Clinico-Virological Outcomes and Mutational Profile of SARS-CoV-2 in Adults Treated with Ribavirin Aerosol for COVID-19 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Clinical and Laboratory Monitoring
2.4. Outcomes
2.5. Sample Collection and Virological Assays
2.6. Statistical Analysis
3. Results
3.1. Clinical Features in the Study Group
3.2. Biochemistry in the Study Group
3.3. Concomitant Treatment
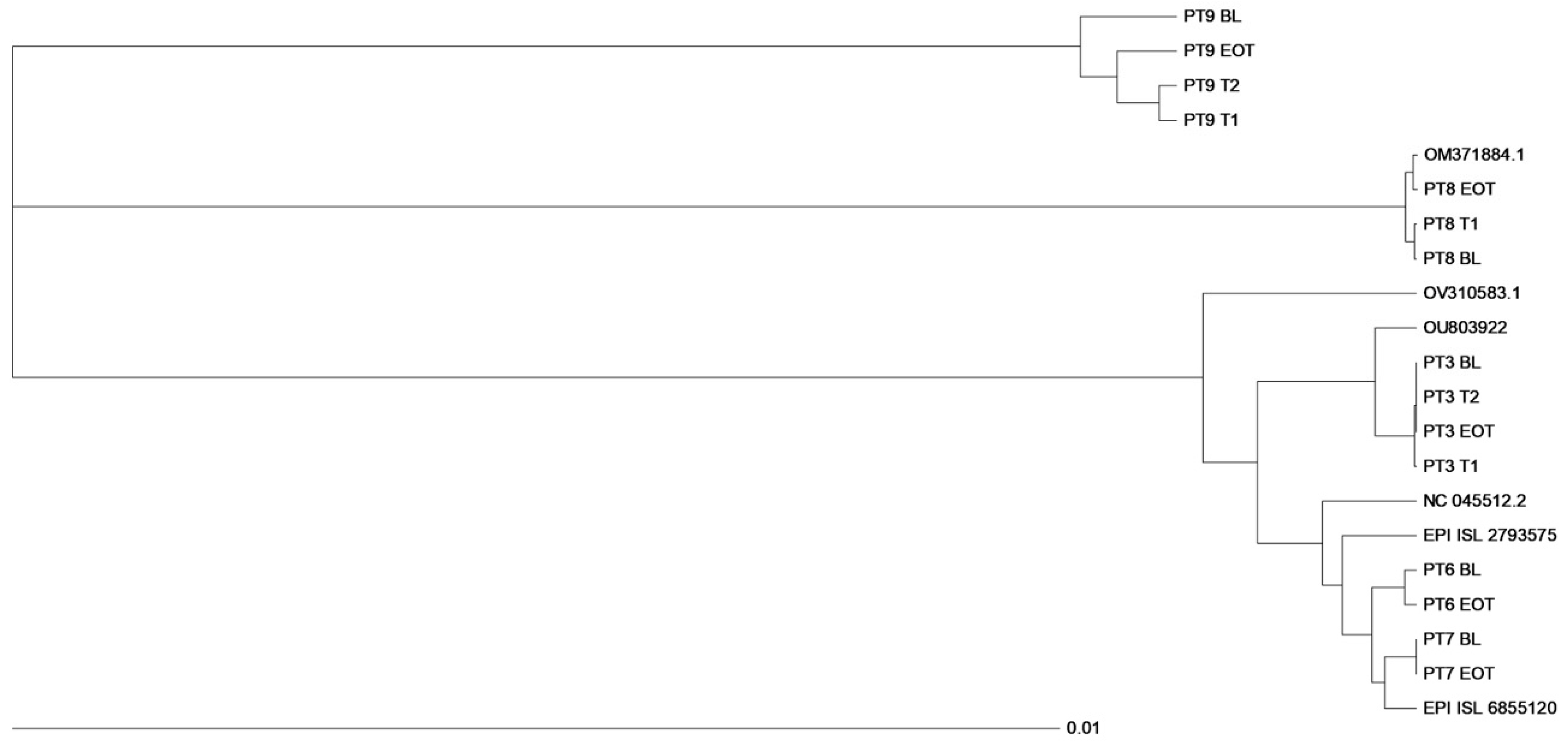
3.4. Virological Findings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; Chu, C.M.; Cheng, V.C.; Chan, K.S.; Hung, I.F.; Poon, L.L.; Law, K.I.; Tang, B.S.; Hon, T.Y.; HKU/UCH SARS Study Group; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.L.; Fernandez-Larsson, R. Molecular mechanisms of action of ribavirin. Rev. Infect. Dis. 1990, 12, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; McClung, H.W.; Wilson, S.Z.; Waters, B.K.; Quarles, J.M.; Cameron, R.W.; Greggs, S.E.; Zerwas, J.M.; Couch, R.B. Ribavirin small-particle aerosol treatment of influenza. Lancet 1981, 2, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.Z.; Gilbert, B.E.; Quarles, J.M.; Knight, V.; McClung, H.W.; Moore, R.V.; Couch, R.B. Treatment of influenza A (H1N1) virus infection with ribavirin aerosol. Antimicrob. Agents Chemother. 1984, 26, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its patho- physiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Messina, E.; Danise, A.; Ferrari, G.; Andolina, A.; Chiurlo, M.; Razanakolona, M.; Barakat, M.; Israel, R.J.; Castagna, A. Ribavirin aerosol in the treatment of SARS-CoV-2: A case series. Infect. Dis. Ther. 2021, 10, 2791–2804. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. 2007, 3, 218–225. [Google Scholar]
- Crotty, S.; Cameron, C.E.; Andino, R. RNA virus error catastrophe: Direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. USA 2001, 98, 6895–6900. [Google Scholar] [CrossRef]
