Construction and Validation of Chicken Immune scFv Antibody Library against Helicobacter pylori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Strains and Culture Conditions
2.2. Construction of the scFv Antibody Library
2.3. Expression and Purification of Recombinant HpaA Protein
2.4. scFv Library Panning
2.5. Phage ELISA
2.6. scFv Antibody Expression in P. pastoris and Purification
2.7. Western Blot
2.8. Affinity of scFv A8 Antibody
3. Results
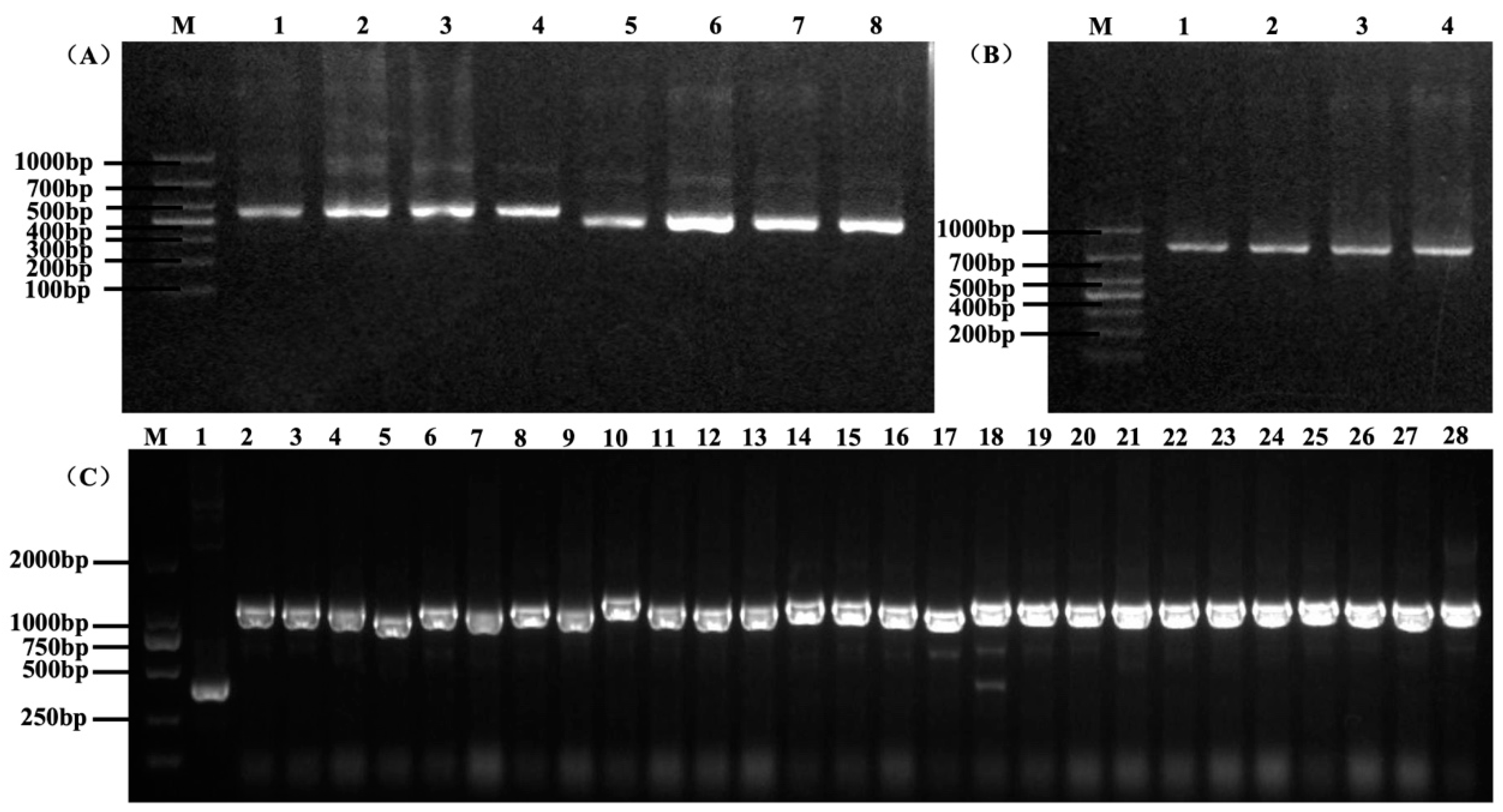
3.1. Construction of scFv Antibody Library
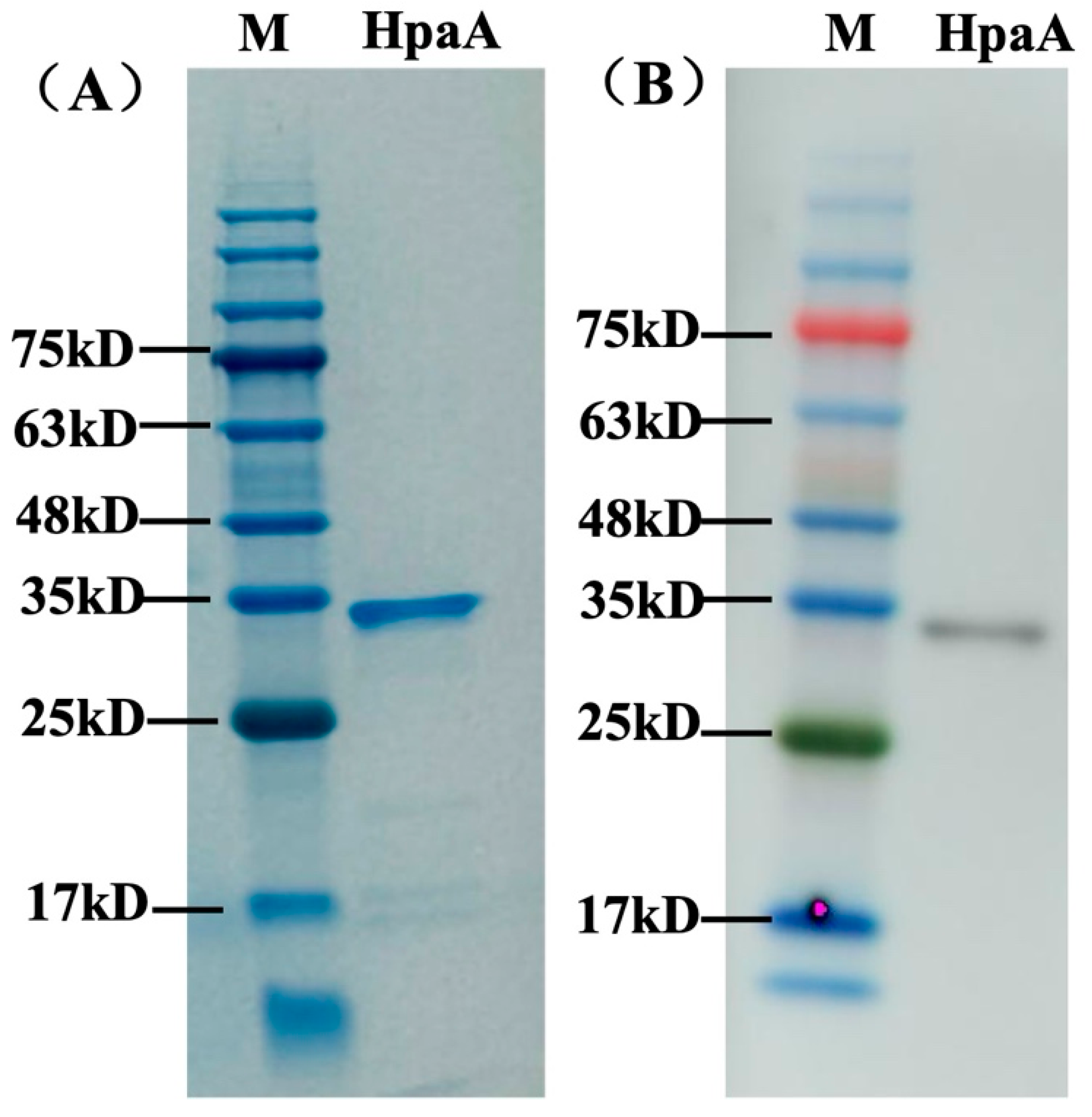
3.2. Expression and Purification of Recombinant HpaA Protein
3.3. Selection of Anti-HpaA scFv Antibodies
3.4. scFv Antibody A8 Expression and Purification
3.5. Binding Activity of scFv Antibody A8
3.6. Cross-Activities of scFv Antibody A8
3.7. scFv Affinity Determination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P.; et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ning, Y.S.; Wang, Y.D.; Luo, J.; Wang, W.; Dong, W.Q.; Li, M. Production of mouse monoclonal antibodies against Helicobacter pylori Catalase and mapping the antigenic epitope by phage display library. Vaccine 2008, 26, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Malekshahi, Z.V.; Gargari, S.L.; Rasooli, I.; Ebrahimizadeh, W. Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microb. Pathog. 2011, 51, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Nam, S.W.; Kim, J.T.; Yoon, J.B.; Bang, W.G.; Roe, I.H. Identification of immunodominant Helicobacter pylori proteins with reactivity to H. pylori-specific egg-yolk immunoglobulin. J. Med. Microbiol. 2003, 52, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Reiche, N.; Jung, A.; Brabletz, T.; Vater, T.; Kirchner, T.; Faller, G. Generation and characterization of human monoclonal scFv antibodies against Helicobacter pylori antigens. Infect. Immun. 2002, 70, 4158–4164. [Google Scholar] [CrossRef]
- Schmausser, B.; Eck, M.; Greiner, A.; Luhrs, H.; Vollmers, H.P.; Muller-Hermelink, H.K. Disparity between mucosal and serum IgA and IgG in Helicobacter pylori infection. Virchows Arch. 2002, 441, 143–147. [Google Scholar] [CrossRef]
