome Mining and Mutasynthesis. Cell Chem. Biol. 2020, 27, 1532–1543.e6. [Google Scholar] [CrossRef]
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An Alternative Bioremediation Strategy? Sci. Total Environ. 2022, 819, 153144. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Imperi, F.; Lamont, I.L. Pyoverdine Siderophores: From Biogenesis to Biosignificance. Trends Microbiol. 2007, 15, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Newman, D. Both Toxic and Beneficial Effects of Pyocyanin Contribute to the Lifecycle of Pseudomonas aeruginosa. Mol. Microbiol. 2018, 110, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, N.C.; Shanks, R.M.Q.; O’Toole, G.A. Rhamnolipids Modulate Swarming Motility Patterns of Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 7351–7361. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Wichmann, R.; Küpper, B.; Zwick, M.; Wilhelm, S.; et al. Growth Independent Rhamnolipid Production from Glucose Using the Non-Pathogenic Pseudomonas putida KT2440. Microb. Cell Fact. 2011, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of Structures, Microbial Origins and Roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.L.; McNally, D.J.; Charchoglyan, A.; Brewer, D.; Field, R.A.; Lam, J.S. Characterization of WbpB, WbpE, and WbpD and Reconstitution of a Pathway for the Biosynthesis of UDP-2,3-Diacetamido-2,3-Dideoxy-D-Mannuronic Acid in Pseudomonas aeruginosa. J. Biol. Chem. 2009, 284, 11854–11862. [Google Scholar] [CrossRef]
- Nasrin, S.; Hegerle, N.; Sen, S.; Nkeze, J.; Sen, S.; Permala-Booth, J.; Choi, M.; Sinclair, J.; Tapia, M.D.; Johnson, J.K.; et al. Distribution of Serotypes and Antibiotic Resistance of Invasive Pseudomonas aeruginosa in a Multi-Country Collection. BMC Microbiol. 2022, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Rojo, F.; Martínez, J.L. Environmental and Clinical Isolates of Pseudomonas aeruginosa Show Pathogenic and Biodegradative Properties Irrespective of Their Origin. Environ. Microbiol. 1999, 1, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Laborda, P.; Sanz-García, F.; Hernando-Amado, S.; Martínez, J.L. Pseudomonas aeruginosa: An Antibiotic Resilient Pathogen with Environmental Origin. Curr. Opin. Microbiol. 