- Hadj Hassine, I.; Ben M’hadheb, M.; Menéndez-Arias, L. Lethal mutagenesis of RNA viruses and approved drugs with antiviral mutagenic activity. Viruses 2022, 14, 841. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.M.; González-Candelas, F.; Moya, A.; Sanjuán, R. Effect of ribavirin on the mutation rate and spectrum of hepatitis C virus in vivo. J. Virol. 2009, 83, 5760–5764. [Google Scholar] [CrossRef]
- Thakur, S.; Sasi, S.; Pillai, S.G.; Nag, A.; Shukla, D.; Singhal, R.; Phalke, S.; Velu, G.S.K. SARS-CoV-2 mutations and their impact on diagnostics, therapeutics and vaccines. Front. Med. 2022, 9, 815389. [Google Scholar] [CrossRef]
- Heinen, N.; Meister, T.L.; Klöhn, M.; Steinmann, E.; Todt, D.; Pfaender, S. Antiviral Effect of Budesonide against SARS-CoV-2. Viruses 2021, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z. Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 2023, 257, 115503. [Google Scholar] [CrossRef]
- Revel, M.P.; Parkar, A.P.; Prosch, H.; Silva, M.; Sverzellati, N.; Gleeson, F.; Brady, A.; European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department—Advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur. Radiol. 2020, 30, 4903–4909. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; GS-US-540-5774 Investigators; et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Lai, A.; Bergna, A.; Della Ventura, C.; Menzo, S.; Bruzzone, B.; Sagradi, F.; Ceccherini-Silberstein, F.; Weisz, A.; Clementi, N.; Sars-CoV-Italian Research Enterprise-Scire Collaborative Group; et al. Epidemiological and clinical features of SARS-CoV-2 variants circulating between April-December 2021 in Italy. Viruses 2022, 14, 2508. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.R.; Rennick, L.J.; Nambulli, S.; Robinson-McCarthy, L.R.; Bain, W.G.; Haidar, G.; Duprex, W.P. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 2021, 371, 1139–1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrés, C.; González-Sánchez, A.; Jiménez, M.; Márquez-Algaba, E.; Piñana, M.; Fernández-Naval, C.; Esperalba, J.; Saubi, N.; Quer, J.; Rando-Segura, A.; et al. Emergence of Delta and Omicron variants carrying resistance-associated mutations in immunocompromised patients undergoing sotrovimab treatment with long-term viral excretion. Clin. Microbiol. Infect. 2023, 29, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhaori, G. Antiviral treatment of SARS: Can we draw any conclusions? CMAJ 2003, 169, 1165–1166. [Google Scholar]
