Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America
Abstract
1. Introduction
Rationale of the Study
2. Materials and Methods
2.1. Sample Collection
2.2. Microbiology of the Larvae
2.3. PCR Methods
2.4. Histopathology
3. Results
3.1. PCR Results
3.2. Microbiology
3.3. Histopathology
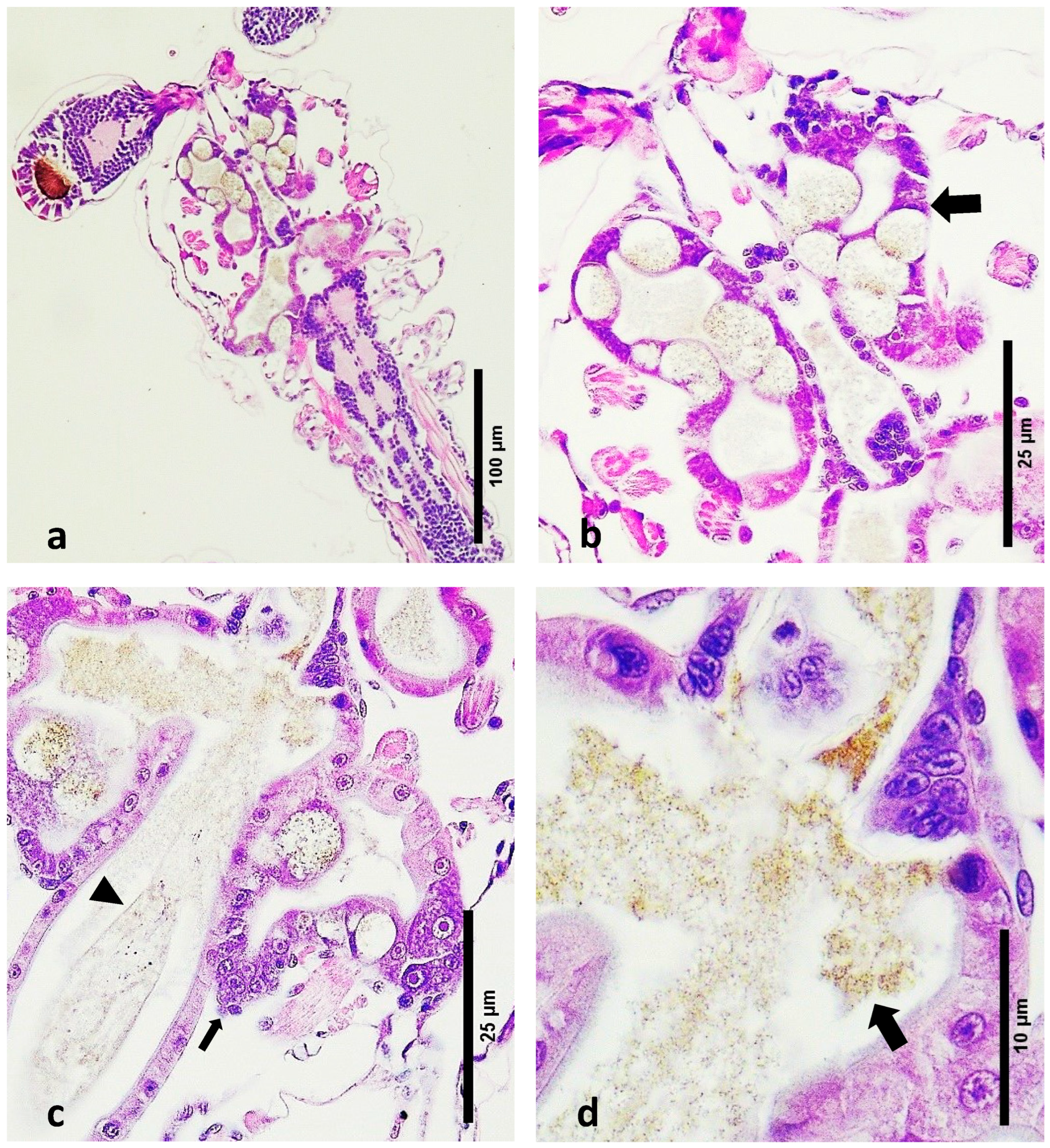
- Digestive Tract Content: Healthy zoea 2–3 larvae exhibited feed debris within their digestive tracts. The digestive system at this stage is still developing, and the presence of feed debris indicates normal feeding and digestive processes.
- Peritrophic Membrane Integrity: An intact peritrophic membrane was observed in healthy larvae. This membrane plays a crucial role in protecting the digestive epithelium and facilitating digestion.
- Algal Material: The algal material consumed by the larvae at this stage was seen as small-sized brown debris within the digestive tract (Figure 2c,d). This indicates that the larvae were actively feeding on algal matter, which is typical for this developmental stage.
- Absence of Detached Cells: No detached cell material was observed in the lumen of the developing digestive system of healthy larvae. The absence of detached cells indicates a lack of tissue degeneration or pathology within the digestive tract (Figure 2c,d).
