Summatory Effects of Anaerobic Exercise and a ‘Westernized Athletic Diet’ on Gut Dysbiosis and Chronic Low-Grade Metabolic Acidosis
Phenotyping the Athletic Gut Microbiota: The Basis of Health and Physical Performance?
)
Abstract
:1. Introduction
2. Methods
3. Effects of Anaerobic Exercise on Acid-Base Homeostasis
4. Effects of Nutrition on Metabolic Acidosis in High-Intensity Sports
5. Gut Microbiota and Systemic Acid-Base Homeostasis in Anaerobic Athletes
6. Influence of Anaerobic Athlete’s Training and Diet on Gut Microbiota
| Authors | Sample and Type of Study | Results | Effects of WAN and/or Exercise on GM Composition? |
|---|---|---|---|
| Scheiman et al. [14] | Runner athletes (n = 15) who ran in the 2015 Boston marathon were compared to a set of sedentary controls (n = 10) sequenced on approximately daily samples collected up to one week before and one week after marathon day. | The link between members of the genus Veillonella and exercise performance: Increases in Veillonella relative abundance in marathon runners postmarathon. Inoculation of this strain into mice significantly increased exhaustive treadmill run time. Veillonella atypica improved run time via its metabolic conversion of exercise-induced lactate into propionate. |
YES Exercise promotes bacterial specialization and can decrease lactate during exercise. |
| Bressa et al. [54] |
Experimental study Two groups: runners were complemented with a protein supplement (whey isolate and beef hydrolysate) (n = 12) or maltodextrin (control) (n = 12) for 10 weeks. | Fecal pH, water content, ammonia, and SCFA concentrations did not change, indicating that protein supplementation did not increase the presence of these fermentation-derived metabolites. Increased abundance of the Bacteroidetes phylum and decreased the presence of health-related taxa, including Roseburia, Blautia, and Bifidobacterium longum. |
YES Protein supplementations affect GM balance and can alter beneficial composition. Long-term protein supplementation may have a negative impact on gut microbiota. |
| Estaki et al. [95] |
Experimental study N = 39 subjects physically fit (22 males and 17 females) | Peak oxygen uptake explained more than 20% of the variation in taxonomic richness after accounting for all other factors, including diet. This higher endurance performance was related to increases in the production of fecal butyrate amongst physically fit participants, identifying increased abundances of key butyrate-producing taxa (Clostridiales, Roseburia, Lachnospiraceae, and Erysipelotrichaceae). |
YES Cardiorespiratory fitness is correlated with increased microbial diversity in healthy humans, and the associated changes are anchored around a set of functional cores rather than specific taxa. The microbial profiles of fit individuals favor the production of butyrate. Increased microbiota diversity and butyrate production are associated with overall host health. |
| Allen et al. [107] |
N = 32 sedentary subjects Two groups: lean (n = 18 [9 female]) and obese (n = 14 [11 female]). Six weeks of supervised, endurance-based exercise training (3 d·wk−1) that progressed from 30 to 60 min·d−1 and from moderate (60% of HR reserve) to vigorous intensity (75% HR reserve). Subsequently, participants returned to a sedentary lifestyle activity for a 6 wk washout period. Fecal samples were collected before and after 6 wk of exercise, as well as after the sedentary washout period, with 3 d dietary controls in place before each collection. | β-diversity analysis revealed that exercise-induce alterations of the gut microbiota. Exercise increased fecal concentrations of short-chain fatty acids in lean, but not obese, participants. Exercise-induced shifts in the metabolic output of the microbiota paralleled changes in bacterial genes and taxa capable of short-chain fatty acid production. Exercise-induced changes in the microbiota were largely reversed once exercise training ceased. |
YES Exercise training induces compositional and functional changes in the human gut microbiota but is reversed if a positive stimulus (exercise and/or diet) does not exist. |
| Fernández-Sanjurjo et al. [111] |
A total of 16 professional cyclists competing in La Vuelta 2019 were recruited. Fecal samples were collected at four time points: the day before the first stage (A), after 9 stages (B), after 15 stages (C), and on the last stage (D). | Bifidobacteriaceae, Coriobacteriaceae, Erysipelotrichaceae, and Sutterellaceae dynamics showed a strong final performance predictive value (r = 0.83, ranking, and r = 0.81, accumulated time). Positive correlations were observed between Coriobacteriaceae with acetate (r = 0.530) and isovalerate (r = 0.664) and between Bifidobacteriaceae with isobutyrate (r = 0.682). No relationship was observed between SCFAs and performance. The abundance of Erysipelotrichaceae at the beginning of La Vuelta was directly related to the previous intake of complex-carbohydrate-rich foods (r = 0.956), while during the competition, the abundance of Bifidobacteriaceae was negatively affected by the intake of simple carbohydrates from supplements (r = −0.650). |
YES An ecological perspective more realistically represents the relationship between gut microbiota composition and performance compared with single-taxon approaches. The composition and periodization of diet and supplementation during a grand tour, particularly carbohydrates, could be designed to modulate gut microbiota composition to allow better performance. |
