Inhibition Mechanism of Water-Soluble Chitosan–Curdlan Composite Coating on the Postharvest Pathogens of Serratia marcescens and Pseudomonas syringae in Cherry Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coatings
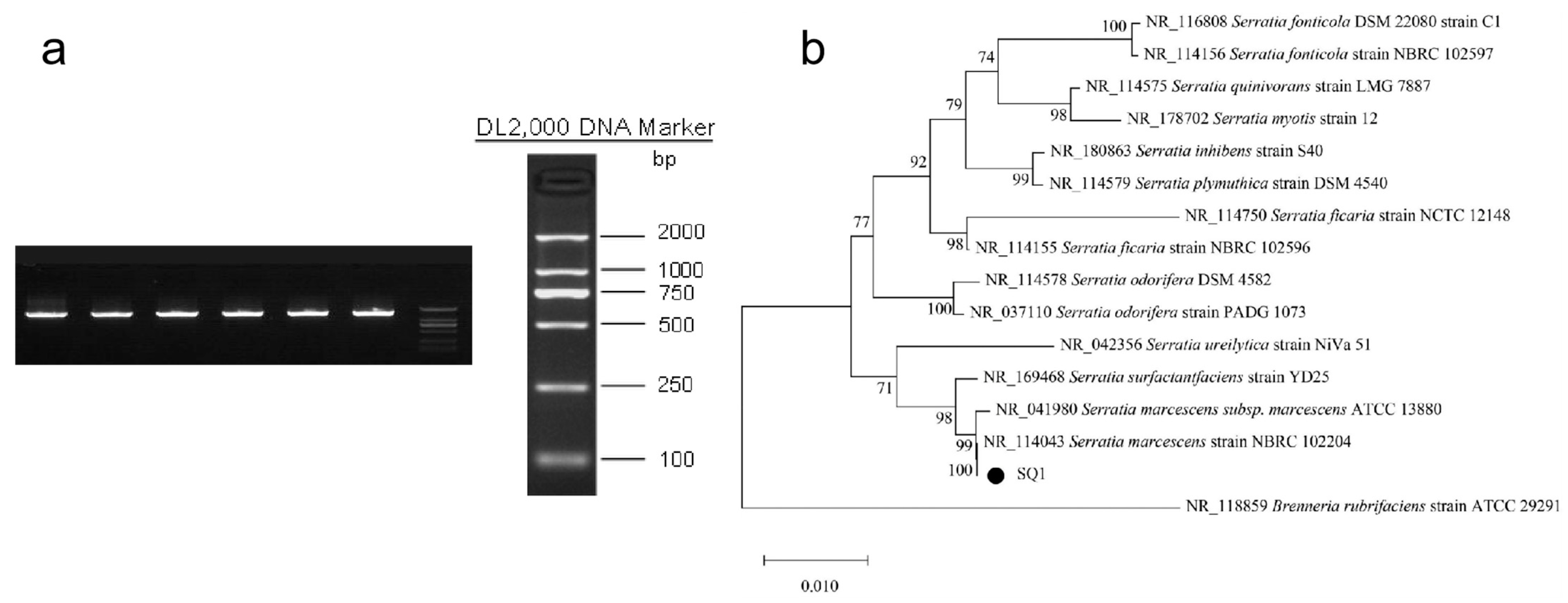
2.3. Isolation of Serratia marcescens
2.4. Cultivation Methods of Serratia marcescens and Pseudomonas syringae
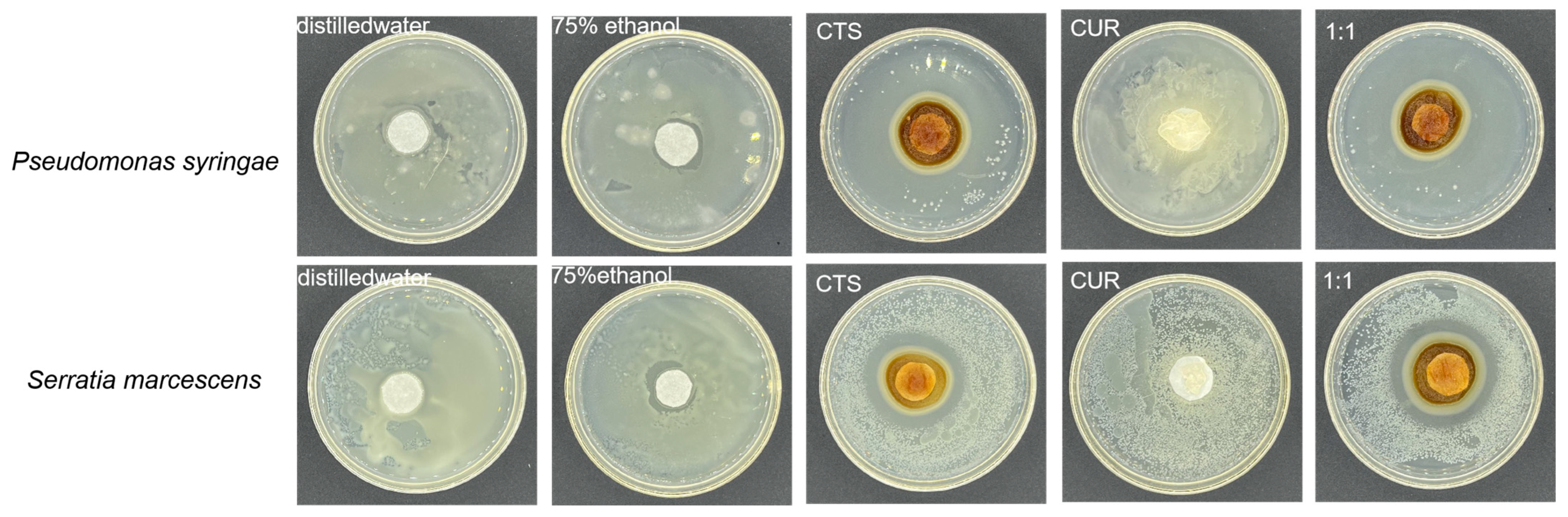
2.5. Inhibition Effect of Composite Coating on Bacteria
2.6. Determination of MIC and MBC of Bacteria Treated with Composite Coating
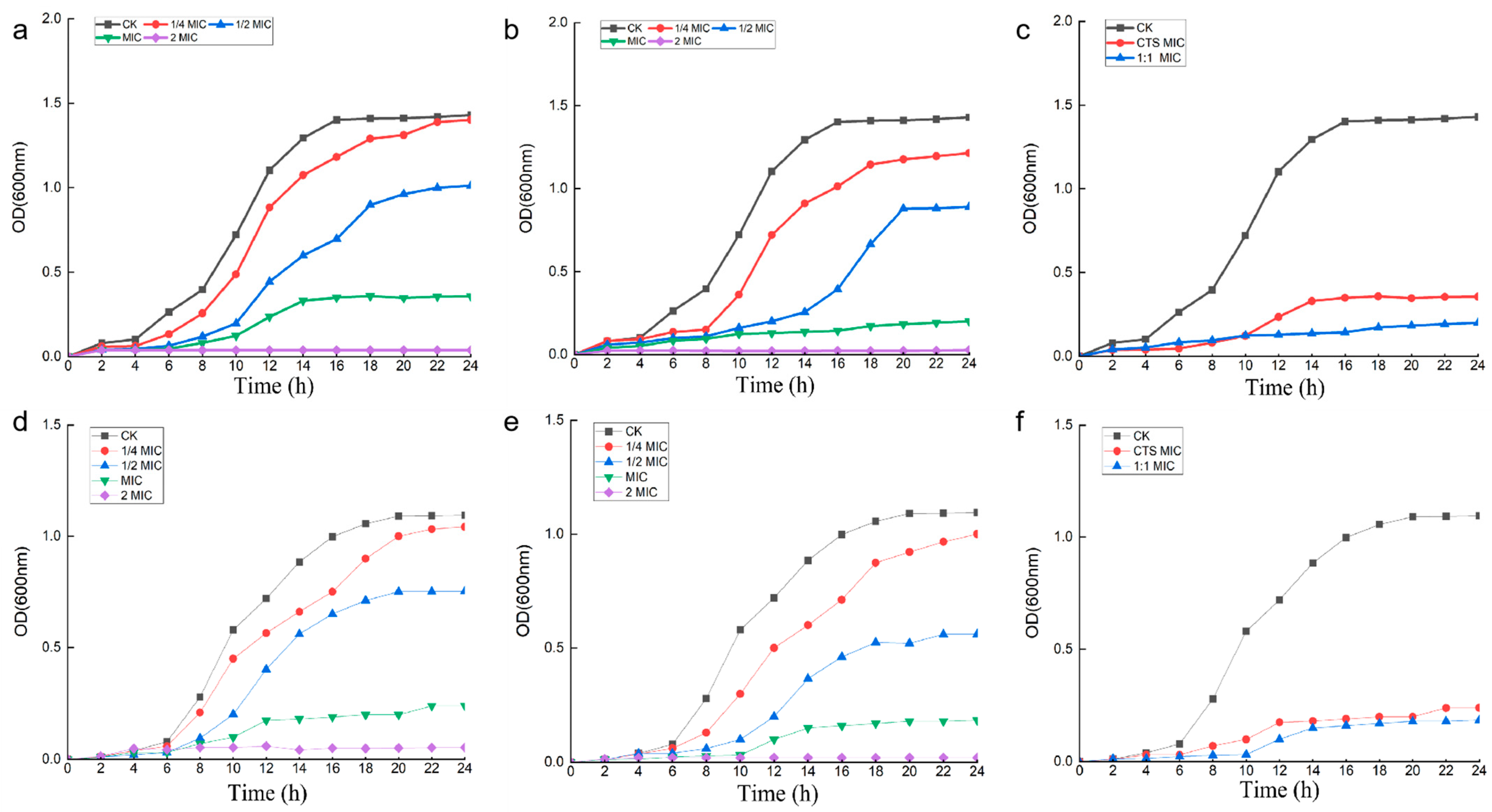
2.7. Effect of Composite Coating on Bacterial Growth Curve
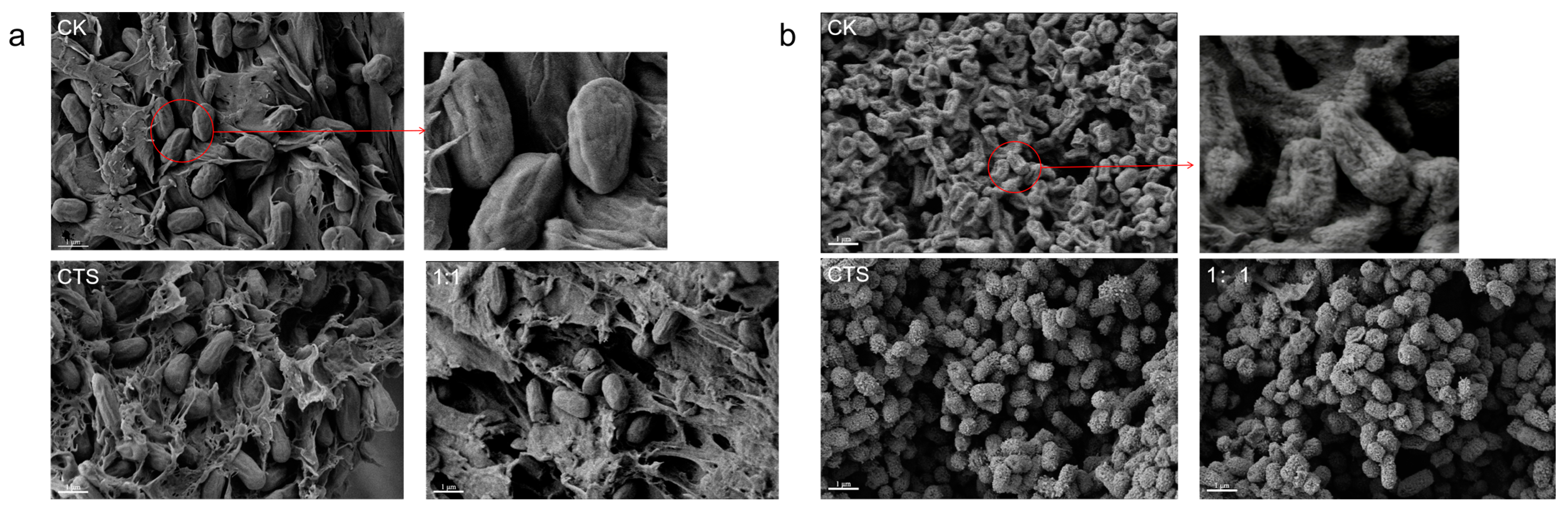
2.8. Cell Morphology by Scanning Electronic Microscopy
2.9. Effect of Composite Coating on Bacterial Cell Membrane Permeability
2.10. Detection of Bacterial Cell Membrane Permeability
2.11. Effect of Composite Coating Solution on Bacterial Cell Leakage
2.12. Bacteriostasis Experiment on Cherry Tomatoes
2.13. Statistical Analysis
3. Results
3.1. Separation Results of Serratia marcescens
3.2. Inhibition Effect of Composite Coating on Bacteria
3.3. Effect of Composite Coating on Bacterial Growth Curve
3.4. Effect of Composite Coating on the Morphology of Serratia marcescens and Pseudomonas syringae