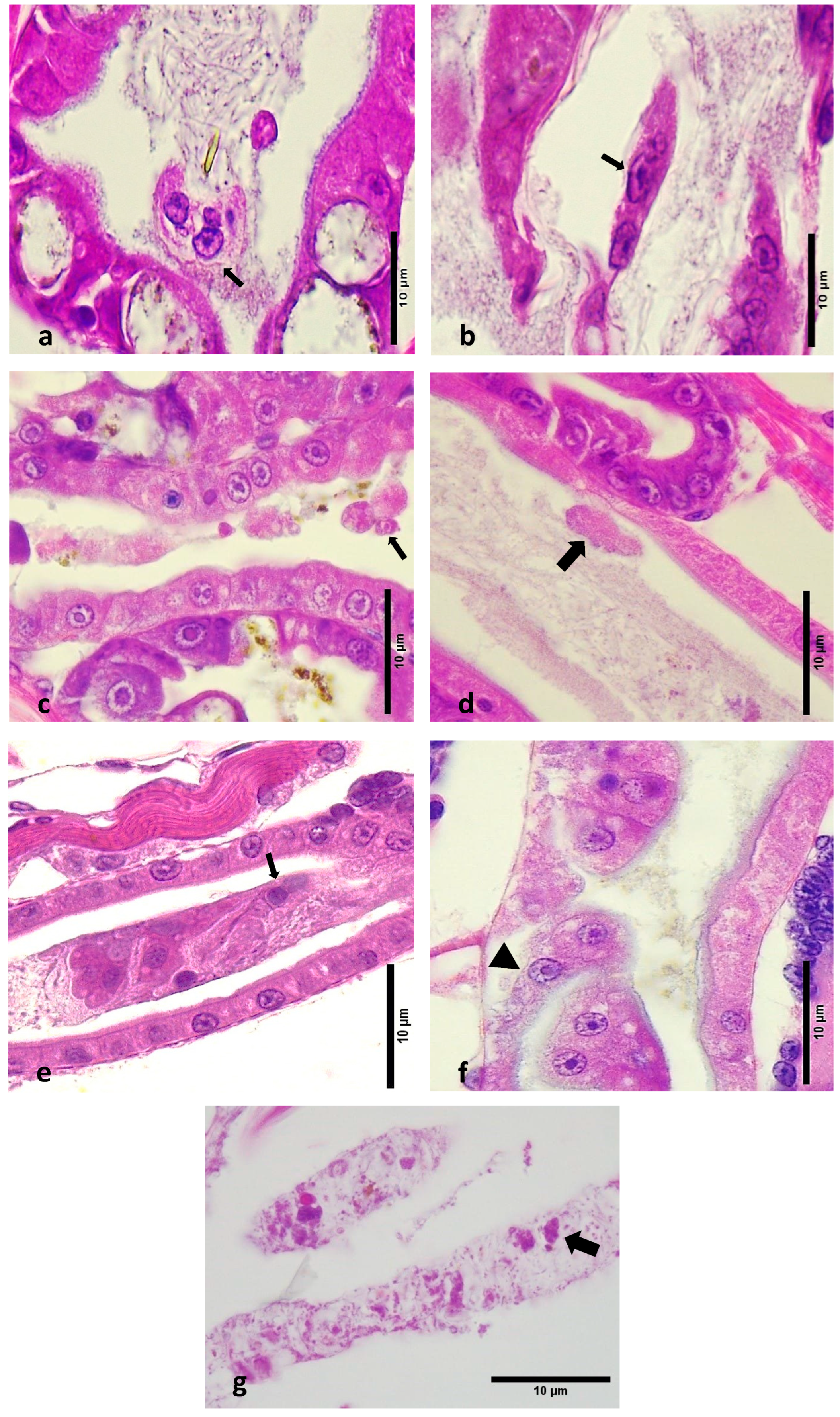
- Epithelial Cell Detachment and Necrosis: Most LBS-affected zoea showed detachment of epithelial cells within the lumen and structural loss due to necrosis in the hepatopancreas (Figure 3a–c). This detachment ranged from minimal to severe and contrasted sharply with the intact epithelia in healthy larvae (Figure 3d–f).
- Midgut Epithelium: In LBS-affected larvae, there was notable detachment of midgut epithelial cells into the lumen. This pathological feature was absent in healthy larvae, where the midgut epithelium remained intact.
- Peritrophic Membrane: Cells were observed inside the peritrophic membrane in LBS-affected larvae (Figure 3g). This is indicative of disruption in the peritrophic membrane’s protective role.