| Barton et al. [108] | Metabolic phenotyping and functional metagenomic analysis of the gut microbiome of professional international rugby union players (n = 40) and controls (n = 46) were carried out, and the results were correlated with lifestyle parameters and clinical measurements (e.g., dietary habit and serum creatine kinase, respectively). | Athletes had relative increases in pathways associated with enhanced muscle turnover (fitness) and overall health when compared with control groups. |
YES Differences in fecal microbiota between athletes and sedentary controls were associated with exercise and diet regimens. |
| Cronin et al. [113] |
N = 90 healthy Irish male and female Caucasian volunteers. Age between 18 to 40 years and with a body mass index (BMI) of between 22 and 35 kg/m2 (predominantly overweight or obese). Two randomized groups were recruited to an exercise-only group (E group) and an exercise plus daily whey protein supplementation group (EP group). A separate parallel group consuming whey protein supplementation but not participating in exercise programs (p group) was included in the study as a control. All participants were observed and measured for 8 weeks (n = 30 for each group). The exercise-only group (E) participated in an 8-week mixed aerobic and resistance exercise training program. The exercise plus whey protein supplementation group (EP) followed the same exercise program in addition to consuming the once-daily whey protein supplement. |
Significant changes in the diversity of the gut virome were evident in participants receiving daily whey protein supplementation. Improved body composition with exercise is not dependent on major changes in the diversity of microbial populations in the gut. The diverse microbial characteristics previously observed in long-term habitual athletes may be a later response to exercise and fitness improvement. |
YES Increasing the fitness levels of physically inactive humans leads to modest but detectable changes in gut microbiota characteristics. Regular whey protein intake leads to significant alterations to the composition of the gut virome. |
| Jang et al. [115] |
Bodybuilders (n = 15), elite distance runners (n = 15), and healthy men in their twenties without regular exercise habits (n = 15). All participants were males. 3-day food diary (2 weekdays and 1 weekend day) that reflected habitual dietary intake. |
Exercise type was associated with athlete diet patterns (bodybuilders: high-protein, high-fat, low-carbohydrate, and low dietary fiber diet; distance runners: low-carbohydrate and low dietary fiber diet). However, athlete type did not differ regarding gut microbiota alpha and beta diversity but was significantly associated with the relative abundance of gut microbiota at the genus and species level. Faecalibacterium , Sutterella, Clostridium, Haemophilus, and Eisenbergiella were the highest (p < 0.05) in bodybuilders, while Bifidobacterium and Parasutterella were the lowest (p < 0.05). At the species level, intestinal beneficial bacteria widely used as probiotics (Bifidobacterium adolescentis group, Bifidobacterium longum group, Lactobacillus sakei group) and those producing short-chain fatty acids (Blautia wexlerae, Eubacterium hallii) were the lowest in bodybuilders and the highest in controls. In addition, aerobic or resistance exercise training with an unbalanced intake of macronutrients and low intake of dietary fiber led to a similar diversity of gut microbiota. Specifically, daily protein intake was negatively correlated with operation taxonomic unit and Shannon index in distance runners. |
YES High-protein diets may have a negative impact on gut microbiota diversity for athletes. |
| Han et al. [116] | A team of professional female rowing athletes in China was recruited, and 306 fecal samples were collected from 19 individuals, which were separated into three cohorts: adult elite athletes (AE), youth elite athletes (YE), and youth non-elite athletes (YN). | The microbial diversities of elite athletes were higher than those of youth non-elite athletes. The taxonomical, functional, and phenotypic compositions of AE, YE, and YN were significantly different. Additionally, three enterotypes with clear separation were identified in athlete’s fecal samples, with the majority of elite athletes stratified into enterotype 3, which is strongly associated with athlete performances. |
YES Direct association between type of exercise regimen and diet: the versatilities of athlete microbial communities of athletes were found to be associated with dietary factors and physical characteristics of GM profile as a biomarker of physical performance and health. |
| Vázquez-Cuesta et al. [119] | The study included 60 patients (51.7% females). Classification of subjects into two groups according to the categories of good (1–4) and medium (5–9). Stratification by age group was as follows: children (0–2 years), teenagers (13–18 years), young adults (19–30 years), middle-aged adults (31–48 years), and older adults (49–76 years). |
The Mediterranean diet (MD), renowned for its potential health benefits, and the influence of adherence thereto on gut microbiota have become a focus of research. Adherence to MD correlated with alpha diversity, and higher values were recorded in good adherers. Good adherers had a higher abundance of Paraprevotella and Bacteroides (p < 0.001). Alpha diversity correlated inversely with fat intake and positively with non-starch polysaccharides. Evenness correlated inversely with red meat intake and positively with NSPs. |
YES Diet has an important influence on GM composition and health, and MD has better prognostic effects on GM than animal protein diets. |
7. The Chronic Impact of Metabolic Acidosis on Systemic Inflammation: Some Role for the Microbiota?
