Molecular Mechanisms of Intestinal Protection by Levilactobacillus brevis 23017 against Salmonella typhimurium C7731-Induced Damage: Role of Nrf2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacterial Strains
2.3. Mice
2.4. Experimental Design
2.5. Determination of S. typhimurium C7731 CFUs
2.6. Detection of Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Antibody Detection
2.8. Histopathological Examinations
2.9. Antioxidant Activity Assay
2.10. Real-Time Quantitative PCR (RT-qPCR)
2.11. Western Blot Analysis of Nrf2
2.12. Statistical Analysis
3. Results
3.1. Improvements in DAI Score and Clinical Symptoms by L. brevis 23017
3.2. L. brevis 23017 Decreases S. typhimurium C7731 in Tissues
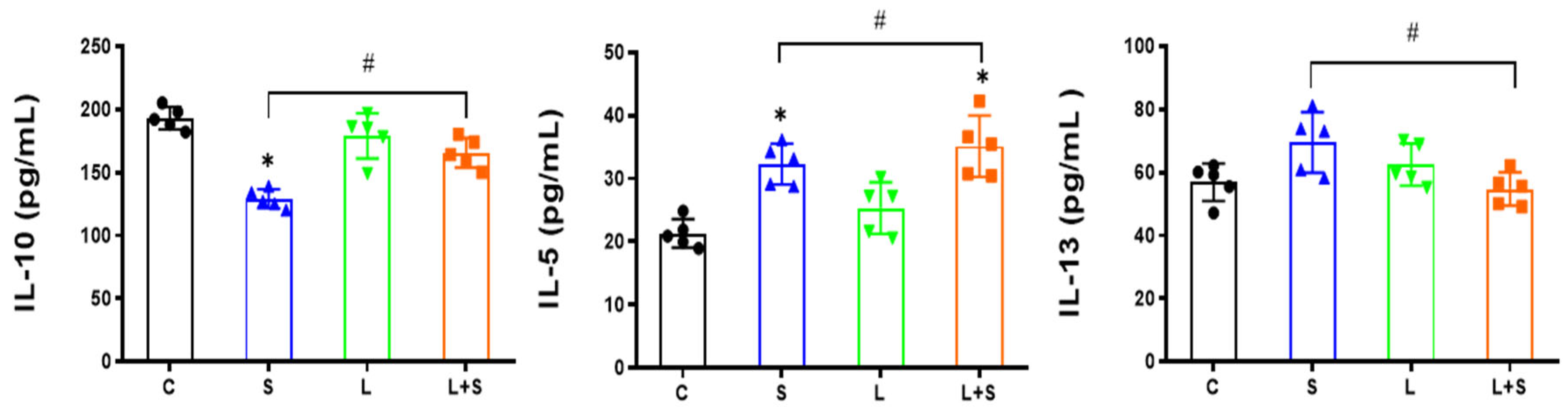
3.3. L. brevis 23017 Regulates Cytokines in Sera
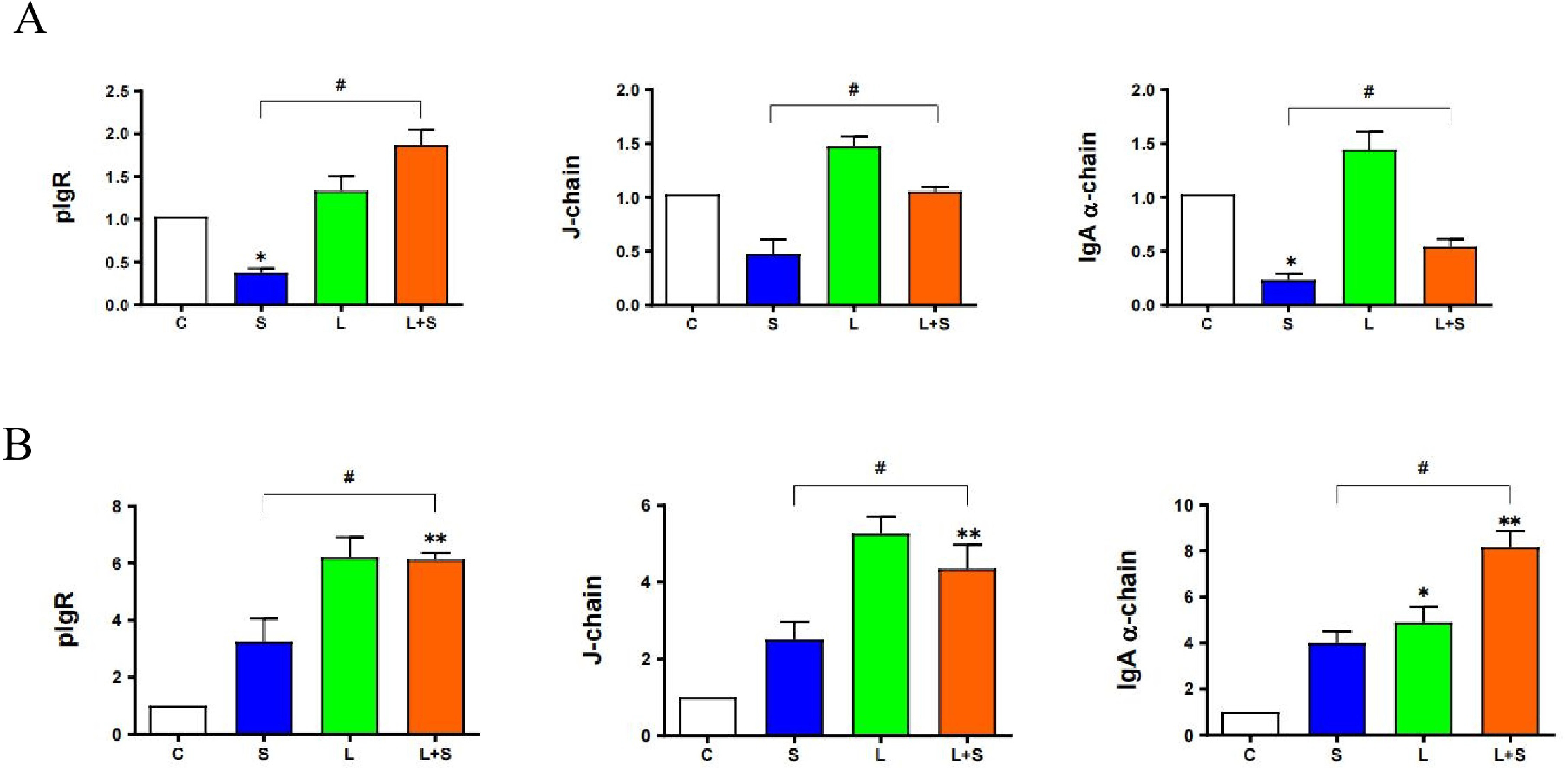
3.4. L. brevis 23017 Prevents S. typhimurium C7731 Intestinal Infection by Improving Intestinal Immunity
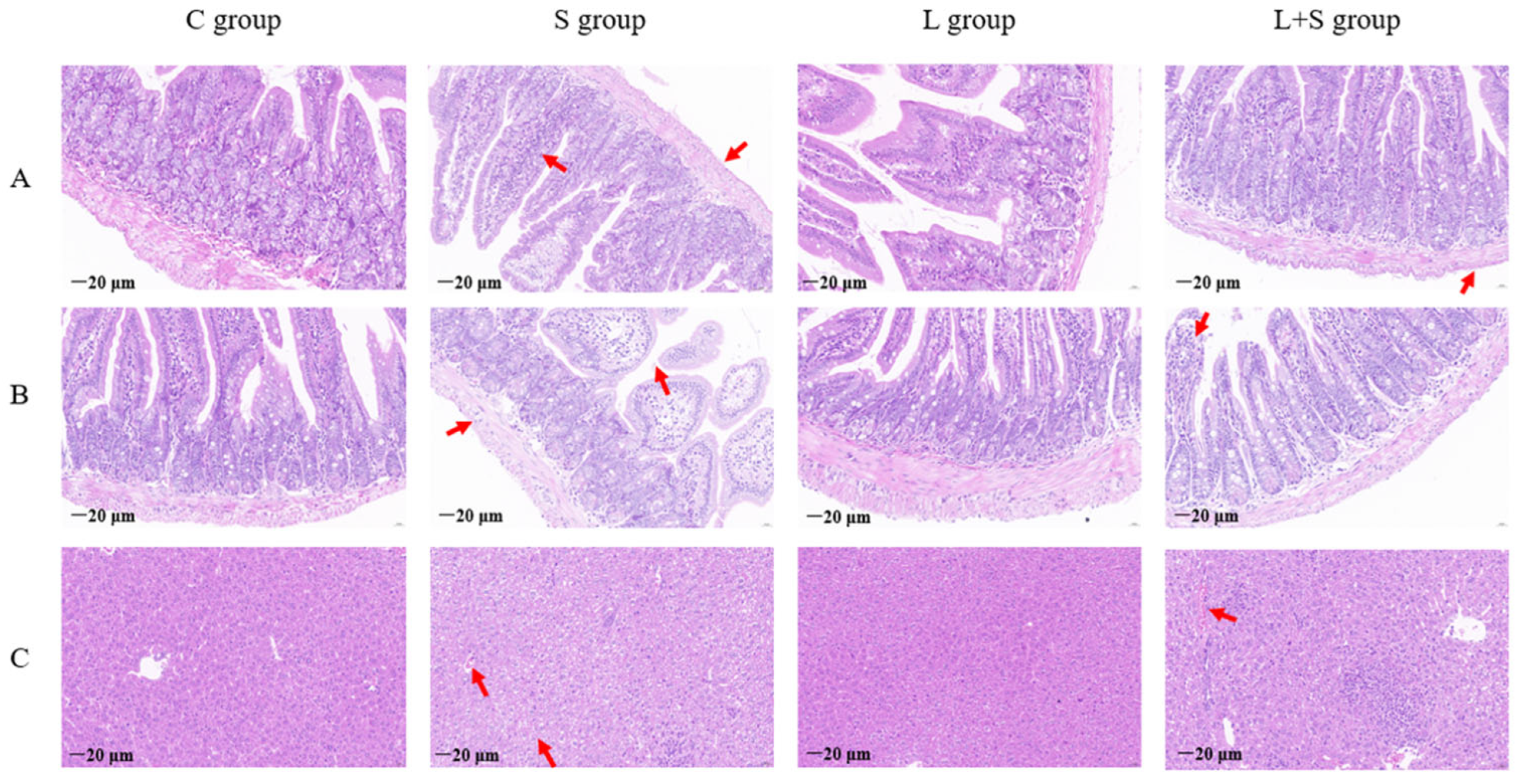
3.5. L. brevis 23017 Inhibits Colitis Induced by S. typhimurium C7731 Infection and Maintains the Integrity of Tissue Morphology
3.6. L. brevis 23017 Effectively Increases the Antioxidant Activity of Intestinal Tissues Infected by S. typhimurium C7731
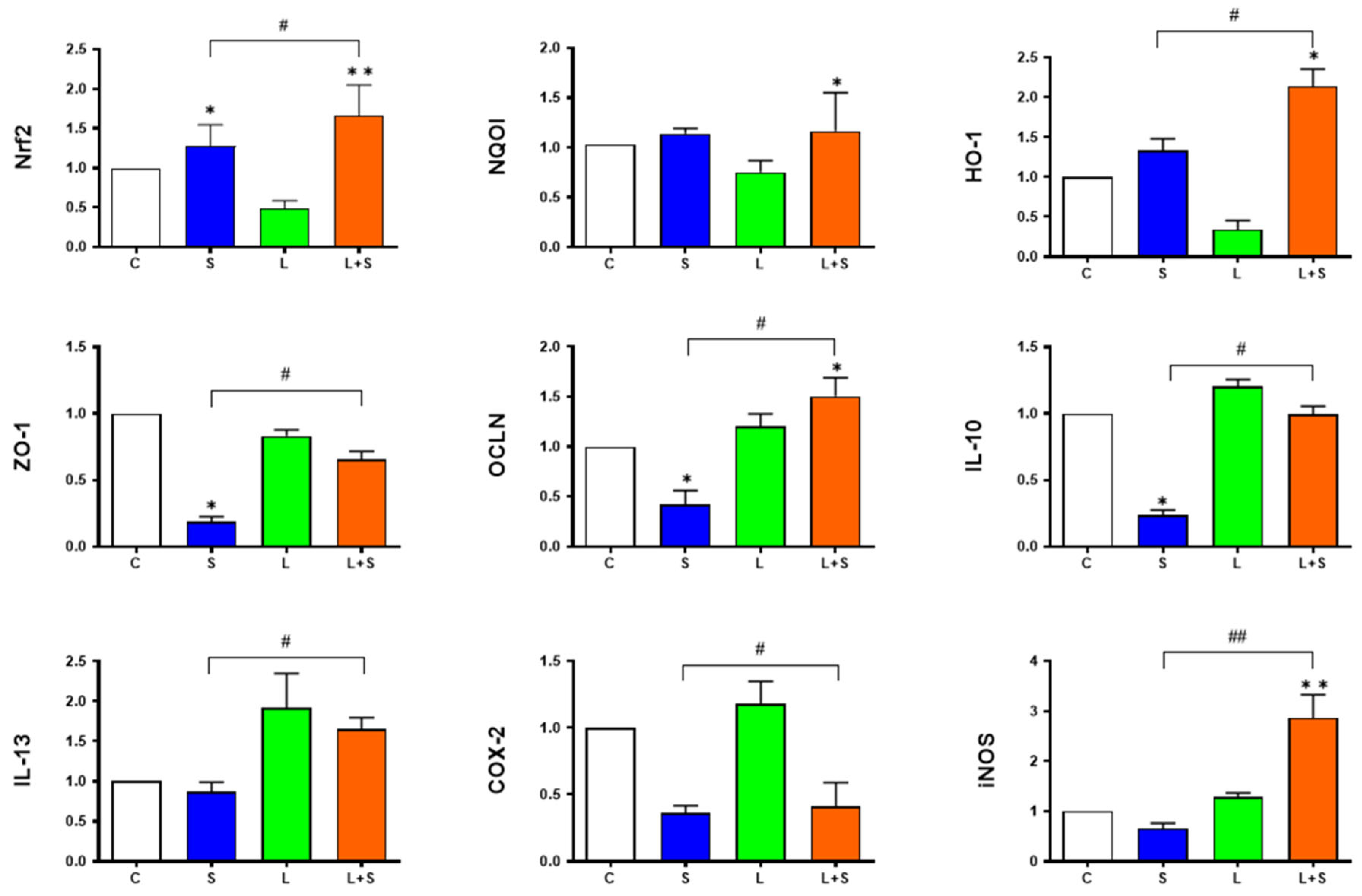
3.7. L. brevis 23017 Regulates mRNA Expression of Genes Related to Intestinal Integrity and Inflammatory Mediators
3.8. L. brevis 23017 Increases mRNA Expression of Nrf2 Signaling Pathway-Related Factors
3.9. L. brevis 23017 Upregulates mRNA Expression of Intestinal Immune-Related Factors
