Development of an Ex Vivo Porcine Eye Model for Exploring the Pathogenicity of Acanthamoeba
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Acanthamoeba Protozoa
2.2. Porcine Eye Pretreatment
2.3. Acanthamoeba Infection
2.4. Slit Lamp Examination
2.5. Histological Evaluation
2.6. Comparison of Acanthamoeba Strain Infections
2.7. Statistical Analysis
3. Results
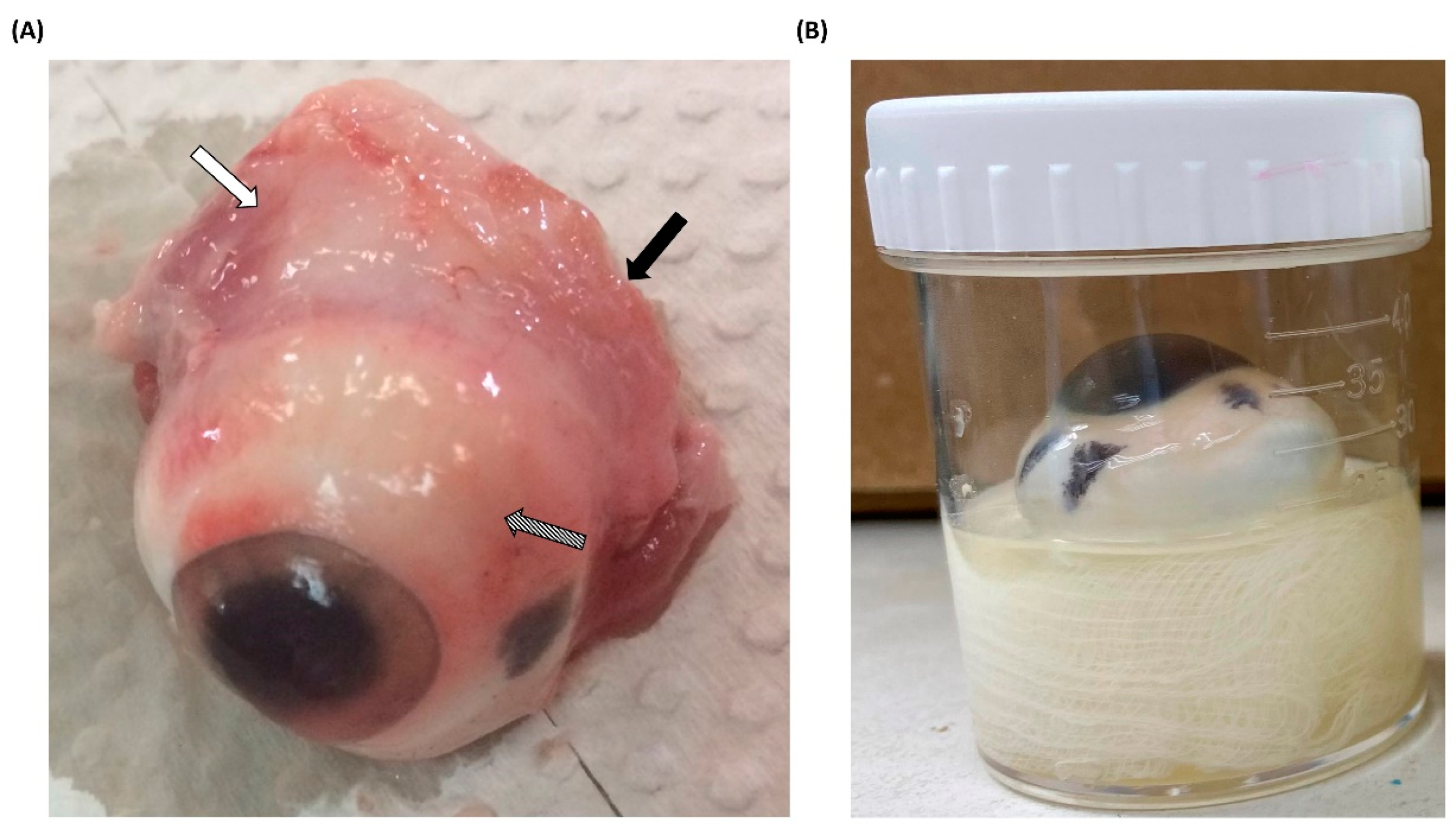
3.1. Processing of Porcine Eyeballs as an Ex Vivo Model for Acanthamoeba Infection
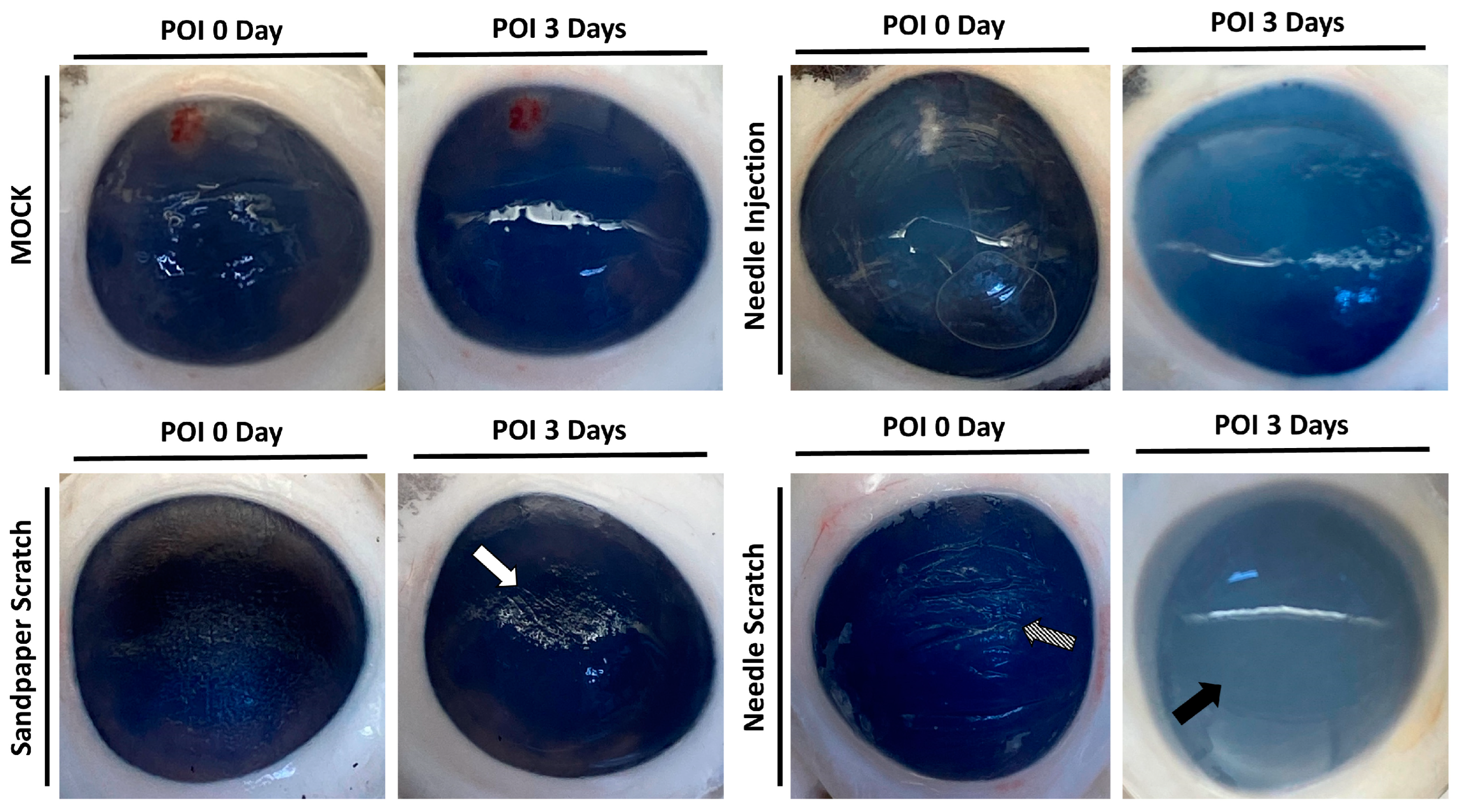
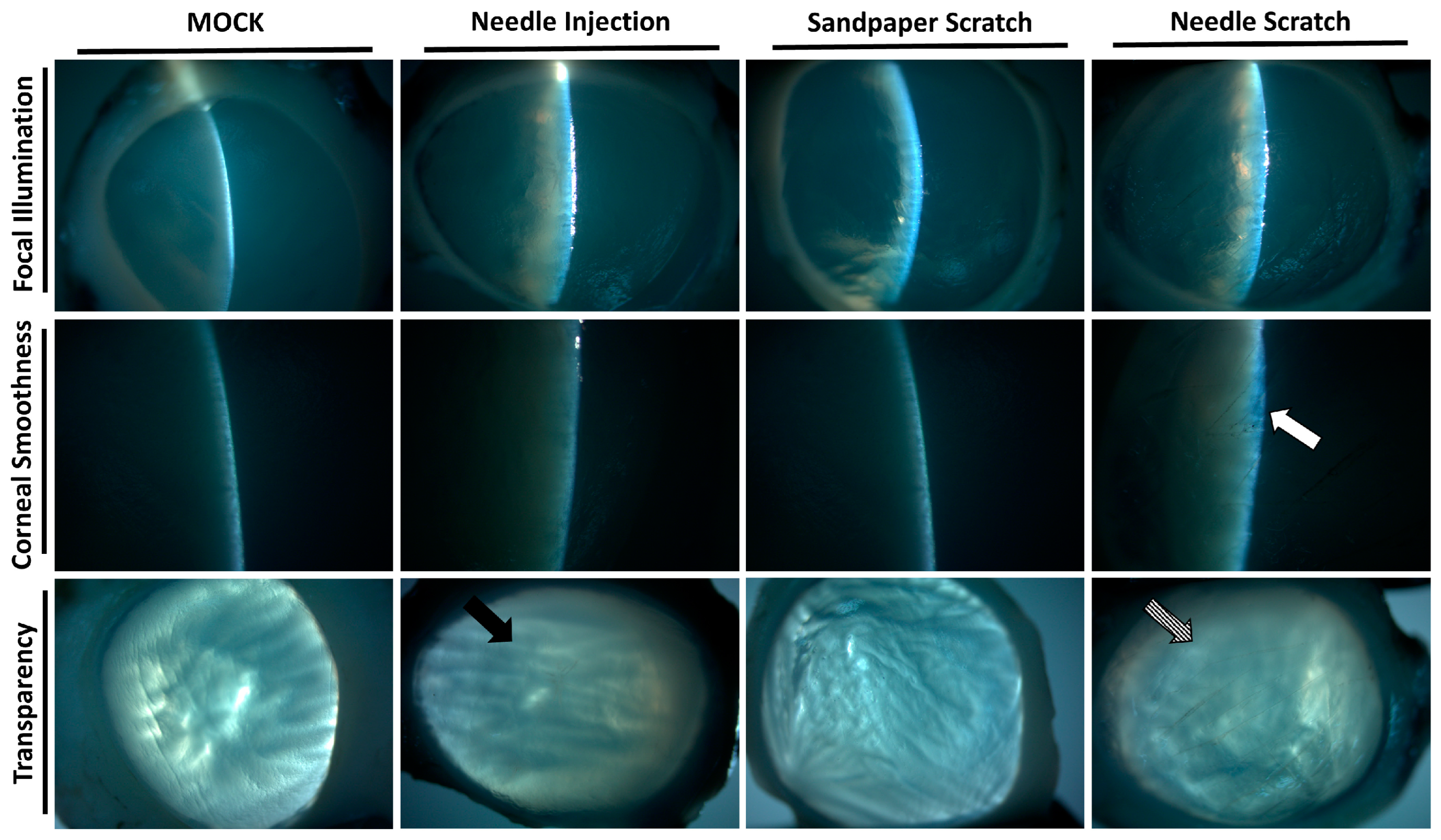
3.2. Needle Scraping as an Effective Method to Simulate Corneal Injury and Infection in Porcine Models
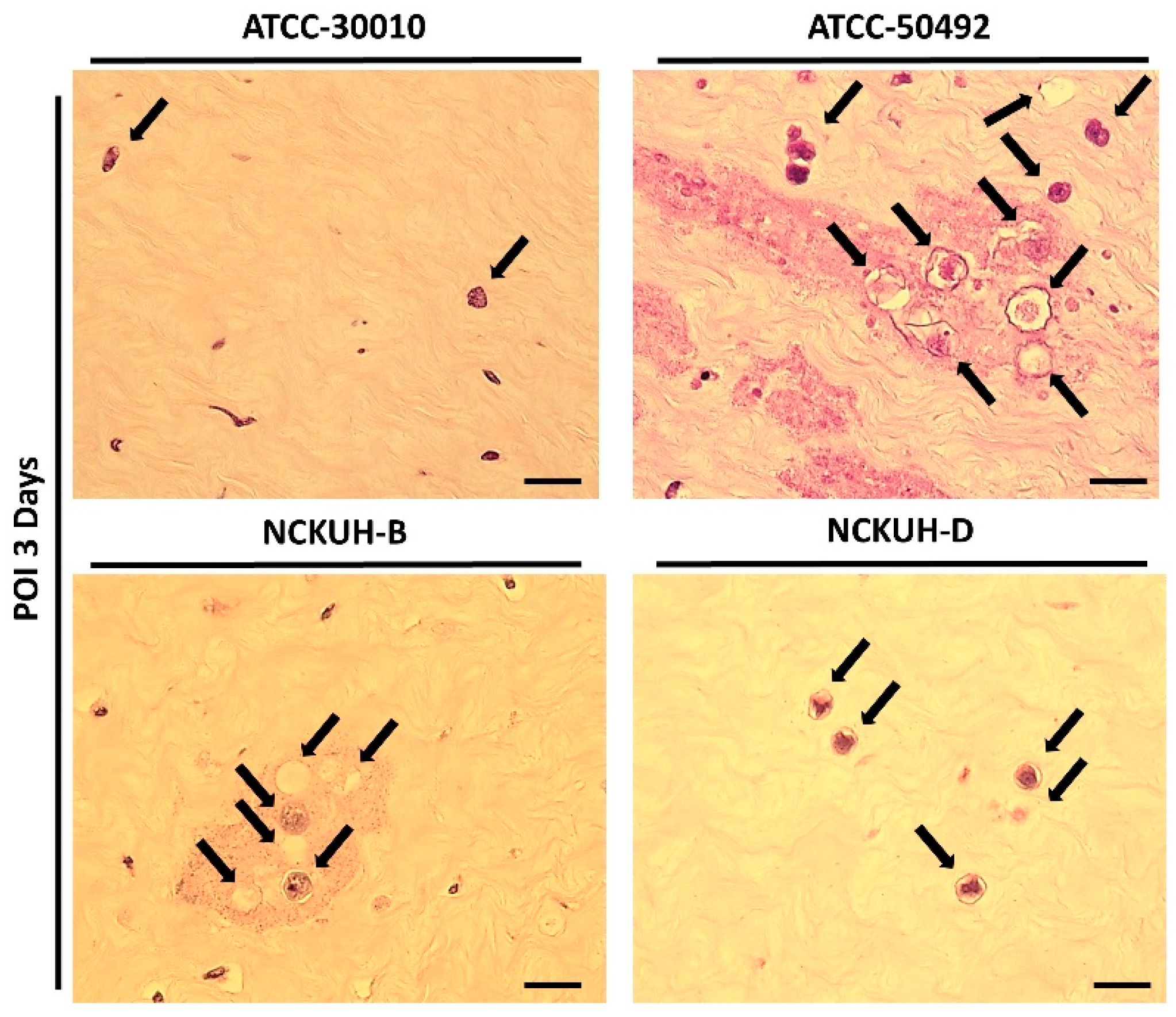
3.3. Histological Confirmation of Acanthamoeba Invasion in Needle-Scraped Corneas
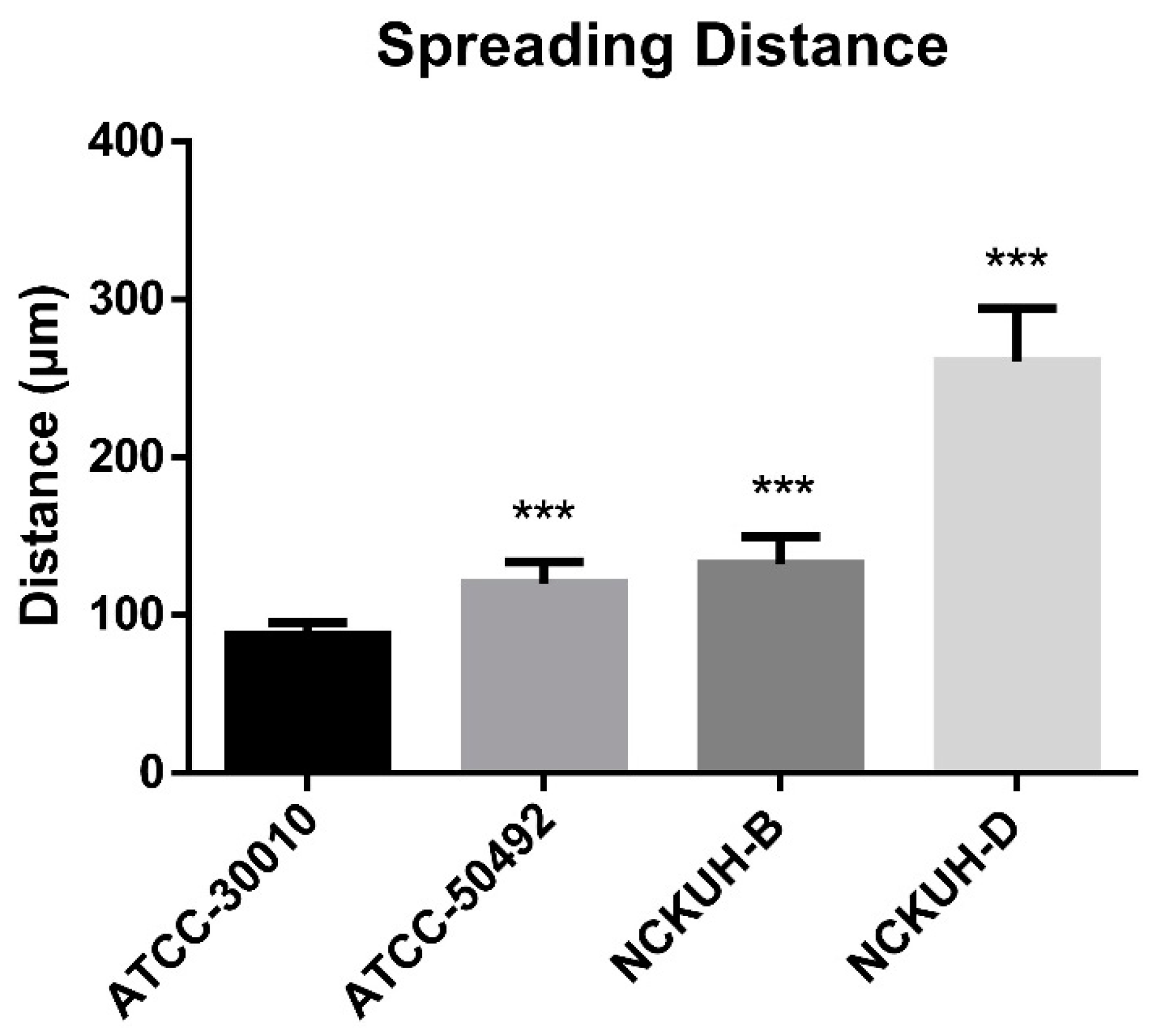
3.4. Evaluation of the Pathogenicity of Acanthamoeba Isolates Using a Needle-Scraped Porcine Eye Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Shoff, M.; Rogerson, A.; Kessler, K.; Schatz, S.; Seal, D. Prevalence of Acanthamoeba and other naked amoebae in South Florida domestic water. J. Water Health 2008, 6, 99–104. [Google Scholar] [CrossRef]
