Gut Microbial Diversity Reveals Differences in Pathogenicity between Metarhizium rileyi and Beauveria bassiana during the Early Stage of Infection in Spodoptera litura Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungi and Media
2.2. Insect Feeding
2.3. Bioassay
2.4. Physiology Experiments
2.5. DNA Extraction and PCR Amplification
2.6. Illumina MiSeq Sequencing
2.7. Data Processing
2.8. Phenotypes of Fungal Stress and Growth
3. Results
3.1. Differences in Virulence in Mixtures of M. rileyi and B. bassiana
3.2. Sequencing Data Statistics
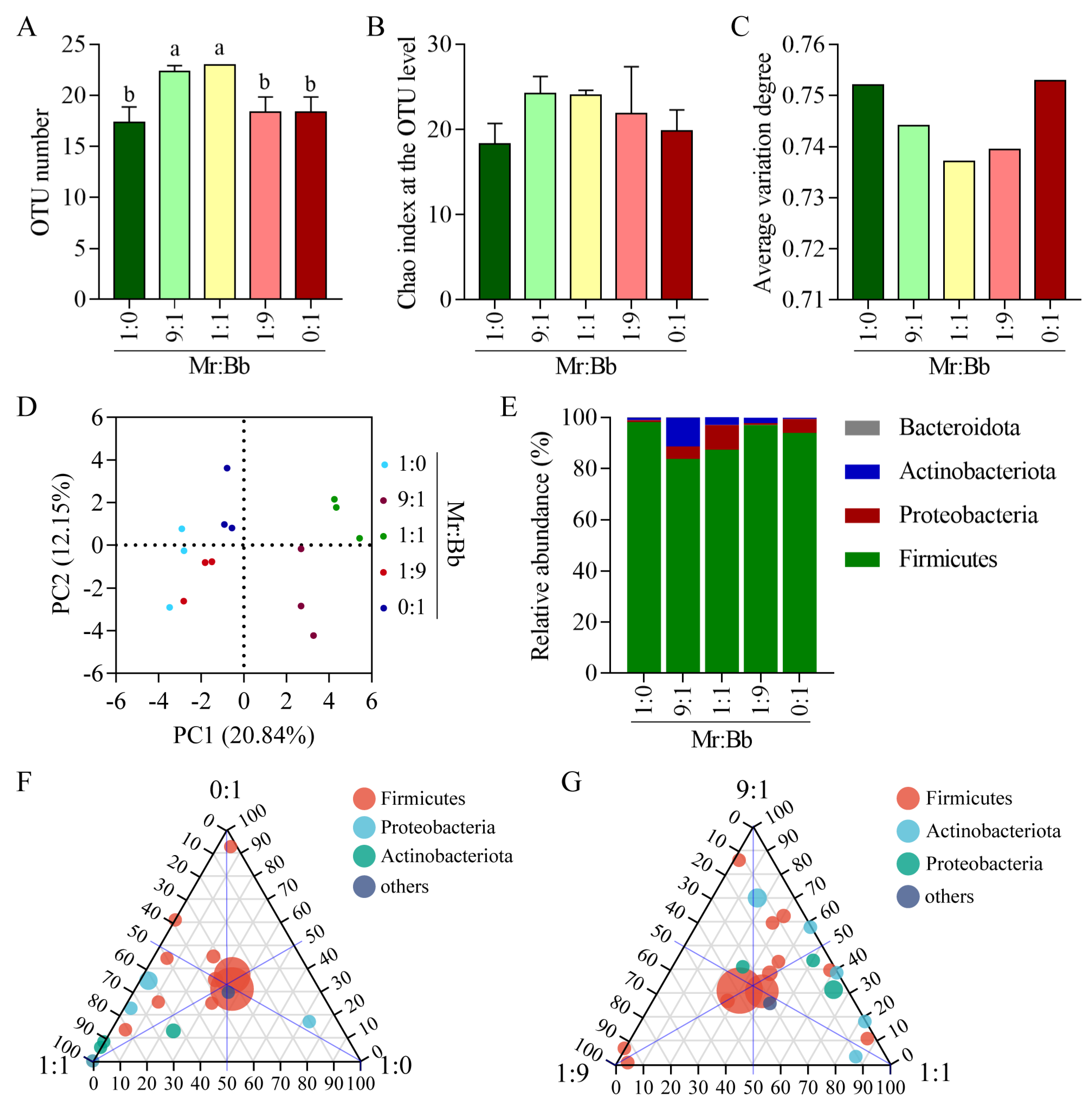
3.3. Diversity and Composition of Gut Bacteria in S. litura
3.4. Abundance and Diversity Analyses of the Gut Bacteria in S. litura
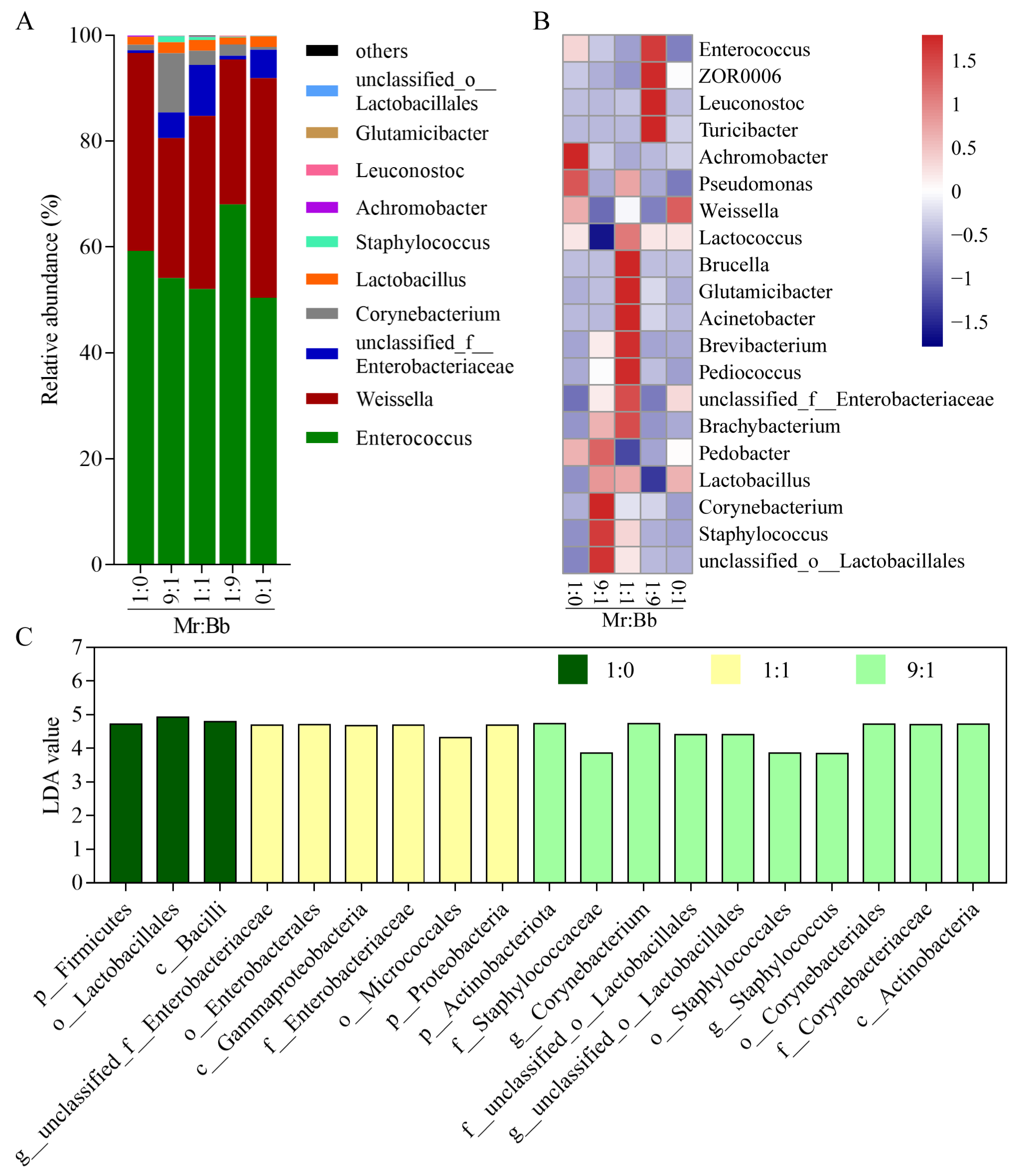
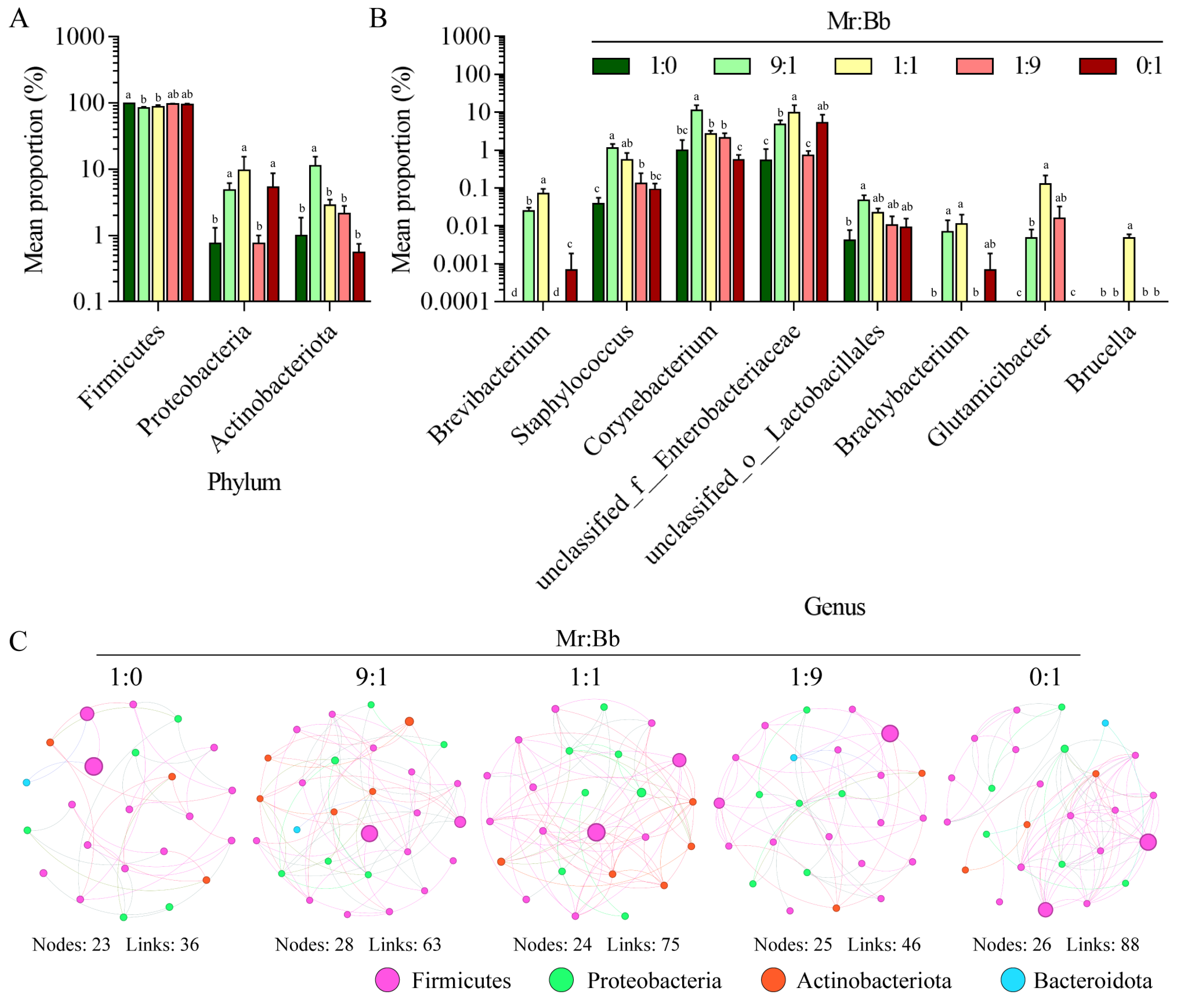
3.5. Analysis of the Microbial Community and Composition of Gut Bacteria in S. litura
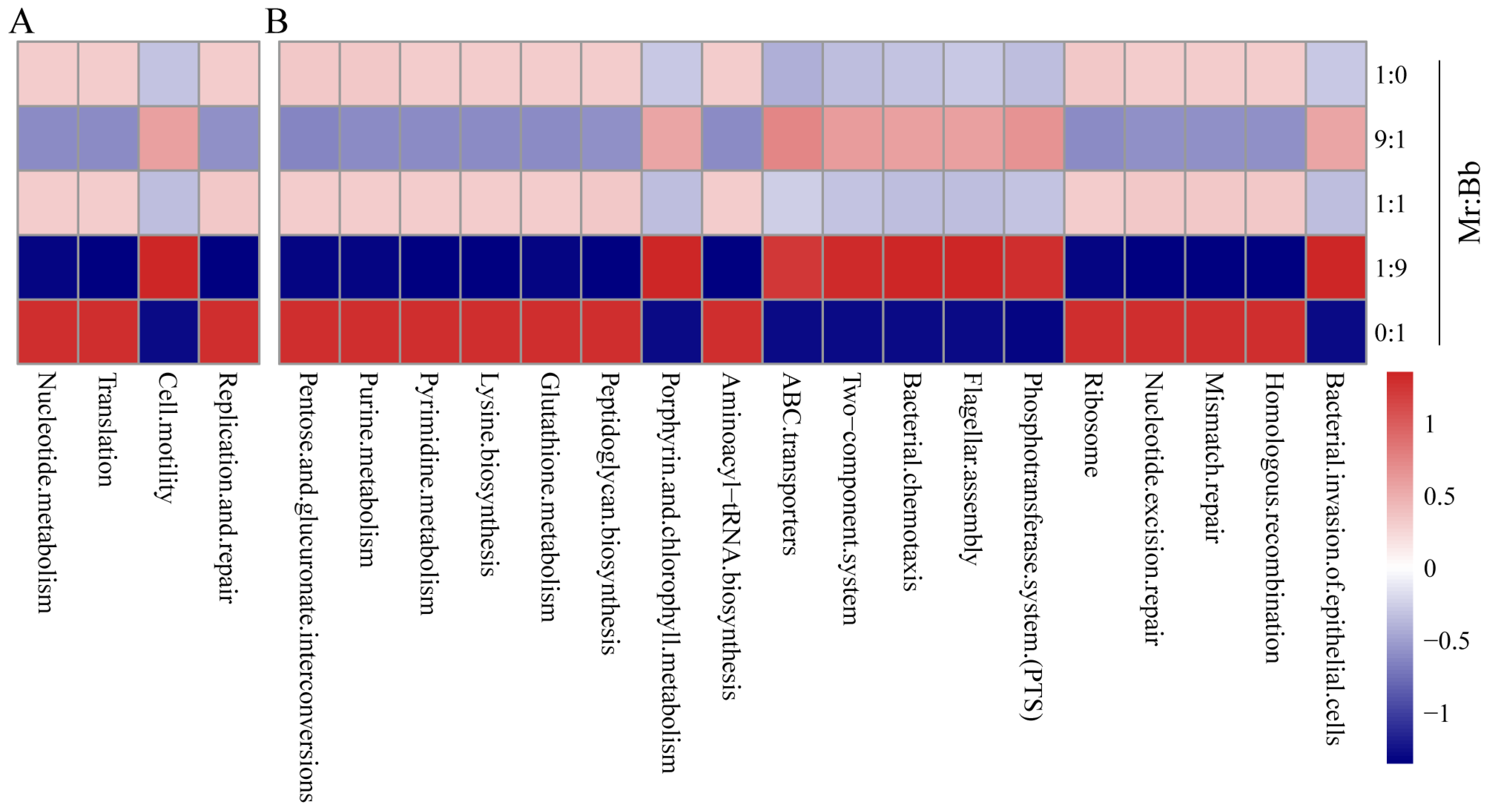
3.6. Gut Microbial Community Functional Prediction for S. litura
3.7. M. rileyi and B. bassiana in Multiple Stress Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.J.; Wang, J.J.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. HapX, an Indispensable bZIP Transcription Factor for Iron Acquisition, Regulates Infection Initiation by Orchestrating Conidial Oleic Acid Homeostasis and Cytomembrane Functionality in Mycopathogen Beauveria bassiana. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Peng, Y.; Yao, Y.; Pang, J.; Di, T.; Du, G.; Chen, B. Genetic Diversity and Virulence Variation of Metarhizium rileyi from Infected Spodoptera frugiperda in Corn Fields. Microorganisms 2024, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, I.J.; Fernandes, E.