2021, 64, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Sayeed, S.; Antalis, P.; Gladitz, J.; Ahmed, A.; Dice, B.; Janto, B.; Dopico, R.; Keefe, R.; Hayes, J.; et al. Extensive Genomic Plasticity in Pseudomonas aeruginosa Revealed by Identification and Distribution Studies of Novel Genes among Clinical Isolates. Infect. Immun. 2006, 74, 5272–5283. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.C.; Sanders, W.E.; Sanders, C.C.; Sanders, W.E. Type I Beta-Lactamases of Gram-Negative Bacteria: Interactions with Beta-Lactam Antibiotics. J. Infect. Dis. 1986, 154, 792–800. [Google Scholar] [CrossRef]
- Castanheira, M.; Doyle, T.B.; Hubler, C.M.; Collingsworth, T.D.; DeVries, S.; Mendes, R.E. The Plethora of Resistance Mechanisms in Pseudomonas aeruginosa: Transcriptome Analysis Reveals a Potential Role of Lipopolysaccharide Pathway Proteins to Novel β-Lactam/β-Lactamase Inhibitor Combinations. J. Glob. Antimicrob. Resist. 2022, 31, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.R.; Kumar, V.; Taracila, M.A.; Mojica, M.F.; Shea, M.O.; Schinabeck, W.; Silver, G.; Hujer, A.M.; Papp-wallace, K.M.; Chen, S.; et al. Natural Protein Engineering in the Ω-Loop: The Role of Y221 in Ceftazidime and Ceftolozane Resistance in Pseudomonas-Derived Cephalosporinase. Antimicrob. Agents Chemother. 2023, 67, e00791-23. [Google Scholar] [CrossRef] [PubMed]
- Berrazeg, M.; Jeannot, K.; Ntsogo Enguéné, V.Y.; Broutin, I.; Loeffert, S.; Fournier, D.; Plésiat, P. Mutations in β-Lactamase AmpC Increase Resistance of Pseudomonas aeruginosa Isolates to Antipseudomonal Cephalosporins. Antimicrob. Agents Chemother. 2015, 59, 6248–6255. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, J.M.; Poirel, L.; Nordmann, P. Extended-Spectrum Cephalosporinases in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009, 53, 1766. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mima, T.; Komori, Y.; Morita, Y.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. A New Member of the Tripartite Multidrug Efflux Pumps, MexVW–OprM, in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2003, 52, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Adamiak, J.W.; Leus, I.V. Making Sense of Drug-Efflux Transporters in the Physiological Environment. Curr. Opin. Microbiol. 2022, 69, 102179. [Google Scholar] [CrossRef] [PubMed]