- Unal, M.A.; Bitirim, C.V.; Summak, G.Y.; Bereketoglu, S.; Cevher Zeytin, I.; Besbinar, O.; Gurcan, C.; Aydos, D.; Goksoy, E.; Kocakaya, E.; et al. Ribavirin shows antiviral activity against SARS-CoV-2 and downregulates the activity of TMPRSS2 and the expression of ACE2 in vitro. Can. J. Physiol. Pharmacol. 2021, 99, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Somovilla, P.; García-Crespo, C.; Martínez-González, B.; Soria, M.E.; de Ávila, A.I.; Gallego, I.; Mínguez, P.; Durán-Pastor, A.; Ferrer-Orta, C.; Salar-Vidal, L.; et al. Atypical mutational spectrum of SARS-CoV-2 replicating in the presence of ribavirin. Antimicrob. Agents Chemother. 2023, 67, e0131522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L.V. Overview of Targets and Potential Drugs of SARS-CoV-2 according to the viral replication. J. Proteome Res. 2021, 20, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L.; Day, C.W.; Bailey, K.; Heiner, M.; Montgomery, R.; Lauridsen, L.; Winslow, S.; Hoopes, J.; Li, J.K.; Lee, J.; et al. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral. Res. 2006, 71, 53–63. [Google Scholar] [CrossRef]
- Gong, W.J.; Zhou, T.; Wu, S.L.; Ye, J.L.; Xu, J.Q.; Zeng, F.; Su, Y.Y.; Han, Y.; Lv, Y.N.; Zhang, Y.; et al. A retrospective analysis of clinical efficacy of ribavirin in adults hospitalized with severe COVID-19. J. Infect. Chemother. 2021, 27, 876–881. [Google Scholar] [CrossRef]
- Tong, S.; Su, Y.; Yu, Y.; Wu, C.; Chen, J.; Wang, S.; Jiang, J. Ribavirin therapy for severe COVID-19: A retrospective cohort study. Int. J. Antimicrob. Agents 2020, 56, 106114. [Google Scholar] [CrossRef]
- Hung, I.F.; Lung, K.C.; Tso, E.Y.; Liu, R.; Chung, T.W.; Chu, M.Y.; Ng, Y.Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Poulakou, G.; Barakat, M.; Israel, R.J.; Bacci, M.R.; Virazole Collaborator Group for COVID-19 Respiratory Distress. Ribavirin aerosol in hospitalized adults with respiratory distress and COVID-19: An open-label trial. Clin. Transl. Sci. 2023, 16, 165–174. [Google Scholar] [CrossRef]
