Significant Factors for Modelling Survival of Escherichia coli in Lake Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Sampling
2.2. Preparation of E. coli Strain
2.3. Laboratory Experiments
2.3.1. Temperature
2.3.2. pH
2.3.3. Water-Extractable Total Dissolved Solids
2.3.4. Coexisting Microbes
2.3.5. Sampling Sites
2.4. Data Analysis
2.4.1. Determination of E. coli Population Change Rate
2.4.2. Relative Maximum E. coli Concentration
2.4.3. Statistical Analysis
3. Results and Discussion
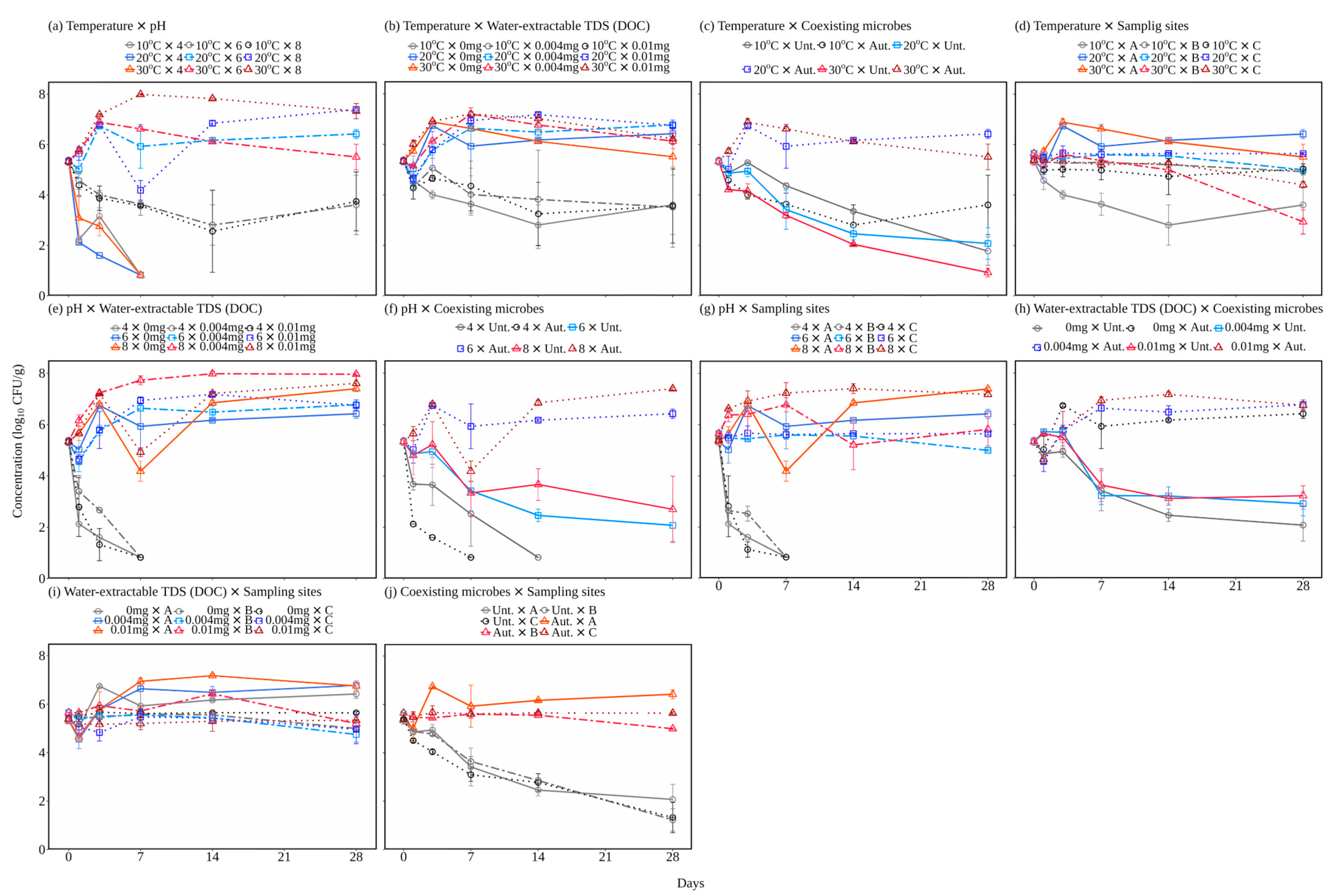
3.1. E. coli Survival under Various Sediment Conditions
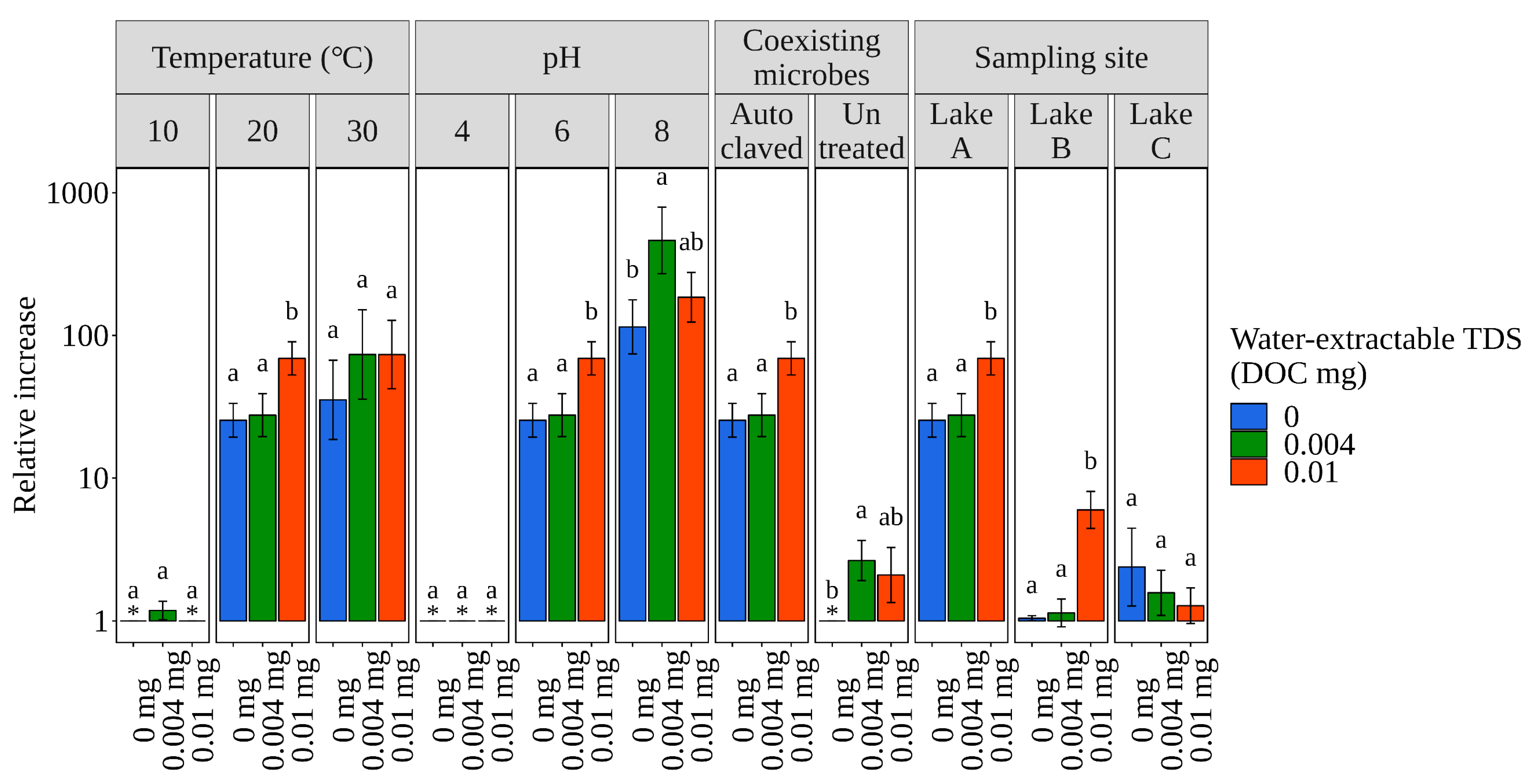
3.2. Quantification of Effects of Factors on E. coli Population Change Rate
3.2.1. Independent Effects of Each Factor
3.2.2. Interactive Effects of Each Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weller, D.L.; Murphy, C.M.; Johnson, S.; Green, H.; Michalenko, E.M.; Love, T.M.T.; Strawn, L.K. Land Use, Weather, and Water Quality Factors Associated with Fecal Contamination of Northeastern Streams That Span an Urban-Rural Gradient. Front. Water 2022, 3, 741676. [Google Scholar] [CrossRef]
- Ma, C.Y.; Sugie, Y.; Yu, Z.; Okuno, Y.; Tanaka, H.; Ihara, M. Occurrence of E. coli and Antibiotic-Resistant E. coli in the Southern Watershed of Lake Biwa, Including in Wastewater Treatment Plant Effluent and Inflow Rivers. Chemosphere 2022, 301, 134372. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Messer, T.; Mittelstet, A.; Berry, E.D.; Bartelt-Hunt, S.; Abimbola, O. Escherichia coli Concentrations in Waters of a Reservoir System Impacted by Cattle and Migratory Waterfowl. Sci. Total Environ. 2020, 705, 135607. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.A.; Shelton, D.R. Escherichia coli and Fecal Coliforms in Freshwater and Estuarine Sediments. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1067–1110. [Google Scholar] [CrossRef]
- Abia, A.L.K.; James, C.; Ubomba-Jaswa, E.; Momba, M.N.B. Microbial Remobilisation on Riverbed Sediment Disturbance in Experimental Flumes and a Human-Impacted River: Implication for Water Resource Management and Public Health in Developing Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2017, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Pachepsky, Y.A.; Kim, J.H.; Guber, A.K.; Shelton, D.R.; Rowland, R. Release of Escherichia coli from the Bottom Sediment in a First-Order Creek: Experiment and Reach-Specific Modeling. J. Hydrol. 2010, 391, 322–332. [Google Scholar] [CrossRef]