8. Could the Gut Microbiota Modulate the Systemic Inflammation and Metabolic Acidosis?
9. Conclusions and Future Perspective
Funding
Data Availability Statement
Conflicts of Interest
References
- Remer, T. Influence of Nutrition on Acid-Base Balance—Metabolic Aspects. Eur. J. Nutr. 2001, 40, 214–220. [Google Scholar] [CrossRef]
- Nechipurenko, Y.D.; Semyonov, D.A.; Lavrinenko, I.A.; Lagutkin, D.A.; Generalov, E.A.; Zaitceva, A.Y.; Matveeva, O.V.; Yegorov, Y.E. The Role of Acidosis in the Pathogenesis of Severe Forms of COVID-19. Biology 2021, 10, 852. [Google Scholar] [CrossRef]
- Chycki, J.; Kurylas, A.; Maszczyk, A.; Golas, A.; Zajac, A. Alkaline Water Improves Exercise-Induced Metabolic Acidosis and Enhances Anaerobic Exercise Performance in Combat Sport Athletes. PLoS ONE 2018, 13, e0205708. [Google Scholar] [CrossRef] [PubMed]
- Messonnier, L.; Kristensen, M.; Juel, C.; Denis, C. Importance of pH Regulation and Lactate/H+ Transport Capacity for Work Production during Supramaximal Exercise in Humans. J. Appl. Physiol. 2007, 102, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.F.; Salvador, A.F.; Hughes, R.L.; Scaroni, S.E.; Alamilla, R.A.; Askow, A.T.; Paluska, S.A.; Dilger, A.C.; Holscher, H.D.; De Lisio, M.; et al. Higher Protein Intake during Resistance Training Does Not Potentiate Strength, but Modulates Gut Microbiota, in Middle-Aged Adults: A Randomized Control Trial. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E900–E913. [Google Scholar] [CrossRef]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A Systematic Review, Meta-Analysis and Meta-Regression of the Effect of Protein Supplementation on Resistance Training-Induced Gains in Muscle Mass and Strength in Healthy Adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a Healthy Human Gut Microbiome: Current Concepts, Future Directions, and Clinical Applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.-J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Herms, J.; González, A.; Corbi, F.; Odriozola, I.; Odriozola, A. Possible Relationship between the Gut Leaky Syndrome and Musculoskeletal Injuries: The Important Role of Gut Microbiota as Indirect Modulator. AIMS Public Health 2023, 10, 710–738. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, Z.; Wu, W.; Lin, Q.; Liang, Y. High Animal Protein Diet and Gut Microbiota in Human Health. Crit. Rev. Food Sci. Nutr. 2022, 62, 6225–6237. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-Omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Rubio, L.A.; Duncan, S.H.; Donachie, G.E.; Holtrop, G.; Lo, G.; Farquharson, F.M.; Wagner, J.; Parkhill, J.; Louis, P.; et al. Pivotal Roles for pH, Lactate, and Lactate-Utilizing Bacteria in the Stability of a Human Colonic Microbial Ecosystem. mSystems 2020, 5, e00645-20. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Anaerobic Metabolism During Exercise. In Exercise Metabolism; McConell, G., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 51–70. ISBN 978-3-030-94305-9. [Google Scholar]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of Exercise-Induced Metabolic Acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Vilmi, N.; Äyrämö, S.; Nummela, A.; Pullinen, T.; Linnamo, V.; Häkkinen, K.; Mero, A.A. Oxygen Uptake, Acid-Base Balance and Anaerobic Energy System Contribution in Maximal 300–400 M Running in Child, Adolescent and Adult Athletes. J. Athl. Enhanc. 2016, 5, 3. [Google Scholar] [CrossRef]
- Hietavala, E.-M.; Stout, J.R.; Frassetto, L.A.; Puurtinen, R.; Pitkänen, H.; Selänne, H.; Suominen, H.; Mero, A.A. Dietary Acid Load and Renal Function Have Varying Effects on Blood Acid-Base Status and Exercise Performance across Age and Sex. Appl. Physiol. Nutr. Metab. 2017, 42, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I. Origins of [H+] Changes in Exercising Skeletal Muscle. Can. J. Appl. Physiol. 1995, 20, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Hanon, C.; Lepretre, P.-M.; Bishop, D.; Thomas, C. Oxygen Uptake and Blood Metabolic Responses to a 400-m Run. Eur. J. Appl. Physiol. 2010, 109, 233–240. [Google Scholar] [CrossRef]
- Lancha Junior, A.H.; de Salles Painelli, V.; Saunders, B.; Artioli, G.G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, G.; Russo, R.; Sofia, A.; Sala, M.R.; Robaudo, C.; Moscatelli, P.; Deferrari, G.; Tizianello, A. Skeletal Muscle Protein Synthesis and Degradation in Patients with Chronic Renal Failure. Kidney Int. 1994, 45, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Q.; Abramowitz, M.K. Clinical Consequences of Metabolic Acidosis—Muscle. Adv. Chronic Kidney Dis. 2022, 29, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. Association Between Estimated Net Endogenous Acid Production and Subsequent Decline in Muscle Mass over Four Years in Ambulatory Older Chinese People in Hong Kong: A Prospective Cohort Study. J. Gerontol. Ser. A 2015, 70, 905–911. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, E.A.L.; Koromani, F.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Rivadeneira, F.; Kiefte-de Jong, J.C. Dietary Acid Load, Trabecular Bone Integrity, and Mineral Density in an Ageing Population: The Rotterdam Study. Osteoporos. Int. 2017, 28, 2357–2365. [Google Scholar] [CrossRef]
- Faure, A.M.; Fischer, K.; Dawson-Hughes, B.; Egli, A.; Bischoff-Ferrari, H.A. Gender-Specific Association between Dietary Acid Load and Total Lean Body Mass and Its Dependency on Protein Intake in Seniors. Osteoporos. Int. 2017, 28, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; MacGregor, A.J.; Skinner, J.; Spector, T.D.; Moayyeri, A.; Cassidy, A. A Higher Alkaline Dietary Load Is Associated with Greater Indexes of Skeletal Muscle Mass in Women. Osteoporos. Int. 2013, 24, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.; Jablonskienė, V.; Abaravičius, J.A.; Samsonienė, L.; Stukas, R. Dietary Acid-Base Balance in High-Performance Athletes. Int. J. Environ. Res. Public Health 2020, 17, 5332. [Google Scholar] [CrossRef]