- Xu, Y.; Li, M.; Zhou, L.; Liu, D.; He, W.; Liang, W.; Sun, Q.; Sun, H.; Li, Y.; Liu, X. Ribavirin treatment for critically ill COVID-19 patients: An observational study. Infect. Drug Resist. 2021, 14, 5287–5291. [Google Scholar] [CrossRef]
- Lee, N.; Allen Chan, K.C.; Hui, D.S.; Ng, E.K.; Wu, A.; Chiu, R.W.; Wong, V.W.; Chan, P.K.; Wong, K.T.; Wong, E.; et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004, 31, 304–309. [Google Scholar] [CrossRef]
- Meng, M.; Chu, Y.; Zhang, S.; Li, X.; Sha, J.; Wang, P.; Cui, Y.; Han, M.; Dong, X.; Sun, W.; et al. Corticosteroid treatment in severe patients with SARS-CoV-2 and chronic HBV co-infection: A retrospective multicenter study. BMC Infect. Dis. 2022, 22, 891. [Google Scholar] [CrossRef]
- Lai, K.L.; Hu, F.C.; Wen, F.Y.; Chen, J.J. Lymphocyte count is a universal predictor of health outcomes in COVID-19 patients before mass vaccination: A meta-analytical study. J. Glob. Health 2022, 12, 05041. [Google Scholar] [CrossRef]
- Eslami, G.; Mousaviasl, S.; Radmanesh, E.; Jelvay, S.; Bitaraf, S.; Simmons, B.; Wentzel, H.; Hill, A.; Sadeghi, A.; Freeman, J.; et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J. Antimicrob. Chemother. 2020, 75, 3366–3372. [Google Scholar] [CrossRef]
- Couroux, P.; Brkovic, A.; Vittitow, J.L.; Israel, R.J.; Pamidi, C.; Patel, J.; Barakat, M. A randomized, placebo-controlled study to evaluate safety and pharmacokinetics of inhaled ribavirin. Clin. Transl. Sci 2022, 15, 2159–2171. [Google Scholar] [CrossRef]
- Morgenstern, B.; Michaelis, M.; Baer, P.C.; Doerr, H.W.; Cinatl, J., Jr. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005, 326, 905–908. [Google Scholar] [CrossRef]
- Nirmalarajah, K.; Yim, W.; Aftanas, P.; Li, A.X.; Shigayeva, A.; Yip, L.; Zhong, Z.; McGeer, A.J.; Maguire, F.; Mubareka, S.; et al. Use of whole genome sequencing to identify low-frequency mutations in SARS-CoV-2 patients treated with remdesivir. Influenza Other Respir. Viruses 2023, 17, e13179. [Google Scholar] [CrossRef]