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous fungi. Virulence 2024, 15, 2299183. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, H.; Wang, G.; Feng, M.; Ying, S. MARVEL family proteins contribute to vegetative growth, development, and virulence of the insect fungal pathogen Beauveria bassiana. J. Invertebr. Pathol. 2024, 203, 108076. [Google Scholar] [CrossRef]
- Pang, J.; Peng, Y.; Di, T.; Du, G.; Chen, B. Virulence of Metarhizium rileyi Is Determined by Its Growth and Antioxidant Stress and the Protective and Detoxifying Enzymes of Spodoptera frugiperda. Insects 2023, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic Fungi: New Insights into Host-Pathogen Interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; St, L.R. Developmental and transcriptional responses to host and nonhost cuticles by the specific locust pathogen Metarhizium anisopliae var. acridum. Eukaryot. Cell 2005, 4, 937–947. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wu, L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem. Pharmacol. 2009, 77, 1709–1716. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Jegorov, A.; Sedmera, P.; Havlicek, V.; Matha, V. Destruxin Ed1 a cyclopeptide from the fungus Metarhizium anisopliae. Phytochemistry 1998, 49, 1815–1817. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sung, G.H. Beauvericin synthetase contains a calmodulin binding motif in the entomopathogenic fungus Beauveria bassiana. J. Gen. Appl. Microbiol. 2018, 64, 145–147. [Google Scholar] [CrossRef]
- Pedrini, N. The Entomopathogenic Fungus Beauveria bassiana Shows Its Toxic Side within Insects: Expression of Genes Encoding Secondary Metabolites during Pathogenesis. J. Fungi 2022, 8, 488. [Google Scholar] [CrossRef]