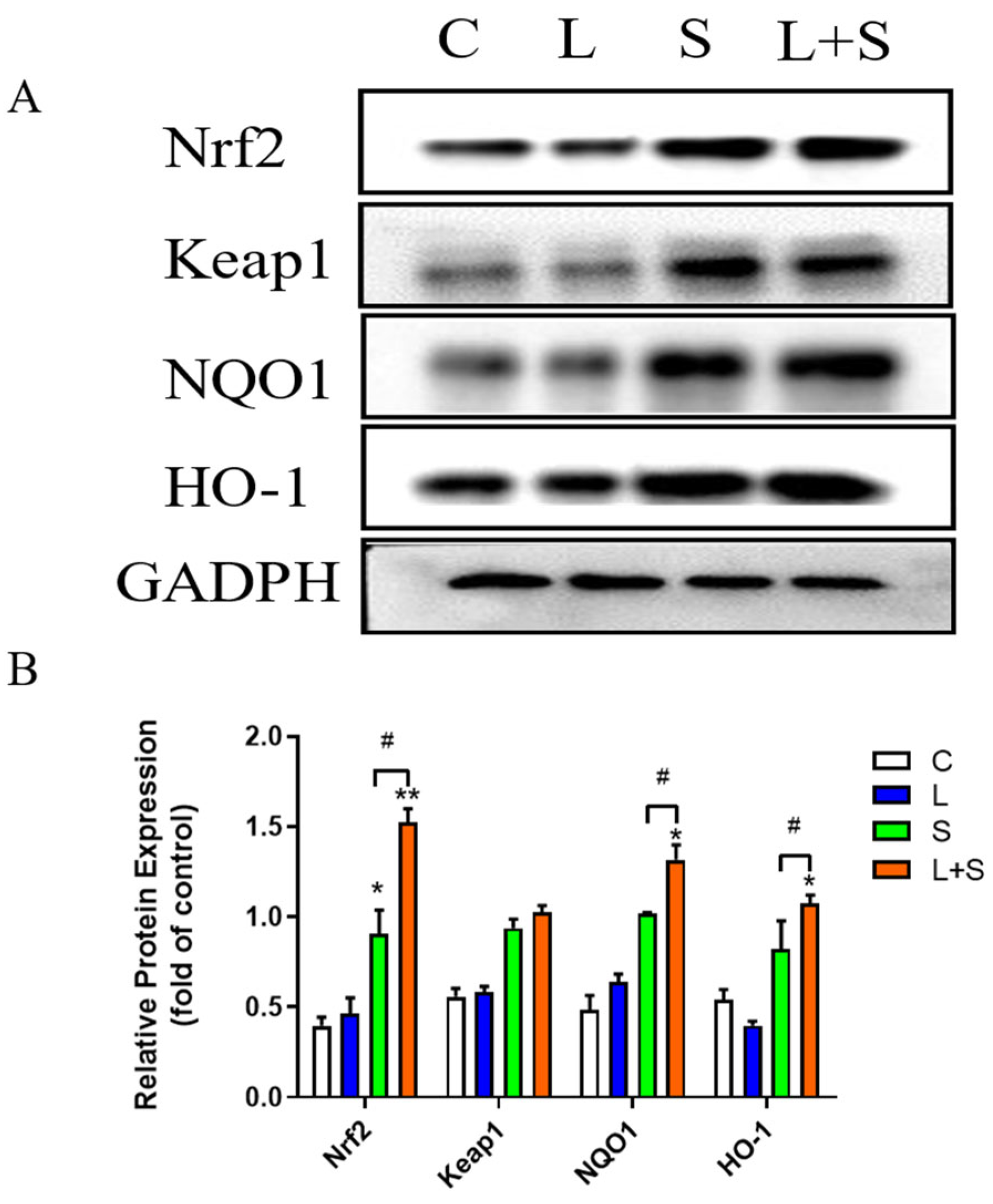
3.10. L. brevis 23017 Protects Mice against Oxidative Stress Induced by S. typhimurium C7731 by Regulating the Nrf2 Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2020, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Bradford, G.; Asgari, B.; Smit, B.; Hatje, E.; Kuballa, A.; Katouli, M. The Efficacy of Selected Probiotic Strains and Their Combination to Inhibit the Interaction of Adherent-Invasive Escherichia coli (AIEC) with a Co-Culture of Caco-2:HT29-MTX Cells. Microorganisms 2024, 12, 502. [Google Scholar] [CrossRef]
- Zhou, L.; Yin, X.; Fang, B.; He, J.; Zhan, J.; Zhang, X.; Wang, R.S. Effects of Bifidobacterium animalis subsp. lactis IU100 on Immunomodulation and Gut Microbiota in Immunosuppressed Mice. Microorganisms 2024, 12, 493. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U. The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-H.; Kim, J.-M.; Park, S.-Y.; Kim, J.-M. Screening lactic acid bacteria from swine origins for multistrain probiotics based on in vitro functional properties. Anaerobe 2010, 16, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Rubayet, U.L.; Alam, A.S.M.; Jahid, I.K. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Aprea, G.; Del Matto, I.; Tucci, P.; Marino, L.; Scattolini, S.; Rossi, F. In Vivo Functional Properties of Dairy Bacteria. Microorganisms 2024, 11, 1787. [Google Scholar] [CrossRef]