- Dias da Silva, W.; Tambourgi, D.V. IgY: A promising antibody for use in immunodiagnostic and in immunotherapy. Vet. Immunol. Immunopathol. 2010, 135, 173–180. [Google Scholar] [CrossRef]
- Spillner, E.; Braren, I.; Greunke, K.; Seismann, H.; Blank, S.; du Plessis, D. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals 2012, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Z.C.; Hu, P.; Ren, H.L.; Li, Y.S.; Zheng, Y.; Wang, C.; Zeng-Shan, L.; Lu, S.Y. Identification of chicken-derived scFv against N-glycolylneuraminic acid retrieved from an immune library by phage display. Protein Expr. Purif. 2021, 182, 105841. [Google Scholar] [CrossRef] [PubMed]
- Solhi, R.; Alebouyeh, M.; Khafri, A.; Rezaeifard, M.; Aminian, M. In vitro evaluation of cross-strain inhibitory effects of IgY polyclonal antibody against H. pylori. Microb. Pathog. 2017, 110, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wu, S.; Guan, L.; Xie, Y.; Yang, X.; Li, Z.; Zhang, Z. Dietary multivalent anti-Helicobacter pylori immunoglobulin Y significantly increase the H. pylori eradication and improve the clinical symptoms in patients. Helicobacter 2021, 26, e12843. [Google Scholar] [CrossRef] [PubMed]
- Haidaris, C.G.; Malone, J.; Sherrill, L.A.; Bliss, J.M.; Gaspari, A.A.; Insel, R.A.; Sullivan, M.A. Recombinant human antibody single chain variable fragments reactive with Candida albicans surface antigens. J. Immunol. Methods 2001, 257, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Mwale, P.F.; Lee, C.H.; Lin, L.T.; Leu, S.J.; Huang, Y.J.; Chiang, L.C.; Mao, Y.C.; Yang, Y.Y. Expression, Purification, and Characterization of Anti-Zika virus Envelope Protein: Polyclonal and Chicken-Derived Single Chain Variable Fragment Antibodies. Int. J. Mol. Sci. 2020, 21, 492. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Liu, J.L.; Li, H.P.; Xing, S.; Xue, S.; Zhang, J.B.; Wang, J.H.; Nölke, G.; Liao, Y.C. Generation of a Highly Reactive Chicken-Derived Single-Chain Variable Fragment against Fusarium verticillioides by Phage Display. Int. J. Mol. Sci. 2012, 13, 7038–7056. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Wang, X.; Li, Y.; Wang, L.; Li, X. Construction and characterization of a highly reactive chicken-derived single-chain variable fragment (scFv) antibody against Staphylococcus aureus developed with the T7 phage display system. Int. Immunopharmacol. 2016, 35, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, F.; Sarhaddi, S.; Fouladi, M.; Samadi, N.; Sadeghi, J.; Golchin, A.; Tohidkia, M.R.; Barar, J.; Omidi, Y. Phage display-derived antibody fragments against conserved regions of VacA toxin of Helicobacter pylori. Appl. Microbiol. Biotechnol. 2018, 102, 6899–6913. [Google Scholar] [CrossRef]
- Hairul Bahara, N.H.; Tye, G.J.; Choong, Y.S.; Ong, E.B.; Ismail, A.; Lim, T.S. Phage display antibodies for diagnostic applications. Biologicals 2013, 41, 209–216. [Google Scholar] [CrossRef]
- Zhai, K.; Gong, Y.; Sun, L.; He, L.; Xue, Z.; Yang, Y.; Fang, M.; Zhang, J. DNA starvation/stationary phase protection protein of Helicobacter pylori as a potential immunodominant antigen for infection detection. Helicobacter 2023, 28, e12955. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Kojoh, K.; Tabata, N.; Doi, N.; Takashima, H.; Miyamoto-Sato, E.; Yanagawa, H. In vitro evolution of single-chain antibodies using mRNA display. Nucleic Acids Res. 2006, 34, e127. [Google Scholar] [CrossRef] [PubMed]
- Valdovino-Navarro, B.J.; Duenas, S.; Flores-Acosta, G.I.; Gasperin-Bulbarela, J.; Bernaldez-Sarabia, J.; Cabanillas-Bernal, O.; Cervantes-Luevano, K.E.; Licea-Navarro, A.F. Neutralizing ability of a single domain vnar antibody: In vitro neutralization of SARS-CoV-2 variants of concern. Int. J. Mol. Sci. 2022, 23, 12267. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, R.; Liu, L.; Feng, S.; Xi, X.; Yu, W.; Gu, Y.; Wang, Y. Identification and characterization of shark VNARs targeting the Helicobacter pylori adhesin HpaA. Artif. Cells Nanomed. Biotechnol. 2023, 51, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.L. Recombinant Monoclonal Antibody Technology. Transfus. Clin. Biol. 2002, 9, 15–22. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Y.; Berglindh, T.; Mellgård, B.; Li, Z.; Mårdh, B.; Mårdh, S. Helicobacter pylori-antigen-binding fragments expressed on the filamentous M13 phage prevent bacterial growth. Biochim. Biophys. Acta 2000, 1474, 107–113. [Google Scholar] [CrossRef]