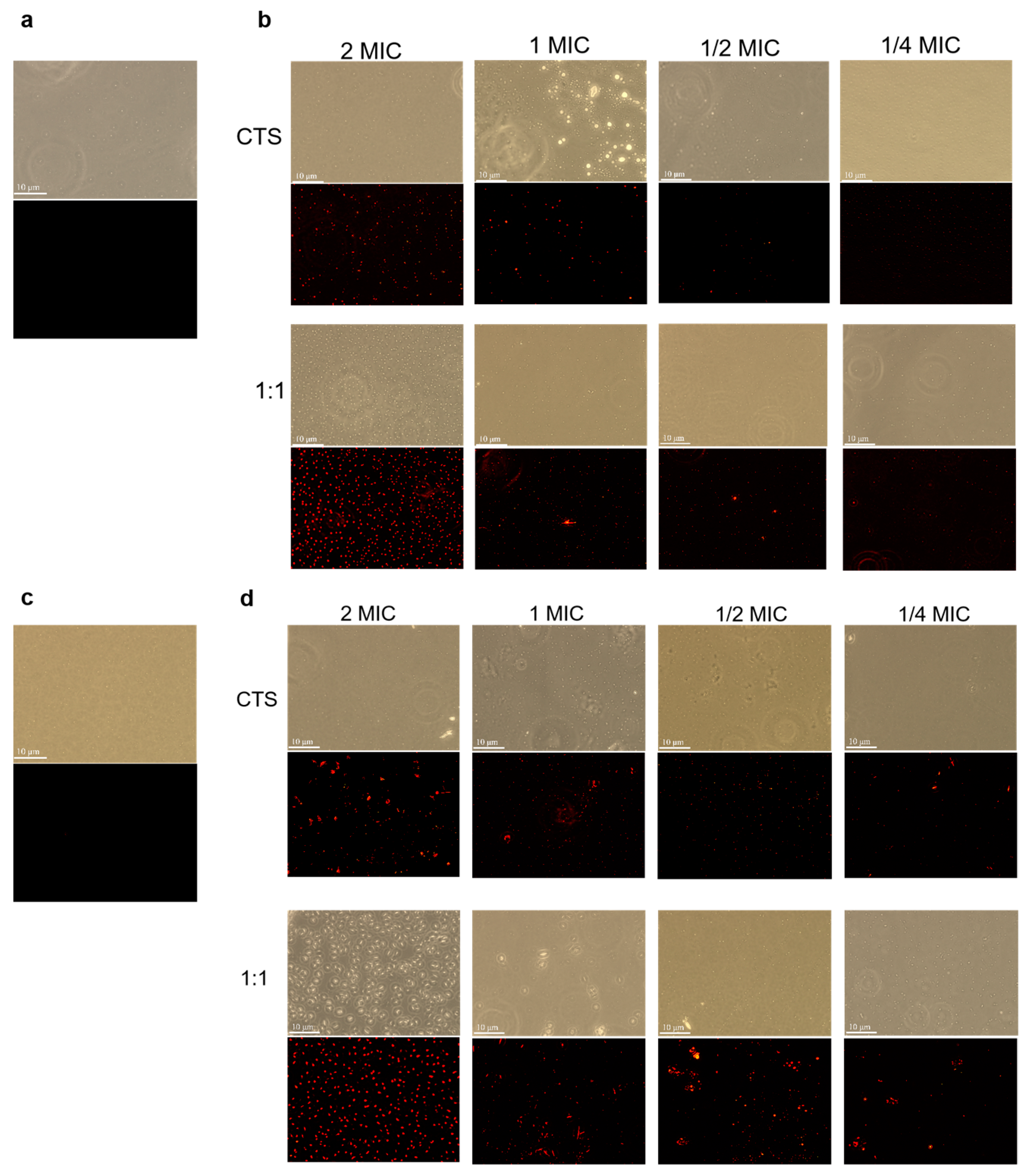
3.5. Effect of Composite Coating on Cell Integrity of Serratia marcescens and Pseudomonas syringae
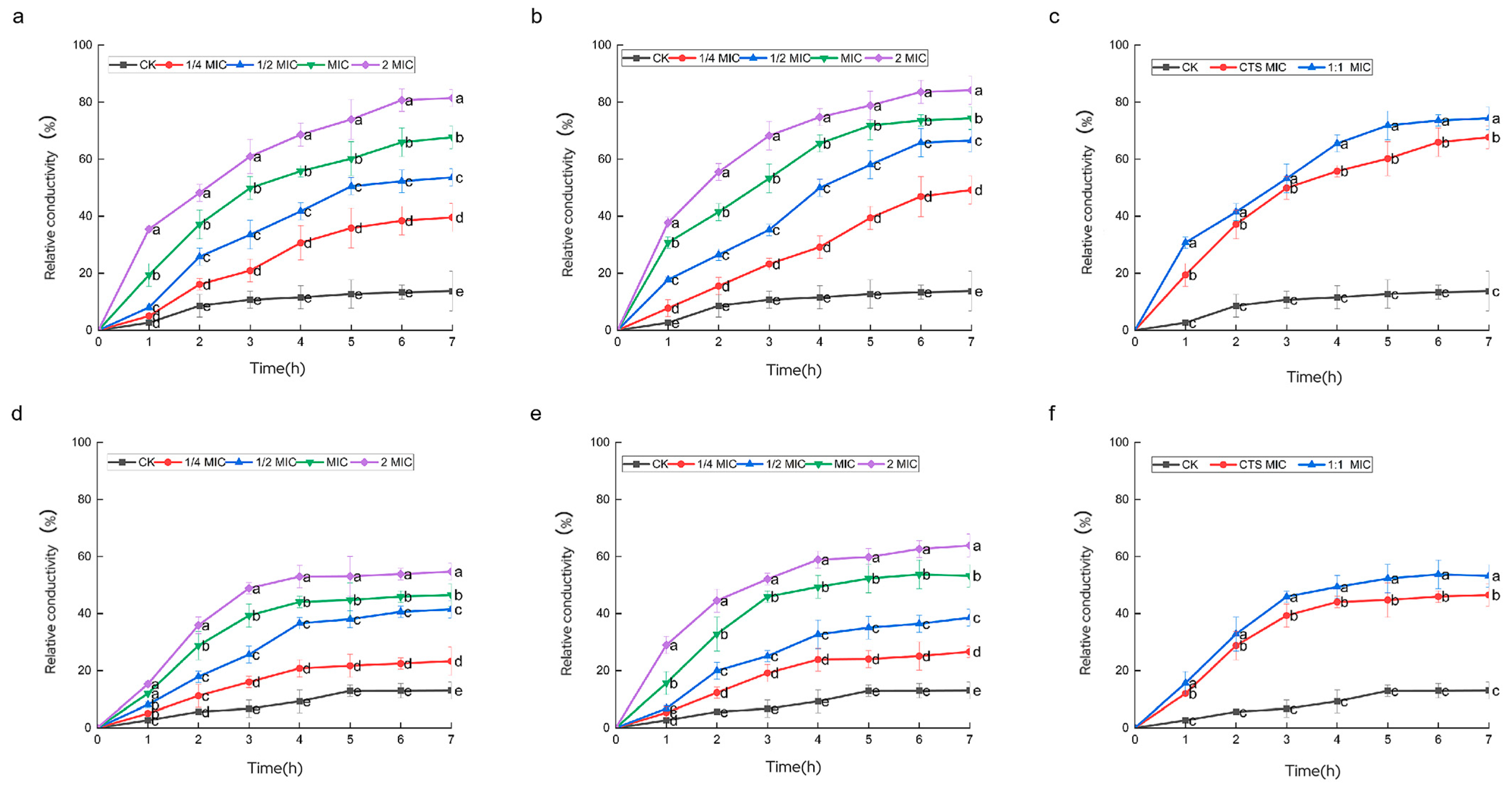
3.6. Effect of Composite Coating on Cell Membrane Permeability of Serratia marcescens and Pseudomonas syringae
3.7. Effect of Composite Coating on the Leakage of Serratia marcescens and Pseudomonas syringae
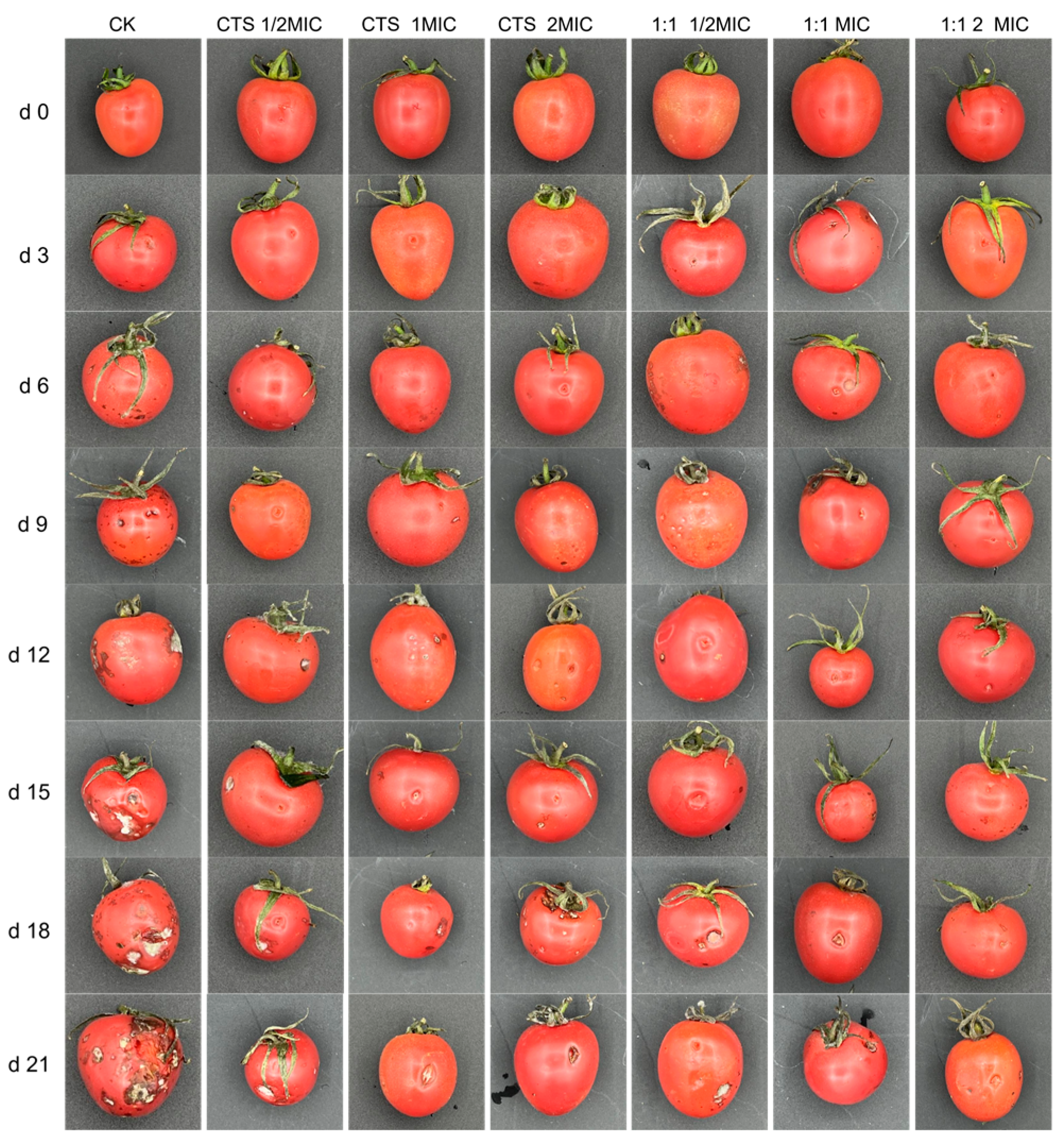
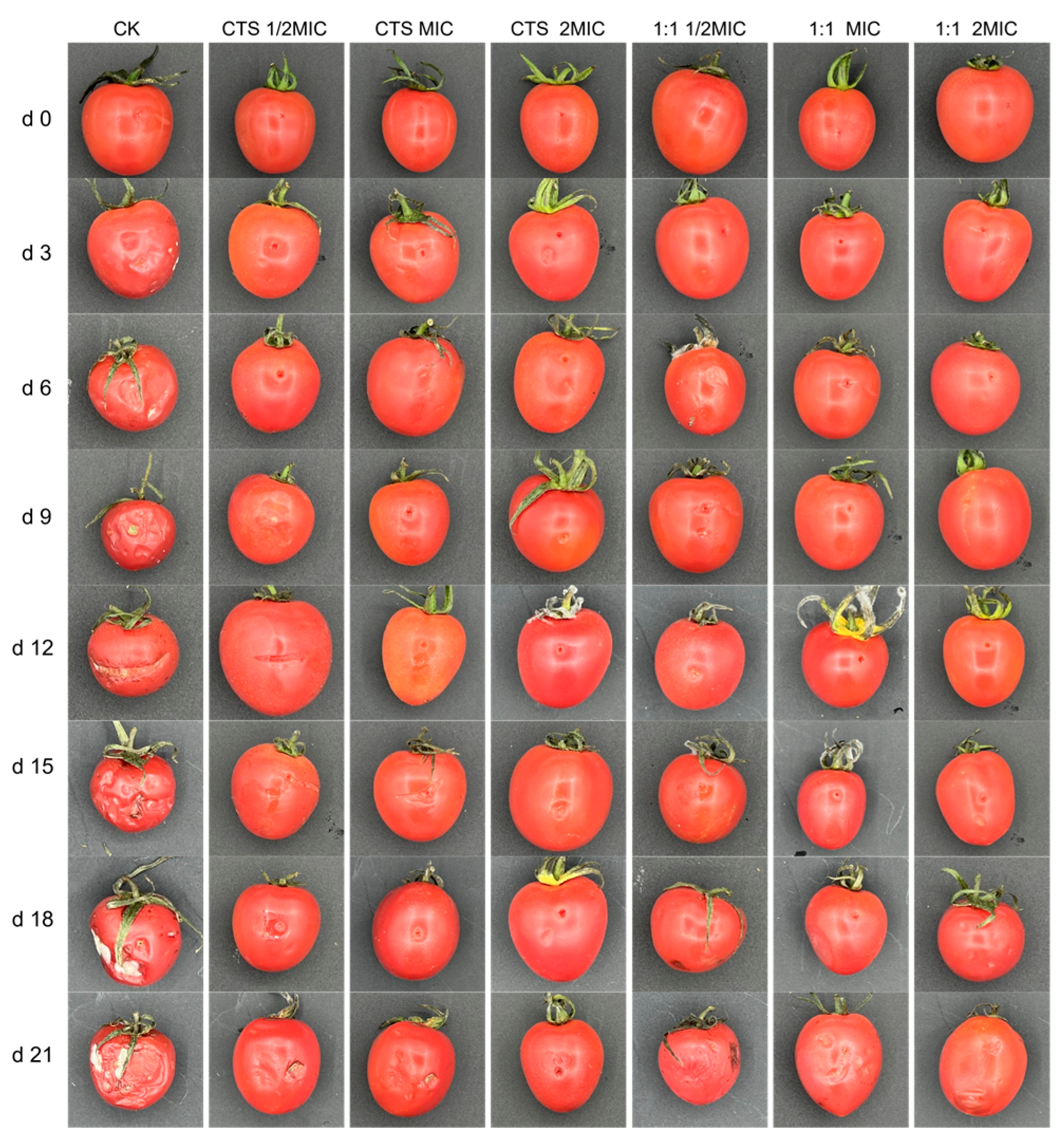
3.8. Bacteriostasis Experiment on Cherry Tomatoes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Gong, L. The Role and Advantages of Rapid Detection Methods in Food Microbiological Safety. Chin. Food Saf. 2023, 36, 160–162. (In Chinese) [Google Scholar]
- Blanca, J.; Montero-Pau, J.; Sauvage, C.; Bauchet, G.; Illa, E.; Díez, M.J.; Francis, D.; Causse, M.; Van der Knaap, E.; Cañizares, J. Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genom. 2015, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Es-sahm, I.; Esserti, S.; Dich, J.; Smaili, A.; Rifai, L.A.; Faize, L.; Koussa, T.; Venisse, J.S.; Benyahia, Y.; Sawadi, N.; et al. Common Bacillus mitigate tomato verticillium wilt and bacterial specks when combined with an essential oil extract. Rhizosphere 2024, 29, 100865. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Hussein, R.M. Evaluation of the antibacterial and antibiofilm activities of sodium benzoate against the soft rot disease of tomato in Iraq. Arch. Phytopathol. Plant Prot. 2022, 55, 636–651. [Google Scholar]
- Huang, K.; Li, H.; Pang, M.; Zou, Y.; Yang, D.; Zhang, W. Serratia marcescens: A key pathogen caused ginger rhizomes soft rot disease. J. Plant Dis. Prot. 2020, 127, 379–391. [Google Scholar] [CrossRef]
- George, A.S.; Cox, C.E.; Desai, P.; Porwollik, S.; Chu, W.; de Moraes, M.H.; McClelland, M.; Brandl, M.T.; Teplitski, M. Interactions of Salmonella enterica Serovar Typhimurium and Pectobacterium carotovorum within a Tomato Soft Rot. Appl. Environ. Microbiol. 2018, 84, e00927-18. [Google Scholar] [CrossRef]
- Khan, M.R.; Di Giuseppe, F.A.; Torrieri, E.; Sadiq, M.B. Recent advances in biopolymeric antioxidant films and coatings for preservation of nutritional quality of minimally processed fruits and vegetables. Food Packag. Shelf Life 2021, 30, 100752. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1912–1935. [Google Scholar] [CrossRef]
- Garcin, M.; Vachard, D. Découverte d’Hermelles fossiles dans le Messiniende San Miguel de Salinas (Annélides du Miocène supérieur du Sud-Est de l’Espagne). Geobios 1987, 20, 407–414. [Google Scholar] [CrossRef]