- Walochnik, J.; Scheikl, U.; Haller-Schober, E.M. Twenty years of Acanthamoeba diagnostics in Austria. J. Eukaryot. Microbiol. 2015, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.W.; Niederkorn, J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006, 22, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Padmavathy, S.; Shivakumar, C.; Srinivasan, M. Microbial keratitis in South India: Influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007, 14, 61–69. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Hashim, F. Visualization on the effect of chlorhexidine gluconate, a biocide on Acanthamoeba sp by electron microscopy. Malays. J. Microsc. 2013, 9, 154–159. [Google Scholar]
- Huang, F.-C.; Shih, M.-H.; Chang, K.-F.; Huang, J.-M.; Shin, J.-W.; Lin, W.-C. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 570–577. [Google Scholar] [CrossRef]
- Potter, J.L.; Weisman, R.A. Correlation of cellulose synthesis in vivo and in vitro during the encystment of Acanthamoeba. Dev. Biol. 1972, 28, 472–479. [Google Scholar] [CrossRef]
- Nakagawa, H.; Koike, N.; Ehara, T.; Hattori, T.; Narimatsu, A.; Kumakura, S.; Goto, H. Corticosteroid eye drop instillation aggravates the development of Acanthamoeba keratitis in rabbit corneas inoculated with Acanthamoeba and bacteria. Sci. Rep. 2019, 9, 12821. [Google Scholar] [CrossRef]
