Sulforaphane Inhibits Oxidative Stress and May Exert Anti-Pyroptotic Effects by Modulating NRF2/NLRP3 Signaling Pathway in Mycobacterium tuberculosis-Infected Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mtb Culture
2.2. Cell Culture
2.3. Primary Peritoneal Macrophages Isolation
2.4. Western Blot Assay
2.5. Cellular Immunofluorescence Assay
2.6. RT-qPCR Assay
2.7. ROS Assay
2.8. MDA Assay
2.9. GSH Assay
2.10. LDH Assay
2.11. ELISA Assay
2.12. Hoechst 33342/PI Double Staining
2.13. CCK8 Assay
2.14. Statistical Analysis
3. Results
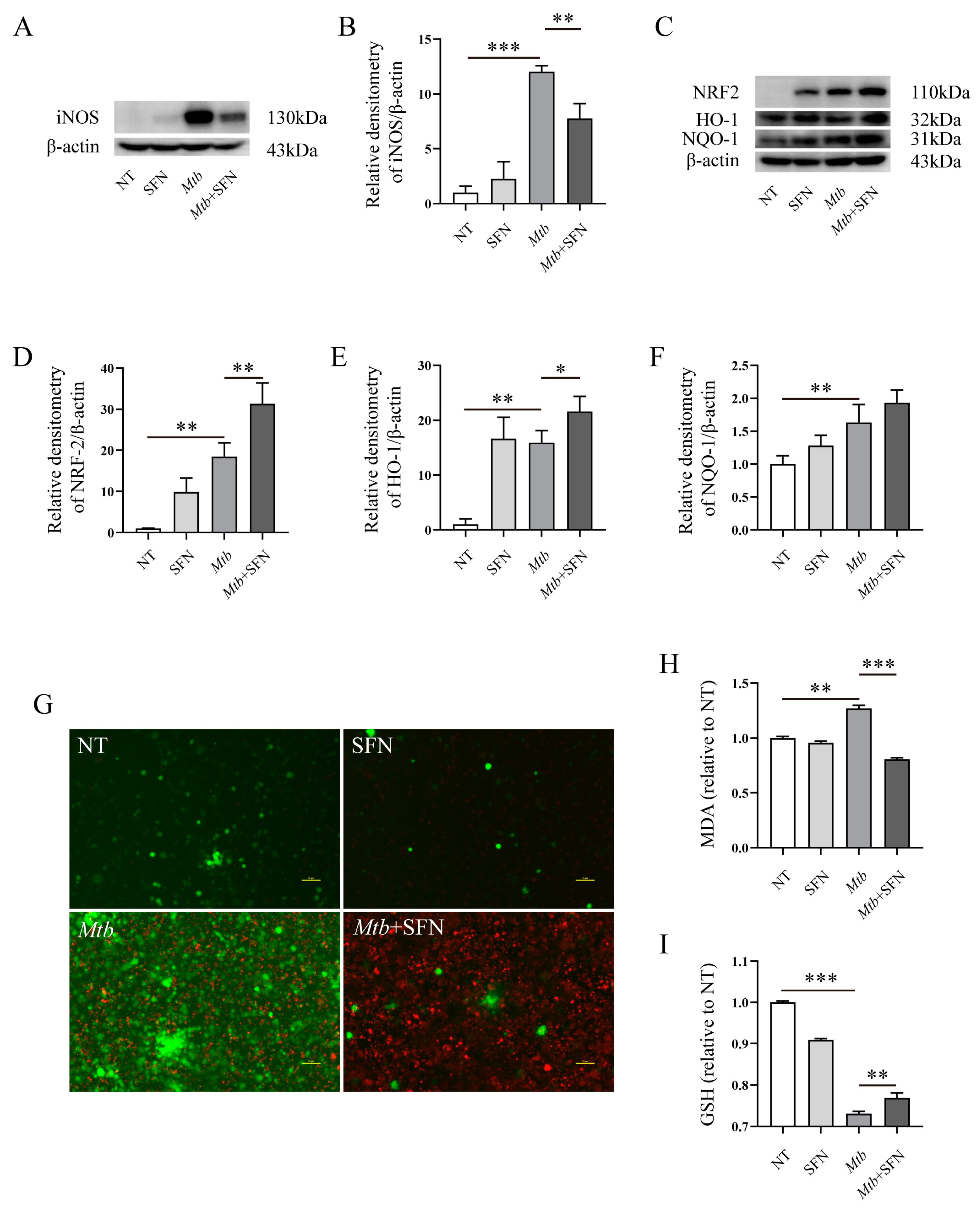
3.1. The Infection of Mtb Induces Oxidative Stress in Macrophages
3.2. SFN Inhibits Oxidative Stress in Mtb-Infected Macrophages by Activating NRF2 Signaling Pathway
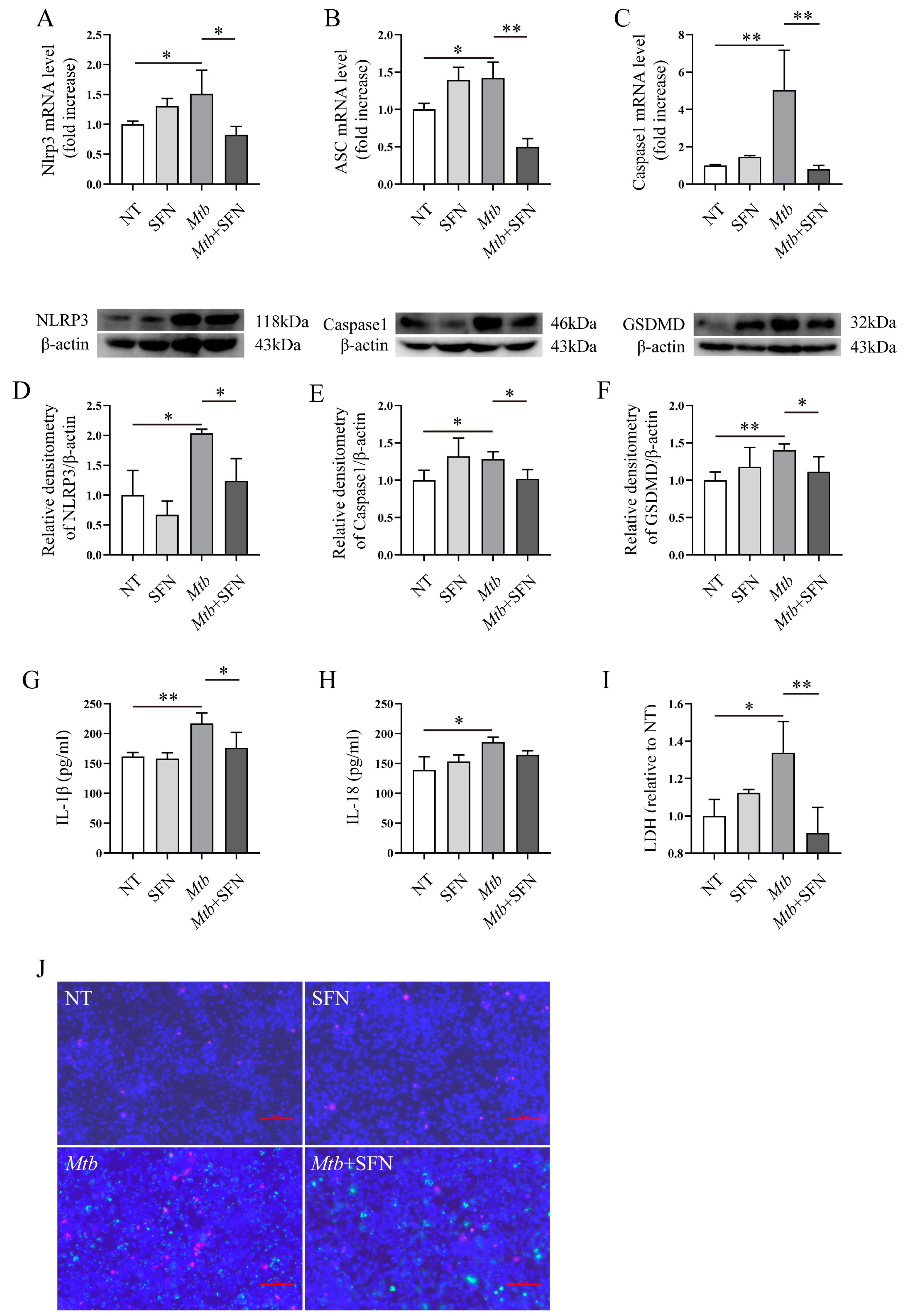
3.3. SFN May Inhibit the Occurrence of Pyroptosis in Mtb-Infected Macrophages
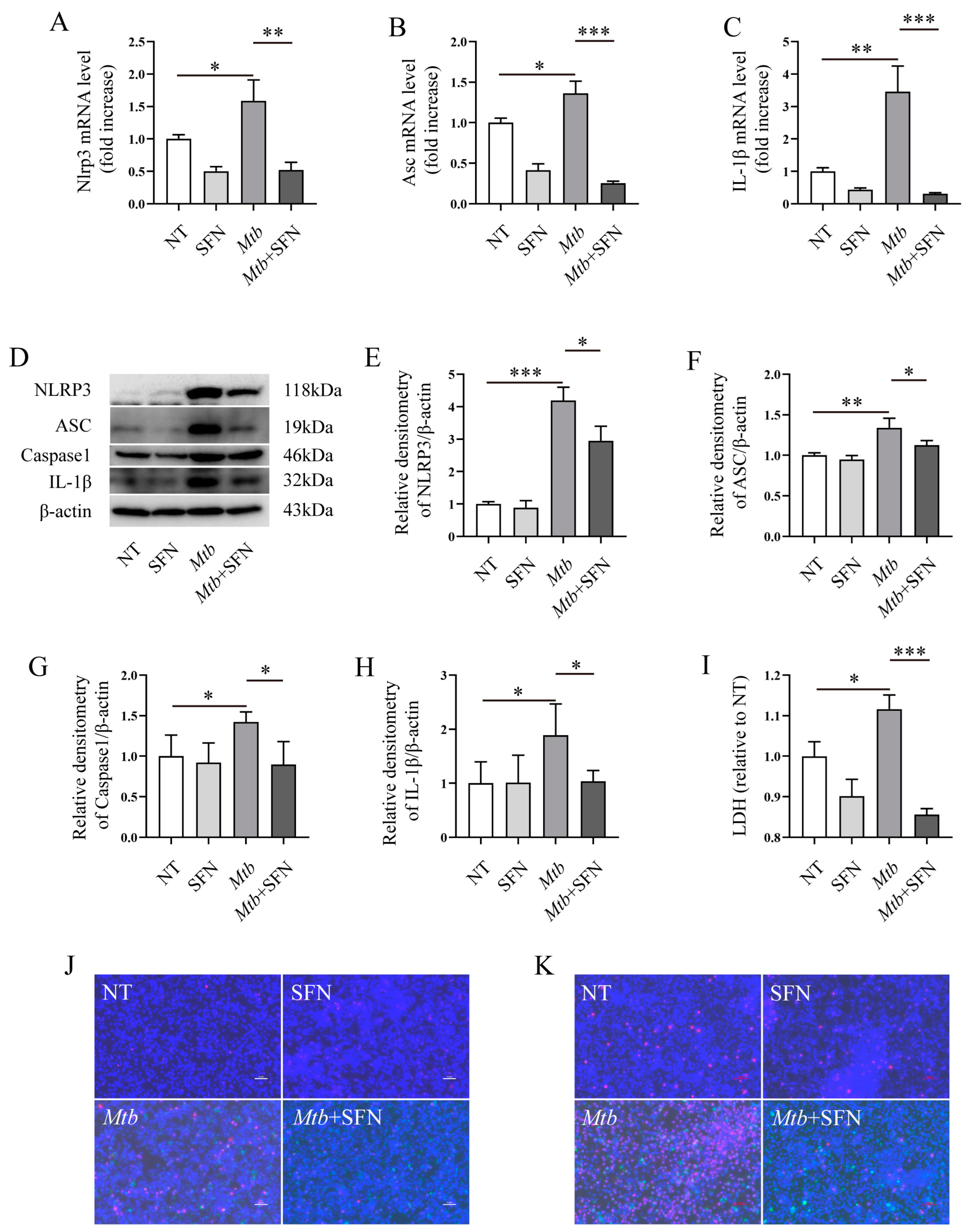
3.4. SFN May Directly Inhibit Pyroptosis by Blocking the Activation of the NLRP3 Signaling Pathway
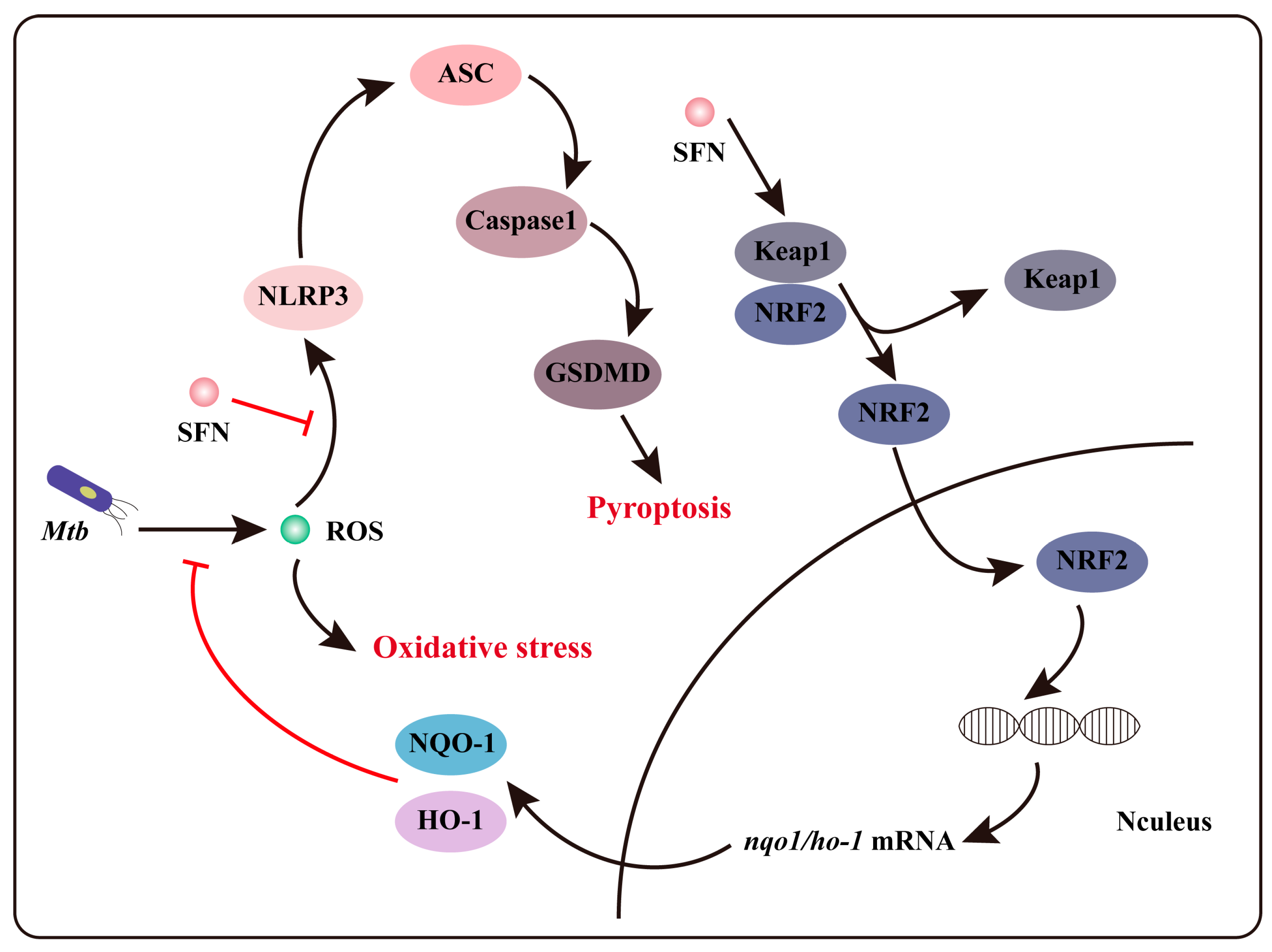
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Segal, L.N.; Clemente, J.C.; Li, Y.; Ruan, C.; Cao, J.; Danckers, M.; Morris, A.; Tapyrik, S.; Wu, B.G.; Diaz, P.; et al. Anaerobic Bacterial Fermentation Products Increase Tuberculosis Risk in Antiretroviral-Drug-Treated HIV Patients. Cell Host Microbe 2017, 21, 530–537.e4. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.A.F.; Silva, K.J.S.; da Costa, E.S.L.M.; de Moura, Y.F.; Zucchi, F.C.R. Tuberculosis: A granulomatous disease mediated by epigenetic factors. Tuberculosis 2020, 123, 101943. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.K.; Chandrasekaran, N.; Priya Doss, C.G. Micro-nanoemulsion and nanoparticle-assisted drug delivery against drug-resistant tuberculosis: Recent developments. Clin. Microbiol. Rev. 2023, 36, e0008823. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Tiemersma, E.W.; van der Werf, M.J.; Borgdorff, M.W.; Williams, B.G.; Nagelkerke, N.J. Natural history of tuberculosis: Duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: A systematic review. PLoS ONE 2011, 6, e17601. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, J.; Gao, G.F.; Liu, C.H. Insights into battles between Mycobacterium tuberculosis and macrophages. Protein Cell 2014, 5, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Ernst, J.D.; Desvignes, L. Beyond macrophages: The diversity of mononuclear cells in tuberculosis. Immunol. Rev. 2014, 262, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.B.; Dill, C.; Bonay, M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Méd. Mal. Infect. 2013, 43, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Cooper, D.A.; Webb, D.R.; Peters, J.C. Evaluation of the potential for olestra to affect the availability of dietary phytochemicals. J. Nutr. 1997, 127, 1699S–1709S. [Google Scholar] [CrossRef] [PubMed]
- Winiwarter, S.; Bonham, N.M.; Ax, F.; Hallberg, A.; Lennernas, H.; Karlen, A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 1998, 41, 4939–4949. [Google Scholar] [CrossRef] [PubMed]
