Rodent Gut Bacteria Coexisting with an Insect Gut Virus in Tapeworm Parasitic Cysts: Metagenomic Evidence of Microbial Selection in Extra-Intestinal Clinical Niches
Abstract
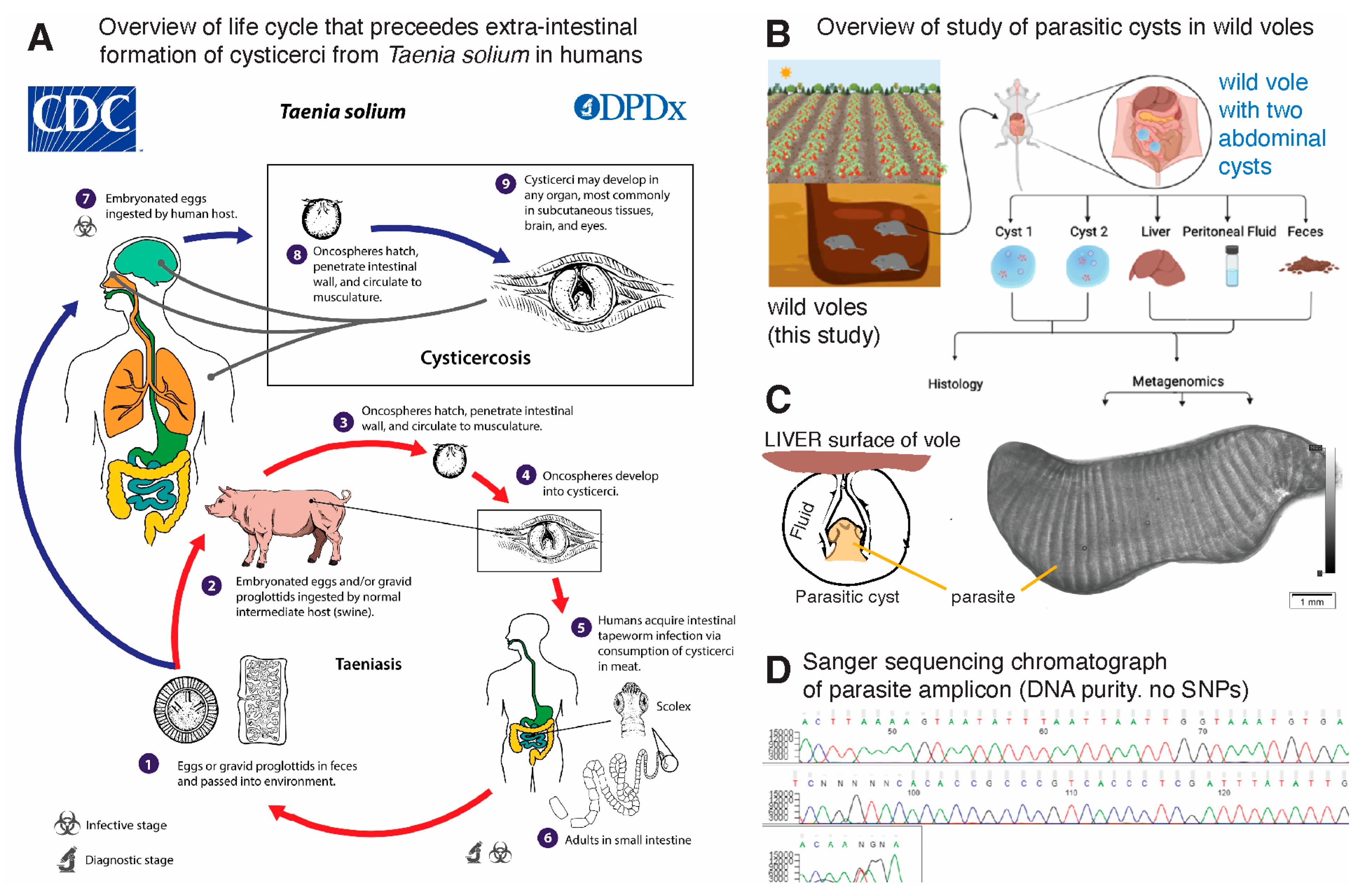
1. Introduction
2. Methods
2.1. Animals and Location
2.2. Identification of the Parasitic Cysts
2.3. Visualization and Anaerobic Culture of Fluid from the Parasitic Cysts
2.4. DNA Extraction and Metagenomics
2.5. Library Preparation and Sequencing
2.6. Bioinformatics Analysis and Metagenome Classification
2.7. Metacestode DNA Extraction and Sequencing
2.8. Statistics
2.9. Data Availability
3. Results
3.1. Nucleotide Sequence Analysis of Metacestode Reveals Hydatigera taeniaeformis
3.2. Visualization and Cultivation of Fluid Yielded Enterococcus faecium
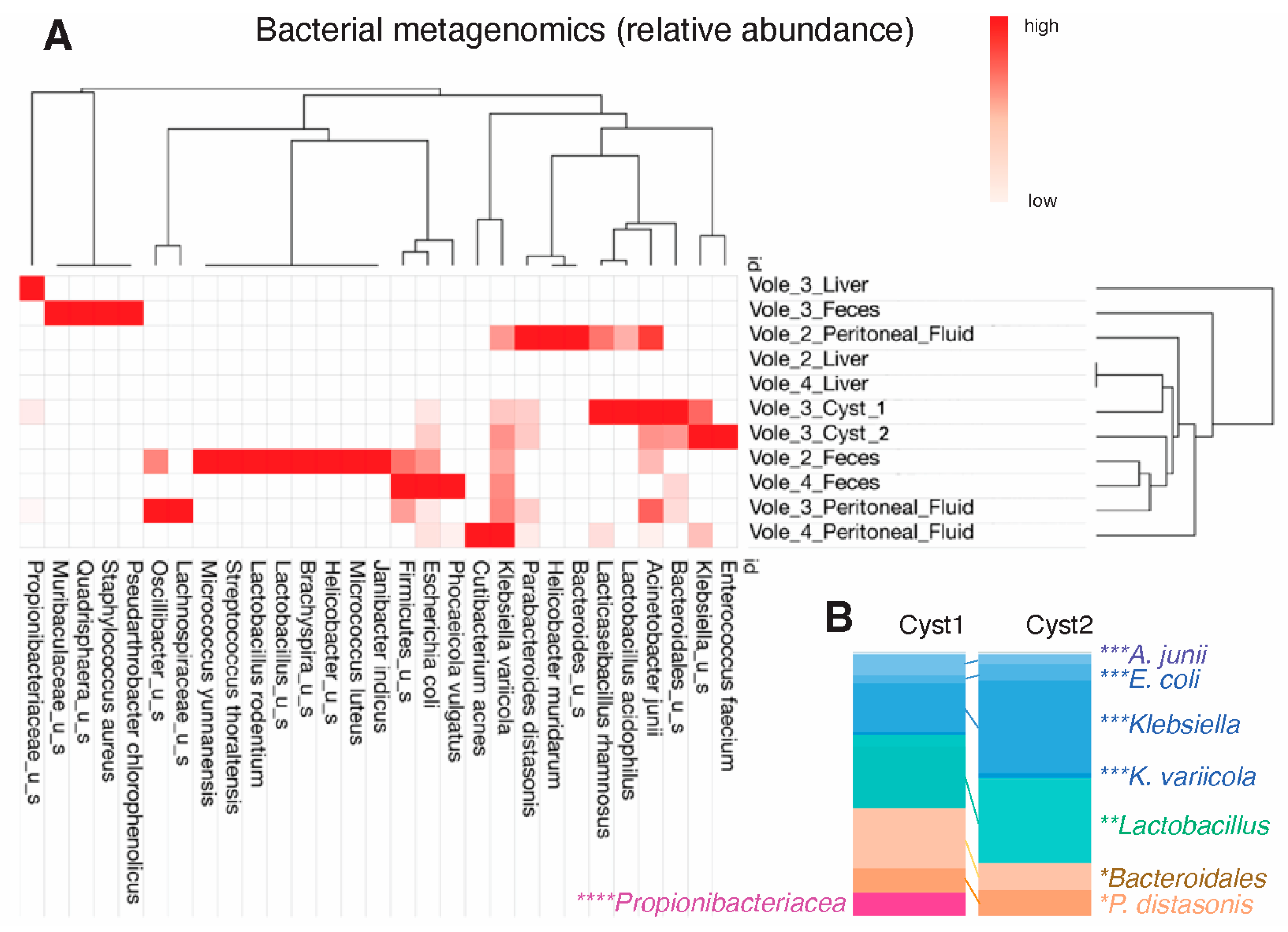
3.3. Metagenomics of the Cystic Fluid Revealed Gut Commensal/Opportunistic Bacteria
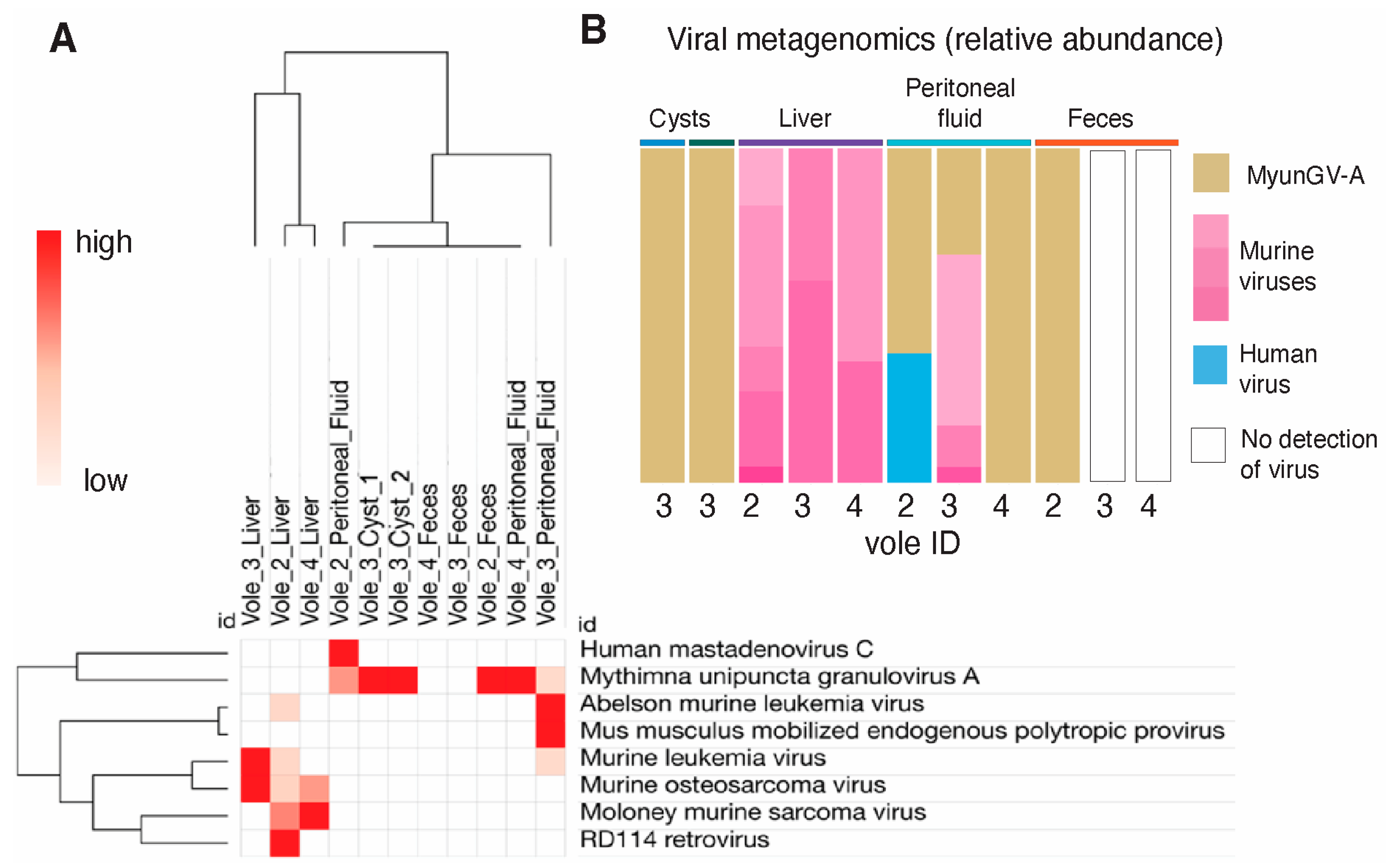
3.4. Identification of Insect Virus Outside Its Natural Habitat
3.5. Metagenomics Suggests Lower Probability of Fungal Detection in the Cysts
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, N.S. Cooperation, communication, and co-evolution: Grand challenges in microbial symbiosis research. Front. Microbiol. 