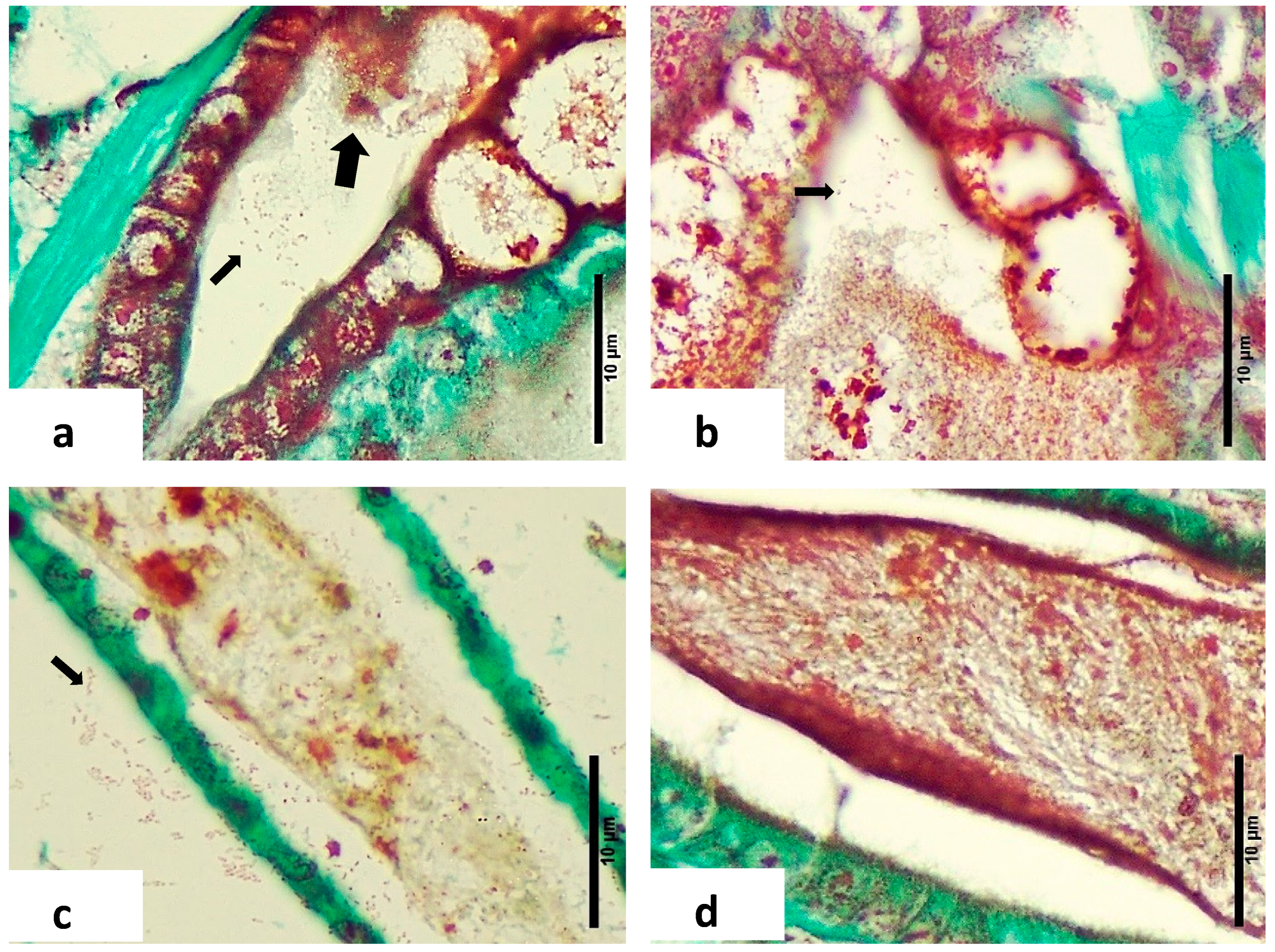
- Gram-Negative Bacteria: Twort’s stain revealed the presence of Gram-negative bacteria, with or without sloughed material, in the affected larvae (Figure 4a,b). This finding was consistent with microbiological analyses showing higher concentrations of Vibrio spp. in diseased zoea.
- Intestinal Differences: Significant differences were noted in the intestine area between normal and LBS-affected larvae. In normal larvae, the intestines appeared healthy with no bacterial overgrowth and intact peritrophic membranes (Figure 2c). In contrast, LBS-affected larvae had an abundance of Gram-negative bacteria and compromised peritrophic membranes (Figure 4d).
- Absence of Bolitas: During this study, no bolitas (i.e., spheres) were observed in any histological slides, and such structures have not been documented in the literature [1,2]. It is suggested that the bolitas may be lipidic in nature, given that tissue sample preparation for histology involves processing through several solvents that could potentially dissolve lipid-based structures.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morales, I. Observaciones sobre el síndrome de descamación del epitelio digestivo “Bolitas” en larvas de Penaeus vannamei en Ecuador. In Memorias del Primer Congreso Ecuatoriano de Acuicultura; Escuela Superior Politécnica del Litoral: Guayaquil, Ecuador, 1992; pp. 203–207. [Google Scholar]
- Robertson, P.A.W.; Calderon, J.; Carrera, L.; Stark, J.K.; Zherdmand, M.; Austin, B. Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Dis. Aquat. Org. 1998, 32, 151–155. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Verdonck, L.; Robles-Arozarena, R.; Rivera, G.; Bolland, A.; Balladares, M.; Gomez-Gil, B.; Calderon, J.; Sorgeloos, P.; Swings, J. Vibrios associated with Litopenaeus vannamei larvae, postlarvae, broodstock, and hatchery probionts. Appl. Environ. Microbiol. 1999, 65, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Baticados, M.C.L.; Lavilla-Pitogo, C.R.; Cruz-Lacierda, E.R.; de la Peña, L.D.; Suñaz, N.A. Studies on the chemical control of luminous bacteria Vibrio harveyi and V. splendidus isolated from diseased Penaeus monodon larvae and rearing water. Dis. Aquat. Org. 1990, 9, 133–139. [Google Scholar] [CrossRef]
- Lavilla-Pitogo, C.R.; Baticados, M.C.L.; Cruz-Lacierda, E.R.; de la Peña, L.D. Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture 1990, 91, 1–13. [Google Scholar] [CrossRef]
- Song, Y.L.; Lee, S.P. Characterization and ecological implication of luminous Vibrio harveyi isolated from tiger shrimp (Penaeus monodon). Bull. Inst. Zool. Acad. Sin. 1993, 32, 217–220. [Google Scholar]
- Nithimathachoke, N.; Pratanpipat, P.; Thongdaeng, K.; Withyachumnarnkul, B.; Nash, G. Luminous bacterial infection in pond reared Penaeus monodon. Asian Shrimp News 1995, 23, 1–4. [Google Scholar]
- Zou, Y.; Xie, G.; Jia, T.; Xu, T.; Wang, C.; Wan, X.; Li, Y.; Luo, K.; Bian, X.; Wang, X.; et al. Determination of the infectious agent of Translucent Post-Larva Disease (TPD) in Penaeus vannamei. Pathogens 2020, 9, 741. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Huang, W.; Li, F. Highly lethal Vibrio parahaemolyticus strains cause acute mortality in Penaeus vannamei post larvae. Aquaculture 2022, 548, 737605. [Google Scholar] [CrossRef]
- Intriago, P.; Medina, A.; Espinoza, J.; Enriquez, X.; Arteaga, K.; Aranguren, L.F.; Shinn, A.P.; Romero, X. Acute mortality of Penaeus vannamei larvae in farm hatcheries associated with the presence of Vibrio sp. carrying the VpPirAB toxin genes. Aquac. Int. 2023, 31, 3363–3382. [Google Scholar] [CrossRef]
- Yang, F.; You, Y.; Lai, Q.; Xu, L.; Li, F. Vibrio parahaemolyticus becomes highly virulent by producing Tc toxins. Aquaculture 2023, 576, 739817. [Google Scholar] [CrossRef]
- Intriago, P.; Jimenez, R. The effect of Vibrio parahaemolyticus, a luminescent bacteria isolated from grow out ponds in Penaeus vannamei larviculture. In Book of Abstracts World Aquaculture ’99: Bridging the Gap, Proceedings of the Annual International Conference and Exposition of the World Aquaculture Society, Sydney, NSW, Australia, 26 April–2 May 1999; World Aquaculture Society: Sydney, NSW, Australia, 1999; p. 353. [Google Scholar]