- Georgikou, C.; Yin, L.; Gladkich, J.; Xiao, X.; Sticht, C.; de la Torre, C.; Gretz, N.; Gross, W.; Schäfer, M.; Karakhanova, S.; et al. Inhibition of miR30a-3p by sulforaphane enhances gap junction intercellular communication in pancreatic cancer. Cancer Lett. 2020, 469, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Yeh, C.T.; Kuo, C.C.; Lee, C.M.; Yen, G.C.; Wang, L.S.; Wu, C.-H.; Yang, W.-C.V.; Wu, A.T.H. Sulforaphane potentiates the efficacy of imatinib against chronic leukemia cancer stem cells through enhanced abrogation of Wnt/beta-catenin function. J. Agric. Food Chem. 2012, 60, 7031–7039. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Rodriguez, T.; Avery, V.M. The Molecular Effects of Sulforaphane and Capsaicin on Metabolism upon Androgen and Tip60 Activation of Androgen Receptor. Int. J. Mol. Sci. 2019, 20, 5384. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Li, Y.; Min, H.; Zhao, X.; Zhang, Y.; Qi, Y.; Shi, J.; Qi, S.; Bao, Y.; et al. Sulforaphane Mediates Glutathione Depletion via Polymeric Nanoparticles to Restore Cisplatin Chemosensitivity. ACS Nano 2019, 13, 13445–13455. [Google Scholar] [CrossRef]
- Ishiura, Y.; Ishimaru, H.; Watanabe, T.; Fujimuro, M. Sulforaphane Exhibits Cytotoxic Effects against Primary Effusion Lymphoma Cells by Suppressing p38MAPK and AKT Phosphorylation. Biol. Pharm. Bull. 2019, 42, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chun, F.K.; Rutz, J.; Blaheta, R.A. Sulforaphane Impact on Reactive