- Balcerek, B.; Steinach, M.; Lichti, J.; Maggioni, M.A.; Becker, P.N.; Labes, R.; Gunga, H.-C.; Persson, P.B.; Fähling, M. A Broad Diversity in Oxygen Affinity to Haemoglobin. Sci. Rep. 2020, 10, 16920. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.E.; Best, K.T.; Muscat, S.N.; Loiselle, A.E. Metabolic Regulation of Tendon Inflammation and Healing Following Injury. Curr. Rheumatol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Hammad, A.A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Saoud, H.A.; et al. Athletes’ Nutritional Demands: A Narrative Review of Nutritional Requirements. Front. Nutr. 2024, 10, 1331854. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-Induced Metabolic Acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.; Sebastian, A. Estimation of Net Endogenous Noncarbonic Acid Production in Humans from Diet Potassium and Protein Contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi-Oyelere, B.L.; Brough, L.; Coad, J.; Roy, N.; Kruger, M.C. The Relationship between Nutrient Patterns and Bone Mineral Density in Postmenopausal Women. Nutrients 2019, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Zanetti, A.; Muser, J.; Hulter, H.N.; Krapf, R. Partial Neutralization of the Acidogenic Western Diet with Potassium Citrate Increases Bone Mass in Postmenopausal Women with Osteopenia. J. Am. Soc. Nephrol. 2006, 17, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Muser, K.; Dickhuth, H.-H.; Berg, A.; Deibert, P. Effect of a Supplement Rich in Alkaline Minerals on Acid-Base Balance in Humans. Nutr. J. 2009, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, Magnesium, and Fruit and Vegetable Intakes Are Associated with Greater Bone Mineral Density in Elderly Men and Women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef] [PubMed]
- van Velden, D.P.; Reuter, H.; Kidd, M.; Müller, F.O. Non-Allopathic Adjuvant Management of Osteoarthritis by Alkalinisation of the Diet. Afr. J. Prim. Health Care Fam. Med. 2015, 7, 780. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary Acid Load, and Bone Effects—A Controversial Subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Konner, M.; Eaton, S.B. Paleolithic Nutrition: Twenty-Five Years Later. Nutr. Clin. Pract. 2010, 25, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Johansson-Persson, A.; Ulmius, M.; Cloetens, L.; Karhu, T.; Herzig, K.-H.; Onning, G. A High Intake of Dietary Fiber Influences C-Reactive Protein and Fibrinogen, but Not Glucose and Lipid Metabolism, in Mildly Hypercholesterolemic Subjects. Eur. J. Nutr. 2014, 53, 39–48. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Egan, B.M.; Geesey, M.E. Relation of Dietary Fat and Fiber to Elevation of C-Reactive Protein. Am. J. Cardiol. 2003, 92, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Williams, C. Muscle Citrate Content and the Regulation of Metabolism in Fed and Fasted Human Skeletal Muscle. Clin. Physiol. 1982, 2, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Piehl, K. Glycogen Storage and Depletion in Human Skeletal Muscle Fibres. Acta Physiol. Scand. Suppl. 1974, 402, 1–32. [Google Scholar] [PubMed]
- Jansson, E.; Kaijser, L. Effect of Diet on Muscle Glycogen and Blood Glucose Utilization during a Short-Term Exercise in Man. Acta Physiol. Scand. 1982, 115, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Spencer, H.G. Predictive Adaptive Responses and Human Evolution. Trends Ecol. Evol. 2005, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K. Is Early Development in Humans a Predictive Adaptive Response Anticipating the Adult Environment? Trends Ecol. Evol. 2006, 21, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K. The Thrifty Phenotype: An Adaptation in Growth or Metabolism? Am. J. Hum. Biol. 2011, 23, 65–75. [Google Scholar] [CrossRef]
- Ortega, E. The “Bioregulatory Effect of Exercise” on the Innate/Inflammatory Responses. J. Physiol. Biochem. 2016, 72, 361–369. [Google Scholar] [CrossRef]
- Álvarez-Herms, J.; González-Benito, A.; Corbi, F.; Odriozola, A. What If Gastrointestinal Complications in Endurance Athletes Were Gut Injuries in Response to a High Consumption of Ultra-Processed Foods? Please Take Care of Your Bugs If You Want to Improve Endurance Performance: A Narrative Review. Eur. J. Appl. Physiol. 2024, 124, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review Article: Insights into Colonic Protein Fermentation, Its Modulation and Potential Health Implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hübscher, G.H. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J. Low-Grade Metabolic Acidosis as a Driver of Chronic Disease: A 21st Century Public Health Crisis. Open Heart 2021, 8, e001730. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar Protein Synthesis Following Ingestion of Soy Protein Isolate at Rest and after Resistance Exercise in Elderly Men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total Volatile Basic Nitrogen and Trimethylamine in Muscle Foods: Potential Formation Pathways and Effects on Human Health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and Their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut Microbiota Role in Dietary Protein Metabolism and Health-Related Outcomes: The Two Sides of the Coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Hussain, M.; Ijaz, M.U.; Ahmad, M.I.; Khan, I.A.; Brohi, S.A.; Shah, A.U.; Shinwari, K.I.; Zhao, D.; Xu, X.; Zhou, G.; et al. Meat Proteins in a High-Fat Diet Have a Substantial Impact on Intestinal Barriers through Mucus Layer and Tight Junction Protein Suppression in C57BL/6J Mice. Food Funct. 