2014, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Raina, J.B.; Eme, L.; Pollock, F.J.; Spang, A.; Archibald, J.M.; Williams, T.A. Symbiosis in the microbial world: From ecology to genome evolution. Biol. Open. 2018, 7, bio032524. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Chia, N.; Yildirim, S.; Miller, M.E.; Kent, A.; Stumpf, R.; Leigh, S.R.; Nelson, K.E. Towards an Evolutionary Model of Animal-Associated Microbiomes. Entropy 2011, 13, 570–594. [Google Scholar] [CrossRef]
- McFall-Ngai, M.J. Giving microbes their due—Animal life in a microbially dominant world. J. Exp. Biol. 2015, 218 Pt 12, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Kodani, T.; Kaydo, L.; Pietropaoli, D.; Corridoni, D.; Howell, S.; Katz, J.; Xin, W.; Pizarro, T.T.; Cominelli, F. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat. Commun. 2015, 6, 7577. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kumar, A.; Davenport, K.W.; Kelliher, J.M.; Ezeji, J.C.; Good, C.E.; Jacobs, M.R.; Conger, M.; West, G.; Fiocchi, C.; et al. Complete Genome Sequence of a Parabacteroides distasonis Strain (CavFT hAR46) Isolated from a Gut Wall-Cavitating Microlesion in a Patient with Severe Crohn’s Disease. Microbiol. Resour. Announc. 2019, 8, e00585-19. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; West, G.; Fiocchi, C.; Good, C.E.; Katz, J.; Jacobs, M.R.; Dichosa, A.E.K.; Flask, C.; Wesolowski, M.; McColl, C.; et al. Clonal Parabacteroides from Gut Microfistulous Tracts as Transmissible Cytotoxic Succinate-Commensal Model of Crohn’s Disease Complications. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Bank, N.C.; Vaidhvi, S.; Grubb, B.; McCourt, B.; Burberry, A.; Roberts, K.D.; Rodriguez-Palacios, A. Antigenic operon fragmentation and diversification mechanism in Bacteroidota impacts gut metagenomics and pathobionts in Crohn’s disease microlesions. Gut Microbes 2024, 16, 2350150, First available as preprint: The basis of antigenic operon fragmentation in Bacteroidota and commensalism. bioRxiv 2023.. [Google Scholar]
- Singh, V.; Rodriguez-Palacios, A. Genomes of Bacteroides ovatus, B. cellulosilyticus, B. uniformis, Phocaeicola vulgatus, and P. dorei isolated from Gut Cavernous Fistulous Tract Micropathologies in Crohn’s disease. Microbiol. Resour. Announc. 2024, 13, e0115223. [Google Scholar] [CrossRef]
- Caira, J.N.; Jensen, K. Electron microscopy reveals novel external specialized organs housing bacteria in eagle ray tapeworms. PLoS ONE 2021, 16, e0244586. [Google Scholar] [CrossRef]
