Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI)
Abstract
1. Introduction
2. Materials and Methods
3. Biofilm
3.1. Biofilm Formation
3.2. Biofilm Formation in Static (Non-Flow) and Flow Conditions
3.3. Biofilm Related Infections in Orthopedy
3.4. Periprosthetic Joint Infections’ Etiology
4. Implant against Biofilm Materials
4.1. Passive Surface Finishing/Modification (PSM)
4.2. Active Surface Finishing/Modification (ASM)
5. Microbiological Methods of Diagnosing Biofilm Formation in Orthopedics
5.1. Direct Microbiological Culturing
5.2. Culture-Negative PJIs
5.3. Culturing Sonication Fluid
5.4. DTT Pre-Treatment Method
5.5. BioTimer Assay (BTA)
5.6. Agar Encasement Culturing Method (AECM) with Candle Dip Method
6. Molecular Diagnostic Methods
6.1. PCR
6.2. Specific PCR
6.3. Broad-Range PCR
6.4. Next Generation Sequencing (NGS)
6.5. Metagenomic Next Generation Sequencing (mNGS)
6.6. Fluorescence In Situ Hybridization (FISH)
7. Microbiological Methods of Visualizing Biofilms
7.1. Scanning Electron Microscopy
7.2. Confocal Laser Scanning Microscopy (CLSM)
7.3. Methylene Blue (MB) as a Disclosing Agent in Biofilm Related Infections Treatment
8. Antibiotic Therapy
8.1. Types of Therapy
8.2. Bone and Joint Penetration
8.3. Treatment Duration
8.4. Antibiofilm Activity
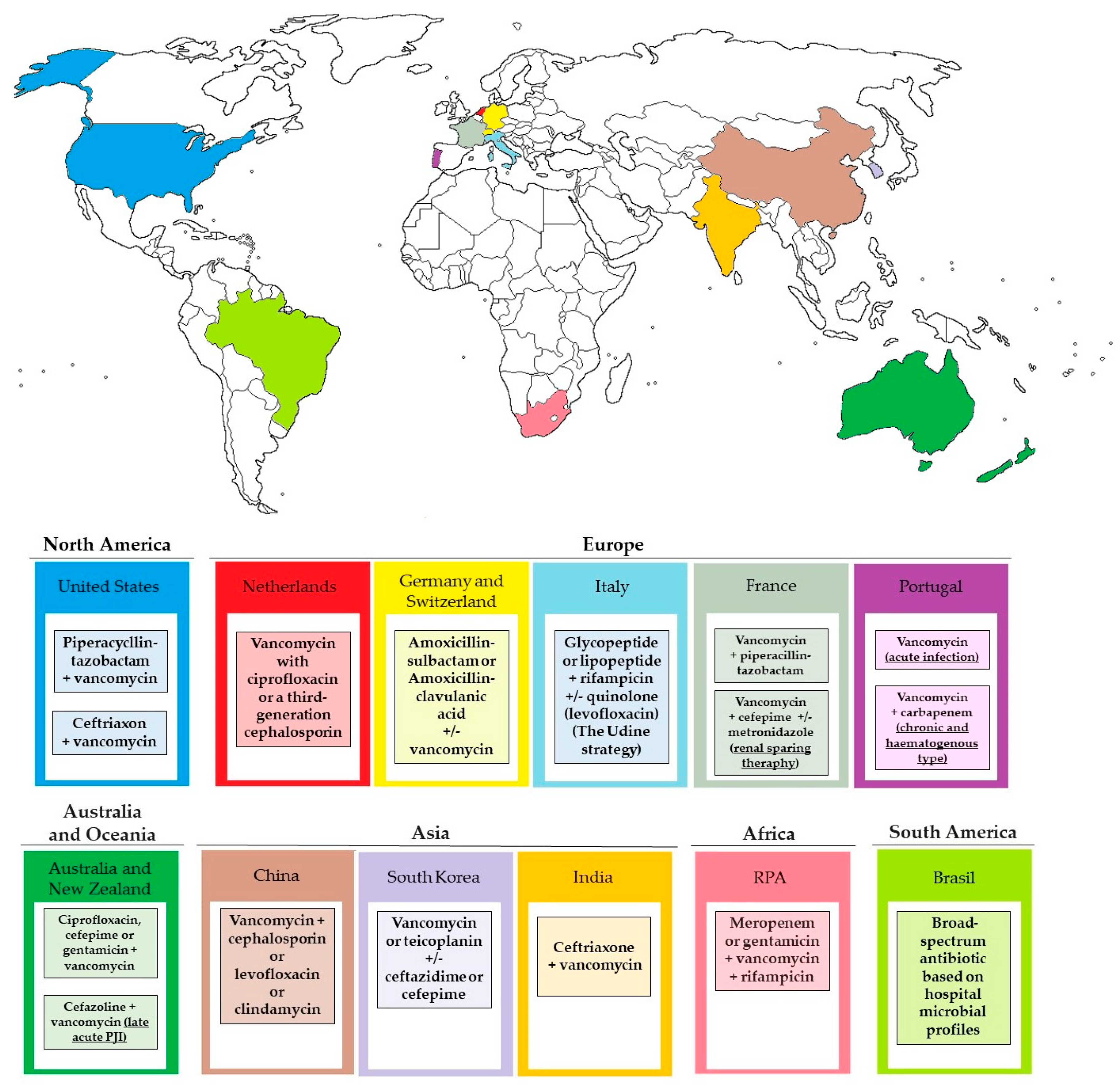
8.5. Empiric Therapy
8.6. Targeted Therapy
8.6.1. Staphylococuus spp.
8.6.2. Streptococcus spp.
8.6.3. Enteroccous spp.
8.6.4. Pseudomonas aeruginosa
8.6.5. Enterobacteriacea
8.6.6. Cutibacterium spp.
8.6.7. Other Anaerobes
| Bacteria | Initial IV Therapy | Possible Oral Switch | References |
|---|---|---|---|
| Methicillin-susceptible Staphylococcus | Oxacillin+ rifampicin Cefazolin+ rifampicin Levofloxacin + rifampicin | Levofloxacin + rifampicin Ciprofloxacin + rifampicin | [99,109] |
| Methicillin-resistant Staphylococcus | Vancomycin (most recommended) + rifampicin or minocycline Daptomycin or linezolid+ rifampicin or minocycline | No oral switch | [103,140,149] |
| Streptococcus spp. | Penicillin Ceftriaxone Amoxicillin | Amoxicillin | [141,142,143] |
| Enterococcus spp. | High-dose penicillin or ampicillin +gentamicin or rifampicin Amoxicillin +/− initial gentamicin | Amoxicillin (worse bioavailability) Clindamycin Linezolid | [99,144,145] |
| Pseudomonas aeruginosa | Cefepime Meropenem Ceftazidime Ciprofloxacin | Ciprofloxacin | [109,146] |
| Enterobacteriaceae | Cefotaxime Ceftriaxone | Ciprofloxacin Levofloxacin | [99,147,148] |
| Ciprofloxacin | [153] | ||
| Cutibacterium spp. | Amoxicillin Clindamycin Cefazolin | Amoxicillin Clindamycin | [99,150,152] |
| Penicillin Ceftriaxon | [151] | ||
| Bacteroides spp. | Metronidazole Imipenem Clindamycin | Metronidazole Imipenem Clindamycin | [150,152] |
| Clostridium spp. | Penicillin Clindamycin Metronidazole | Clindamycin Metronidazole | [153] |
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erivan, R.; Villatte, G.; Dartus, J.; Reina, N.; Descamps, S.; Boisgard, S. Progression and projection for hip surgery in France, 2008–2070: Epidemiologic study with trend and projection analysis. Orthop. Traumatol. Surg. Res. 2019, 105, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Zimmerli, W. Prosthetic joint infections: Update in diagnosis and treatment. Swiss Med. Wkly. 2005, 135, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Sobierajska, P.; Okińczyc, P.; Widelski, J.; Duda-Madej, A.; Krzyżanowska, B.; Krzyżek, P.; Ogórek, R.; Szperlik, J.; Chmielowiec, J.; et al. Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA. Int. J. Mol. Sci. 2022, 23, 1533. [Google Scholar] [CrossRef]