- Moeller, N.H.; Shi, K.; Demir, Ö.; Belica, C.; Banerjee, S.; Yin, L.; Durfee, C.; Amaro, R.E.; Aihara, H. Structure and dynamics of SARS-CoV-2 proofreading exoribonuclease ExoN. Proc. Natl. Acad. Sci. USA 2022, 119, e2106379119. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Popa, H.; Stapon, A.; Bouda, E.; Garcia-Diaz, M. Fidelity of ribonucleotide incorporation by the SARS-CoV-2 replication complex. J. Mol. Biol. 2023, 435, 167973. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Abe, H.; Yasuda, J. Comparison of genome replication fidelity between SARS-CoV-2 and influenza A virus in cell culture. Sci. Rep. 2023, 13, 13105. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Aliota, M.T.; Bonnac, L.F. Broad-spectrum antiviral strategies and nucleoside analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Kosakovsky Pond, S.L.; Ioannidis, J.P.A.; Shafer, R.W. Susceptibility of SARS-CoV-2 Omicron variants to therapeutic monoclonal antibodies: Systematic review and meta-analysis. Microbiol. Spectr. 2022, 10, e0092622. [Google Scholar] [CrossRef]
- Bager, P.; Wohlfahrt, J.; Bhatt, S.; Stegger, M.; Legarth, R.; Møller, C.H.; Skov, R.L.; Valentiner-Branth, P.; Voldstedlund, M.; Fischer, T.K.; et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: An observational cohort study. Lancet Infect. Dis. 2022, 22, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the clinical course of COVID-19 in a retrospective analysis of a large real-world database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef]
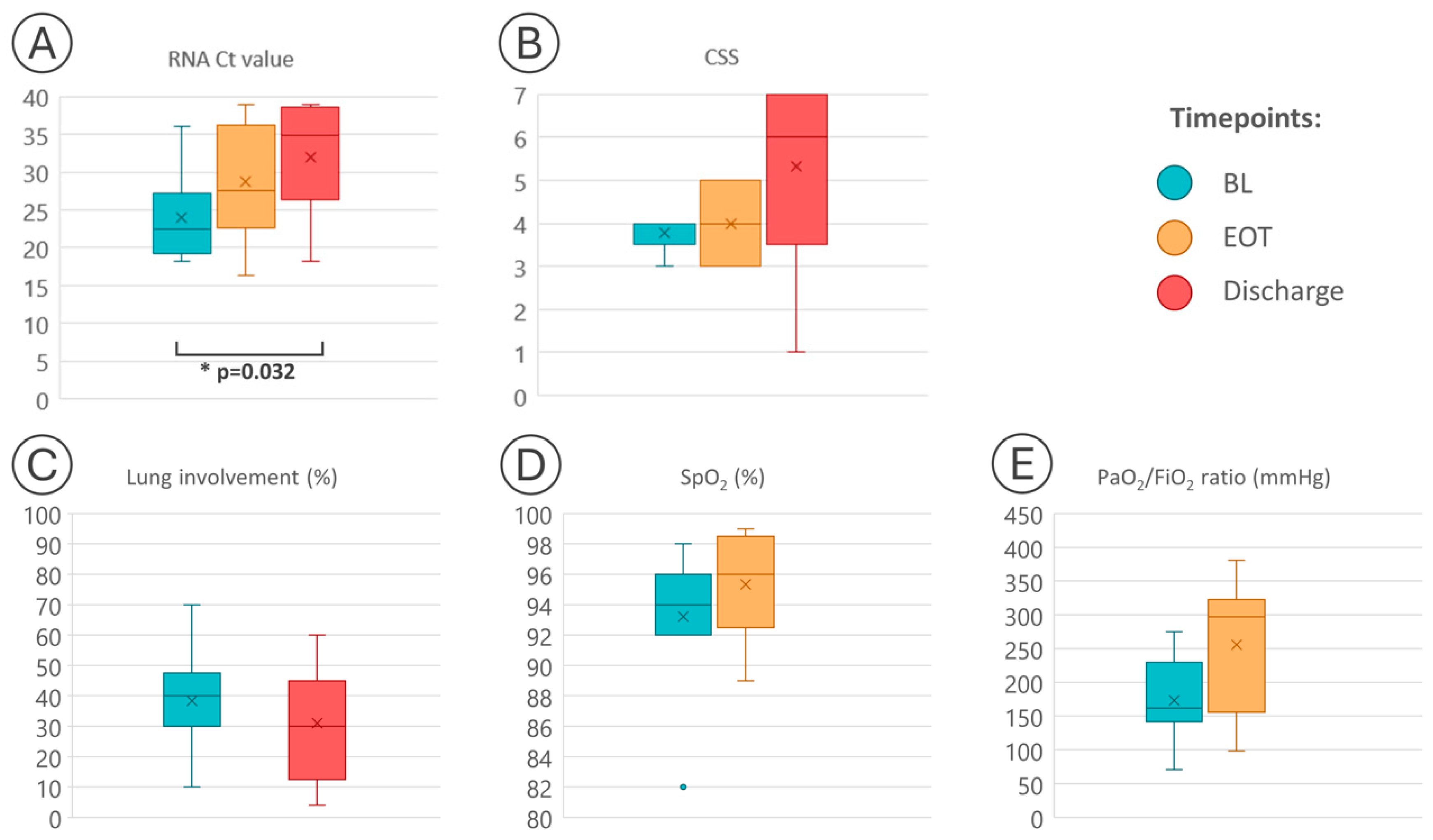
| PT | Age (yrs) | Sex | BL | EOT | Discharge | Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA Ct | Lung Involvement (%) | CSS | SpO2 (%) | PaO2/FiO2 Ratio (mmHg) | RNA Ct Value | CSS | SpO2 (%) | PaO2/FiO2 Ratio (mmHg) | RNA Ct Value | Lung Involvement (%) | CSS | ||||
| 1 | 66 | M | 22.40 | 40 | 4 | 96 | 275 | 34.85 | 5 | 98 | 335 | 38.00 | 10 | 7 | No |
| 2 | 73 | M | 29.29 | 10 | 4 | 94 | 145 | 39.00 1 | 5 | 94 | 300 | 37.44 | 4 | 7 | No |