- de LeBlanc, A.d.M.; Castillo, N.A.; Perdigon, G. Anti-infective mechanisms induced by a probiotic Lactobacillus strain against Salmonella enterica serovar Typhimurium infection. Int. J. Food Microbiol. 2010, 138, 223–231. [Google Scholar] [CrossRef]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef]
- Ge, W.; Li, Z.; Yang, Y.; Liu, X.; Zhu, Z.; Bai, L.; Qin, Z.; Xu, X.; Li, J.; Li, S. Synthesis and antibacterial activity of FST and its effects on inflammatory response and intestinal barrier function in mice infected with Escherichia coli O78. Int. Immunopharmacol. 2024, 127, 111386. [Google Scholar] [CrossRef]
- Lemme-Dumit, J.M.; Cazorla, S.I.; Perdigón, G.D.V.; Maldonado-Galdeano, C. Probiotic Bacteria and Their Cell Walls Induce Th1-Type Immunity against Salmonella Typhimurium Challenge. Front Immunol. 2021, 12, 660854. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.; Isolauri, E.; Salminen, S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 2002, 41, I32–I37. [Google Scholar] [CrossRef] [PubMed]
- De Montijo-Prieto, S.; Moreno, E.; Bergillos-Meca, T.; Lasserrot, A.; Ruiz-López, M.-D.; Ruiz-Bravo, A.; Jiménez-Valera, M. A Lactobacillus plantarum strain isolated from kefir protects against intestinal infection with Yersinia enterocolitica O9 and modulates immunity in mice. Res. Microbiol. 2015, 166, 626–632. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Qin, H.L.; Zhang, M.; Shen, T.Y.; Chen, H.Q.; Ma, Y.L.; Chu, Z.X.; Zhang, P.; Liu, Z.H. Effects of Lactobacillus plantarum on gut barrier function in experimental obstructive jaundice. World J. Gastroenterol. 2012, 18, 3977–3991. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef]