- Boucias, D.G.; Zhou, Y.; Huang, S.; Keyhani, N.O. Microbiota in insect fungal pathology. Appl. Microbiol. Biot. 2018, 102, 5873–5888. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Wang, Y.; Stata, M.; Wang, W.; Stajich, J.E.; White, M.M.; Moncalvo, J.M. Comparative Genomics Reveals the Core Gene Toolbox for the Fungus-Insect Symbiosis. Mbio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zaneveld, J.R.; McMinds, R.; Vega Thurber, R. Stress and stability: Applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2017, 2, 17121. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wen, S.; Wang, G.; Zhang, X.; Di, T.; Du, G.; Chen, B.; Zhang, L. Reconstruction of Gut Bacteria in Spodoptera frugiperda Infected by Beauveria bassiana Affects the Survival of Host Pest. J. Fungi 2023, 9, 906. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef]
- Wang, J.L.; Sun, J.; Song, Y.J.; Zheng, H.H.; Wang, G.J.; Luo, W.X.; Li, L.; Liu, X.S. An entomopathogenic fungus exploits its host humoral antibacterial immunity to minimize bacterial competition in the hemolymph. Microbiome 2023, 11, 116. [Google Scholar] [CrossRef]
- Cerf-Bensussan, N.; Gaboriau-Routhiau, V. The immune system and the gut microbiota: Friends or foes? Nat. Rev. Immunol. 2010, 10, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.B.; Thacker, R.W.; Gochfeld, D.J. Molecular community profiling reveals impacts of time, space, and disease status on the bacterial community associated with the Caribbean sponge Aplysina cauliformis. FEMS Microbiol. Ecol. 2014, 87, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Kannan, S.; Alford, L.; Pittarate, S.; Geedi, R.; Elangovan, D.; Marimuthu, R.; Krutmuang, P. First report on the enzymatic and immune response of Metarhizium majus bag formulated conidia against Spodoptera frugiperda: An ecofriendly microbial insecticide. Front. Microbiol. 