- European Environment Agency. Quality of Bathing Water—2009 Bathing Season; EEA: Copenhagen, Denmark, 2010. [Google Scholar]
- U.S. Environmental Protection Agency. Bacterial Water Quality Standards for Recreational Waters (Freshwater and Marine Waters); U.S. Environmental Protection Agency: Washington, DC, USA, 2003.
- Cho, S.; Jackson, C.R.; Frye, J.G. The Prevalence and Antimicrobial Resistance Phenotypes of Salmonella, Escherichia coli and Enterococcus Sp. in Surface Water. Lett. Appl. Microbiol. 2020, 71, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, J.; de Been, M.; Weerdenburg, E.; Zomer, A.; McNally, A.; Poolman, J. Genomics and Pathotypes of the Many Faces of Escherichia coli. FEMS Microbiol. Rev. 2022, 46, fuac031. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Lin, Z.; Yuan, T.; Zhou, L.; Cheng, S.; Qu, X.; Lu, P.; Feng, Q. Impact Factors of the Accumulation, Migration and Spread of Antibiotic Resistance in the Environment. Environ. Geochem. Health 2021, 43, 1741–1758. [Google Scholar] [CrossRef]
- Abia, A.L.K.; Ubomba-Jaswa, E.; Momba, M.N.B. Competitive Survival of Escherichia coli, Vibrio cholerae, Salmonella typhimurium and Shigella dysenteriae in Riverbed Sediments. Microb. Ecol. 2016, 72, 881–889. [Google Scholar] [CrossRef]
- Garzio-Hadzick, A.; Shelton, D.R.; Hill, R.L.; Pachepsky, Y.A.; Guber, A.K.; Rowland, R. Survival of Manure-Borne E. coli in Streambed Sediment: Effects of Temperature and Sediment Properties. Water Res. 2010, 44, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, C.D.W.; Curtis, L.M.; Humpheson, L.; Billon, C.; McClure, P.J. Development of Thermal Inactivation Models for Salmonella enteritidis and Escherichia coli O157:H7 with Temperature, PH and NaCl as Controlling Factors. Int. J. Food Microbiol. 1997, 38, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Parhad, N.M.; Rao, N.U. Effect of PH on Survival of Escherichia coli. J. (Water Pollut. Control. Fed.) 1974, 46, 980–986. [Google Scholar]
- Chandran, A.; Varghese, S.; Kandeler, E.; Thomas, A.; Hatha, M.; Mazumder, A. An Assessment of Potential Public Health Risk Associated with the Extended Survival of Indicator and Pathogenic Bacteria in Freshwater Lake Sediments. Int. J. Hyg. Environ. Health 2011, 214, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Haller, L.; Amedegnato, E.; Poté, J.; Wildi, W. Influence of Freshwater Sediment Characteristics on Persistence of Fecal Indicator Bacteria. Water. Air. Soil Pollut. 2009, 203, 217–227. [Google Scholar] [CrossRef]