| 3 | 52 | F | 19.84 | 70 | 4 | 92 | 232 | 24.48 | 5 | 89 | 267 | 27.92 | 40 | 6 | No |
| 4 | 53 | M | 36.09 | 45 | 4 | 96 | 143 | 33.79 | 5 | 95 | 381 | 39.00 1 | 15 | 7 | No |
| 5 | 51 | M | 24.94 | 30 | 4 | 93 | 163 | 21.21 | 3 | 96 | 102 | 34.78 | 40 | 6 | No |
| 6 | 34 | M | 25.07 | 30 | 3 | 98 | 162 | 37.45 | 3 | 99 | 210 | 27.52 | 30 | 6 | No |
| 7 | 64 | M | 18.59 | 30 | 3 | 92 | 71 | 27.52 | 3 | 99 | 311 | 39.00 1 | 30 | 7 | No |
| 8 | 54 | M | 21.82 | 40 | 4 | 96 | 228 | 16.30 | 3 | 91 | 98 | 18.24 | 60 | 1 | Yes |
| 9 | 31 | M | 18.13 | 50 | 4 | 82 | 141 | 24.05 | 4 | 97 | 297 | 25.35 | 50 | 1 | Yes |
| Q2 | 53 | NA | 22.40 | 40 | 4 | 94 | 162 | 26.00 | 4 | 96.5 | 297 | 27.92 | 30 | 6 | NA |
| Q1 | 51 | NA | 19.84 | 30 | 4 | 92 | 143 | 23.34 | 3 | 94.75 | 210 | 26.43 | 15 | 6 | NA |
| Q3 | 64 | NA | 25.07 | 45 | 4 | 96 | 228 | 34.06 | 5 | 98.25 | 311 | 36.11 | 40 | 7 | NA |
| p-value | - | - | 0.405 2 | 0.110 2 | 0.032 3 | 0.875 2 | 0.091 3 | ||||||||
| PT | Baseline Date | WBCs (×109/L) | Total Lymphocytes (×109/L) | Ferritin (ng/mL) | IL-6 (pg/mL) | Lactate Dehydrogenase (U/L) | C-Reactive Protein (mg/L) | Fibrinogen (ng/dL) | D-Dimer (µg/mL) | Date of Discharge | WBC (×109/L) | Total Lymphocytes ×109/L) | Ferritin (ng/mL) | IL-6 (pg/mL) | Lactate Dehydrogenase (U/L) | C-Reactive Protein (mg/L) | Fibrinogen (ng/dL) | D-Dimer (µg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24/03/2021 | 7.5 | 0.7 | 2266 | 42.7 | 544 | 84.2 | 723 | 0.75 | 31/03/2021 | 4.9 | 1.5 | 1484 | 8 | 250 | 1.1 | 477 | 0.96 |
| 2 | 15/04/2021 | 8.1 | 1.4 | 1719 | 9.7 | 386 | 51.4 | 597 | 2.61 | 23/04/2021 | 8.6 | 2.4 | 700 | 4.2 | 207 | 1.4 | 360 | 0.52 |
| 3 | 04/06/2021 | 6.4 | 2.3 | 497 | 20.4 | 176 | 17.4 | 634 | 1.11 | 17/06/2021 | 5.6 | 2.4 | 200 | 5.9 | 216 | 2.9 | 481 | 0.63 |
| 4 | 10/09/2021 | 7 | 1.7 | 3259 | 14.7 | 354 | 19.1 | 491 | 0.56 | 24/09/2021 | 6.7 | 1.8 | 806 | 2.7 | 215 | 1.3 | 437 | 0.27 |
| 5 | 27/10/2021 | 2 | 0.6 | 11,685 | 10 | 1187 | 12.7 | 529 | 0.66 | 11/11/2021 | 6.4 | 1.9 | 1272 | 347 | 267 | 0.2 | 333 | 0.27 |