Oxygen Species (ROS) in Bladder Carcinoma. Int. J. Mol. Sci. 2021, 22, 5938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Miao, Q.W.; Zhu, C.X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid beta deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimer’s Dis. Other Dement. 2015, 30, 183–191. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Djemil, A.; D’amico, M.; Pruccoli, L.; Cantelli-Forti, G.; Hrelia, P.; Tarozzi, A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and its Interconversion Product Erucin in in Vitro and in Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018, 66, 856–865. [Google Scholar] [CrossRef]
- Pu, Y.; Qu, Y.; Chang, L.; Wang, S.M.; Zhang, K.; Ushida, Y.; Suganuma, H.; Hashimoto, K. Dietary intake of glucoraphanin prevents the reduction of dopamine transporter in the mouse striatum after repeated administration of MPTP. Neuropsychopharmacol. Rep. 2019, 39, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.-H.; Ge, X.-L.; Zhang, J.; Song, X.-J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-M.; Lee, Y.-S.; Kim, W.; Kim, S.J.; Shin, K.-O.; Yu, J.-Y.; Lee, M.K.; Lee, Y.-M.; Hong, J.T.; Yun, Y.-P.; et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J. Nutr. Biochem. 2014, 25, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Shen, Y.; Wang, A.; Chen, S.; Zhang, H.; Chen, F.; Chen, Z.; Wei, H.; Zou, Z.; Shan, Y.; et al. Sulforaphane induces apoptosis in adipocytes via Akt/p70s6k1/Bad inhibition and ERK activation. Biochem. Biophys. Res. Commun. 2015, 465, 696–701. [Google Scholar] [CrossRef]