2023, 14, 1104079. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Yang, K.; Fan, L.; Wang, Z.; Yin, Y. Regulation of conidiation, polarity growth, and pathogenicity by MrSte12 transcription factor in entomopathogenic fungus, Metarhizium rileyi. Fungal Genet. Biol. 2021, 155, 103612. [Google Scholar] [CrossRef] [PubMed]
- Celar, F.A.; Kos, K. Effects of selected herbicides and fungicides on growth, sporulation and conidial germination of entomopathogenic fungus Beauveria bassiana. Pest. Manag. Sci. 2016, 72, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Asshauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Petlamul, W.; Prasertsan, P. Evaluation of Strains of Metarhizium anisopliae and Beauveria bassiana against Spodoptera litura on the Basis of Their Virulence, Germination Rate, Conidia Production, Radial Growth and Enzyme Activity. Mycobiology 2012, 40, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sui, L.; Tian, Y.; Lu, Y.; Xia, X.; Liu, W.; Cheng, K.; Li, Q.; Shi, W. Metarhizium rileyi with broad-spectrum insecticidal ability confers resistance against phytopathogens and insect pests as a phytoendophyte. Pest. Manag. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, B.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-Derived Antimicrobials Contribute to the Control of the Lepidopteran Gut Microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Wolbachia and the insect immune system: What reactive oxygen species can tell us about the mechanisms of Wolbachia-host interactions. Front. Microbiol. 