- Fouladi, M.; Sarhadi, S.; Tohidkia, M.; Fahimi, F.; Samadi, N.; Sadeghi, J.; Barar, J.; Omidi, Y. Selection of a fully human single domain antibody specific to Helicobacter pylori urease. Appl. Microbiol. Biotechnol. 2019, 103, 3407–3420. [Google Scholar] [CrossRef]
- Zhang, G.M.; Chen, Y.P.; Guan, Y.Z.; Wang, Y.; An, Y.Q. Modification and identification of a vector for making a large phage antibody library. Chin. Med. J. 2007, 120, 2011–2016. [Google Scholar] [CrossRef]
- Fan, R.; Han, X.; Xiao, D.; He, L.; Gong, Y.; Sun, L.; Fan, D.; You, Y.; Wang, T.; Yan, X.; et al. Identification of Functional Interactome of Gastric Cancer Cells with Helicobacter pylori Outer Membrane Protein HpaA by HPLC-MS/MS. Biomed. Res. Int. 2020, 2020, 1052926. [Google Scholar] [CrossRef]
- Evans, D.G.; Queiroz, D.M.M.; Mendes, E.N.; Svennerholm, A.M.; Evans, D.J., Jr. Differences Among Helicobacter pylori strains isolated from three different populations and demonstrated by restriction enzyme analysis of an internal fragment of the conserved gene hpaA. Helicobacter 1999, 4, 82–88. [Google Scholar] [CrossRef]
- Flach, C.F.; Svensson, N.; Blomquist, M.; Ekman, A.; Raghavan, S.; Holmgren, J. A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine 2011, 29, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S. Noninvasine diagnostic tests for Helicobacter pylori infection in children. Can. J. Gastroenterol. 2005, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Pourakbari, B.; Mirsalehian, A.; Maleknejad, P.; Mamishi, S.; Azhdarkosh, H.; Daryani, N.E.; Najafi, M.; Kazemi, B.; Paknejad, M.; Mahmoudi, S.; et al. Evaluation of a New Antigen for Diagnosis of Helicobacter pylori Infection in Stool of Adult and Children. Helicobacter 2011, 16, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, T.; Okuda, M.; Hashiguchi, K.; Imamura, I.; Nakayama, A.; Matsuo, M. Evaluation of a Novel Stool Antigen Rapid Test Kit for Detection of Helicobacter pylori Infection. J. Clin. Microbiol. 