- Laroche, C.; Michaud, P. New developments and prospective applications for β (1, 3) glucans. Recent Pat. Biotechnol. 2007, 1, 59–73. [Google Scholar] [CrossRef]
- Xiang, F.; Xia, Y.; Wang, Y.; Wang, Y.; Wu, K.; Ni, X. Preparation of konjac glucomannan based films reinforced with nanoparticles and its effect on cherry tomatoes preservation. Food Packag. Shelf Life 2021, 29, 100701. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Zhang, S.; Zhang, L.; Chang, J.; Jing, Z. Characterizations of Water-Soluble Chitosan/Curdlan Edible Coatings and the Inhibitory Effect on Postharvest Pathogenic Fungi. Foods 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Identification, Functional Analysis and Construction of Big Data Analysis Platform of Noncoding RNAs Related to Cannabinoid Biosynthesis. Ph.D. Thesis, Peking Union Medical College, Beijing, China, 2022. (In Chinese). [Google Scholar]
- Aureli, P.; Costantini, A.; Zolea, S. Antimicrobial activity of some plant essential oils against Listeria monocytogenes. J. Food Prot. 1992, 55, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.M.; Markham, J.L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, Y.; Shi, H. Effect of the Ethanol Extract of Oxalis corniculata L on the Growth Curve of Bacterium. North. Hortic. 2007, 3, 113–115. (In Chinese) [Google Scholar]