2019, 10, 6903–6914. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L.; Gleeson, M.; Maughan, R.J. The Effects of Dietary Manipulation on Blood Acid-Base Status and the Performance of High Intensity Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L.; Gleeson, M.; Maughan, R.J. The Effects of Diet on Muscle pH and Metabolism during High Intensity Exercise. Europ. J. Appl. Physiol. 1988, 57, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Caciano, S.L.; Inman, C.L.; Gockel-Blessing, E.E.; Weiss, E.P. Effects of Dietary Acid Load on Exercise Metabolism and Anaerobic Exercise Performance. J. Sports Sci. Med. 2015, 14, 364–371. [Google Scholar] [PubMed]
- Niekamp, K.; Zavorsky, G.S.; Fontana, L.; McDaniel, J.L.; Villareal, D.T.; Weiss, E.P. Systemic Acid Load from the Diet Affects Maximal Exercise Respiratory Exchange Ratio. Med. Sci. Sports Exerc. 2012, 44, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Limmer, M.; Eibl, A.D.; Platen, P. Enhanced 400-m Sprint Performance in Moderately Trained Participants by a 4-Day Alkalizing Diet: A Counterbalanced, Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2018, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.; Choue, R. Metabolic Responses to High Protein Diet in Korean Elite Bodybuilders with High-Intensity Resistance Exercise. J. Int. Soc. Sports Nutr. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.; Greenhaff, P.L.; Maughan, R.J. The Acute Reversal of a Diet-Induced Metabolic Acidosis Does Not Restore Endurance Capacity during High-Intensity Exercise in Man. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Maukonen, J.; Saarela, M. Human Gut Microbiota: Does Diet Matter? Proc. Nutr. Soc. 2015, 74, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Merriam, R.L.; Morris, R.C. Estimation of the Net Acid Load of the Diet of Ancestral Preagricultural Homo Sapiens and Their Hominid Ancestors123. Am. J. Clin. Nutr. 2002, 76, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.; Shirreffs, S. Exercise in the Heat: Challenges and Opportunities. J. Sports Sci. 2004, 22, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J. Intraluminal pH of the Human Gastrointestinal Tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar] [PubMed]
- Ilhan, Z.E.; Marcus, A.K.; Kang, D.-W.; Rittmann, B.E.; Krajmalnik-Brown, R. pH-Mediated Microbial and Metabolic Interactions in Fecal Enrichment Cultures. mSphere 2017, 2, e00047-17. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Geypens, B.; Claus, D.; Evenepoel, P.; Hiele, M.; Maes, B.; Peeters, M.; Rutgeerts, P.; Ghoos, Y. Influence of Dietary Protein Supplements on the Formation of Bacterial Metabolites in the Colon. Gut 1997, 41, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Nobile, M.; Sartori, L.; Dalle Carbonare, L.; Ciuffreda, M.; Corrò, P.; D’Angelo, A.; Calò, L.; Crepaldi, G. Acute Effects of Moderate Dietary Protein Restriction in Patients with Idiopathic Hypercalciuria and Calcium Nephrolithiasis. Am. J. Clin. Nutr. 1999, 69, 267–271. [Google Scholar] [CrossRef]
- Yancy, W.S.; Olsen, M.K.; Dudley, T.; Westman, E.C. Acid-Base Analysis of Individuals Following Two Weight Loss Diets. Eur. J. Clin. Nutr. 2007, 61, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Buclin, T.; Cosma, M.; Appenzeller, M.; Jacquet, A.F.; Décosterd, L.A.; Biollaz, J.; Burckhardt, P. Diet Acids and Alkalis Influence Calcium Retention in Bone. Osteoporos. Int. 2001, 12, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Potential Renal Acid Load of Foods and Its Influence on Urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Durk, R.P.; Bagley, J.R. Rapid Gut Microbiome Changes in a World-Class Ultramarathon Runner. Physiol. Rep. 2019, 7, e14313. [Google Scholar] [CrossRef] [PubMed]
- Olbricht, H.; Twadell, K.; Sandel, B.; Stephens, C.; Whittall, J.B. Is There a Universal Endurance Microbiota? Microorganisms 2022, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Tidjani Alou, M.; Lagier, J.-C.; Raoult, D. Diet Influence on the Gut Microbiota and Dysbiosis Related to Nutritional Disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the Human Gut Microbiota by Dietary Fibres Occurs at the Species Level. BMC Biol. 2016, 14, 3. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of Diet to the Composition of the Human Gut Microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Fuster-Botella, D. Endurance Exercise and Gut Microbiota: A Review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Stewart, C.J.; Nelson, A.; Campbell, M.D.; Walker, M.; Stevenson, E.J.; Shaw, J.A.; Cummings, S.P.; West, D.J. Gut Microbiota of Type 1 Diabetes Patients with Good Glycaemic Control and High Physical Fitness Is Similar to People without Diabetes: An Observational Study. Diabet. Med. 2017, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wiklund, P.; Tan, X.; Wu, N.; Zhang, X.; Tikkanen, O.; Zhang, C.; Munukka, E.; Cheng, S. The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients 2017, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanjurjo, M.; Fernández, J.; Tomás-Zapico, C.; Fernández-García, B.; Villar, C.J.; Lombó, F.; Iglesias-Gutiérrez, E. Is Physical Performance (in Mice) Increased by Veillonella atypica or Decreased by Lactobacillus bulgaricus? J. Sport Health Sci. 2020, 9, 197–200. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The Mucus and Mucins of the Goblet Cells and Enterocytes Provide the First Defense Line of the Gastrointestinal Tract and Interact with the Immune System. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Caldarini, M.I.; Pons, S.; D’Agostino, D.; Depaula, J.A.; Greco, G.; Negri, G.; Ascione, A.; Bustos, D. Abnormal Fecal Flora in a Patient with Short Bowel Syndrome. Dig. Dis. Sci. 1996, 41, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Barton, W.; Cronin, O.; Garcia-Perez, I.; Whiston, R.; Holmes, E.; Woods, T.; Molloy, C.B.; Molloy, M.G.; Shanahan, F.; Cotter, P.D.; et al. The Effects of Sustained Fitness Improvement on the Gut Microbiome: A Longitudinal, Repeated Measures Case-Study Approach. Transl. Sports Med. 2021, 4, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.; Cox, A.J.; Bellinger, P.; Desbrow, B.; Irwin, C.; Buchan, J.; McCartney, D.; Sabapathy, S. The Influence of Exercise Training Volume Alterations on the Gut Microbiome in Highly-Trained Middle-Distance Runners. Eur. J. Sport Sci. 2022, 22, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.-G.; Choi, G.; Kim, S.-W.; Kim, B.-Y.; Lee, S.; Park, H. The Combination of Sport and Sport-Specific Diet Is Associated with Characteristics of Gut Microbiota: An Observational Study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, K.; Yang, P.; Zhong, C.; Chen, C.; Wang, S.; Lu, Q.; Ning, K. Stratification of Athletes’ Gut Microbiota: The Multifaceted Hubs Associated with Dietary Factors, Physical Characteristics and Performance. Gut Microbes 2020, 12, 1842991. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Gianfranceschi, G.; Liguori, R.; Antinozzi, M.; Mederer, B.; Liguori, G.; Romano Spica, V. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients 2020, 12, 2164. [Google Scholar] [CrossRef]
- Vázquez-Cuesta, S.; Lozano García, N.; Rodríguez-Fernández, S.; Fernández-Avila, A.I.; Bermejo, J.; Fernández-Avilés, F.; Muñoz, P.; Bouza, E.; Reigadas, E. Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals. Nutrients 2024, 16, 793. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Kellum, J.A.; Park, M.; Ranzani, O.T.; Barbeiro, H.V.; de Souza, H.P.; da Cruz Neto, L.M.; Pinheiro da Silva, F. Relationship between Acid–Base Status and Inflammation in the Critically Ill. Crit. Care 2014, 18, R154. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Song, M.; Li, J. Lactic and Hydrochloric Acids Induce Different Patterns of Inflammatory Response in LPS-Stimulated RAW 264.7 Cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R686–R692. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Effects of Hyperchloremic Acidosis on Arterial Pressure and Circulating Inflammatory Molecules in Experimental Sepsis. Chest 2004, 125, 243–248. [Google Scholar] [CrossRef]
- Brooks, G.A. The Lactate Shuttle during Exercise and Recovery. Med. Sci. Sports Exerc. 1986, 18, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Medbø, J.I.; Jebens, E.; Noddeland, H.; Hanem, S.; Toska, K. Lactate Elimination and Glycogen Resynthesis after Intense Bicycling. Scand. J. Clin. Lab. Investig. 2006, 66, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Flint, H.J.; Lobley, G.E. Quantitative Analysis of Microbial Metabolism in the Human Large Intestine. Curr. Nutr. Food Sci. 2008, 4, 109–126. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Acidosis: An Old Idea Validated by New Research. Integr. Med. 2015, 14, 8–12. [Google Scholar]
- Oishi, Y.; Manabe, I. Macrophages in Inflammation, Repair and Regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. The Hypoxia-Lactate Axis Tempers Inflammation. Nat. Rev. Immunol. 2020, 20, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Melamed, P.; Melamed, F. Chronic Metabolic Acidosis Destroys Pancreas. JOP 2014, 15, 552–560. [Google Scholar] [CrossRef]
- van der Togt, V.; Rossman, J.S. Hypothesis: Inflammatory Acid-Base Disruption Underpins Long COVID. Front. Immunol. 2023, 14, 1150105. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hoffman, K.L.; Chen, J.-S.; Shivappa, N.; Sood, A.; Browman, G.J.; Dirba, D.D.; Hanash, S.; Wei, P.; Hebert, J.R.; et al. Dietary Inflammatory Potential in Relation to the Gut Microbiome: Results from a Cross-Sectional Study. Br. J. Nutr. 2020, 124, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Ganzel, B.L.; Morris, P.A.; Wethington, E. Allostasis and the Human Brain: Integrating Models of Stress from the Social and Life Sciences. Psychol. Rev. 2010, 117, 134–174. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stressed or Stressed out: What Is the Difference? J. Psychiatry Neurosci. 2005, 30, 315–318. [Google Scholar] [PubMed]
- Ashizawa, N.; Ouchi, G.; Fujimura, R.; Yoshida, Y.; Tokuyama, K.; Suzuki, M. Effects of a Single Bout of Resistance Exercise on Calcium and Bone Metabolism in Untrained Young Males. Calcif. Tissue Int. 1998, 62, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Berardi, J.M.; Logan, A.C.; Rao, A.V. Plant Based Dietary Supplement Increases Urinary pH. J. Int. Soc. Sports Nutr. 2008, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, L.; Orheim, A.; Sejersted, O.M. Metabolic Acidosis and Changes in Water and Electrolyte Balance in Relation to Fatigue during Maximal Exercise of Short Duration. Int. J. Sports Med. 1984, 5, S110–S115. [Google Scholar] [CrossRef]
- Robergs, R.; Hutchinson, K.; Hendee, S.; Madden, S.; Siegler, J. Influence of Pre-Exercise Acidosis and Alkalosis on the Kinetics of Acid-Base Recovery Following Intense Exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Requena, B.; Zabala, M.; Padial, P.; Feriche, B. Sodium Bicarbonate and Sodium Citrate: Ergogenic Aids? J. Strength Cond. Res. 2005, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Hadzic, M.; Eckstein, M.L.; Schugardt, M. The Impact of Sodium Bicarbonate on Performance in Response to Exercise Duration in Athletes: A Systematic Review. J. Sports Sci. Med. 2019, 18, 271–281. [Google Scholar] [PubMed]