- Dibartola, A.C.; Swearingen, M.C.; Granger, J.F.; Stoodley, P.; Dusane, D.H. Biofilms in orthopedic infections: A review of laboratory methods. APMIS 2017, 125, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Post, J.C.; Ehrlich, G.D.; Hu, F.Z.; Kreft, R.; Nistico, L.; Kathju, S.; Stoodley, P.; Hall-Stoodley, L.; Maale, G.; et al. New methods for the detection of orthopedic and other biofilm infections. FEMS Immunol. Med. Microbiol. 2011, 61, 133–140. [Google Scholar] [CrossRef]
- Ronin, D.; Boyer, J.; Alban, N.; Natoli, R.M.; Johnson, A.; Kjellerup, B.V. Current and novel diagnostics for orthopedic implant biofilm infections: A review. APMIS 2022, 130, 59–81. [Google Scholar] [CrossRef]
- Hernández-Jiménez, E.; Del Campo, R.; Toledano, V.; Vallejo-Cremades, M.T.; Muñoz, A.; Largo, C.; Arnalich, F.; García-Rio, F.; Cubillos-Zapata, C.; López-Collazo, E. Biofilm vs. planktonic bacterial mode of growth: Which do human macrophages prefer? Biochem. Biophys. Res. Commun. 2013, 441, 947–952. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Paluch, E.; Szperlik, J.; Lamch, Ł.; Wilk, K.A.; Obłak, E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci. Rep. 2021, 11, 8896. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Romo, J.A. Fungal Biofilms 2020. J. Fungi 2021, 7, 603. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Okińczyc, P.; Zwyrzykowska-Wodzińska, A.; Szperlik, J.; Żarowska, B.; Duda-Madej, A.; Bąbelewski, P.; Włodarczyk, M.; Wojtasik, W.; Kupczyński, R.; et al. Composition and Antimicrobial Activity of Ilex Leaves Water Extracts. Molecules 2021, 26, 7442. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation-Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Marzec-Grządziel, A.; Paluch, E.; Pilarski, K.; Wolna-Maruwka, A.; Kubiak, A.; Kałuża, T.; Kulupa, T. Biofilm Formation and Genetic Diversity of Microbial Communities in Anaerobic Batch Reactor with Polylactide (PLA) Addition. Int. J. Mol. Sci. 2023, 24, 10042. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan Thirunavukkarasu, G.; Bacova, J.; Monfort, O.; Dworniczek, E.; Paluch, E.; Bilal Hanif, M.; Rauf, S.; Motlochova, M.; Capek, J.; Hensel, K.; et al. Critical Comparison of Aerogel TiO2 and P25 Nanopowders: Cytotoxic Properties, Photocatalytic Activity and Photoinduced Antimicrobial/Antibiofilm Performance. Appl. Surf. Sci. 2022, 579, 152145. [Google Scholar] [CrossRef]
- Stoodley, P.; Ehrlich, G.D.; Sedghizadeh, P.P.; Hall-Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; Costerton, J.W.; Demeo, P. Orthopaedic biofilm infections. Curr. Orthop. Pract. 2011, 22, 558–563. [Google Scholar] [CrossRef]
- Colston, J.; Atkins, B. Bone and joint infection. Clin. Med. 2018, 18, 150–154. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Cobo, J.; Fernández-Sampedro, M.; Del Toro, M.D.; Guío, L.; et al. The Different Microbial Etiology of Prosthetic Joint Infections according to Route of Acquisition and Time after Prosthesis Implantation, Including the Role of Multidrug-Resistant Organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Ayoade, F.; Li, D.D.; Mabrouk, A.; Todd, J.R. Periprosthetic Joint Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sambri, A.; Zunarelli, R.; Fiore, M.; Bortoli, M.; Paolucci, A.; Filippini, M.; Zamparini, E.; Tedeschi, S.; Viale, P.; De Paolis, M. Epidemiology of Fungal Periprosthetic Joint Infection: A Systematic Review of the Literature. Microorganisms 2022, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial Coating of Implants in Orthopaedics and Trauma: A Classification Proposal in an Evolving Panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant Materials and Prosthetic Joint Infection: The Battle with the Biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Cochis, A.; Azzimonti, B.; Caravaca, C.; Chevalier, J.; Kuntz, M.; Porporati, A.A.; Streicher, R.M.; Rimondini, L. Reduced Bacterial Adhesion on Ceramics Used for Arthroplasty Applications. J. Eur. Ceram. Soc. 2018, 38, 963–970. [Google Scholar] [CrossRef]