- Wiradana, P.A.; Sani, M.D.; Mawli, R.E.; Ashshoffa, F.N.D.; Widhiantara, I.G.; Mukti, A. Monitoring the occurrence of Zoea Syndrome (ZS) in Pacific white shrimp (Litopenaeus vannamei) larval from several hatcheries in East Java, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1036, 012003. [Google Scholar] [CrossRef]
- Kumar, S.T.; Vidya, R.; Kumar, S.; Alavandi, S.V.; Vijayan, K.K. Zoea-2 syndrome of Penaeus vannamei in shrimp hatcheries. Aquaculture 2017, 479, 759–767. [Google Scholar] [CrossRef]
- Buller, N.B. Bacteria from Fish and Other Aquatic Animals: A Practical Identification Manual; CABI Publishing: Cambridge, MA, USA, 2004; eISBN 978-0-85199. [Google Scholar]
- Phromjai, J.; Boonsaeng, V.; Withyachumnarnkul, B.; Flegel, T.W. Detection of hepatopancreatic parvovirus in Thai shrimp Penaeus monodon by in situ hybridization, dot blot hybridization and PCR amplification. Dis. Aquat. Org. 2002, 51, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gangnonngiw, W.; Bunnontae, M.; Kayansamruaj, P.; Senapin, S.; Srisala, J.; Flegel, T.W.; Wongprasert, K. A novel ssDNA Bidnavirus in the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 2023, 568, 739340. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Wang, R.Y.; Cheng, D.Y.; Dong, X.; Yang, B.; Wang, X.H.; et al. Characterization of a new member of Iridoviridae, Shrimp Hemocyte Iridescent Virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Sci. Rep. 2017, 7, 11834. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.F.; Ho, C.H.; Peng, S.E.; Chen, C.H.; Hsu, H.C.; Chiu, Y.L.; Chang, C.F.; Liu, K.F.; Su, M.S.; Wang, C.H.; et al. White Spot Syndrome Baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis. Aquat. Organ. 1996, 27, 215–225. [Google Scholar] [CrossRef]
- Nunan, L.M.; Poulos, B.T.; Lightner, D.V. Use of polymerase chain reaction (PCR) for the detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in penaeid shrimp. Mar. Biotechnol. 2000, 2, 319–328. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Durand, S.V.; White, B.L.; Redman, R.M.; Pantoja, C.R.; Lightner, D.V. Postlarvae and juveniles of a selected line of Penaeus stylirostris are resistant to infectious hypodermal and hematopoietic necrosis virus infection. Aquaculture 2000, 190, 203–210. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Lightner, D.V. A yellow-head virus gene probe: Application to in situ hybridization and determination of its nucleotide sequence. Dis. Aquat. Organ. 1999, 35, 165–173. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Navarro, S.A.; Lightner, D.V. PCR assay for discriminating between infectious hypodermal and hematopoietic necrosis virus (IHHNV) and the virus-related sequences in the genome of Penaeus monodon. Dis. Aquat. Organ. 2007, 74, 165–170. [Google Scholar] [CrossRef]
- Srisala, J.; Thaiue, D.; Sanguanrut, P.; Aldama-Cano, D.J.; Flegel, T.W.; Sritunyalucksana, K. Potential universal PCR method to detect decapod hepanhamaparvovirus (DHPV) in crustaceans. Aquaculture 2021, 541, 736782. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Pantoja, C.R.; Redman, R.M.; Lightner, D.V. Development of in situ hybridization and RT-PCR assay for the detection of a nodavirus (PvNV) that causes muscle necrosis in Penaeus vannamei. Dis. Aquat. Organ. 2007, 75, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, T.; Wan, X.; Liu, S.; Wang, X.; Li, X.; Dong, X.; Yang, B.; Huang, J. Prevalence and distribution of covert mortality nodavirus (CMNV) in cultured crustacean. Virus Res. 2017, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Poulos, B.T.; Lightner, D.V. Detection of infectious myonecrosis virus (IMNV) of penaeid shrimp by reverse-transcriptase polymerase chain reaction (RT-PCR). Dis. Aquat. Org. 2006, 73, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.G.; Moody, N.J.; Hoad, J.; Williams, L.M.; Bowater, R.O.; Cummins, D.M.; Cowley, J.A.; Crane, M.S. New yellow head virus genotype (YHV7) in giant tiger shrimp Penaeus monodon indigenous to northern Australia. Dis. Aquat. Org. 2015, 115, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Nunan, L.M.; Poulos, B.T.; Lightner, D.V. Reverse transcription polymerase chain reaction (RT-PCR) used for the detection of Taura Syndrome Virus (TSV) in experimentally infected shrimp. Dis. Aquat. Organ. 1998, 34, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.A.; Tang, K.F.J.; Lightner, D.V. An improved Taura syndrome virus (TSV) RT-PCR using newly designed primers. Aquaculture 2009, 293, 290–292. [Google Scholar] [CrossRef]