- Mündel, T. Sodium Bicarbonate Ingestion Improves Repeated High-Intensity Cycling Performance in the Heat. Temperature 2018, 5, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Naude MTech (Hom), D.F. Chronic Sub-Clinical Systemic Metabolic Acidosis—A Review with Implications for Clinical Practice. J. Evid. Based Integr. Med. 2022, 27, 2515690X221142352. [Google Scholar] [CrossRef] [PubMed]
- Vormann, J.; Worlitschek, M.; Goedecke, T.; Silver, B. Supplementation with Alkaline Minerals Reduces Symptoms in Patients with Chronic Low Back Pain. J. Trace Elem. Med. Biol. 2001, 15, 179–183. [Google Scholar] [CrossRef] [PubMed]
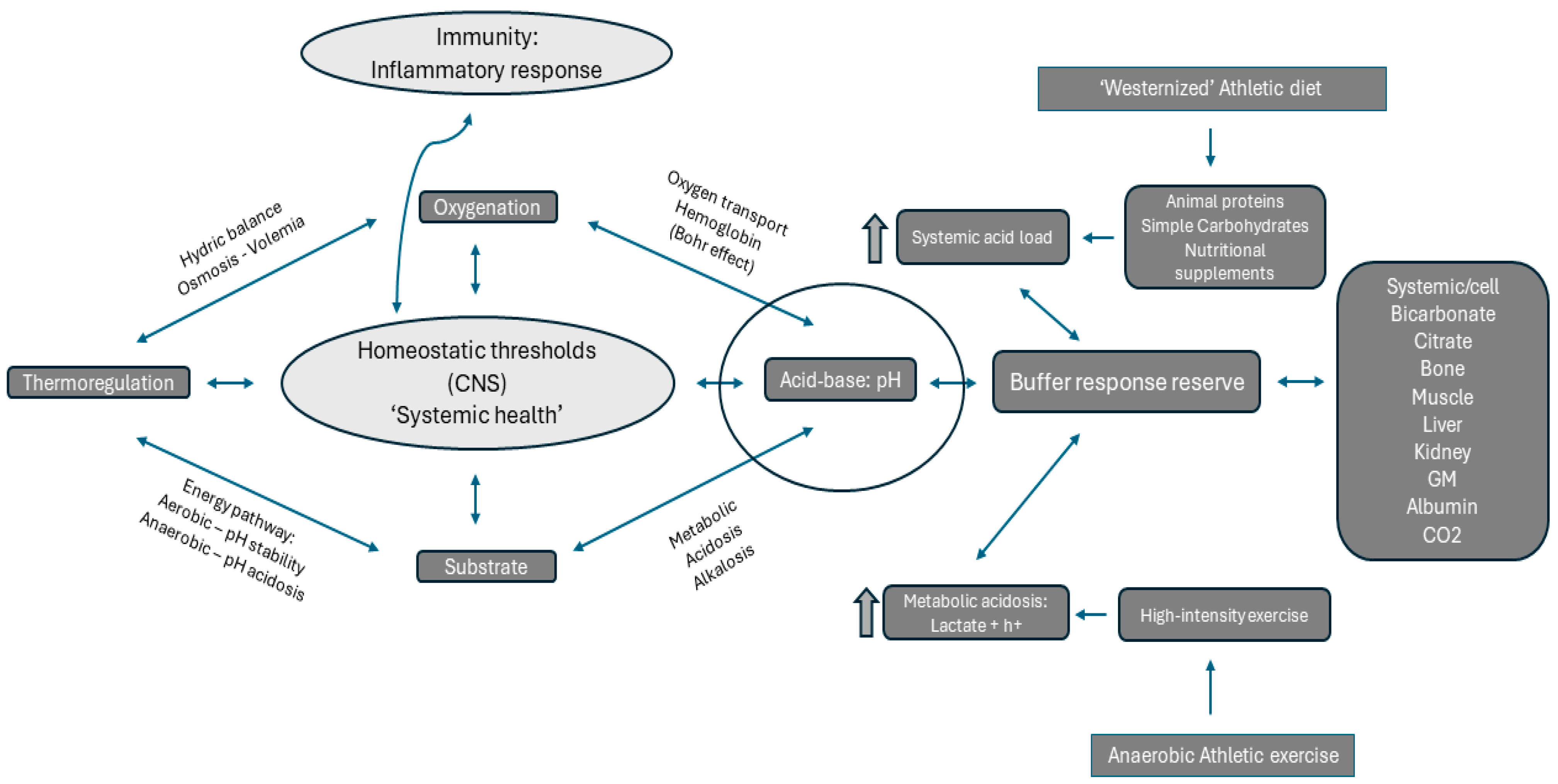
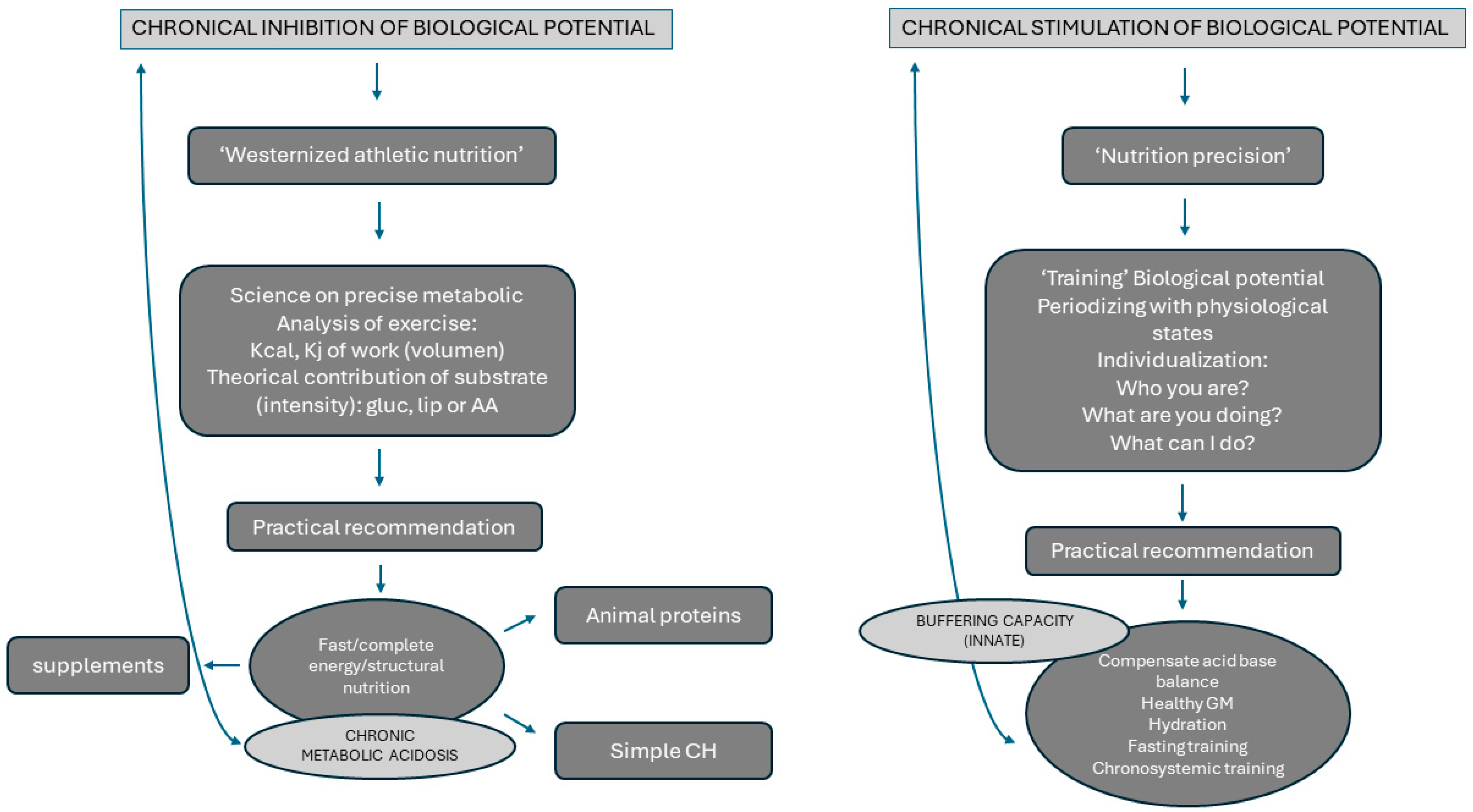
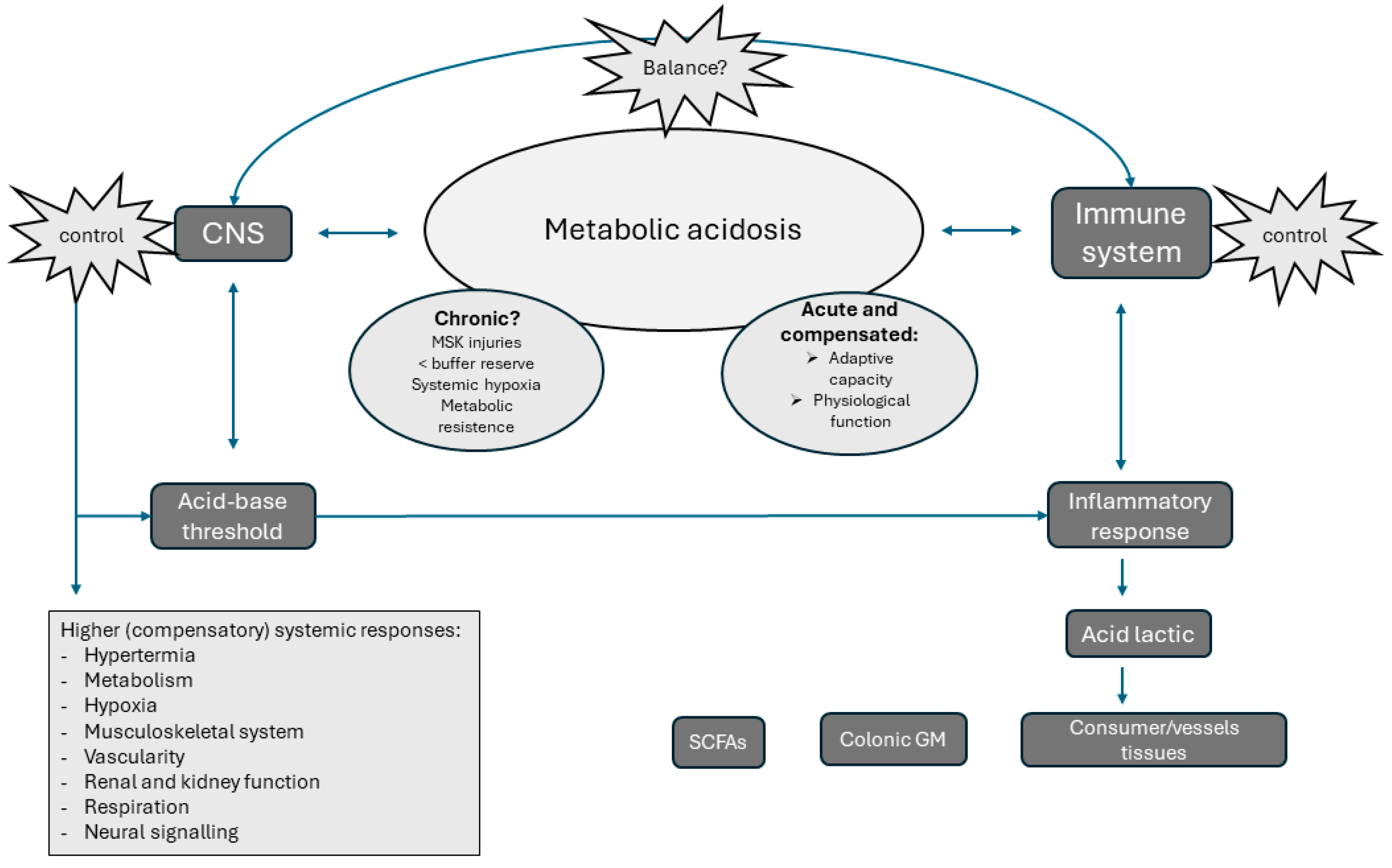
| Authors | Type of Study and Sample | Protocol/Intervention | Results |
|---|---|---|---|
| Greenhaff et al. [67] | Experimental design N = 6 Physically active subjects | Four diet protocols for 4 days following a maximal test until exhaustion. Three groups with intervention: (carbs–lipids–proteins) Normal diet (45–41–14%) Low carbs–high fat (3–71–26%) High carbs (73–12–15%) High fat–protein (47–27–26%) | Dietary composition influences acid-base balance by affecting the plasma buffer base and circulating non-volatile weak acids and, by doing so, may influence the time taken to reach exhaustion during high-intensity exercise. High-protein diet elevates metabolic acidosis and alters acid-base balance. |
| Greenhaff et al. [68] | Randomized study N = 5 Physically active subjects | A total of 3 min at 100% of maximal oxygen uptake on two separate occasions post 4-day diet interventions (cycle ergometer): (a) low carbs, 3%; high fat, 73%; high protein, 24%; or (b) high CHO, 82%; low fat, 8%; and low protein, 10%. |
There were no differences between the two treatments in blood acid-base status at rest prior to dietary manipulation. Muscle glycogen content increased by 23% on the (b) diet but was unchanged after the (a) diet. The decline in muscle glycogen content during exercise was 50% greater on the (b) diet. Low-CHO diet could induce metabolic acidosis and may reduce pre-exercise muscle buffering capacity, which may then influence subsequent exercise performance. |
| Caciano et al. [69] | Cross-over trial randomized and counterbalanced N = 10 Physically active subjects | Graded treadmill test to exhaustion and an anaerobic exercise test on two occasions: after following a low- and high-potential renal load diet (diets were continued as long as needed to achieve an alkaline (4 days) or acid (9 days) fasted morning urine pH state). Anaerobic test until exhaustion lasting 1–4 min. |
Maximal exercise Respiratory exhalation ratio (RER) was lower in the alkaline trial compared to the acidic trial (1.10 ± 0.02 vs. 1.20 ± 0.05, p = 0.037). The alkaline diet also resulted in a 21% greater time to exhaustion during anaerobic exercise (2.56 ± 0.36 vs. 2.11 ± 0.31 s, p = 0.044) and a strong tendency for lower RER values during submaximal exercise at 70% VO2max (0.88 ± 0.02 vs. 0.96 ± 0.04, p = 0.060). Alkaline-promoting diet resulted in lower RER values during maximal-intensity exercise, and also increased anaerobic exercise time to exhaustion may favor lipid oxidation. |
| Kim et al. [72] |
Experimental study N = 8 Elite Korean bodybuilders |
The study investigated the metabolic response to high protein consumption in elite bodybuilders: Diet regimen: protein (4.3 ± 1.2 g/kg body weight/day) and calories (5621.7 ± 1354.7 kcal/day) recorded during three days (breakfast, lunch, dinner, and snacks). |
Serum creatinine (1.3 ± 0.1 mg/dL) and potassium (5.9 ± 0.8 mmol/L), and urinary urea nitrogen (24.7 ± 9.5 mg/dL) and creatinine (2.3 ± 0.7 mg/dL) were observed to be higher than the normal reference ranges. Increased urinary excretion of urea nitrogen and creatinine might be due to the high rates of protein metabolism that follow high protein intake and muscle turnover. |
| Hietavala et al. [19] |
Experimental study; randomized N = 88 Three groups: adolescents (12–15 years), young adults (20–35 years), and old subjects (60–75 years) Physically active |
A 7-day high-vegetable (alkaline) and a 7-day high-protein diet with no vegetables and fruits in a randomized order. After each diet intervention, incremental cycle ergometer tests were performed until 100% of maximal individual intensity. | In young and old subjects, capillary-pH (p ≤ 0.038) and urine-pH (p < 0.001) were higher at rest after a high-vegetable diet compared with a high-protein diet. During cycling, capillary-pH was higher (p ≤ 0.034) after high vegetable compared with high protein at submaximal workloads in young subjects at 75% of maximal oxygen consumption and older subjects. Older subjects may be more sensitive to the diet-induced acid-base changes. |
| Niekamp et al. [70] |
Experimental study N = 47 sedentary men and women (47–63) |
Maximal graded treadmill exercise tests (100% maximal oxygen uptake). Habitual diet was assessed for its long-term effect on systemic acid-base status. |
A more alkaline diet promoted higher respiratory exchange ratio values (1.21 ± 0.01, p ≤ 0.05) than the middle (1.17 ± 0.01) and highest acidic diet (1.15 ± 0.01). There were no significant differences (all p ≥ 0.30) among diets for submaximal exercise intensities of 70%, 80%, or 90% of maximal oxygen consumption. After controlling for age, sex, VO2max, and maximal heart rate, regression analysis demonstrated that 19% of the variability in RER was attributed to renal load diets (r = −0.43, p = 0.001). Alkaline diets were associated with the attainment of higher peak values for respiratory exchange ratio during maximal-intensity exercise testing. |