- Bhatia, R.; Sharma, S.; Bhadada, S.K.; Bishnoi, M.; Kondepudi, K.K. Lactic Acid Bacterial Supplementation Ameliorated the Lipopolysaccharide-Induced Gut Inflammation and Dysbiosis in Mice. Front. Microbiol. 2024, 13, 930928. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Guan, N.; Sun, W.; Sun, T.; Niu, L.; Li, J.; Ge, J. Protective Effect of Levilactobacillus brevis Against Yersinia enterocolitica Infection in Mouse Model via Regulating MAPK and NF-κB Pathway. Probiotics Antimicrob. Proteins 2022, 14, 830–844. [Google Scholar] [CrossRef]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic-Stanojevic, N.; Miloradović, Z.; Ivanov, M.; Bugarski, B.; Jovović, Đ.; Karanović, D.; Vajić, U.J.; Komes, D.; Grujić-Milanović, J. Upregulation of Heme Oxygenase-1 in Response to Wild Thyme Treatment Protects against Hypertension and Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 1458793. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes. 2020, 12, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Shang, Z.; Qiao, J.; Wang, Y.; Li, H. Clostridium butyricum Protects IPEC-J2 Cells from ETEC K88-Induced Oxidative Damage by Activating the Nrf2/ARE Signaling Pathway. Oxid. Med. Cell Longev. 2021, 2021, 4464002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Fu, A.; Gong, L.; Li, W.; Li, Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Prajapati, K.D.; Sonara, B.M.; Sharma, M.M.; Patel, H.M.; Pawar, V.D.; Jain, M.R. Ameliorative potential of aliskiren in experimental colitis in mice. Eur. J. Pharmacol. 2014, 737, 70–76. [Google Scholar] [CrossRef]