- Paulitsch-Fuchs, A.H.; Bödendorfer, B.; Wolrab, L.; Eck, N.; Dyer, N.P.; Lohberger, B. Effect of Cobalt-Chromium-Molybdenum Implant Surface Modifications on Biofilm Development of S. aureus and S. epidermidis. Front. Cell. Infect. Microbiol. 2022, 12, 837124. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured Titanium Surfaces Exhibit Recalcitrance towards Staphylococcus epidermidis Biofilm Formation. Sci. Rep. 2018, 8, 1071. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion Behaviors on Superhydrophobic Surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Yin, C.; Dai, S.; Fan, X.; Jiang, Y.; Liu, X.; Fang, J.; Yi, B.; Zhou, Q.; et al. Recent Progress in Antibacterial Hydrogel Coatings for Targeting Biofilm to Prevent Orthopedic Implant-Associated Infections. Front. Microbiol. 2023, 14, 1343202. [Google Scholar] [CrossRef] [PubMed]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-Adhesion Activity of Two Biosurfactants Produced by Bacillus spp. Prevents Biofilm Formation of Human Bacterial Pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial Coating of Implants: Are We Missing Something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Hailer, N.P. The Role of Silver Coating for Arthroplasty Components. Bone Jt. J. 2021, 103-B, 423–429. [Google Scholar] [CrossRef]
- Sevencan, A.; Kartal Doyuk, E.; Köse, N. Silver Ion Doped Hydroxyapatite-Coated Titanium Pins Prevent Bacterial Colonization. Jt. Dis. Relat. Surg. 2021, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Yu, W.X.; Hwang, P.A.; Huang, S.S.; Lin, H.M.; Hsu, Y.W.; Hsu, F.Y. Fabrication and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CaCO₃ Nanofibers with a Mesoporous Structure as Drug Delivery Carriers. Pharmaceutics 2018, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Tsuchiya, H.; Nishida, H.; Yamamoto, N.; Watanabe, K.; Nakase, J.; Terauchi, R.; Arai, Y.; Fujiwara, H.; Kubo, T. Antimicrobial Megaprostheses Supported with Iodine. J. Biomater. Appl. 2014, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Srimaneepong, V.; Skallevold, H.E.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Sapkota, J. Graphene for Antimicrobial and Coating Application. Int. J. Mol. Sci. 2022, 23, 499. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Stange, R.; Schmidmaier, G.; Raschke, M.J. The Use of Gentamicin-Coated Nails in the Tibia: Preliminary Results of a Prospective Study. Arch. Orthop. Trauma Surg. 2011, 131, 1419–1425. [Google Scholar] [CrossRef]
- Almasri, D.; Dahman, Y. Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option. Pharmaceutics 2023, 15, 1401. [Google Scholar] [CrossRef]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and Hydroxyapatite Based Biomaterials to Prevent Periprosthetic Joint Infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Lin, C.C.; Liao, J.W.; Yen, S.K. Vancomycin-Chitosan Composite Deposited on Post Porous Hydroxyapatite Coated Ti6Al4V Implant for Drug Controlled Release. Mater. Sci. Eng. C 2013, 33, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Kheir, M.M.; Tan, T.L.; Ackerman, C.T.; Modi, R.; Foltz, C.; Parvizi, J. Culturing Periprosthetic Joint Infection: Number of Samples, Growth Duration, and Organisms. J. Arthroplast. 2018, 33, 3531–3536.e1. [Google Scholar] [CrossRef] [PubMed]
- National Periprosthetic Joint Infection Sampling and Culture Guide, Health Quality and Safety Commission New Zealand, March 2018. Available online: https://www.hqsc.govt.nz/assets/Our-work/Infection-Prevention-Control/Publications-resources/National_PJI_sampling_guide_Mar_2018.pdf (accessed on 1 May 2024).