- Bonelli, P.; Serra, E.; Dei Giudici, S.; Peruzzu, A.; Crotti, S.; Danesi, P.; Carvelli, A.; Piseddu, T.; Masala, G. Molecular phylogenetic analysis of Echinococcus granulosus sensu lato infecting sheep in Italy. Acta Trop. 2024, 252, 107151. [Google Scholar] [CrossRef] [PubMed]
- Romig, T.; Wassermann, M. Echinococcus species in wildlife. Int. J. Parasitol. Parasites Wildl. 2024, 23, 100913. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Selcuk, M.A.; Kilinc, S.G.; Kesik, H.K.; Ahmed, H.; Wang, Y.; Simsek, S.; Cao, J. Molecular discrimination of G1 and G3 genotypes of Echinococcus granulosus sensu stricto obtained from human, cattle, and sheep using the mitochondrial NADH dehydrogenase subunit 5 marker. Acta Trop. 2024, 252, 107124. [Google Scholar] [CrossRef] [PubMed]
- Casulli, A.; Pane, S.; Randi, F.; Scaramozzino, P.; Carvelli, A.; Marras, C.E.; Carai, A.; Santoro, A.; Santolamazza, F.; Tamarozzi, F.; et al. Primary cerebral cystic echinococcosis in a child from Roman countryside: Source attribution and scoping review of cases from the literature. PLoS Negl. Trop. Dis. 2023, 17, e0011612. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zheng, H.; Wang, Y.; Zheng, X.; He, L.; Qi, W.; Wang, T.; Guo, B.; Guo, G.; Zhang, Z.; et al. Echinococcus granulosus Infection Results in an Increase in Eisenbergiella and Parabacteroides Genera in the Gut of Mice. Front. Microbiol. 2018, 9, 2890. [Google Scholar] [CrossRef] [PubMed]