2019, 57, e01825-18. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Wakasugi, M.; Nakaya, S.; Okada, K.; Mochida, R.; Sato, M.; Kajiyama, H.; Takahashi, R.; Hirata, H.; Ezure, Y.; et al. Production and application of new monoclonal antibodies specific for a fecal Helicobacter pylori antigen. Clin. Diagn. Lab. Immunol. 2002, 9, 75–78. [Google Scholar]



| Gene | Primer |
|---|---|
| hpaA | F-CGCGGATCCATGTGCAGCCCGCATATTATT R-CGCCTCGAGTTATCGGTTTCTTTTGCCTTTTA |
| VH | F-GTCCTCGCAACTGCCCATGGCTGATGGCGGCGTGA R-CCGCCAGAGCCACCTCCACCTGAACCGCCTCCACCGGAGGAGACGATGACTTCGG |
| VL | F-TCAGGTGGAGGTGGCTCTGGCGGAGGCGGATCGGCGCTGACTCAGCCGTCCTCGG R-TGATGGTGGCGGCCGCATTGGGCTG |
| scFv-1 | F-GCCGGCCATGGCTGATGGCGGCCGTGACG R-ATGTGCGGCCGCATTGGGCTGGCCTAGGACGGT |
| scFv-2 | F-GCCGGGAATTCCTGATGGCGGCCGTGACG R-ATGTGCGGCCGCATTGGGCTGGCCTAGGACGGT |
| scFv-3 | F-TGGAATTGTGAGCGGATAACAATT R-GTAAATGAATTTTCTGTATGAGG |
| Round | Input (pfu) | Output (pfu) | Ratio (%) |
|---|---|---|---|
| 1 | 9 × 1012 | 6 × 106 | 6 × 10−7 |
| 2 | 1 × 1012 | 2 × 106 | 2 × 10−6 |
| 3 | 1 × 1012 | 1 × 109 | 1 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Chen, X.; Fan, J.; Sun, L.; He, L.; Wang, H.; Yan, X.; Zhang, J. Construction and Validation of Chicken Immune scFv Antibody Library against Helicobacter pylori. Microorganisms 2024, 12, 1148. https://doi.org/10.3390/microorganisms12061148
Gong Y, Chen X, Fan J, Sun L, He L, Wang H, Yan X, Zhang J. Construction and Validation of Chicken Immune scFv Antibody Library against Helicobacter pylori. Microorganisms. 2024; 12(6):1148. https://doi.org/10.3390/microorganisms12061148
Chicago/Turabian StyleGong, Yanan, Xiaoli Chen, Jiaming Fan, Lu Sun, Lihua He, Hairui Wang, Xiaomei Yan, and Jianzhong Zhang. 2024. "Construction and Validation of Chicken Immune scFv Antibody Library against Helicobacter pylori" Microorganisms 12, no. 6: 1148. https://doi.org/10.3390/microorganisms12061148