- Martin, F.; van Deursen, J.M.; Shivdasani, R.A.; Jackson, C.W.; Troutman, A.G.; Ney, P.A. Erythroid maturation and globin gene expression in mice with combined deficiency of NF-E2 and nrf-2. Blood 1998, 91, 3459–3466. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef] [PubMed]
- Pieters, J. Mycobacterium tuberculosis and the macrophage: Maintaining a balance. Cell Host Microbe 2008, 3, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef]
- Kurthkoti, K.; Varshney, U. Distinct mechanisms of DNA repair in mycobacteria and their implications in attenuation of the pathogen growth. Mech. Ageing Dev. 2012, 133, 138–146. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, N.; Trivedi, A.; Kansal, P.; Gupta, P.; Kumar, A. The mechanism of redox sensing in Mycobacterium tuberculosis. Free Radic. Biol. Med. 2012, 53, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Martin, C.J.; Booty, M.G.; Nishimura, T.; Zhao, X.; Gan, H.-X.; Divangahi, M.; Remold, H.G. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Farhana, A.; Guidry, L.; Saini, V.; Hondalus, M.; Steyn, A.J. Redox homeostasis in mycobacteria: The key to tuberculosis control? Expert Rev. Mol. Med. 2011, 13, e39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lan, P.; Hou, X.; Han, Q.; Lu, N.; Li, T.; Jiao, C.; Zhang, J.; Zhang, C.; Tian, Z. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J. Hepatol. 2017, 66, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yeon, S.H.; Lee, H.E.