- Geduhn, A.; Schlötelburg, A.; Kalle, A.; Fleischer, S. Testing Animal Welfare of House Mouse (Mus musculus) Snap and Electrocution Traps. In Mammal Trapping—Wildlife Management, Animal Welfare & International Standards; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 2022; pp. 69–80. [Google Scholar]
- Sikes, R. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Animal Care and Use Committee of the American Society of Mammalogists. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Browne, H.P.; Forster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Szabó, B.G.; Kiss, R.; Makra, N.; Pénzes, K.; Vad, E.; Kamotsay, K.; Szalbó, D.; Ostorházi, E. Composition and changes of blood microbiota in adult patients with community-acquired sepsis: A. Front. Cell Infect. Microbiol. 2022, 12, 1067476. [Google Scholar] [CrossRef] [PubMed]
- Thoendel, M.; Jeraldo, P.; Greenwood-Quaintance, K.E.; Yao, J.; Chia, N.; Hanssen, A.D.; Abdel, M.P.; Patel, R. Comparison of Three Commercial Tools for Metagenomic Shotgun Sequencing Analysis. J. Clin. Microbiol. 2020, 58, e00981-19. [Google Scholar] [CrossRef]
- CosmosID. Metagenomics Cloud, CosmosID Inc. 2024. Available online: https://www.cosmosid.com (accessed on 3 May 2024).