- Gangnonngiw, W.; Bunnontae, M.; Phiwsaiya, K.; Senapin, S.; Dhar, A.K. In experimental challenge with infectious clones of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), MrNV alone can cause mortality in freshwater prawn (Macrobrachium rosenbergii). Virology 2020, 540, 30–37. [Google Scholar] [CrossRef]
- Senapin, S.; Jaengsanong, C.; Phiwsaiya, K.; Prasertsri, S.; Laisutisan, K.; Chuchird, N.; Limsuwan, C.; Flegel, T.W. Infections of MrNV (Macrobrachium rosenbergii nodavirus) in cultivated whiteleg shrimp Penaeus vannamei in Asia. Aquaculture 2012, 338–341, 41–46. [Google Scholar] [CrossRef]
- Nunan, L.M.; Pantoja, C.R.; Salazar, M.; Aranguren, F.; Lightner, D.V. Characterization and molecular methods for detection of a novel Spiroplasma pathogenic to Penaeus vannamei. Dis. Aquat. Organ. 2004, 62, 255–264. [Google Scholar] [CrossRef]
- Yong, L.; Guanpin, Y.; Hualei, W.; Jixiang, C.; Xianming, S.; Guiwei, Z.; Qiwei, W.; Sun, X. Design of Vibrio 16S rRNA gene specific primers and their application in the analysis of seawater Vibrio community. J. Ocean. Univ. China 2006, 5, 157–164. [Google Scholar] [CrossRef]
- Khimmakthong, U.; Sukkarun, P. The spread of Vibrio parahaemolyticus in tissues of the Pacific white shrimp Litopenaeus vannamei analyzed by PCR and histopathology. Microb. Pathog. 2017, 113, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Jia, T.; Xin, L.; Xu T-t Wang, C.; Xie, G.; Luo, K.; Li, J.; Kong, J.; Zhang, Q. Vibrio parahaemolyticus becomes lethal to post-larvae shrimp via acquiring novel virulence factors. Microbiol. Spectr. 2023, 11, e0049223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aweya, J.J.; Yao, D.; Zheng, Z.; Wang, C.; Zhao, Y.; Li, S.; Zhang, Y. Taurine metabolism is modulated in Vibrio-infected Penaeus vannamei to shape shrimp antibacterial response and survival. Microbiome 2022, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Nunan, L.M.; Poulos, B.; Redman, R.; Groumellec, M.L.; Lightner, D.V. Molecular detection methods developed for a systemic Rickettsia-like bacterium (RLB) in Penaeus monodon (Decapoda: Crustacea). Dis. Aquat. Organ. 2003, 53, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Aranguren, L.F.; Tang, K.F.J.; Lightner, D.V. Quantification of the bacterial agent of necrotizing hepatopancreatitis (NHP-B) by real-time PCR and comparison of survival and NHP load of two shrimp populations. Aquaculture 2010, 307, 187–192. [Google Scholar] [CrossRef]
- Jaroenlak, P.; Sanguanrut, P.; Williams, B.A.P.; Stentiford, G.D.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. A nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei (EHP) in environmental samples in shrimp farms. PLoS ONE 2016, 11, e0166320. [Google Scholar] [CrossRef] [PubMed]
- Pasharawipas, T.; Flegel, T.W. A specific DNA probe to identify the intermediate host of a common microsporidian parasite of Penaeus merguiensis and P. monodon. Asian Fish. Sci. 1994, 7, 157–167. [Google Scholar] [CrossRef]
- Dangtip, S.; Sirikharin, R.; Sanguanrut, P.; Thitamadee, S.; Sritunyalucksana, K.; Taengchaiyaphum, S.; Mavichak, R.; Proespraiwong, P.; Flegel, T.W. AP4 method for two-tube nested PCR detection of AHPND isolates of Vibrio parahaemolyticus. Aquacult Rep. 2015, 2, 158–162. [Google Scholar] [CrossRef]
- Utari, H.B.; Senapin, S.; Jaengsanong, C.; Flegel, T.W.; Kruatrachue, M. A haplosporidian parasite associated with high mortality and slow growth in Penaeus (Litopenaeus) vannamei cultured in Indonesia. Aquaculture 2012, 366–367, 85–89. [Google Scholar] [CrossRef]