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Suppression of NLRP3 inflammasome by oral treatment with sulforaphane alleviates acute gouty inflammation. Rheumatology 2018, 57, 727–736. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, H.; Hong, E.J.; An, B.S.; Jeung, E.B.; Lee, G.S. Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell. Immunol. 2016, 306–307, 53–60. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Shen, L.; Hu, H.; Feng, Y.; Wen, D.; Liu, Y.; Zhai, H.; Sun, W.; Wang, M.; Lei, X.; et al. Sulforaphane Inhibits Oxidative Stress and May Exert Anti-Pyroptotic Effects by Modulating NRF2/NLRP3 Signaling Pathway in Mycobacterium tuberculosis-Infected Macrophages. Microorganisms 2024, 12, 1191. https://doi.org/10.3390/microorganisms12061191
Chen G, Shen L, Hu H, Feng Y, Wen D, Liu Y, Zhai H, Sun W, Wang M, Lei X, et al. Sulforaphane Inhibits Oxidative Stress and May Exert Anti-Pyroptotic Effects by Modulating NRF2/NLRP3 Signaling Pathway in Mycobacterium tuberculosis-Infected Macrophages. Microorganisms. 2024; 12(6):1191. https://doi.org/10.3390/microorganisms12061191
Chicago/Turabian StyleChen, Guangxin, Lin Shen, Hong Hu, Yazhi Feng, Da Wen, Yiyao Liu, Huizhe Zhai, Wei Sun, Meifen Wang, Xinghua Lei, and et al. 2024. "Sulforaphane Inhibits Oxidative Stress and May Exert Anti-Pyroptotic Effects by Modulating NRF2/NLRP3 Signaling Pathway in Mycobacterium tuberculosis-Infected Macrophages" Microorganisms 12, no. 6: 1191. https://doi.org/10.3390/microorganisms12061191