- Hu, J.; Zhao, X.; Zhu, C.; Hu, F.; Hui, J. Inhibitory Effect and Mechanisms of Sophorolipids against Staphyloccocus aureus. Food Sci. 2012, 33, 33–36. (In Chinese) [Google Scholar]
- Yan, F.; Dang, Q.; Liu, C.; Yan, J.; Wang, T.; Fan, B.; Cha, D.; Li, X.; Liang, S.; Zhang, Z. 3, 6-O-[N-(2-Aminoethyl)-acetamide-yl]-chitosan exerts antibacterial activity by a membrane damage mechanism. Carbohydr. Polym. 2016, 149, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Diao, W.R.; Zhang, L.L.; Feng, S.S.; Xu, J.G. Chemical composition, antibacterial activity, and mechanism of action of the essential oil from Amomum kravanh. J. Food Prot. 2014, 77, 1740–1746. [Google Scholar] [CrossRef]
- Wilson, K.H.; Blitchington, R.B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 1996, 62, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, C.P.; Persing, D.H. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr. Opin. Microbiol. 1999, 2, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, P.; Pot, B.; Gillis, M.; De Vos, P.; Kersters, K.; Swings, J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 1996, 60, 407–438. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Pan, L.; Du, H.; Chen, J.; Zhao, L. ERIC-PCR fingerprinting-based community DNA hybridization to pinpoint genome-specific fragments as molecular markers to identify and track populations common to healthy human guts. J. Microbiol. Methods 2004, 59, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Yan, C.; Shi, C.; Xu, Y.; Ma, Y.; Zhang, C.; Peng, X.; Xia, X. Inhibitory effect of coenzyme Q0 on the growth of Staphylococcus aureus. Foodborne Pathog. Dis. 2019, 16, 317–324. [Google Scholar] [CrossRef]
- He, Y.; Sang, S.; Tang, H.; Ou, C. In vitro mechanism of antibacterial activity of eucalyptus essential oil against specific spoilage organisms in aquatic products. J. Food Process. Preserv. 2022, 46, e16349. [Google Scholar] [CrossRef]
- Zhou, J.W.; Ji, P.C.; Wang, C.Y.; Yang, Y.J.; Zhao, X.Y.; Tang, H.Z.; Tang, S.R. Synergistic effect of propyl gallate and antibiotics against biofilms of Serratia marcescens and Erwinia carotovora in vitro. LWT 2023, 173, 114258. [Google Scholar] [CrossRef]