- Aguilera-Rivera, D.; Prieto-Davó, A.; Rodríguez-Fuentes, G.; Escalante-Herrera, K.S.; Gaxiola, G. A vibriosis outbreak in the Pacific white shrimp, Litopenaeus vannamei reared in biofloc and clear seawater. J. Invertebr. Pathol. 2019, 167, 17246. [Google Scholar] [CrossRef]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; The World Aquaculture Society: Baton Rouge, LA, USA, 1998; ISBN 0-935868-37-2. [Google Scholar]
- Thompson, J.R.; Randa, M.A.; Marcelino, L.A.; Tomita-Mitchell, A.; Lim, E.; Polz, M.F. Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl. Environ. Microbiol. 2004, 70, 4103–4110. [Google Scholar] [CrossRef]
- Abubakr, M.A.; Jones, D.A. Functional morphology and ultrastructure of the anterior mid-gut diverticulae of larvae of Penaeus monodon Fabricius, 1798 (Decapoda, Natantia). Crustaceana 1992, 62, 142–158. [Google Scholar] [CrossRef]
- Intriago, P.; Medina, A.; Cercado, N.; Arteaga, K.; Montenegro, A.; Burgos, M.; Espinoza, J.; Brock, J.A.; McIntosh, R.; Flegel, T. Passive surveillance for shrimp pathogens in Penaeus vannamei submitted from 3 Regions of Latin America. Aquac. Rep. 2023, 36, 102092. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Lozano-Olvera, R.; Palacios-Gonzalez, D.A.; Bolan-Mejia, C.; Rendon-Aguilar, K.G. Characterization and growth conditions of Vibrio parahaemolyticus strains with different virulence degrees that cause acute hepatopancreatic necrosis disease in Litopenaeus vannamei. J. World Aquac. Soc. 2019, 50, 1002–1015. [Google Scholar] [CrossRef]
- Overman, T.L.; Kessler, J.F.; Seabolt, J.P. Comparison of API 20E, API rapid E, and API rapid NFT for identification of members of the family Vibrionaceae. J. Clin. Microbiol. 1985, 22, 778–781. [Google Scholar] [CrossRef]
- Croci, L.; Suffredini, E.; Cozzi, L.; Toti, L.; Ottaviani, D.; Pruzzo, C.; Serratore, P.; Fischetti, R.; Goffredo, E.; Vibrio parahaemolyticus Working Group. Comparison of different biochemical and molecular methods for the identification of Vibrio parahaemolyticus. J. Appl. Microbiol. 2007, 102, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, C.; Cataletto, B.; Del Negro, P. Detection of pathogenic Vibrio parahaemolyticus through biochemical and molecular-based methodologies in coastal waters of the Gulf of Trieste (North Adriatic Sea). FEMS Microbiol. Lett. 2010, 307, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lydon, K.A.; Kinsey, T.; Le, C.; Gulig, P.A.; Jones, J.L. Biochemical and virulence characterization of Vibrio vulnificus isolates from clinical and environmental sources. Front. Cell Infect. Microbiol. 2021, 11, 637019. [Google Scholar] [CrossRef]
- Vivijs, B.; Moons, P.; Aertsen, A.; Michiels, C.W. Acetoin synthesis acquisition favors Escherichia coli growth at low pH. App. Environ. Microb. 2014, 80, 6054–6061. [Google Scholar] [CrossRef]
- Oh, Y.T.; Kim, H.Y.; Kim, E.J.; Go, J.; Hwang, W.; Kim, H.R.; Kim, D.W.; Yoon, S.S. Selective and efficient elimination of Vibrio cholerae with a chemical modulator that targets glucose metabolism. Front. Cell. Infect. Microbiol. 2016, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Zhang, Z.F.; Shao, M.Y.; Dong, Y.P.; Muhammad, S. Ontogenesis of digestive system in Litopenaeus vannamei (Boone, 1931) (Crustacea: Decapoda). Ital. J. Zool. 2012, 79, 77–85. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.Y.; Yang, Q.; Xie, G.S.; Dong, X.; Huang, J. Case studies: Pathogenic agent and microbiome analysis of zoea of Litopenaeus vannamei suffering from an unknown disease. Progress. Fish. Sci. 2019, 40, 134–144. (In Chinese) [Google Scholar] [CrossRef]
- Wolkenfelt, B.; Blonk, R. Balanced shrimp breeding in Ecuador, for now and the future. In Proceedings of the Aqua Expo 2021, World Aquaculture Conference and Trade Show, Cámara Nacional de Acuacultura, Guayaquil, Ecuador, 25–28 October 2021. [Google Scholar]
- Hameed, A.S.S. A study of the aerobic heterotrophic bacterial flora of hatchery-reared eggs, larvae and postlarvae of Penaeus indicus. Aquaculture 1993, 117, 195–204. [Google Scholar] [CrossRef]
- Garcia, A.T.; Olmos, J.S. Quantification by fluorescent in situ hybridization of bacteria associated with Litopenaeus vannamei larvae in Mexican shrimp hatchery. Aquaculture 2007, 262, 211–218. [Google Scholar] [CrossRef]