- Pontel, L.B.; Audero, M.E.P.; Espariz, M.; Checa, S.K.; Soncini, F.C. GolS Controls the Response to Gold by the Hierarchical Induction of Salmonella-Specific Genes That Include a CBA Efflux-Coding Operon. Mol. Microbiol. 2007, 66, 814–825. [Google Scholar] [CrossRef]
- Smith, H.E.; Blair, J.M.A. Redundancy in the Periplasmic Adaptor Proteins AcrA and AcrE Provides Resilience and an Ability to Export Substrates of Multidrug Efflux. J. Antimicrob. Chemother. 2014, 69, 982–987. [Google Scholar] [CrossRef]
- Morita, Y.; Tomida, J.; Kawamura, Y. Mexxy Multidrug Efflux System of Pseudomonas aeruginosa. Front. Microbiol. 2012, 3, 408. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.H.; Berghuis, A.M. Structural Basis of APH(3′)-IIIa-Mediated Resistance to N1-Substituted Aminoglycoside Antibiotics. Antimicrob. Agents Chemother. 2009, 53, 3049–3055. [Google Scholar] [CrossRef]
- Mella, M.S.; Sepúlveda, A.M.; González, R.G.; Bello, T.H.; Domínguez, Y.M.; Zemelman, Z.R.; Ramírez, G.C. Aminoglucósidos-Aminociclitoles: Características Estructurales y Nuevos Aspectos Sobre Su Resistencia. Rev. Chil. Infectología 2004, 21, 330–338. [Google Scholar] [CrossRef]
- Papadovasilaki, M.; Oberthür, D.; Gessmann, R.; Sarrou, I.; Betzel, C.; Scoulica, E.; Petratos, K. Biophysical and Enzymatic Properties of Aminoglycoside Adenylyltransferase AadA6 from Pseudomonas aeruginosa. Biochem. Biophys. Rep. 2015, 4, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Martínez, L.; López-Jiménez, L.; Fusté, E.; Vinuesa, T.; Martínez, J.P.; Viñas, M. Class 1 Integrons in Environmental and Clinical Isolates of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2011, 38, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Glen, K.A.; Lamont, I.L. β-Lactam Resistance in Pseudomonas aeruginosa: Current Status, Future Prospects. Pathogens 2021, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.; Robert, B. Extended Spectrum Beta Lactamases: A Critical Update. In Multidrug Resistance: A Global Concern; Shakil, S., Ali, H.M., Zarrilli, R., Khan, A.U., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; Volume 18, pp. 115–129. ISBN 9781608052554. [Google Scholar]
- Peymani, A.; Naserpour-Farivar, T.; Zare, E.; Azarhoosh, K. Distribution of BlaTEM, BlaSHV, and BlaCTX-M Genes among ESBL-Producing P. Aeruginosa Isolated from Qazvin and Tehran Hospitals, Iran. J. Prev. Med. Hyg. 2017, 58, E155–E160. [Google Scholar] [PubMed]