- Chorawala, M.R.; Chauhan, S.; Patel, R.; Shah, G. Cell Wall Contents of Probiotics Lactobacillus species Protect Against Lipopolysaccharide LPS-Induced Murine Colitis by Limiting Immuno-inflammation and Oxidative Stress. Probiotics Antimicrob. Proteins. 2021, 13, 1005–1017. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Liang, Z.; Liu, A.; Chen, Z.; Tang, Y.; Xiao, M.; Chu, F.; Liu, W.; Jin, X.; et al. Musca domestica Cecropin Mdc Alleviates Salmonella typhimurium-Induced Colonic Mucosal Barrier Impairment: Associating With Inflammatory and Oxidative Stress Response, Tight Junction as Well as Intestinal Flora. Front. Microbiol. 2019, 10, 522. [Google Scholar] [CrossRef]
- Jiang, X.; Gu, S.; Liu, D.; Zhao, L.; Xia, S.; He, X.; Chen, H.; Ge, J. Lactobacillus brevis 23017 Relieves Mercury Toxicity in the Colon by Modulation of Oxidative Stress and Inflammation Through the Interplay of MAPK and NF-κB Signaling Cascades. Front. Microbiol. 2018, 9, 2425. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Fresno, A.; Olsen, J.E. Salmonella Typhimurium metabolism affects virulence in the host—A mini-review. Food Microbiol. 2018, 71, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.J.; Hill, J.; Blohmke, C.J.; Pollard, A.J. Host restriction, pathogenesis and chronic carriage of typhoidal Salmonella. FEMS Microbiol. Rev. 2021, 45, 14. [Google Scholar]