- Intriago, P.; Espinoza, J.; Medina, A.; Altamirano, L.; Enriquez, X.; Sanchez, A.; Navarrete, A.; Alvarez, E.; Forestieri, J. Microbiological dynamics of a Litopenaeus vannamei hatchery in Ecuador. In Proceedings of the Aqua Expo 2018, World Aquaculture Conference and Trade Show, Cámara Nacional de Acuacultura, Guayaquil, Ecuador, 15–18 October 2018. [Google Scholar]
- Kurmaly, K.; Jones, D.A.; Yule, A.B.; East, J. Comparative analysis of the growth and survival of Penaeus monodon (Fabricius) larvae, from protozoea 1 to postlarva 1, on live feeds, artificial diets and a combination of both. Aquaculture 1989, 81, 27–45. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Simoes, N.; Jones, D.A.; Roque, A.; Gómez-Gil, B. Assessment of fluorescent labeled bacteria (FLB) for evaluation of in vivo uptake of bacteria (Vibrio spp.) by crustacean larvae. J. Microbiol. Methods 2003, 52, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Guzman, G.; Vazquez-Juarez, R.; Ascencio, F. Differences in the susceptibility of American white shrimp larval substages (Litopenaeus vannamei) to four Vibrio species. J. Invert. Pathol. 2001, 78, 215–219. [Google Scholar] [CrossRef]
- Intriago, P.; Jimenez, R. The Effect of Bacteria Isolated from an Outbreak of Haemocytic Enteritis in Pond Reared Shrimp in Litopenaeus vannamei (Boone) larvae in Ecuador; Unpublished Internal Report Aqualab 1997; Ayangue-Peninsula de Santa Elena, Ecuador, 1997.
- Carella, F.; Sirri, R. Editorial: Fish and shellfish pathology. Front. Mar. Sci. 2017, 4, 375. [Google Scholar] [CrossRef]
- Reyes, G.; Andrade, B.; Betancourt, I.; Panchana, F.; Solorzano, R.; Preciado, C.; Sorroza, L.; Trujillo, L.E.; Bayot, B. Microbial profiles of Penaeus vannamei larvae in low survival hatchery tanks affected by vibriosis. Peer J. 2023, 11, e15795. [Google Scholar] [CrossRef]

| DNA Virus | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV 1 | MrBdv 2 | DIV1 3 | WSSV 4 | IHHNV 5 | Virus *6 | EVE **6 | |||||||
| 309 FR | 392 F/R | 389 F/R | 77012/773 | ||||||||||
| LBS | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 1/2 (50%) | 1/2 (50%) | 1/2 (50%) | 0/2 (0%) | 0/2 (0%) | 1/2 (50%) | |||
| Healthy | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 2/2 (100%) | 2/2 (100%) | 1/2 (50%) | 0/2 (0%) | 0/2 (0%) | 2/2 (100%) | |||
| RNA Virus | |||||||||||||
| WzSV8 7 | PvNV 8 | CMNV 9 | IMNV 10 | YHV 11 | GAV 12 | TSV 13 | MrNV 14 | XSV 15 | |||||
| LBS | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | ||||
| Healthy | 2/2 (100%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | ||||
| Bacteria/Microsporidia/Haplosporidia | |||||||||||||
| Spirop 16 | Vibrio 17 | Vp 18 | Vp(tdh) 19 | Vp(vhp 1) 20 | Vp(vhp 2) 20 | Vh (vhh) 21 | AHPND 22 | RLB 23 | NHP 24 | EHP 25 | Microsp 26 | Haplosp 27 | |
| LBS | 0/2 (0%) | 2/2 (100%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 2/2 (100%) | 0/2 (0%) | 0/2 (0%) | 1/2 (50%) | 0/2 (0%) |
| Healthy | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 1/2 (50%) | 0/2 (0%) | 0/2 (0%) | 1/2 (50%) | 0/2 (0%) |
| CFU/gr Larvae | ||||||
|---|---|---|---|---|---|---|
| TSA 1 | TCBS 2 | Green 3 | CHROMagar 4 | Purple 5 | ||
| LBS | Tank 7 | 1.20 × 107 | 4.40 × 105 | 1.00 × 104 | 4.80 × 105 | 2.40 × 105 |
| Tank 19 | 1.36 × 107 | 4.00 × 106 | 0.00 × 100 | 3.90 × 106 | 3.40 × 106 | |
| Average | 1.28 × 107 | 2.22 × 106 | 5.00 × 103 | 2.19 × 106 | 1.82 × 106 | |
| std dev | 1.13 × 106 | 2.52 × 106 | 7.07 × 103 | 2.42 × 106 | 2.23 × 106 | |
| 17.3% 6 | 0.2% 7 | 83.1% 8 | ||||
| Healthy | Room 2 | 1.46 × 106 | 7.60 × 104 | 7.60 × 104 | 6.20 × 104 | 2.00 × 103 |
| Room 3 | 8.90 × 105 | 6.10 × 104 | 0.00 × 100 | 5.00 × 104 | 1.00 × 100 | |
| Average | 1.18 × 106 | 6.85 × 104 | 3.80 × 104 | 5.60 × 104 | 1.00 × 103 | |
| std dev | 4.03 × 105 | 1.06 × 104 | 5.37 × 104 | 8.49 × 103 | 1.41 × 103 | |
| 5.8% 6 | 55.5% 7 | 1.8% 8 | ||||
| (a) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Date | AHPND 1 | toxR 2 | Vibrio 3 | Vibrio 4 | Vhp-1 5 | Vhp-2 5 | V.p 6 | V.hh 7 | TCBS | CHROMagarTM | API 20NE Code | Identified as |
| LBS-8 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4047124 | V. alginolyticus 97.8% |
| LBS-1 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4047124 | V. alginolyticus 97.8% |