- Smith, J.E.; Stocker, M.D.; Hill, R.L.; Pachepsky, Y.A. The Effect of Temperature Oscillations and Sediment Texture on Fecal Indicator Bacteria Survival in Sediments. Water. Air. Soil Pollut. 2019, 230, 270. [Google Scholar] [CrossRef]
- Rumball, N.A.; Mayer, H.R.C.; McLellan, S.L. Selective Survival of Escherichia coli Phylotypes in Freshwater Beach Sand. Appl. Environ. Microbiol. 2021, 87, e02473-20. [Google Scholar] [CrossRef] [PubMed]
- Wanjugi, P.; Harwood, V.J. The Influence of Predation and Competition on the Survival of Commensal and Pathogenic Fecal Bacteria in Aquatic Habitats. Environ. Microbiol. 2013, 15, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Japan Water Works Association. Water Supply in Japan; Japan Water Works Association: Tokyo, Japan, 2017. [Google Scholar]
- Van, L.A.; Nguyen, K.D.; Le Marrec, F.; Jairy, A. Development of a Tool for Modeling the Fecal Contamination in Rivers with TurbulentfFlows—Application to the Seine et Marne Rivers (Parisian Region, France). Water 2022, 14, 1191. [Google Scholar] [CrossRef]
- Huang, G.; Falconer, R.A.; Lin, B. Integrated Hydro-Bacterial Modelling for Predicting Bathing Water Quality. Estuar. Coast. Shelf Sci. 2017, 188, 145–155. [Google Scholar] [CrossRef]
- Sokolova, E.; Pettersson, T.J.R.; Bergstedt, O.; Hermansson, M. Hydrodynamic Modelling of the Microbial Water Quality in a Drinking Water Source as Input for Risk Reduction Management. J. Hydrol. 2013, 497, 15–23. [Google Scholar] [CrossRef]
- Shi, X.; Jovanovic, D.; Meng, Z.; Hipsey, M.R.; McCarthy, D. Modelling Faecal Microbe Dynamics within Stormwater Constructed Wetlands. Water Res. 2024, 248, 120855. [Google Scholar] [CrossRef] [PubMed]
- DeVilbiss, S.E.; Steele, M.K.; Krometis, L.A.H.; Badgley, B.D. Freshwater Salinization Increases Survival of Escherichia coli and Risk of Bacterial Impairment. Water Res. 2021, 191, 116812. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sutton, N.B.; Wagner, T.V.; Rijnaarts, H.H.M.; van der Wielen, P.W.J.J. Influence of Combined Abiotic/Biotic Factors on Decay of P. aeruginosa and E. coli in Rhine River Water. Appl. Microbiol. Biotechnol. 2024, 108, 294. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Hashimoto, R.; Xie, H.; Nishimura, E.; Nishiyama, M.; Nukazawa, K.; Ishii, S. Growth and Antibiotic Resistance Acquisition of Escherichia coli in a River That Receives Treated Sewage Effluent. Sci. Total Environ. 2019, 690, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ateia, M.; Hatano, Y.; Yoshimura, C. Regrowth of Escherichia coli in Environmental Waters after Chlorine Disinfection: Shifts in Viability and Culturability. Environ. Sci. Water Res. Technol. 2022, 8, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Ksoll, W.B.; Hicks, R.E.; Sadowsky, M.J. Presence and Growth of Naturalized Escherichia coli in Temperate Soils from Lake Superior Watersheds. Appl. Environ. Microbiol. 2006, 72, 612–621. [Google Scholar] [CrossRef]
- Shinohara, R.; Tsuchiya, K.; Kohzu, A. Warming of Water Temperature in Spring and Nutrient Release from Sediment in a Shallow Eutrophic Lake. J. Water Clim. Chang. 2021, 12, 3187–3199. [Google Scholar] [CrossRef]
- Blaga, C.I.; Reichart, G.J.; Schouten, S.; Lotter, A.F.; Werne, J.P.; Kosten, S.; Mazzeo, N.; Lacerot, G.; Sinninghe Damsté, J.S. Branched Glycerol Dialkyl Glycerol Tetraethers in Lake Sediments: Can They Be Used as Temperature and PH Proxies? Org. Geochem. 2010, 41, 1225–1234. [Google Scholar] [CrossRef]
- Lin, J.; Smith, M.P.; Chapin, K.C.; Baik, H.S.; Bennett, G.N.; Foster, J.W. Mechanisms of Acid Resistance in Enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 1996, 62, 3094–3100. [Google Scholar] [CrossRef]
- NandaKafle, G.; Christie, A.A.; Vilain, S.; Brözel, V.S. Growth and Extended Survival of Escherichia coli O157: H7 in Soil Organic Matter. Front. Microbiol. 2018, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shengrui, W.; Haichao, Z.; Yanping, L.; Shouliang, H.; Weibin, Q.; Yanli, Y.; Jie, C. Using Multiple Combined Analytical Techniques to Characterize Water Extractable Organic Nitrogen from Lake Erhai Sediment. Sci. Total Environ. 2016, 542, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Prayoga, W.; Nishiyama, M.; Praise, S.; Pham, D.V.; Van Duong, H.; Pham, L.K.; Dang, L.T.T.; Watanabe, T. Tracking Fecal Bacterial Dispersion from Municipal Wastewater to Peri-Urban Farms during Monsoon Rains in Hue City, Vietnam. Int. J. Environ. Res. Public Health 2021, 18, 9580. [Google Scholar] [CrossRef] [PubMed]