- Brown, E.W.; Bell, R.; Zhang, G.; Timme, R.; Zheng, J.; Hammack, T.S.; Allard, M.W. Salmonella Genomics in Public Health and Food Safety. EcoSal Plus 2021, 9, eESP00082020. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Perdigón, G.; de Moreno de Leblanc, A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.B.; Fagarasan, S. IgA synthesis: A form of functional immune adaptation extending beyond gut. Curr. Opin. Immunol. 2012, 24, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.A.L.; de Jesus, L.C.L.; de Castro, C.P.; Batista, V.L.; Ferreira, Ê.; Fernandes, R.S.; de Barros, A.L.B.; Leclerq, S.Y.; Azevedo, V.; Mancha-Agresti, P.; et al. Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis. Microorganisms 2021, 9, 107. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, H.; Liu, G.; Chen, S.; Tan, B.; Ren, W.; Bazer, F.W.; Wu, G.; Yin, Y. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 2016, 60, 1637–1648. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, X.; Chen, J.; Luo, D.; Xie, J.; Su, Z.; Huang, X.; Yi, X.; Wei, L.; Cai, J.; et al. Protective Effect of Bruguiera gymnorrhiza L. Lam. Fruit on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice: Role of Keap1/Nrf2 Pathway and Gut Microbiota. Front. Pharmacol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Schmidt, H.; Braubach, P.; Schilpp, C.; Lochbaum, R.; Neuland, K.; Thompson, K.; Jonigk, D.; Frick, M.; Dietl, P.; Wittekindt, O.H. IL-13 Impairs Tight Junctions in Airway Epithelia. Int. J. Mol. Sci. 2019, 20, 3222. [Google Scholar] [CrossRef]