| 6 | 20/11/2021 | 5.4 | 1 | 1038 | 13.1 | 391 | 30.2 | 645 | 0.58 | 03/12/2021 | 9.5 | 3.3 | 637 | 15.9 | 239 | 0.6 | 271 | 0.27 |
| 7 | 24/11/2021 | 6.6 | 0.9 | 814 | 2.4 | 525 | 77.5 | 644 | 0.78 | 04/12/2021 | 7.1 | 2.2 | 471 | 108 | 307 | 0.6 | 224 | 0.27 |
| 8 | 12/05/2022 | 9.9 | 0.1 | 3391 | 15.9 | 405 | 8.7 | 653 | 0.3 | 02/06/2022 | 10.8 | 0.1 | 32,388 | 15.9 | 900 | 146.1 | 736 | 4.41 |
| 9 | 11/08/2022 | 3.3 | 0.2 | 6448 | 10.8 | 831 | 30.4 | 313 | 1.83 | 27/08/2022 | 3.2 | 0.2 | 5834 | 30.2 | 598 | 332.3 | 532 | 2.82 |
| Q2 | 6.6 | 0.9 | 2266 | 13.1 | 405 | 30.2 | 634 | 0.75 | 6.7 | 1.9 | 806 | 15.9 | 250 | 1.3 | 437 | 0.52 | ||
| Q1 | 5.4 | 0.6 | 1038 | 10 | 386 | 17.4 | 529 | 0.58 | 5.6 | 1.5 | 637 | 5.9 | 216 | 0.6 | 333 | 0.27 | ||
| Q3 | 7.5 | 1.4 | 3391 | 15.9 | 544 | 51.4 | 645 | 1.11 | 8.6 | 2.4 | 1484 | 30.2 | 307 | 2.9 | 481 | 0.96 | ||
| p-value (baseline vs. discharge) 1 | 0.173 | 0.012 | 0.110 | 0.906 | 0.109 | 0.515 | 0.066 | 0.515 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsica, G.; Messina, E.; Bagaglio, S.; Galli, L.; Lolatto, R.; Sampaolo, M.; Barakat, M.; Israel, R.J.; Castagna, A.; Clementi, N. Clinico-Virological Outcomes and Mutational Profile of SARS-CoV-2 in Adults Treated with Ribavirin Aerosol for COVID-19 Pneumonia. Microorganisms 2024, 12, 1146. https://doi.org/10.3390/microorganisms12061146
Morsica G, Messina E, Bagaglio S, Galli L, Lolatto R, Sampaolo M, Barakat M, Israel RJ, Castagna A, Clementi N. Clinico-Virological Outcomes and Mutational Profile of SARS-CoV-2 in Adults Treated with Ribavirin Aerosol for COVID-19 Pneumonia. Microorganisms. 2024; 12(6):1146. https://doi.org/10.3390/microorganisms12061146
Chicago/Turabian StyleMorsica, Giulia, Emanuela Messina, Sabrina Bagaglio, Laura Galli, Riccardo Lolatto, Michela Sampaolo, Maxime Barakat, Robert J. Israel, Antonella Castagna, and Nicola Clementi. 2024. "Clinico-Virological Outcomes and Mutational Profile of SARS-CoV-2 in Adults Treated with Ribavirin Aerosol for COVID-19 Pneumonia" Microorganisms 12, no. 6: 1146. https://doi.org/10.3390/microorganisms12061146