- Palan, J.; Nolan, C.; Sarantos, K.; Westerman, R.; King, R.; Foguet, P. Culture-Negative Periprosthetic Joint Infections. EFORT Open Rev. 2019, 4, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wouthuyzen-Bakker, M. Cultures in periprosthetic joint infections, the imperfect gold standard? EFORT Open Rev. 2023, 8, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.F.; Riedel, S. A Case Illustrating the Practical Application of the AAOS Clinical Practice Guideline: Diagnosis and Prevention of Periprosthetic Joint Infection. J. Am. Acad. Orthop. Surg. 2020, 28, e1081–e1085. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.L.; Kheir, M.M.; Shohat, N.; Tan, D.D.; Kheir, M.; Chen, C.; Parvizi, J. Culture-Negative Periprosthetic Joint Infection: An Update on What to Expect. JB JS Open Access 2018, 3, e0060. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.S.; Parvizi, J. Diagnosis and Treatment of Culture-Negative Periprosthetic Joint Infection. J. Arthroplast. 2022, 37, 1488–1493. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. The Function of Sonication in the Diagnosis of Periprosthetic Joint Infection After Total Knee Arthroplasty. Arch. Bone Jt. Surg. 2022, 10, 735–740. [Google Scholar] [CrossRef]
- Sebastian, S.; Malhotra, R.; Sreenivas, V.; Kapil, A.; Chaudhry, R.; Dhawan, B. Sonication of orthopaedic implants: A valuable technique for diagnosis of prosthetic joint infections. J. Microbiol. Methods 2018, 146, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.E.; Jacobson, M.J.; Cofield, R.H.; Sperling, J.W.; Sanchez-Sotelo, J.; Osmon, D.R.; McDowell, A.; Patrick, S.; Steckelberg, J.M.; Mandrekar, J.N.; et al. Microbiologic Diagnosis of Prosthetic Shoulder Infection by Use of Implant Sonication. J. Clin. Microbiol. 2009, 47, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Fu, J.; Yu, B.; Sun, W.; Chen, J.; Hao, L. Meta-Analysis of Sonication Prosthetic Fluid PCR for Diagnosing Periprosthetic Joint Infection. PLoS ONE 2018, 13, e0196418. [Google Scholar] [CrossRef] [PubMed]
- Ale, J.S.; Castelo, F.S.; Barros, B.S.; Ribau, A.C.; Carvalho, A.D.; Sousa, R.J.G. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection-A Systematic Review and Meta-Analysis of Their Diagnostic Accuracy According to Different Definitions. J. Arthroplast. 2023, 38, 2731–2738.e3. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.E.; Salvadó, M.; Trampuz, A.; Siverio, A.; Alier, A.; Sorli, L.; Martínez, S.; Pérez-Prieto, D.; Horcajada, J.P.; Puig-Verdie, L. Improved Diagnosis of Orthopedic Implant-Associated Infection by Inoculation of Sonication Fluid into Blood Culture Bottles. J. Clin. Microbiol. 2015, 53, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Romanò, D.; Fidanza, A.; Giannetti, A.; Erasmo, R.; Mavrogenis, A.F.; Romanò, C.L. Dithiothreitol Pre-Treatment of Synovial Fluid Samples Improves Microbiological Counts in Peri-Prosthetic Joint Infection. Int. Orthop. 2023, 47, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Signori, V.; De Vecchi, E.; Vassena, C.; Palazzi, E.; Cappelletti, L.; Romanò, D.; Romanò, C.L. Use of Dithiothreitol to Improve the Diagnosis of Prosthetic Joint Infections. J. Orthop. Res. 2013, 31, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Cadossi, M.; Giannini, S.; Pignatti, G.; Marcacci, M.; Neri, M.P.; Maso, A.; Storni, E.; Gamberini, S.; Naldi, S.; et al. Is Treatment With Dithiothreitol More Effective Than Sonication for the Diagnosis of Prosthetic Joint Infection? Clin. Orthop. Relat. Res. 2018, 476, 137–145. [Google Scholar] [CrossRef]
- Silva, N.D.S.; De Melo, B.S.T.; Oliva, A.; de Araújo, P.S.R. Sonication Protocols and Their Contributions to the Microbiological Diagnosis of Implant-Associated Infections: A Review of the Current Scenario. Front. Cell Infect. Microbiol. 2024, 14, 1398461. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Frioni, A.; Natalizi, T.; Coltella, L.; Berlutti, F. BioTimer Assay, a New Method for Counting Staphylococcus spp. in Biofilm without Sample Manipulation Applied to Evaluate Antibiotic Susceptibility of Biofilm. J. Microbiol. Methods 2008, 75, 478–484. [Google Scholar] [CrossRef]
- Moley, J.P.; McGrath, M.S.; Granger, J.F.; Sullivan, A.C.; Stoodley, P.; Dusane, D.H. Mapping bacterial biofilms on recovered orthopaedic implants by a novel agar candle dip method. APMIS 2019, 127, 123–130. [Google Scholar] [CrossRef]
- Khehra, N.; Padda, I.S.; Swift, C.J. Polymerase Chain Reaction (PCR). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gatti, G.; Taddei, F.; Brandolini, M.; Mancini, A.; Denicolò, A.; Congestrì, F.; Manera, M.; Arfilli, V.; Battisti, A.; Zannoli, S.; et al. Molecular Approach for the Laboratory Diagnosis of Periprosthetic Joint Infections. Microorganisms 2022, 10, 1573. [Google Scholar] [CrossRef]
- Saeed, K.; Ahmad-Saeed, N. The impact of PCR in the management of prosthetic joint infections. Expert Rev. Mol. Diagn. 2015, 15, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Prinz, J.; Schmid, B.; Zbinden, R.; Zingg, P.O.; Uçkay, I.; Achermann, Y.; Bosshard, P.P. Fast and Sensitive Multiplex Real-Time Quantitative PCR to Detect Cutibacterium Periprosthetic Joint Infections. J. Mol. Diagn. 2022, 24, 666–673. [Google Scholar] [CrossRef]