2015, 6, 1201. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. Roy. Soc. B-Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Mullens, N.; Hendrycks, W.; Bakengesa, J.; Kabota, S.; Tairo, J.; Svardal, H.; Majubwa, R.; Mwatawala, M.; De Meyer, M.; Virgilio, M. Anna Karenina as a promoter of microbial diversity in the cosmopolitan agricultural pest Zeugodacus cucurbitae (Diptera, Tephritidae). PLoS ONE 2024, 19, e300875. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raffa, K.F.; Goodman, R.M.; Handelsman, J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microb. 2004, 70, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, C.; Baixeras, J.; Latorre, A.; Porcar, M. The Generalist Inside the Specialist: Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus sp. Front. Microbiol. 2016, 7, 1005. [Google Scholar] [CrossRef] [PubMed]
- Mereghetti, V.; Chouaia, B.; Limonta, L.; Locatelli, D.P.; Montagna, M. Evidence for a conserved microbiota across the different developmental stages of Plodia interpunctella. Insect Sci. 2019, 26, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Mason, C.J.; Felton, G.W.; Hoover, K. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci. Rep. 2019, 9, 2792. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, V.L.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial Symbionts in Lepidoptera: Their Diversity, Transmission, and Impact on the Host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Luo, Z.; Keyhani, N.O. Improving mycoinsecticides for insect biological control. Appl. Microbiol. Biot. 2015, 99, 1057–1068. [Google Scholar] [CrossRef]