- Almeer, R.S.; Mahmoud, S.M.; Amin, H.K.; Abdel, M.A.E. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 115, 49–62. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Li, H.; Tuo, Y.; Gao, Y.; Zhang, Y. Antioxidative effect of Lactobacillus plantarum Y44 on 2,2’-azobis2-methylpropionamidine dihydrochloride ABAP-damaged Caco-2 cells. J. Dairy Sci. 2019, 102, 6863–6875. [Google Scholar] [CrossRef]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox Role of Lactobacillus casei Shirota Against the Cellular Damage Induced by 2,2’-Azobis 2-Amidinopropane Dihydrochloride-Induced Oxidative and Inflammatory Stress in Enterocytes-Like Epithelial Cells. Front. Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Sun, F.; Wang, Z.; Liu, T.; Gong, J.; Kan, X.; Zou, Y.; Zhao, X. Lactobacillus fermentum CQPC08 protects rats from lead-induced oxidative damage by regulating the Keap1/Nrf2/ARE pathway. Food Funct. 2021, 12, 6029–6044. [Google Scholar] [CrossRef] [PubMed]
- Marius, M.; Amadou, D.; Donatien, A.A.; Gilbert, A.; William, Y.N.; Rauf, K.; Arif, M.; Adeline, F.Y.S.; Saint, N.I.; Dar, H.; et al. In Vitro Antioxidant, Anti-inflammatory, and In Vivo Anticolitis Effects of Combretin A and Combretin B on Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. Gastroenterol. Res. Pract. 2020, 2020, 4253174. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E.; et al. Control of lupus nephritis by changes of gut microbiota. Microbiome 2017, 5, 73. [Google Scholar] [CrossRef]



| Intestinal Segments | Groups | MDA (nmol/mg Protein) | SOD (U/mg Protein) | GSH-Px (μmol/g Protein) | T-AOC (μmol/g Protein) |
|---|---|---|---|---|---|
| duodenum | C | 4.25 ± 1.58 | 11.07 ± 4.67 | 10.16 ± 1.09 | 0.69 ± 0.08 |