- Ren, M.; Wu, X. Evaluation of three different methods to establish animal models of Acanthamoeba keratitis. Yonsei Med. J. 2009, 51, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Neelam, S.; Hurt, M.; Niederkorn, J.Y. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect. Immun. 2005, 73, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Moral, M.; García-Posadas, L.; López-García, A.; Diebold, Y. Histological and immunohistochemical characterization of the porcine ocular surface. PLoS ONE 2020, 15, e0227732. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S. Porcine ophthalmology. Vet. Clin. Food Anim. Pract. 2010, 26, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.; Gillespie, S.R.; Bernstein, A.M. Ex vivo corneal organ culture model for wound healing studies. JoVE (J. Vis. Exp.) 2019, 144, e58562. [Google Scholar]
- Pacella, E.; La Torre, G.; De Giusti, M.; Brillante, C.; Lombardi, A.M.; Smaldone, G.; Lenzi, T.; Pacella, F. Results of case-control studies support the association between contact lens use and Acanthamoeba keratitis. Clin. Ophthalmol. 2013, 7, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, Y.; Siddiqui, R.; Iftikhar, H.; Khan, N.A. The effect of different environmental conditions on the encystation of Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2013, 135, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Shivakumar, C.; Raj, D.L. Analysis of the risk factors predisposing to fungal, bacterial & Acanthamoeba keratitis in south India. Indian J. Med. Res. 2009, 130, 749–757. [Google Scholar] [PubMed]