- Fan, G.; Xiao, Q.; Li, Q.; Xia, Y.; Feng, H.; Ma, X.; Cai, L.; Sun, X. Antimicrobial mechanisms of ZnO nanoparticles to phytopathogen Pseudomonas syringae: Damage of cell envelope, suppression of metabolism, biofilm and motility, and stimulation of stomatal immunity on host plant. Pestic. Biochem. Physiol. 2023, 194, 105455. [Google Scholar] [CrossRef]
- Li, J. Antioxidant Activity, Antibacterial Activity and Mechanism of Action of the Eugenol and Isoeugeno. Master’s Thesis, Shanxi Normal University, Linfen, China, 2017. (In Chinese). [Google Scholar]
- Mumtaz, T. Membranes and Transport Mechanisms. Pak. J. Health Sci. 2022, 2, 2. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, R.; Yue, T.; Gao, Z.; Yuan, Y.; Wang, Z. Application of nanostructures as antimicrobials in the control of foodborne pathogen. Crit. Rev. Food Sci. Nutr. 2022, 62, 3951–3968. [Google Scholar] [CrossRef]
- Shao, S.Y.; Shi, Y.G.; Wu, Y.; Bian, L.Q.; Zhu, Y.J.; Huang, X.Y.; Pan, Y.; Zeng, L.Y.; Zhang, R.R. Lipase-catalyzed synthesis of sucrose monolaurate and its antibacterial property and mode of action against four pathogenic bacteria. Molecules 2018, 23, 1118. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Du, J.; Sun, Y.; Xie, J. Insight into the antibacterial activity and mechanism of chitosan caffeic acid graft against Pseudomonas fluorescens. Int. J. Food Sci. Technol. 2023, 58, 1317–1325. [Google Scholar] [CrossRef]