- Uchida, R.; Imasato, R.; Yamaguchi, Y.; Masuma, R.; Shiomi, K.; Tomoda, H.; Ōmura, S. New Insecticidal Antibiotics, Hydroxyfungerins A and B, Produced by Metarhizium sp. FKI-1079. J. Antibiot. 2005, 58, 804–809. [Google Scholar] [CrossRef]
- Prompiboon, P.; Bhumiratana, A.; Ruchirawat, S.; Boucias, D.G.; Wiwat, C. Isolation of ergosterol peroxide from Nomuraea rileyi infected larvae of tobacco cutworm. World J. Microb. Biot. 2008, 24, 2909–2917. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhang, H.; Feng, M.G.; Ying, S.H. SterylAcetyl Hydrolase 1 (BbSay1) Links Lipid Homeostasis to Conidiogenesis and Virulence in the Entomopathogenic Fungus Beauveria bassiana. J. Fungi 2022, 8, 292. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Xu, S.; Chen, L.; Zhan, T.; Zhang, X.; Liang, H.; Chen, B.; Peng, Y. Gut Microbial Diversity Reveals Differences in Pathogenicity between Metarhizium rileyi and Beauveria bassiana during the Early Stage of Infection in Spodoptera litura Larvae. Microorganisms 2024, 12, 1129. https://doi.org/10.3390/microorganisms12061129
Wang G, Xu S, Chen L, Zhan T, Zhang X, Liang H, Chen B, Peng Y. Gut Microbial Diversity Reveals Differences in Pathogenicity between Metarhizium rileyi and Beauveria bassiana during the Early Stage of Infection in Spodoptera litura Larvae. Microorganisms. 2024; 12(6):1129. https://doi.org/10.3390/microorganisms12061129
Chicago/Turabian StyleWang, Guang, Sicai Xu, Laiyan Chen, Tianjiao Zhan, Xu Zhang, Honghui Liang, Bin Chen, and Yuejin Peng. 2024. "Gut Microbial Diversity Reveals Differences in Pathogenicity between Metarhizium rileyi and Beauveria bassiana during the Early Stage of Infection in Spodoptera litura Larvae" Microorganisms 12, no. 6: 1129. https://doi.org/10.3390/microorganisms12061129