- Wang, Y.-J.; Li, S.-C.; Lin, W.-C.; Huang, F.-C. Intracellular microbiome profiling of the Acanthamoeba clinical isolates from lens associated keratitis. Pathogens 2021, 10, 266. [Google Scholar] [CrossRef]
- Huang, J.-M.; Liao, C.-C.; Kuo, C.-C.; Chen, L.-R.; Huang, L.L.; Shin, J.-W.; Lin, W.-C. Pathogenic Acanthamoeba castellanii secretes the extracellular aminopeptidase M20/M25/M40 family protein to target cells for phagocytosis by disruption. Molecules 2017, 22, 2263. [Google Scholar] [CrossRef]
- Polat, Z.A.; Ozcelik, S.; Vural, A.; Yıldız, E.; Cetin, A. Clinical and histologic evaluations of experimental Acanthamoeba keratitis. Parasitol. Res. 2007, 101, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Klink, F.V.; Leher, H.; Jager, M.; Alizadeh, H.; Taylor, W.; Niederkorn, J. Systemic immune response to Acanthamoeba keratitis in the Chinese hamster. Ocul. Immunol. Inflamm. 1997, 5, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; He, Y.; McCulley, J.P.; Ma, D.; Stewart, G.L.; Via, M.; Haehling, E.; Niederkorn, J.Y. Successful immunization against Acanthamoeba keratitis in a pig model. Cornea 1995, 14, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Vural, A.; Polat, Z.A.; Topalkara, A.; Toker, M.I.; Erdogan, H.; Arici, M.K.; Cetin, A. The effect of propolis in experimental Acanthamoeba keratitis. Clin. Exp. Ophthalmol. 