| Chycki et al. [3] |
Randomized study N = 16 trained sport athletes Two groups: the experimental group (EG; n = 8), which ingested highly alkaline water for three weeks, and the control group (CG; n = 8), which received regular table water |
Anaerobic performance was evaluated by two double 30 s Wingate tests for lower and upper limbs, with a passive rest interval of 3 min between the bouts of exercise. In addition, acid-base equilibrium and electrolyte status were evaluated. Urine samples were evaluated for specific gravity and pH. |
Lactate after the Wingate test was drawn 3 min of recovery and
revealed statistically significant decreases in concentration at rest (from 1.99 mmol/L to 1.30 mmol/L with p = 0.008) and a significant increase in post-exercise concentration (from 19.09 mmol/L to 21.20 mmol/L with p = 0.003) in the experimental group ingesting alkaline water. Additionally, a significant increase in blood pH at rest (from 7.36 to 7.44 with p = 0.001), bicarbonate at rest (from 23.87 to 26.76 with p = 0.001), and post-exercise (from 12.90 to 13.88 with p = 0.002) were observed in the experimental group. The results indicated that drinking alkalized water enhances hydration and improves acid-base balance and anaerobic exercise performance. |
| Baranauskas [29] | N = 323 competitive Lithuanian high-performance athletes |
The actual diet was investigated using the 24 h recall dietary survey method. The potential renal acid load of the diets and net endogenous acid production of athletes were calculated. |
A total of 10.2% of athletes exceed endogenous acid production of 100 mEq
·
day−1, and on average 126.1 ± 32.7 mEq
·
day−1 is associated with lower muscle mass (β −1.2% of body weight,
p
< 0.001) but has no effect on the amount of minerals in the body (β 0.01% of body weight,
p
= 0.073). Overall, 25–30% of Lithuanian high-performance athletes use high-protein diets (2.0–4.8 g · kg−1 · day−1), leading to a dietary acid-base imbalance as well as an excessive production of endogenous acids in the body. |
| Ball et al. [73] |
Experimental study N = 6 Males cycled to exhaustion at a workload equivalent to 95 percent of maximum oxygen uptake on four separate occasions. |
Exercise tests were performed after an overnight fast, and each test was preceded by one of four experimental conditions. Two experimental diets were designed, either to replicate each subject’s own normal diet [mean (SD) daily energy intake (E) = 14.5 (0.8), percent protein (Pro), 37.5 (2.2) percent fat (Fat), and 47.5 (2.1) percent carbohydrate (CHO)], or a low-carbohydrate diet [33.6 (1.3) percent Pro, 64.4 (1.5) percent fat, and 2.2 (0.4) percent CHO]. These diets were prepared and consumed within the department over a 3-day period. |
Exercise time following the low-CHO diet was less than on the normal diet conditions (p < 0.05). Post-exercise blood pH bicarbonate was higher following the ingestion of sodium bicarbonate irrespective of the pre-exercise diet (p < 0.05). Blood lactate concentration was higher 2 min after exercise following the N diet with sodium bicarbonate when compared with the low-CHO diets with either sodium bicarbonate or placebo (p < 0.05). Plasma ammonia accumulation was not significantly different between experimental conditions. Low-CHO diet reduces the capacity to perform high-intensity exercise, but it appears that the metabolic acidosis induced by the low-CHO diet is not the cause of the reduced exercise capacity observed during high-intensity exercise under these conditions. |
| Limmer et al. [71] |
Experimental study N = 11 Recreationally active participants (8 men, 3 women) |
One trial under each individual’s unmodified diet and subsequently two trials following either 4 days of an alkalizing (BASE) or acidizing (ACID) diet. Trials consisted of 400 m runs at intervals of 1 week on a tartan track in a randomized order. |
A 400 m performance time for the BASE trial (65.8 ± 7.2 s) compared with the ACID trial (67.3 ± 7.1 s; p = 0.026). BASE diet blood lactate (BASE: 16.3 ± 2.7; ACID: 14.4 ± 2.1 mmol/L; p = 0.32) and urinary pH (BASE: 7.0 ± 0.7; ACID: 5.5 ± 0.7; p = 0.001) were different. A short-term alkalizing diet may improve 400 m performance time in moderately trained participants. Higher blood lactate concentrations under the alkalizing diet suggest an enhanced blood or muscle buffer capacity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Herms, J. Summatory Effects of Anaerobic Exercise and a ‘Westernized Athletic Diet’ on Gut Dysbiosis and Chronic Low-Grade Metabolic Acidosis. Microorganisms 2024, 12, 1138. https://doi.org/10.3390/microorganisms12061138
Álvarez-Herms J. Summatory Effects of Anaerobic Exercise and a ‘Westernized Athletic Diet’ on Gut Dysbiosis and Chronic Low-Grade Metabolic Acidosis. Microorganisms. 2024; 12(6):1138. https://doi.org/10.3390/microorganisms12061138
Chicago/Turabian StyleÁlvarez-Herms, Jesús. 2024. "Summatory Effects of Anaerobic Exercise and a ‘Westernized Athletic Diet’ on Gut Dysbiosis and Chronic Low-Grade Metabolic Acidosis" Microorganisms 12, no. 6: 1138. https://doi.org/10.3390/microorganisms12061138
APA StyleÁlvarez-Herms, J. (2024). Summatory Effects of Anaerobic Exercise and a ‘Westernized Athletic Diet’ on Gut Dysbiosis and Chronic Low-Grade Metabolic Acidosis. Microorganisms, 12(6), 1138. https://doi.org/10.3390/microorganisms12061138