- Como, M.; Reddy, R.P.; Hankins, M.L.; Kane, G.E.; Ma, D.; Alexander, P.G.; Urish, K.L.; Karimi, A.; Lin, A. Quantitative Real-Time Polymerase Chain Reaction May Serve as a Useful Adjunct to Conventional Culture in The Detection of Cutibacterium acnes in the Glenohumeral Joint: A Study of 100 Consecutive Patients. Arch. Bone Jt. Surg. 2024, 12, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cho, Y.J. Current Guideline for Diagnosis of Periprosthetic Joint Infection: A Review Article. Hip Pelvis 2021, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Cazanave, C.; Cunningham, S.A.; Greenwood-Quaintance, K.E.; Steckelberg, J.M.; Uhl, J.R.; Hanssen, A.D.; Karau, M.J.; Schmidt, S.M.; Osmon, D.R.; et al. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J. Clin. Microbiol. 2012, 50, 3501–3508. [Google Scholar] [CrossRef]
- Hartley, J.C.; Harris, K.A. Molecular techniques for diagnosing prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. S1), i21–i24. [Google Scholar] [CrossRef]
- Prieto-Borja, L.; Rodriguez-Sevilla, G.; Auñon, A.; Pérez-Jorge, C.; Sandoval, E.; Garcia-Cañete, J.; Gadea, I.; Fernandez-Roblas, R.; Blanco, A.; Esteban, J. Evaluation of a commercial multi-plex PCR (Unyvero i60®) designed for the diagnosis of bone and joint infections using prosthetic-joint sonication. Enfermedades Infecc. Microbiol. Clin. 2017, 35, 236–242. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Ivy, M.I.; Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Hanssen, A.D.; Abdel, M.P.; Chia, N.; Yao, J.Z.; Tande, A.J.; Mandrekar, J.N.; et al. Direct Detection and Identification of Prosthetic Joint Infection Pathogens in Synovial Fluid by Metagenomic Shotgun Sequencing. J. Clin. Microbiol. 2018, 56, e00402-18. [Google Scholar] [CrossRef]
- Mei, J.; Hu, H.; Zhu, S.; Ding, H.; Huang, Z.; Li, W.; Yang, B.; Zhang, W.; Fang, X. Diagnostic Role of mNGS in Polymicrobial Periprosthetic Joint Infection. J. Clin. Med. 2023, 12, 1838. [Google Scholar] [CrossRef]
- Nath, J.; Johnson, K.L. A review of fluorescence in situ hybridization (FISH): Current status and future prospects. Biotech Histochem. 2000, 75, 54–78. [Google Scholar] [CrossRef]
- Shakoori, A.R. Fluorescence In Situ Hybridization (FISH) and Its Applications. In Chromosome Structure and Aberrations; Springer: Berlin/Heidelberg, Germany, 2017; pp. 343–367. Available online: https://link.springer.com/chapter/10.1007/978-81-322-3673-3_16 (accessed on 11 May 2024). [CrossRef]
- Barbosa, A.; Miranda, S.; Azevedo, N.F.; Cerqueira, L.; Azevedo, A.S. Imaging biofilms using fluorescence in situ hybridization: Seeing is believing. Front. Cell Infect. Microbiol. 2023, 13, 1195803. [Google Scholar] [CrossRef]
- Karygianni, L.; Hellwig, E.; Al-Ahmad, A. Multiplex fluorescence in situ hybridization (M-FISH) and confocal laser scanning microscopy (CLSM) to analyze multispecies oral biofilms. Methods Mol. Biol. 2014, 1147, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G. Scanning electron microscopy: Preparation and imaging for SEM. Methods Mol. Biol. 2012, 915, 1–20. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning electron microscopy. Curr. Protoc Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Jhass, A.K.; Johnston, D.A.; Gulati, A.; Anand, R.; Stoodley, P.; Sharma, S. A scanning electron microscope characterisation of biofilm on failed craniofacial osteosynthesis miniplates. J. Craniomaxillofac. Surg. 2014, 42, e372–e378. [Google Scholar] [CrossRef]
- Moore, K.; Gupta, N.; Gupta, T.T.; Patel, K.; Brooks, J.R.; Sullivan, A.; Litsky, A.S.; Stoodley, P. Mapping Bacterial Biofilm on Features of Orthopedic Implants In Vitro. Microorganisms 2022, 10, 586. [Google Scholar] [CrossRef]
- Nistico, L.; Hall-Stoodley, L.; Stoodley, P. Imaging bacteria and biofilms on hardware and periprosthetic tissue in orthopedic infections. Methods Mol. Biol. 2014, 1147, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Paddock, S.W. Confocal laser scanning microscopy. Biotechniques 1999, 27, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. Investigation of microbial biofilm structure by laser scanning microscopy. Adv. Biochem. Eng. Biotechnol. 2014, 146, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Canette, A.; Briandet, R. MICROSCOPY|Confocal Laser Scanning Microscopy. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 676–683. [Google Scholar]
- Reichhardt, C.; Parsek, M.R. Confocal Laser Scanning Microscopy for Analysis of Pseudomonas aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Agrappi, S.; Bortolin, M.; Toscano, M.; Romanò, C.L.; De Vecchi, E. How to Study Biofilms after Microbial Colonization of Materials Used in Orthopaedic Implants. Int. J. Mol. Sci. 2016, 17, 293. [Google Scholar] [CrossRef]
- Pushparajah Mak, R.S.; Liebelt, E.L. Methylene Blue: An Antidote for Methemoglobinemia and Beyond. Pediatr. Emerg. Care. 2021, 37, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. The development of phenothiazinium photosensitisers. Photodiagn. Photodyn. Ther. 2005, 2, 263–272. [Google Scholar] [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Szczęsny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A Review on Biomaterials for Orthopaedic Surgery and Traumatology: From Past to Present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef]
- Shaw, J.D.; Brodke, D.S.; Williams, D.L.; Ashton, N.N. Methylene Blue Is an Effective Disclosing Agent for Identifying Bacterial Biofilms on Orthopaedic Implants. J. Bone Jt. Surg. Am. 2020, 102, 1784–1791. [Google Scholar] [CrossRef]