2007, 35, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Said, N.A.; Shoeir, A.T.; Panjwani, N.; Garate, M.; Cao, Z. Local and systemic humoral immune response during acute and chronic Acanthamoeba keratitis in rabbits. Curr. Eye Res. 2004, 29, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P. Antimicrobial tear lipids in the ocular surface defense. Front. Cell. Infect. Microbiol. 2022, 12, 866900. [Google Scholar] [CrossRef] [PubMed]
- Bouten, M.; Elsheikha, H.M. Diagnosis and Management of Acanthamoeba Keratitis: A Continental Approach. Parasitologia 2022, 2, 167–197. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jo, H.-J.; Sohn, H.-J.; Shin, H.-J.; Quan, F.-S.; Kong, H.-H.; Moon, E.-K. Evaluating the Diagnostic Potential of Chorismate Mutase Poly-Clonal Peptide Antibody for the Acanthamoeba Keratitis in an Animal Model. Pathogens 2023, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Dor, M.; Eperon, S.; Lalive, P.H.; Guex-Crosier, Y.; Hamedani, M.; Salvisberg, C.; Turck, N. Investigation of the global protein content from healthy human tears. Exp. Eye Res. 2019, 179, 64–74. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-depth analysis of the human tear proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef]
- Butovich, I.A. Tear film lipids. Exp. Eye Res. 2013, 117, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Wang, Y.-J.; Huang, J.-M.; Huang, F.-C.; Lin, W.-C. Inhibitory effect of host ocular microenvironmental factors on chlorhexidine digluconate activity. Antimicrob. Agents Chemother. 2021, 65, e02066-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Sung, K.-C.; Lin, W.-C.; Huang, F.-C. Comparison of Microbial Sampling Sites and Donor-Related Factors on Corneal Graft Contamination. J. Clin. Med. 2022, 11, 6236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Chen, C.-H.; Chen, J.-W.; Lin, W.-C. Commensals Serve as Natural Barriers to Mammalian Cells during Acanthamoeba castellanii Invasion. Microbiol. Spectr. 2021, 9, e00512-21. [Google Scholar]
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Lorget, F.; Parenteau, A.; Carrier, M.; Lambert, D.; Gueorguieva, A.; Schuetz, C.; Bantseev, V.; Thackaberry, E. Characterization of the pH and temperature in the rabbit, pig, and monkey eye: Key parameters for the development of long-acting delivery ocular strategies. Mol. Pharm. 2016, 13, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.; Hicks, D. Distribution and density of medium-and short-wavelength selective cones in the domestic pig retina. Exp. Eye Res. 2002, 74, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Funke, S.; Markowitsch, S.; Schmelter, C.; Perumal, N.; Mwiiri, F.K.; Gabel-Scheurich, S.; Pfeiffer, N.; Grus, F.H. In-depth