| Strains | Water-Soluble Chitosan (μg/mL) | Composite Coating (1:1) (μg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Serratia marcescens | 23.438 | 46.875 | 15.000 | 30.000 |
| Pseudomonas syringae | 18.750 | 37.500 | 11.719 | 23.438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Liu, K.; Chang, J.; Jing, Z.; Li, J.; Yu, Y.; Zhang, S. Inhibition Mechanism of Water-Soluble Chitosan–Curdlan Composite Coating on the Postharvest Pathogens of Serratia marcescens and Pseudomonas syringae in Cherry Tomatoes. Microorganisms 2024, 12, 1149. https://doi.org/10.3390/microorganisms12061149
Yan K, Liu K, Chang J, Jing Z, Li J, Yu Y, Zhang S. Inhibition Mechanism of Water-Soluble Chitosan–Curdlan Composite Coating on the Postharvest Pathogens of Serratia marcescens and Pseudomonas syringae in Cherry Tomatoes. Microorganisms. 2024; 12(6):1149. https://doi.org/10.3390/microorganisms12061149
Chicago/Turabian StyleYan, Kejing, Kunyu Liu, Jiaqi Chang, Ziyu Jing, Jiasi Li, Youwei Yu, and Shaoying Zhang. 2024. "Inhibition Mechanism of Water-Soluble Chitosan–Curdlan Composite Coating on the Postharvest Pathogens of Serratia marcescens and Pseudomonas syringae in Cherry Tomatoes" Microorganisms 12, no. 6: 1149. https://doi.org/10.3390/microorganisms12061149