| S | 5.95 ± 1.09 # | 4.15 ± 1.93 *# | 14.78 ± 3.84 * | 0.25 ± 0.11 *# | |
| L | 4.31 ± 1.82 | 10.97 ± 3.32 | 12.17 ± 1.49 | 0.43 ± 0.03 | |
| L+S | 4.54 ± 0.56 ^ | 10.52 ± 3.25 #^^ | 14.85 ± 4.12 *^ | 0.39 ± 0.09 *^ | |
| jejunum | C | 4.25 ± 1.38 | 10.77 ± 1.25 | 12.856 ± 0.86 | 0.62 ± 0.06 |
| S | 5.88 ± 0.31 *# | 4.51 ± 1.57 *# | 7.81 ± 0.32 **# | 0.36 ± 0.11 *# | |
| L | 4.16 ± 0.97 | 9.27 ± 2.02 * | 10.92 ± 0.57 * | 0.42 ± 0.02 | |
| L+S | 4.31 ± 0.32 ^ | 8.75 ± 0.75 *#^ | 8.31 ± 3.21 *#^ | 0.51 ± 0.10 #^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Nan, Y.; Zhou, X.; Zhang, W.; Zhang, Z.; Zhang, C.; Duan, H.; Ge, J.; Zhao, L. Molecular Mechanisms of Intestinal Protection by Levilactobacillus brevis 23017 against Salmonella typhimurium C7731-Induced Damage: Role of Nrf2. Microorganisms 2024, 12, 1135. https://doi.org/10.3390/microorganisms12061135
Shi Z, Nan Y, Zhou X, Zhang W, Zhang Z, Zhang C, Duan H, Ge J, Zhao L. Molecular Mechanisms of Intestinal Protection by Levilactobacillus brevis 23017 against Salmonella typhimurium C7731-Induced Damage: Role of Nrf2. Microorganisms. 2024; 12(6):1135. https://doi.org/10.3390/microorganisms12061135
Chicago/Turabian StyleShi, Ziqi, Yongchao Nan, Xinyao Zhou, Wenzhi Zhang, Zheng Zhang, Chuankun Zhang, Haoyuan Duan, Junwei Ge, and Lili Zhao. 2024. "Molecular Mechanisms of Intestinal Protection by Levilactobacillus brevis 23017 against Salmonella typhimurium C7731-Induced Damage: Role of Nrf2" Microorganisms 12, no. 6: 1135. https://doi.org/10.3390/microorganisms12061135