proteomic analysis of the porcine retina by use of a four step differential extraction bottom up LC MS platform. Mol. Neurobiol. 2017, 54, 7262–7275. [Google Scholar] [CrossRef] [PubMed]
- Menduni, F.; Davies, L.N.; Madrid-Costa, D.; Fratini, A.; Wolffsohn, J.S. Characterisation of the porcine eyeball as an in-vitro model for dry eye. Contact Lens Anterior Eye 2018, 41, 13–17. [Google Scholar] [CrossRef]
- Notara, M.; Schrader, S.; Daniels, J.T. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng. Part A 2011, 17, 741–750. [Google Scholar] [CrossRef]
- Henker, R.; Scholz, M.; Gaffling, S.; Asano, N.; Hampel, U.; Garreis, F.; Hornegger, J.; Paulsen, F. Morphological features of the porcine lacrimal gland and its compatibility for human lacrimal gland xenografting. PLoS ONE 2013, 8, e74046. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, M.K.; Lee, H.J.; Ko, J.H.; Wee, W.R.; Lee, J.H. Comparative observation of freeze–thaw-induced damage in pig, rabbit, and human corneal stroma. Vet. Ophthalmol. 2009, 12, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pumidonming, W.; Koehsler, M.; Leitsch, D.; Walochnik, J. Protein profiles and immunoreactivities of Acanthamoeba morphological groups and genotypes. Exp. Parasitol. 2014, 145, S50–S56. [Google Scholar] [CrossRef]