- Chick, H. An Investigation of the Laws of Disinfection. J. Hyg. 1908, 8, 92–158. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, E.O.; Quilliam, R.S.; Oliver, D.M. Persistence of E. coli in Streambed Sediment Contaminated with Faeces from Dairy Cows, Geese, and Deer: Legacy Risks to Environment and Health. Int. J. Environ. Res. Public Health 2023, 20, 5375. [Google Scholar] [CrossRef] [PubMed]
- LaLiberte, P.; Grimes, D.J. Survival of Escherichia coli in Lake Bottom Sediment. Appl. Environ. Microbiol. 1982, 43, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Orruño, M.; Kaberdin, V.R.; Arana, I. Survival Strategies of Escherichia coli and Vibrio Spp.: Contribution of the Viable but Nonculturable Phenotype to Their Stress-Resistance and Persistence in Adverse Environments. World J. Microbiol. Biotechnol. 2017, 33, 45. [Google Scholar] [CrossRef]
- Ismail, N.S.; Blokker, B.M.; Feeney, T.R.; Kohn, R.H.; Liu, J.; Nelson, V.E.; Ollive, M.C.; Price, S.B.L.; Underdah, E.J. Impact of Metazooplankton Filter Feeding on Escherichia coli under Variable Environmental Conditions. Appl. Environ. Microbiol. 2019, 85, e02006-19. [Google Scholar] [CrossRef] [PubMed]
- Sherr, B.F.; Sherr, E.B.; Rassoulzadegan, F. Rates of Digestion of Bacteria by Marine Phagotrophic Protozoa: Temperature Dependence. Appl. Environ. Microbiol. 1988, 54, 1091–1095. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Datta, A.R. Acid Tolerance of Enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 1995, 61, 1669–1672. [Google Scholar] [CrossRef]
- Ansa, E.D.O.; Lubberding, H.J.; Ampofo, J.A.; Gijzen, H.J. The Role of Algae in the Removal of Escherichia coli in a Tropical Eutrophic Lake. Ecol. Eng. 2011, 37, 317–324. [Google Scholar] [CrossRef]
- Liang, C.; Yao, Z.; Du, S.; Hong, M.; Wang, K.; Zhang, D. Sediment PH, Not the Bacterial Diversity, Determines Escherichia coli O157:H7 Survival in Estuarine Sediments. Environ. Pollut. 2019, 252, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wei, G.; Wang, H.; Wu, L.; Wu, J.; Xu, J. Survival of Escherichia coli O157: H7 in Soils from Vegetable Fields with Different Cultivation Patterns. Appl. Environ. Microbiol. 2013, 79, 1755–1756. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, T.; Wei, G.; Wu, L.; Wu, J.; Xu, J. Survival of Escherichia coli O157:H7 in Soils under Different Land Use Types. Environ. Sci. Pollut. Res. 2014, 21, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and Public Health Implications—A Review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Wanjugi, P.; Fox, G.A.; Harwood, V.J. The Interplay between Predation, Competition, and Nutrient Levels Influences the Survival of Escherichia coli in Aquatic Environments. Microb. Ecol. 2016, 72, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Barman, U.; Choudhury, R.D. Soil texture classification using multi class support vector machine. Inf. Process. Agric. 2020, 7, 318–332. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, R.; Ji, M.; Ren, Z.J. Organic content influences sediment microbial fuel cell performance and community structure. Bioresour. Technol. 2016, 220, 549–556. [Google Scholar] [CrossRef]


| Parameter | Levels | ||
|---|---|---|---|
| Temperature (°C) | 10 | 20 | 30 |
| pH | 4 | 6 | 8 |
| Added water-extractable TDSs (concentration of DOC: mg/g-wet) | 0 | 0.004 | 0.01 |
| Coexisting microbes | Untreated sediment | Autoclaved sediment (no protozoa and bacteria) | |
| Sampling site | Lake A | Lake B | Lake C |
| Factors (#1 × #2) | Effect Size (η2: %) | ||
|---|---|---|---|
| Factor #1 | Factor #2 | Interaction #1 × #2 | |
| Temperature × pH | 16.2 * | 68.9 * | 13.4 * |
| Temperature × Water-extractable TDSs | 65.9 * | 14.4 * | 16.5 * |
| Temperature × Coexisting microbes | 25.8 * | 46.0 * | 26.4 * |
| Temperature × Sampling site | 22.5 * | 36.1 * | 36.9 * |
| pH × Water-extractable TDSs | 89.0 * | 3.2 * | 7.4 * |
| pH × Coexisting microbes | 59.5 * | 9.0 * | 31.1 * |
| pH × Sampling site | 84.1 * | 4.8 * | 9.9 * |
| Water-extractable TDSs × Coexisting microbes | 0.4 | 1.7 | 85.4 * |
| Water-extractable TDSs × Sampling site | 20.9 * | 44.3 * | 30.2 * |
| Coexisting microbes × Sampling site | 48.4 * | 26.6 * | 22.3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, I.; Nishiyama, M.; Watanabe, T. Significant Factors for Modelling Survival of Escherichia coli in Lake Sediments. Microorganisms 2024, 12, 1192. https://doi.org/10.3390/microorganisms12061192
Yoneda I, Nishiyama M, Watanabe T. Significant Factors for Modelling Survival of Escherichia coli in Lake Sediments. Microorganisms. 2024; 12(6):1192. https://doi.org/10.3390/microorganisms12061192
Chicago/Turabian StyleYoneda, Ichiro, Masateru Nishiyama, and Toru Watanabe. 2024. "Significant Factors for Modelling Survival of Escherichia coli in Lake Sediments" Microorganisms 12, no. 6: 1192. https://doi.org/10.3390/microorganisms12061192