- Kraftova, L.; Finianos, M.; Studentova, V.; Chudejova, K.; Jakubu, V.; Zemlickova, H.; Papagiannitsis, C.C.; Bitar, I.; Hrabak, J. Evidence of an Epidemic Spread of KPC-Producing Enterobacterales in Czech Hospitals. Sci. Rep. 2021, 11, 15732. [Google Scholar] [CrossRef] [PubMed]
- Cuzon, G.; Naas, T.; Villegas, M.V.; Correa, A.; Quinn, J.P.; Nordmann, P. Wide Dissemination of Pseudomonas aeruginosa Producing β-Lactamase BlaKPC-2 Gene in Colombia. Antimicrob. Agents Chemother. 2011, 55, 5350. [Google Scholar] [CrossRef] [PubMed]
- Santella, G.; Cittadini, R.; Papalia, M.; Vera Ocampo, C.; Del Castillo, M.; Vay, C.; Gutkind, G.; Radice, M. First Clonal Spread of KPC-Producing Pseudomonas aeruginosa in Buenos Aires, Argentina. Infect. Genet. Evol. 2012, 12, 2003–2005. [Google Scholar] [CrossRef] [PubMed]
- Haghighifar, E.; Dolatabadi, R.K.; Norouzi, F. Prevalence of BlaVEB and BlaTEM Genes, Antimicrobial Resistance Pattern and Biofilm Formation in Clinical Isolates of Pseudomonas aeruginosa from Burn Patients in Isfahan, Iran. Gene Rep. 2021, 23, 101157. [Google Scholar] [CrossRef]
- Haider, M.H.; McHugh, T.D.; Roulston, K.; Arruda, L.B.; Sadouki, Z.; Riaz, S. Detection of Carbapenemases BlaOXA48-BlaKPC-BlaNDM-BlaVIM and Extended-Spectrum-β-Lactamase BlaOXA1-BlaSHV-BlaTEM Genes in Gram-Negative Bacterial Isolates from ICU Burns Patients. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 18. [Google Scholar] [CrossRef]
- Militza Guzmán, L.; Rodríguez, E.; Karen Antón, C.; Silva, S.; Navarro, J.; Lastra, L.; de Elsa Salazar, V.; Alonso, G. Genes BlaTEM, BlaSHV y BlaCTX-M En Enterobacterias Productoras de β-Lactamasas de Espectro Extendido Aisladas de Pacientes Con Infección Intrahospitalaria. Investig. Clin. 2013, 54, 235–245. [Google Scholar]
- Maurya, A.P.; Das Talukdar, A.; Dhar Chanda, D.; Chakravarty, A.; Bhattacharjee, A. Genetic Environment of OXA-2 Beta-Lactamase Producing Gram-Negative Bacilli from a Tertiary Referral Hospital. Indian J. Med. Res. 2015, 141, 368. [Google Scholar] [CrossRef] [PubMed]
- Jara, M. Tetraciclinas: Un Modelo de Resistencia Antimicrobiana. Av. Cienc. Vet. 2010, 22, 49–55. [Google Scholar] [CrossRef]
- Hernández, D.V.; Hernández, A. Resistencia a Los Antibióticos, Una Amenaza Latente. Rev. Aire Libr. 2016, 4, 29–40. [Google Scholar]