- Yan, Q.; Wi, Y.M.; Thoendel, M.J.; Raval, Y.S.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Jeraldo, P.R.; Chia, N.; Patel, R. Evaluation of the CosmosID Bioinformatics Platform for Prosthetic Joint-Associated Sonicate Fluid Shotgun Metagenomic Data Analysis. J. Clin. Microbiol. 2019, 57, e01182-18. [Google Scholar] [CrossRef]
- Oberli, A.; Furrer, L.; Skoko, L.; Müller, N.; Gottstein, B.; Bittel, P. A novel multiplex real-time polymerase chain reaction for the molecular diagnosis of metacestode infections in human patients. Clin. Microbiol. Infect. 2023, 29, 1451.e1–1451.e5. [Google Scholar] [CrossRef] [PubMed]
- Martin, W. Linking causal concepts, study design, analysis and inference in support of one epidemiology for population health. Prev. Vet. Med. 2008, 86, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Aladyshkina, N.; Ezeji, J.C.; Erkkila, H.L.; Conger, M.; Ward, J.; Webster, J.; Cominelli, F. ‘Cyclical Bias’ in Microbiome Research Revealed by A Portable Germ-Free Housing System Using Nested Isolation. Sci. Rep. 2018, 8, 3801. [Google Scholar] [CrossRef] [PubMed]
- Raffner Basson, A.; Gomez-Nguyen, A.; LaSalla, A.; Butto, L.; Kulpins, D.; Warner, A.; Di Martino, L.; Ponzani, G.; Osme, A.; Rodriguez-Palacios, A.; et al. Replacing Animal Protein with Soy-Pea Protein in an “American Diet” Controls Murine Crohn Disease-Like Ileitis Regardless of Firmicutes: Bacteroidetes Ratio. J. Nutr. 2021, 151, 579–590. [Google Scholar] [CrossRef]
- Basson, A.R.; Gomez-Nguyen, A.; Menghini, P.; Buttó, L.F.; Di Martino, L.; Aladyshkina, N.; Osme, A.; Lasalla, A.; Fischer, D.; Ezeji, J.C.; et al. Human Gut Microbiome Transplantation in Ileitis Prone Mice: A Tool for the Functional Characterization of the Microbiota in Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2020, 26, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Mowery, J.D.; Bauchan, G.R.; Theilmann, D.A.; Erlandson, M.A. The complete genome sequence of a second alphabaculovirus from the true armyworm, Mythimna unipuncta: Implications for baculovirus phylogeny and host specificity. Virus Genes. 2019, 55, 104–116. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Ortego, F.; Hernández-Crespo, P.; Farinós, G.P.; Castañera, P. Inheritance, fitness costs, incomplete resistance and feeding preferences in a laboratory-selected MON810-resistant strain of the true armyworm Mythimna unipuncta. Pest. Manag. Sci. 2015, 71, 1631–1639. [Google Scholar] [CrossRef]
- Gomez-Puerta, L.A.; Vargas-Calla, A.; Garcia-Leandro, M.; Jara-Vila, J.; Rojas-Anticona, W.; Pacheco, J.I.; Angulo-Tisoc, J.M.; Silva, W.; Gonzalez, A.E. Identification of wild rodents as intermediate hosts for Hydatigera taeniaeformis in Peru. Parasitol. Res. 2023, 122, 1915–1921. [Google Scholar] [CrossRef]
- Jia, W.; Yan, H.; Lou, Z.; Ni, X.; Dyachenko, V.; Li, H.; Littlewood, D.T.J. Mitochondrial genes and genomes support a cryptic species of tapeworm within Taenia taeniaeformis. Acta Trop. 2012, 123, 154–163. [Google Scholar] [CrossRef]
- Cook, R.W.; Trapp, A.L.; Williams, J.F. Pathology of Taenia taeniaeformis infection in the rat: Hepatic, lymph node and thymic changes. J. Comp. Pathol. 1981, 91, 219–226. [Google Scholar] [CrossRef]
- Mahesh Kumar, J.; Reddy, P.L.; Aparna, V.; Srinivas, G.; Nagarajan, P.; Venkatesan, R.; Sreekumar, C.; Sesikaran, B. Strobilocercus fasciolaris infection with hepatic sarcoma and gastroenteropathy in a Wistar colony. Vet. Parasitol. 2006, 141, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, C.; Yang, S.; Du, X.; Zhu, Y.; Zhang, T.; Lv, Y.; Zhao, W. Alterations in Gut Microbiota Profiles of Mice Infected with Echinococcus granulosus sensu lato Microbiota Profiles of Mice Infected with E. granulosus s.l. Acta Parasitol. 2022, 67, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Pang, M.; Wu, D.; Chen, G.; Peng, X.; Xu, K.; Fan, H. Alterations in the Gut Microbiota of Tibetan Patients with Echinococcosis. Front. Microbiol. 2022, 13, 860909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, B. Alterations in the Gut Microbial Composition and Diversity of Tibetan Sheep Infected with Echinococcus granulosus. Front. Vet. Sci. 2021, 8, 778789. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, Y.; Zhou, L.; Lu, Y.; Feng, T.; Dai, M.; Liu, J.; Xu, W.; Cheng, W.; Sun, F.; et al. Parasite-Derived Excretory-Secretory Products Alleviate Gut Microbiota Dysbiosis and Improve Cognitive Impairment Induced by a High-Fat Diet. Front. Immunol. 2021, 12, 710513. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.J.; Passarelli, A.L. Baculoviruses: Sophisticated pathogens of insects. PLoS Pathog. 2013, 9, e1003729. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, G.F. Baculovirus Molecular Biology; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2013.