- Shaw, J.D.; Miller, S.; Plourde, A.; Shaw, D.L.; Wustrack, R.; Hansen, E.N. Methylene Blue-Guided Debridement as an Intraoperative Adjunct for the Surgical Treatment of Periprosthetic Joint Infection. J. Arthroplast. 2017, 32, 3718–3723. [Google Scholar] [CrossRef]
- Bužga, M.; Machytka, E.; Dvořáčková, E.; Švagera, Z.; Stejskal, D.; Máca, J.; Král, J. Methylene blue: A controversial diagnostic acid and medication? Toxicol. Res. 2022, 11, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Infectious Diseases Society of America. Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Triffault-Fillit, C.; Ferry, T.; Laurent, F.; Pradat, P.; Dupieux, C.; Conrad, A.; Becker, A.; Lustig, S.; Fessy, M.H.; Chidiac, C.; et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 353–358. [Google Scholar] [CrossRef]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorli, L.; Fresco, G.; Fernandez-Sampedro, M.; Del Toro, M.D.; Guıo, L.; et al. Time trends in the etiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef]
- Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Metten, M.A.; Desplaces, N.; Marmor, S. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J. Infect. 2018, 76, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Zimmerli, W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin. Microbiol. Infect. 2012, 18, 1176–1184. [Google Scholar] [CrossRef]
- Tanaka, G.; Shigeta, M.; Komatsuzawa, H.; Sugai, M.; Suginaka, H.; Usui, T. Effect of the growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents: Beta-lactams and fluoroquinolones. Chemotherapy 1999, 45, 28–36. [Google Scholar] [CrossRef]
- Zeller, V.; Magreault, S.; Heym, B.; Salmon, D.; Kitzis, M.D.; Billaud, E.; Marmor, S.; Jannot, A.S.; Salomon, L.; Jullien, V. Influence of the clindamycin administration route on the magnitude of clindamycin-rifampicin interaction: A prospective pharmacokinetic study. Clin. Microbiol. Infect. 2021, 27, 1857.e1–1857.e7. [Google Scholar] [CrossRef] [PubMed]
- Fischbacher, A.; Borens, O. Prosthetic-joint Infections: Mortality Over the Last 10 Years. J. Bone Jt. Infect. 2019, 4, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Shrayteh, Z.M.; Rahal, M.K.; Malaeb, D.N. Practice of switch from intravenous to oral antibiotics. Springerplus 2014, 3, 717. [Google Scholar] [CrossRef]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Forest, G.N.; Tamura, K. Rifampin combination therapy for nonmycobacterial infections. Clin. Microbiol. Rev. 2010, 23, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, H.; Uçkay, I.; Vuagnat, A.; Druon, J.; Gras, G.; Rosset, P.; Lipsky, B.A.; Bernard, L. Antibiotic therapy duration for prosthetic joint infections treated by Debridement and Implant Retention (DAIR): Similar long-term remission for 6 weeks as compared to 12 weeks. Int. J. Infect. Dis. 2017, 63, 37–42. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Euba, G.; Cobo, J.; Lora-Tamayo, J.; Euba, G.; Cobo, J.; Horcajada, J.P.; Soriano, A.; Sandoval, E.; Pigrau, C.; et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: A randomised clinical trial. Int. J. Antimicrob. Agents 2016, 48, 310–316. [Google Scholar] [CrossRef]
- Benkabouche, M.; Racloz, G.; Spechbach, H.; Lipsky, B.A.; Gaspoz, J.M.; Uçkay, I. Four versus six weeks of antibiotic therapy for osteoarticular infections after implant removal: A randomized trial. J. Antimicrob. Chemother. 2019, 74, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Aboltins, C.; Daffy, J.; Choong, P.; Stanley, P. Current concepts in the management of prosthetic joint infection. Intern. Med. J. 2014, 44, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Arvieux, C.; Brunschweiler, B.; Touchais, S.; Ansart, S.; Bru, J.P.; Oziol, E.; Boeri, C.; Gras, G.; Druon, J.; et al. Antibiotic Therapy for 6 or 12 Weeks for Prosthetic Joint Infection. N. Engl. J. Med. 2021, 384, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Macià, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Gellert, M.; Hardt, S.; Köder, K.; Renz, N.; Perka, C.; Trampuz, A. Biofilm-active antibiotic treatment improves the outcome of knee periprosthetic joint infection: Results from a 6-year prospective cohort study. Int. J. Antimicrob. Agents 2020, 55, 105904. [Google Scholar] [CrossRef]
- Parra-Ruiz, J.; Bravo-Molina, A.; Peña-Monje, A.; Hernández-Quero, J. Activity of linezolid and high-dose daptomycin, alone or in combination, in an in vitro model of Staphylococcus aureus biofilm. J. Antimicrob. Chemother. 2012, 67, 2682–2685. [Google Scholar] [CrossRef]
- Aktas, G.; Derbentli, S. In vitro activity of daptomycin combined with dalbavancin and linezolid, and dalbavancin with linezolid against MRSA strains. J. Antimicrob. Chemother. 2017, 72, 441–443. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Z.; Hu, Y.; Deng, C.; Liao, L.; Sun, L.; Wang, C. Systematic review and meta-analysis of the efficacy and safety of vancomycin combined with β-lactam antibiotics in the treatment of methicillin-resistant Staphylococcus aureus bloodstream infections. J. Glob. Antimicrob. Resist. 2020, 23, 303–310. [Google Scholar] [CrossRef]
- University of Nebraska Medical Center. Joint Panel Guidance for Antimicrobial Stewardship in Orthopaedic Surgery. Available online: https://www.unmc.edu/intmed/_documents/id/asp/jointpanel_guidance.pdf (accessed on 15 March 2024).