- Maldonado, N.A.; Múnera, M.I.; López, J.A.; Sierra, P.; Robledo, C.; Robledo, J.; Germen, G. Tendencias de La Resistencia a Antibióticos En Medellín y En Los Municipios Del Área Metropolitana Entre 2007 y 2012. Biomédica 2014, 34, 433–446. [Google Scholar] [CrossRef] [PubMed]
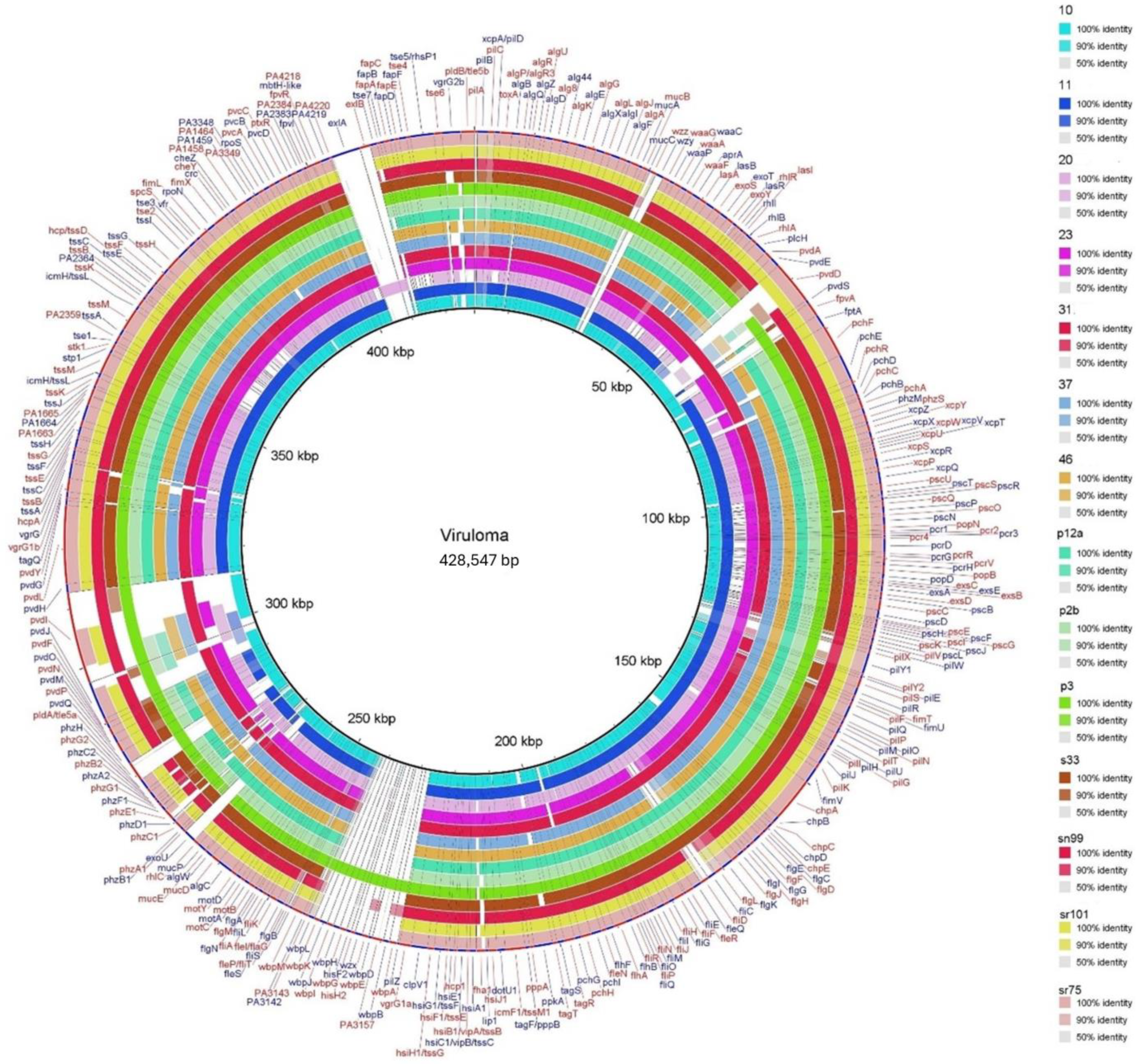
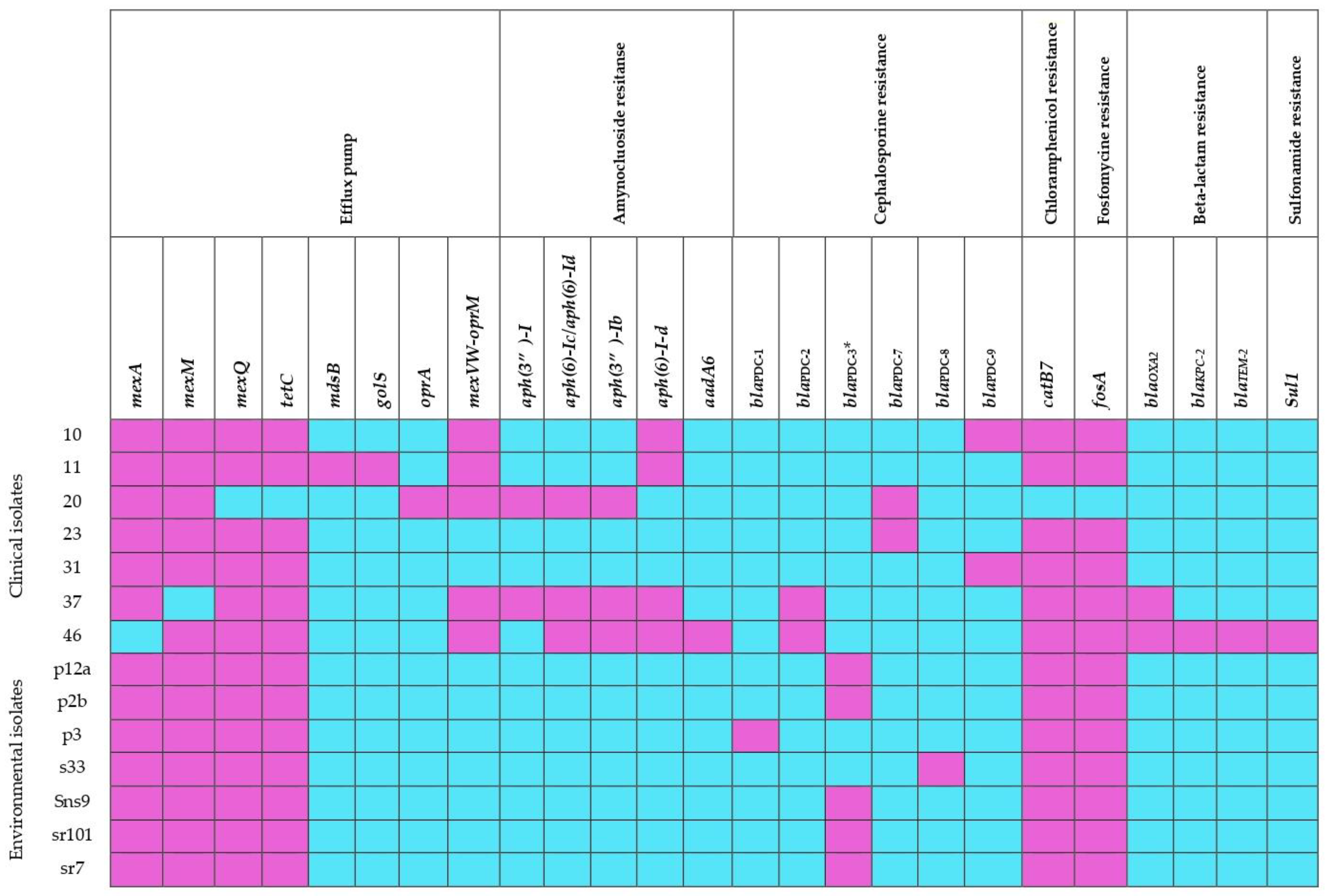
| Clinical Isolates | Environmental Isolates | ||
|---|---|---|---|
| ID | origin | ID | origin |
| 10 | Acute clinical infection/not identified | p12a | Water, inlet of the facultative lagoon of the domestic wastewater treatment plant. |
| 11 | Acute clinical infection/blood sample | p2b | Water, outlet of the anaerobic lagoon of the domestic wastewater treatment plant |
| 20 | Acute clinical infection/blood sample | p3 | Water, outlet of the facultative lagoon of the domestic wastewater treatment plant. |
| 23 | Acute clinical infection/blood sample | s33 | Soil, sugarcane crop, located south of Cali. |
| 31 | Acute clinical infection/respiratory sample | sn99 | Soil, Experiment station of the Valle University |
| 37 | Acute clinical infection/not identified | sr101 | Soil, riverbank Pance |
| 46 | Acute clinical infection/gastrointestinal sample | sr75 | Soil, riverbank Pance |
| Source | Isolate | Monobact | Cephalosporin | Fluoroq | Carbapenems | Comb β-lact | Aminogluc | ||
|---|---|---|---|---|---|---|---|---|---|
| AZT | CPE | CAZ | CP | IMP | MER | P/T | AK | ||
| Water | p12a | S | S | S | S | S | S | S | S |
| p2b | S | S | S | S | S | S | S | S | |
| p3 | R | S | S | R | S | S | S | R | |
| Soil | s33 | R | S | S | S | S | S | S | S |
| sn99 | S | S | S | S | S | S | S | S | |
| sr101 | S | S | S | S | S | S | S | S | |
| sr75 | S | S | S | S | S | S | S | S | |
| Hospital environment | 37 | S | S | R | R | R | R | R | R |
| 46 | R | R | R | R | R | R | R | R | |
| 31 | R | R | R | R | R | R | R | R | |
| 23 | R | S | R | R | R | R | R | S | |
| 20 | S | S | S | R | S | S | S | S | |
| 10 | S | S | S | S | S | S | S | S | |
| 11 | S | S | S | S | S | S | S | S | |
| Source | ID | Genome Length (Mbp) | CD | Content of G + C (%) | MLST |
|---|---|---|---|---|---|
| Clinic | 10 | 6.9 | 6633 | 66.0 | 253 |
| 11 | 7.0 | 6845 | 66.0 | 111 | |
| 20 | 6.3 | 6087 | 66.6 | 1978 | |
| 23 | 6.4 | 6017 | 66.5 | 3236 | |