- Weldon, L.; Abolins, S.; Lenzi, L.; Bourne, C.; Riley, E.M.; Viney, M. The Gut Microbiota of Wild Mice. PLoS ONE 2015, 10, e0134643. [Google Scholar] [CrossRef] [PubMed]
- Viney, M. The gut microbiota of wild rodents: Challenges and opportunities. Lab. Anim. 2019, 53, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Tang, P.; Zhang, H.; Qin, Q.; Zhang, Z. Genome sequence and organization of the Mythimna (formerly Pseudaletia) unipuncta granulovirus Hawaiian strain. Sci. Rep. 2021, 11, 414. [Google Scholar] [CrossRef]
- Mukawa, S.; Goto, C. In vivo characterization of two granuloviruses in larvae of Mythimna separata (Lepidoptera: Noctuidae). J. Gen. Virol. 2008, 89 Pt 4, 915–921. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- van Oers, M.M.; Pijlman, G.P.; Vlak, J.M. Thirty years of baculovirus-insect cell protein expression: From dark horse to mainstream technology. J. Gen. Virol. 2015, 96 Pt 1, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Y.; Cao, L.; Li, J.; Wu, C.; Tian, M.; Zhang, Z.; Zhang, C.; Zhang, W.; Li, Y. A Diverse Virome Is Identified in Parasitic Flatworms of Domestic Animals in Xinjiang, China. Microbiol. Spectr. 2023, 11, e0070223. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, A.; Singh, V.; Ilic, S.; Samiksha, F.; Marsh, A.; Rodriguez-Palacios, A. Rodent Gut Bacteria Coexisting with an Insect Gut Virus in Tapeworm Parasitic Cysts: Metagenomic Evidence of Microbial Selection in Extra-Intestinal Clinical Niches. Microorganisms 2024, 12, 1130. https://doi.org/10.3390/microorganisms12061130
Ammar A, Singh V, Ilic S, Samiksha F, Marsh A, Rodriguez-Palacios A. Rodent Gut Bacteria Coexisting with an Insect Gut Virus in Tapeworm Parasitic Cysts: Metagenomic Evidence of Microbial Selection in Extra-Intestinal Clinical Niches. Microorganisms. 2024; 12(6):1130. https://doi.org/10.3390/microorganisms12061130
Chicago/Turabian StyleAmmar, Amro, Vaidhvi Singh, Sanja Ilic, Fnu Samiksha, Antoinette Marsh, and Alexander Rodriguez-Palacios. 2024. "Rodent Gut Bacteria Coexisting with an Insect Gut Virus in Tapeworm Parasitic Cysts: Metagenomic Evidence of Microbial Selection in Extra-Intestinal Clinical Niches" Microorganisms 12, no. 6: 1130. https://doi.org/10.3390/microorganisms12061130
APA StyleAmmar, A., Singh, V., Ilic, S., Samiksha, F., Marsh, A., & Rodriguez-Palacios, A. (2024). Rodent Gut Bacteria Coexisting with an Insect Gut Virus in Tapeworm Parasitic Cysts: Metagenomic Evidence of Microbial Selection in Extra-Intestinal Clinical Niches. Microorganisms, 12(6), 1130. https://doi.org/10.3390/microorganisms12061130