- University of Michigan Health System. Antibiotic Guidelines: Orthopaedic Infections. Available online: https://www.med.umich.edu/asp/pdf/adult_guidelines/Bone-Joint_ADULT.pdf (accessed on 15 March 2024).
- Scholten, R.; Klein Klouwenberg, P.M.C.; Gisolf, J.E.H.; van Susante, J.L.C.; Somford, M.P. Empiric antibiotic therapy in early periprosthetic joint infection: A retrospective cohort study. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 29–35. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Leone, S.; Bassetti, M.; Borrè, S.; Leoncini, F.; Meani, E.; Venditti, M.; Mazzotta, F. Italian guidelines for the diagnosis and infectious disease management of osteomyelitis and prosthetic joint infections in adults. Infection 2009, 37, 478–496. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Cadeo, B.; Villa, G.; Sartor, A.; Cainero, V.; Causero, A. Current antibiotic management of prosthetic joint infections in Italy: The ‘Udine strategy’. J. Antimicrob. Chemother. 2014, 69 (Suppl. S1), i41–i45. [Google Scholar] [CrossRef]
- Triffault-Fillit, C.; Valour, F.; Guillo, R.; Tod, M.; Goutelle, S.; Lustig, S.; Fessy, M.H.; Chidiac, C.; Ferry, T.; Lyon, B.J.I. Prospective Cohort Study of the Tolerability of Prosthetic Joint Infection Empirical Antimicrobial Therapy. Antimicrob. Agents Chemother. 2018, 62, e00163-18. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Pereira, A.; Massada, M.; da Silva, M.V.; Lemos, R.; Costa e Castro, J. Empirical antibiotic therapy in prosthetic joint infections. Acta Orthop. Belg. 2010, 76, 254–259. Available online: http://www.actaorthopaedica.be/assets/1772/16-Sousa_et_al.pdf (accessed on 1 May 2024). [PubMed]
- Davis, J.S.; Metcalf, S.; Paterson, D.L.; Robinson, J.O.; Clarke, B.; Manning, L. Proposed empiric antibiotic therapy for prosthetic joint infections: An analysis of the Prosthetic Joint Infection in Australia and New Zealand, Observational (PIANO) cohort. Intern. Med. J. 2022, 52, 322–325. [Google Scholar] [CrossRef]
- Yu, Y.; Kong, Y.; Ye, J.; Wang, A.; Si, W. Microbiological pattern of prosthetic hip and knee infections: A high-volume, single-centre experience in China. J. Med. Microbiol. 2021, 70, 001305. [Google Scholar] [CrossRef]
- Lai, Y.H.; Xu, H.; Li, X.Y.; Zhao, W.X.; Lv, N.; Zhou, Z.K. Outcomes of culture-negative or -positive periprosthetic joint infections: A systematic review and meta-analysis. Jt. Dis. Relat. Surg. 2024, 35, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Korean Society for Chemotherapy; Korean Society of Infectious Diseases; Korean Orthopedic Association. Clinical guidelines for the antimicrobial treatment of bone and joint infections in Korea. Infect. Chemother. 2014, 46, 125–138. [Google Scholar] [CrossRef]
- Subbiah, V.B. Principles of Antibiotic Therapy in Orthopedic Surgery. J. Orthop. Jt. Surg. 2021, 3, 51–53. [Google Scholar] [CrossRef]
- Hiddema, J.S. Periprosthetic joint infection: A South African perspective. S. Afr. Med. J. 2023, 113, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pecora, J.R.; Lima, A.L.M.; Helito, C.P.; Gobbi, R.G.; Demange, M.K.; Camanho, G.L. Protocol for Treating Acute Infections in Cases of Total Knee Arthroplasty. Acta Ortop. Bras. 2019, 27, 27–30. [Google Scholar] [CrossRef]
- Scheper, H.; Gerritsen, L.M.; Pijls, B.G.; Van Asten, S.A.; Visser, L.G.; De Boer, M.G.J. Outcome of Debridement, Antibiotics, and Implant Retention for Staphylococcal Hip and Knee Prosthetic Joint Infections, Focused on Rifampicin Use: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2021, 8, ofab298. [Google Scholar] [CrossRef]
- Alves-Barroco, C.; Paquete-Ferreira, J.; Santos-Silva, T.; Fernandes, A.R. Singularities of Pyogenic Streptococcal Biofilms—From Formation to Health Implication. Front. Microbiol. 2020, 11, 584947. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Sante; Prothèse de Hanche ou de Genou: Diagnostic et Prise en Charge de L’infection Dans le Mois Suivant L’implantation, HAS 2014. Available online: https://www.has-sante.fr/jcms/c_1228574/fr/prothese-de-hanche-ou-de-genoudiagnostic-et-prise-en-charge-de-l-infection-dans-le-mois-suivant-l-implantation (accessed on 1 May 2024).