| LBS-10 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4047124 | V. alginolyticus 97.8% |
| LBS-11 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4146124 | V. alginolyticus 96.5% |
| LBS-6 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4147124 | V. alginolyticus 85.9% |
| LBS-5 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4045120 | V. alginolyticus 80.2% |
| LBS-2 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | Clear | 1040127 | V. fluvialis 76.9% |
| LBS-12 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | Purple | 0040027 | V. fluvialis 63.9% |
| LBS-4 | 18 September 2023 | - | - | - | + | - | - | - | - | Green | Torquise | 5046005 | V. vulnificus 99.9% |
| LBS-9 | 18 September 2023 | + | - | + | + | - | - | - | - | Yellow | White | 0044427 | Aeromonas hydrophila/caviae/sobria 1 71.1% |
| LBS-7 | 18 September 2023 | - | - | + | + | - | - | - | - | Yellow | Purple | 0044526 | Pasteurella multocida 2 98.4% |
| H-1 | 23 September 2023 | - | - | + | + | - | - | - | - | Yellow | White | 4147125 | V. alginolyticus 92.1% |
| H-2 | 23 September 2023 | + | - | + | + | - | - | - | - | Yellow | White | 1042025 | V. fluvialis 84.1% |
| H-3 | 23 September 2023 | - | - | + | + | - | - | - | + | Yellow | White | 5044165 | Aeromonas hydrophila/caviae/sobria 1 90.6% |
| (b) | |||||||||||||
| LBS zoea | Healthy | ||||||||||||
| API 20 E | V. alginolyticus | Vibrio spp. | Vibrio spp. | ||||||||||
| (6/11) a | (5/11) a | (3/3) a | |||||||||||
| ONPG | ONPG | beta-galactosidase | 0% | 40% | 67% | ||||||||
| ADH | arginine | arginine dihydrolase | 0% | 0% | 0% | ||||||||
| LDC | lysine | lysine decarboxylase | 100% | 20% | 67% | ||||||||
| ODC | ornithine | ornithine decarboxylase yellow | 33% | 0% | 33% | ||||||||
| CIT | citrate | citrate utilization | 0% | 0% | 0% | ||||||||
| H2S | Na thiosulfate | H2S production | 0% | 0% | 0% | ||||||||
| URE | urea | urea hydrolysis | 0% | 0% | 0% | ||||||||
| TDA | tryptophan | deaminase | 0% | 0% | 0% | ||||||||
| IND | tryptophan | indole production | 100% | 100% | 100% | ||||||||
| VP | Na pyruvate | acetoin production | 100% | 0% | 33% | ||||||||
| GEL | charcoal gelatin | gelatinase | 67% | 20% | 67% | ||||||||
| GLU | glucose | fermentation/oxidation | 100% | 60% | 67% | ||||||||
| MAN | mannitol | fermentation/oxidation | 100% | 60% | 67% | ||||||||
| INO | inositol | fermentation/oxidation | 0% | 0% | 0% | ||||||||
| SOR | sorbitol | fermentation/oxidation | 0% | 0% | 0% | ||||||||
| RHA | rhamnose | fermentation/oxidation | 0% | 0% | 0% | ||||||||
| SAC | sucrose | fermentation/oxidation | 100% | 80% | 100% | ||||||||
| MEL | melibiose | fermentation/oxidation | 0% | 0% | 0% | ||||||||
| AMY | amygdalin | fermentation/oxidation | 0% | 80% | 100% | ||||||||
| ARA | arabinose | fermentation/oxidation | 0% | 80% | 0% | ||||||||
| oxidase | oxidase | 83% | 100% | 100% | |||||||||
| Catalase | Catalase | 100% | 100% | 100% | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intriago, P.; Montiel, B.; Valarezo, M.; Romero, X.; Arteaga, K.; Cercado, N.; Burgos, M.; Shinn, A.P.; Montenegro, A.; Medina, A.; et al. Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America. Microorganisms 2024, 12, 1186. https://doi.org/10.3390/microorganisms12061186
Intriago P, Montiel B, Valarezo M, Romero X, Arteaga K, Cercado N, Burgos M, Shinn AP, Montenegro A, Medina A, et al. Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America. Microorganisms. 2024; 12(6):1186. https://doi.org/10.3390/microorganisms12061186
Chicago/Turabian StyleIntriago, Pablo, Bolivar Montiel, Mauricio Valarezo, Xavier Romero, Kelly Arteaga, Nicole Cercado, Milena Burgos, Andrew P. Shinn, Alejandra Montenegro, Andrés Medina, and et al. 2024. "Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America" Microorganisms 12, no. 6: 1186. https://doi.org/10.3390/microorganisms12061186
APA StyleIntriago, P., Montiel, B., Valarezo, M., Romero, X., Arteaga, K., Cercado, N., Burgos, M., Shinn, A. P., Montenegro, A., Medina, A., & Gallardo, J. (2024). Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America. Microorganisms, 12(6), 1186. https://doi.org/10.3390/microorganisms12061186