- Huang, J.-M.; Lin, W.-C.; Li, S.-C.; Shih, M.-H.; Chan, W.-C.; Shin, J.-W.; Huang, F.-C. Comparative proteomic analysis of extracellular secreted proteins expressed by two pathogenic Acanthamoeba castellanii clinical isolates and a non-pathogenic ATCC strain. Exp. Parasitol. 2016, 166, 60–67. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.-D.; Sung, K.-C.; Huang, J.-M.; Chen, C.-H.; Wang, Y.-J. Development of an Ex Vivo Porcine Eye Model for Exploring the Pathogenicity of Acanthamoeba. Microorganisms 2024, 12, 1161. https://doi.org/10.3390/microorganisms12061161
Shi M-D, Sung K-C, Huang J-M, Chen C-H, Wang Y-J. Development of an Ex Vivo Porcine Eye Model for Exploring the Pathogenicity of Acanthamoeba. Microorganisms. 2024; 12(6):1161. https://doi.org/10.3390/microorganisms12061161
Chicago/Turabian StyleShi, Ming-Der, Ko-Chiang Sung, Jian-Ming Huang, Chun-Hsien Chen, and Yu-Jen Wang. 2024. "Development of an Ex Vivo Porcine Eye Model for Exploring the Pathogenicity of Acanthamoeba" Microorganisms 12, no. 6: 1161. https://doi.org/10.3390/microorganisms12061161
APA StyleShi, M. -D., Sung, K. -C., Huang, J. -M., Chen, C. -H., & Wang, Y. -J. (2024). Development of an Ex Vivo Porcine Eye Model for Exploring the Pathogenicity of Acanthamoeba. Microorganisms, 12(6), 1161. https://doi.org/10.3390/microorganisms12061161