| 31 | 6.8 | 6535 | 66.0 | 253 | |
| 37 | 6.6 | 6406 | 66.0 | 235 | |
| 46 | 6.7 | 6488 | 66.1 | 235 | |
| Average | 6.67 | 6430 | 66.2 | ||
| Water | p12a | 6.5 | 6099 | 66.2 | 1427 |
| p2b | 6.3 | 5949 | 66.5 | 1993 | |
| p3 | 6.2 | 5860 | 66.6 | 549 | |
| Soil | s33 | 6.2 | 5841 | 66.6 | 3579 |
| sn99 | 6.2 | 5892 | 66.5 | 252 | |
| sr101 | 6.3 | 5890 | 66.5 | 282 | |
| sr75 | 6.2 | 5907 | 66.5 | 282 | |
| Average | 6.27 | 5920 | 66.5 |
| Resistance Mechanism | Genes |
|---|---|
| Target antibiotic | alr, ddl, dxr, EF-G, EF-Tu, folA, dfr, folP, inhA, fabI iso-tRNA, kasA, murA, rho, rpoC, s10p, rpoB. |
| Efflux pump | macA, macB, mdtABC-OMF, mdtABC-tolC, mexAB-oprM mexCD-oprJ, mexEF-OprN, mexHI-opmD, mexJK-oprM/opmH. mexPQ-opmE, mexXY-oMP, tolC/opmH, triABC-opmH, amrA, amrB, mexB, mexC, mexD, mexE, mexF, mexG, mexH, mexI, mexJ, mexK, mexL, mexN, mexP, mexR, mexS, mexV, mexW, mexZ, nalC, nalD, nfxB, opmD, opmE, opmH, oprJ, oprM, oprN, phoP, phoQ, triA, triB, triC, emrE. |
| Changes in membrane permeability | occD2/opdC, occD3/opdP, occD4/opdT, occD5/opdI, occD6/oprQ, occD7/opdB, occD8/opdJ, occK1/opdK, occK10/opdN, occK11/opdR, occK3/opdO, occK4/opdL, occK5/opdH, occK6/opdQ, occK8/oprE, occK9/opdG, oprB, porF, oprD, pgsA, pmrA, pmrB. gdpD. |
| Antibiotic inactivation | aph(3′)-II/aph(3′)-XV, blaOXA-50 aph(3′)-IIb. |
| Antibiotic target modification | gyrA, gyrB, parC, parE. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aroca Molina, K.J.; Gutiérrez, S.J.; Benítez-Campo, N.; Correa, A. Genomic Differences Associated with Resistance and Virulence in Pseudomonas aeruginosa Isolates from Clinical and Environmental Sites. Microorganisms 2024, 12, 1116. https://doi.org/10.3390/microorganisms12061116
Aroca Molina KJ, Gutiérrez SJ, Benítez-Campo N, Correa A. Genomic Differences Associated with Resistance and Virulence in Pseudomonas aeruginosa Isolates from Clinical and Environmental Sites. Microorganisms. 2024; 12(6):1116. https://doi.org/10.3390/microorganisms12061116
Chicago/Turabian StyleAroca Molina, Kelly J., Sonia Jakeline Gutiérrez, Neyla Benítez-Campo, and Adriana Correa. 2024. "Genomic Differences Associated with Resistance and Virulence in Pseudomonas aeruginosa Isolates from Clinical and Environmental Sites" Microorganisms 12, no. 6: 1116. https://doi.org/10.3390/microorganisms12061116