- Lora-Tamayo, J.; Senneville, É.; Ribera, A.; Bernard, L.; Dupon, M.; Zeller, V.; Li, H.K.; Arvieux, C.; Clauss, M.; Uçkay, I.; et al. The Not-So-Good Prognosis of Streptococcal Periprosthetic Joint Infection Managed by Implant Retention: The Results of a Large Multicenter Study. Clin. Infect. Dis. 2017, 64, 1742–1752. [Google Scholar] [CrossRef]
- Thompson, O.; Rasmussen, M.; Stefánsdóttir, A.; Christensson, B.; Åkesson, P. A population-based study on the treatment and outcome of enterococcal prosthetic joint infections. A consecutive series of 55 cases. J. Bone Jt. Infect. 2019, 4, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Veerman, K.; Goosen, J.; Spijkers, K.; Jager, N.; Heesterbeek, P.; Telgt, D. Prolonged use of linezolid in bone and joint infections: A retrospective analysis of adverse effects. J. Antimicrob Chemother. 2023, 78, 2660–2666. [Google Scholar] [CrossRef]
- Cerioli, M.; Batailler, C.; Conrad, A.; Roux, S.; Perpoint, T.; Becker, A.; Triffault-Fillit, C.; Lustig, S.; Fessy, M.H.; Laurent, F.; et al. Pseudomonas aeruginosa Implant-Associated Bone and Joint Infections: Experience in a Regional Reference Center in France. Front. Med. 2020, 7, 513242. [Google Scholar] [CrossRef]
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; del Toro, M.D.; Cobo, J.; Palomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-negative prosthetic joint infection: Outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin. Microbiol. Infect. 2014, 20, O911–O919. [Google Scholar] [CrossRef]
- Grossi, O.; Asseray, N.; Bourigault, C.; Corvec, S.; Valette, M.; Navas, D.; Happi-Djeukou, L.; Touchais, S.; Bémer, P.; Boutoille, D. Gram-negative prosthetic joint infections managed according to a multidisciplinary standardized approach: Risk factors for failure and outcome with and without fluoroquinolones. J. Antimicrob. Chemother. 2016, 71, 2593–2597. [Google Scholar] [CrossRef]
- Peel, T.N.; de Steiger, R. How to manage treatment failure in prosthetic joint infection. Clin. Microbiol. Infect. 2020, 26, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- SPILF. Recommandations Pour la Pratique Clinique. Infections Ostéo-Articulaires sur Matériel (Prothèse, Implant, Ostéosynthèse). 2009. Available online: www.infectiologie.com (accessed on 1 May 2024).
- Kusejko, K.; Auñón, Á.; Jost, B.; Natividad, B.; Strahm, C.; Thurnheer, C.; Pablo-Marcos, D.; Slama, D.; Scanferla, G.; Uckay, I.; et al. The Impact of Surgical Strategy and Rifampin on Treatment Outcome in Cutibacterium Periprosthetic Joint Infections. Clin. Infect. Dis. 2021, 72, e1064–e1073. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Osmon, D.; Tande, A.J.; Steckelberg, J.; Sierra, R.; Walker, R.; Berbari, E.F. Clinical and Microbiological Characteristics of Bacteroides Prosthetic Joint Infections. J. Bone Jt. Infect. 2017, 2, 122–126. [Google Scholar] [CrossRef]
- Manceau, L.; Bémer, P.; Decroo, J.; Jolivet-Gougeon, A.; Plouzeau, C.; Lartigue, M.F.; Bouard, L.; Chenouard, R.; Mazuet, C.; Leroy, A.G. Clostridial prosthetic joint infections: A series of 16 cases and literature review. Infect. Dis. Now 2023, 53, 104776. [Google Scholar] [CrossRef] [PubMed]




| Diagnostic Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Direct microbiological culturing |
|
| [45,46,47,48,49,50,51] |
| Culturing sonication fluid |
|
| [52,53,54,55,56,57,58] |
| DTT pre-treatment method |
|
| [59,60,61] |
| BioTimer Assay (BTA) method |
|
| [62,63] |
| Agar encasement culturing method (AECM) with Candle dip method |
|
| [64] |
| Specific PCR |
|
| [65,66,67,68,69] |
| Broad-range PCR |
|
| [70,71,72,73] |
| Next generation sequencing |
|
| [74,75,76] |
| Fluorescence in situ hybridization (FISH) |
|
| [77,78,79,80] |
| Visualization Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Scanning electron microscopy (SEM) |
|
| [81,82,83,84] |
| Confocal laser scanning microscopy (CLSM) |
|
| [85,86,87,88,89,90,91] |
| Methylene blue (MB) |
|
| [92,93,94,95,96,97,98,99] |
| Bacteria | Antibiotic | Penetration through the Biofilm Extracellular Matrix | Penetration into the Bone and Joint |
|---|---|---|---|
| S. aures | Amikacin | + | + |
| Cefotaxime | Reduced | ||
| Ciprofloaxacin | + | ||
| Oxacillin | Reduced | ||
| Vancomycin | +/Reduced | ||
| Linezolid | + | ||
| S. epidermidis | Amikacin | + | |
| Cefotiam | + | ||
| Cefotaxime | Reduced | ||
| Ciprofloaxacin | + | ||
| Daptomycin | + | ||
| Linezolid | + | ||
| Ofloxacin | + | ||
| Oxacillin | Reduced | ||
| Rifampicin | + | ||
| Vancomycin | Reduced | ||
| P. aeruginosa | Amikacin | Reduced | |
| Amoxicillin- clavulanic acid | + | ||
| Ciprofloaxacin | + | ||
| Gentamicin | Reduced | ||
| Imipenem | + | ||
| Levofloxacin | + | ||
| Piperacillin | +/Reduced | ||
| Fosfomycin | + | ||
| E.coli | Amoxicillin- clavulanic acid | + | |
| Ciprofloaxacin | + | ||
| Fosfomycin | + | ||
| References: | [110,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikziński, P.; Kraus, K.; Widelski, J.; Paluch, E. Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms 2024, 12, 1198. https://doi.org/10.3390/microorganisms12061198
Mikziński P, Kraus K, Widelski J, Paluch E. Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms. 2024; 12(6):1198. https://doi.org/10.3390/microorganisms12061198
Chicago/Turabian StyleMikziński, Paweł, Karolina Kraus, Jarosław Widelski, and Emil Paluch. 2024. "Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI)" Microorganisms 12, no. 6: 1198. https://doi.org/10.3390/microorganisms12061198
APA StyleMikziński, P., Kraus, K., Widelski, J., & Paluch, E. (2024). Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms, 12(6), 1198. https://doi.org/10.3390/microorganisms12061198







